Abstract
Arterial stiffness is associated with increased cardiovascular disease risk. Previous sex-based investigations of local and central stiffness report inconsistent findings and have not controlled for menstrual cycle phase in women. There is also evidence that sex hormones influence the vasculature, but their impact on arterial stiffness across a natural menstrual (NAT) or oral contraceptive pill (OCP) cycle has been understudied. This study sought to 1) examine potential sex differences in local and central stiffness, 2) compare stiffness profiles between NAT and OCP cycles, and 3) investigate the relationship between duration of OCP use and arterial stiffness. Sex hormone concentrations, β-stiffness index (local stiffness), and carotid-femoral pulse wave velocity [cfPWV (central stiffness)] were assessed in 53 healthy adults (22 ± 3 yr old, 20 men, 15 NAT women, and 18 OCP women). All participants were tested three times: men on the same day and time 1 wk apart, NAT women in menstrual, midfollicular and luteal phases of the menstrual cycle, and OCP women in placebo, early active and late active pill phases. β-Stiffness was higher in men than NAT and OCP women (P < 0.001), whereas cfPWV was similar between groups (P = 0.09). β-Stiffness and cfPWV did not differ across or between NAT and OCP cycles (P > 0.05 for both) and were not associated with duration of OCP use (β-stiffness: r = 0.003, P = 0.99; cfPWV: r = −0.26, P = 0.30). The apparent sex differences in local, but not central, stiffness highlight the importance of assessing both indexes in comparisons between men and women. Furthermore, fluctuating sex hormone levels do not appear to influence β-stiffness or cfPWV. Therefore, these stiffness indexes may need to be assessed during only one cycle phase in women in future investigations.
NEW & NOTEWORTHY We observed higher local, but not central, arterial stiffness in men than women. We also demonstrated that there are no differences in arterial stiffness between naturally cycling women and women who use monophasic oral contraceptive pills, and that the duration of oral contraceptive pill use does not influence arterial stiffness.
Keywords: β-stiffness index, carotid-femoral pulse wave velocity, menstrual cycle phase, oral contraceptive pill use, sex differences
INTRODUCTION
Arterial stiffness is an independent predictor of future cardiovascular disease (CVD) events (19, 26, 50, 57a) and can be assessed centrally, at the aorta, or locally, at the carotid artery (20). Stiffness of the aorta is of clinical interest, particularly because the main function of the aorta is to buffer the pressure of blood ejected from the left ventricle (18, 35); therefore, increased aortic stiffness may result in many cardiovascular and other pathophysiological complications (7, 20, 59). Measurement of local arterial stiffness at the carotid artery also provides important clinical information, as the carotid artery is a common site of atheroma formation and atherosclerosis (20). Additionally, measurements of aortic stiffness and carotid stiffness are not always associated with one another because of the differences in the structural composition of the segments of the arterial tree that are evaluated by these different methodologies (22, 35, 43, 51). Therefore, it is important to examine both central and local stiffness in research studies, as one measure is not representative of the stiffness of the entire arterial tree.
Biological sex has been shown to have an effect on arterial stiffness in some (8, 24), but not all (54), previous studies; however, these discrepancies seem to exist, in part, because of differences in the methods used to assess arterial stiffness. Studies that assessed central arterial stiffness have not reported a sex difference in young adults (54), whereas studies that assessed arterial stiffness locally at the carotid artery typically showed sex differences, with higher stiffness reported in men than women (8, 24). Higher arterial stiffness in men than women may be due to two critical sex hormones, estrogen and testosterone. Premenopausal women experience long-term cyclical elevations in estrogen (46, 48), whereas men do not, and estrogen is thought to provide direct beneficial arterial effects (27, 29, 33, 37). Higher circulating testosterone concentrations in men than women have the potential to increase stiffness indirectly through increases in sympathetic nervous system and renin-angiotensin system (RAS) activation (23). Unfortunately, none of the previous sex-based studies included simultaneous assessments of both local and central arterial stiffness and, furthermore, did not control for menstrual cycle phase in women.
Menstrual cycle phase may affect arterial stiffness because of the fluctuations in sex hormones throughout a cycle (14). These hormonal fluctuations have the potential to influence vascular structure and function through both nongenomic and genomic mechanisms that occur after estrogen binding to endothelial and smooth muscle estrogen receptors (estrogen receptor-α and -β) (6, 28, 37, 45). Nongenomic effects occur rapidly and cause smooth muscle cell relaxation (5, 29, 30), whereas genomic effects are longer-lasting and include reduction of arterial intimal thickening, reduction of smooth muscle cell proliferation, and increases in the elastin-to-collagen ratio in the arterial wall (4, 29, 30, 34). The evidence for an effect of menstrual cycle phase on arterial stiffness is inconclusive but also seems to be dependent on whether arterial stiffness is assessed centrally or locally. Studies that assessed central arterial stiffness across the menstrual cycle have not reported fluctuations in arterial stiffness (1, 38, 57, 58), while a study that assessed local arterial stiffness at the carotid artery reported higher stiffness in the follicular phase and at ovulation than during the menstrual and luteal phases (11). No study has comprehensively compared both local and central arterial stiffness between men and women throughout different menstrual cycle phases.
Oral contraceptive pills (OCPs) are in widespread use by women worldwide (3, 46); however, their effect on arterial stiffness is relatively unknown. No study has assessed the effect of OCP use on local arterial stiffness or compared arterial stiffness in women who use OCPs with arterial stiffness in age-matched men. Only two studies have investigated the effect of OCP use on central arterial stiffness: one study reported increased central arterial stiffness in OCP users compared with naturally cycling (NAT) women (13), and the other study reported no differences (58). The former study did not control for menstrual cycle phase, nor did it report when vascular measures were acquired in women who used OCPs (13). Furthermore, no previous study controlled for OCP formulation (i.e., monophasic vs. multiphasic), which could potentially alter the findings because of the variations in hormone concentrations with different timings of dosing. Additionally, previous investigations reported a positive (39), a negative (21, 49), or no association (12) between the duration of OCP use and other CVD risk factors; however, despite OCP use by many women for many years throughout young adulthood, the effect of duration of OCP use on central and local arterial stiffness is unknown.
The purpose of this study was to examine the effect of sex, menstrual cycle phase, and monophasic OCP use on local and central arterial stiffness in young adults. We hypothesized that 1) local arterial stiffness would be higher in men than women, but there would be no differences in central arterial stiffness between men and women, 2) local arterial stiffness would be lower during the follicular phase than the menstrual and luteal phases of the natural menstrual cycle and all phases of an OCP cycle but central arterial stiffness would not be different between or within a natural menstrual cycle or an OCP cycle, and 3) there would be no association between duration of OCP use and local or central arterial stiffness.
METHODS
Participants
Fifty-six young, healthy, recreationally active participants (20 men, 18 NAT women, and 18 OCP women) were recruited from McMaster University through poster advertisements. Endothelium-dependent and -independent dilation of the same participants was reported in a parallel manuscript (45a). All participants were between 18 and 32 yr of age during enrollment in the study. The women in the NAT group were included in the study if they experienced regular menstrual cycles and did not use any method of hormonal contraception (e.g., pill, patch, ring, or intrauterine device). The women in the OCP group were included in the study if they regularly used a combined, cyclical, low-dose monophasic OCP that was a second-, third-, or fourth-generation pill. All women who used this OCP type were included in the study regardless of duration of use, but duration of use was recorded before study enrollment. Women using a first-generation pill, a progestin-only pill, a combined multiphasic pill, a continuous pill, or any other hormonal contraceptives were excluded from the study. Additional exclusion criteria for all participants included active CVD, cerebrovascular disease, or any other diseases requiring long-term drug prescriptions, including statins and vasoactive drugs. The study was approved by the Hamilton Integrated Research Ethics Board and conformed with the Declaration of Helsinki.
Study Design
All study visits took place in the Vascular Dynamics Laboratory at McMaster University. Participants attended one familiarization visit, when they were screened for eligibility, provided written informed consent to participate in the study, and were familiarized with the vascular measures. Testing for men took place on three visits 1 wk apart scheduled at the same day and time of day (i.e., 3 consecutive Friday mornings); testing for women took place on three visits scheduled at the same time of day (morning) at specific times during their cycle. For the NAT group, visit 1 occurred during the menstrual phase (2–4 days after the onset of menstruation), visit 2 occurred during the midfollicular phase (9–13 days after the onset of menstruation), and visit 3 occurred during the luteal phase (20–32 days after the onset of menstruation and 7 ± 2 days after a positive ovulation). To confirm an ovulatory cycle and to standardize scheduling of the luteal phase visits, participants were required to test for ovulation using ovulation kits (BFP Ovulation Tests, Fairhaven Health, Bellingham, WA). For the OCP group, the three testing visits were scheduled to allow for direct comparison with the NAT group: visit 1 occurred 1–5 days after the onset of withdrawal bleeding (placebo pill or no pill), visit 2 occurred 6–12 days after the onset of withdrawal bleeding (“early” active hormonal pills), and visit 3 occurred 19–27 days after the onset of withdrawal bleeding (“late” active hormonal pills). All testing was conducted in a quiet, temperature-controlled room (22.4 ± 0.7°C, 21 ± 8% relative humidity) after a 10-h overnight fast, during which the participants abstained from food, alcohol, and caffeine; participants also refrained from strenuous physical activity for 24 h before testing. Anthropometric measures including height and weight were collected at visit 1. Hemodynamic and vascular measures were acquired at each visit.
Venous Blood Draw
A venous blood sample was collected at the beginning of all testing visits in women and randomized to one of the three testing visits in men. Blood was collected into two 4-ml serum blood collection tubes (BD Vacutainer Plus and Red BD Hemogard Closure, Becton Dickinson, Franklin Lakes, NJ). Tubes were set aside for ~45 min to allow sufficient time for the samples to clot. After coagulation, tubes were centrifuged (Sorvall Legend XTR, ThermoFisher Scientific, Waltham, MA) at 4,000 rpm at 4°C for 10 min. Serum aliquots were added to three polypropylene tubes (Falcon, Corning Science, Corning, NY) and frozen at −20°C. Frozen serum samples were transported to the Core Laboratory in the McMaster University Medical Centre for analysis of endogenous concentrations of estradiol, progesterone, and testosterone. Estradiol was analyzed using an Architect estradiol chemiluminescent microparticle immunoassay (Abbott Diagnostics, Abbott Park, IL, sensitivity < 92 pmol/l). This assay is unable to detect the synthetic estrogen in OCPs, as there is no cross-reactivity with ethinyl estradiol. Progesterone was analyzed using an Architect progesterone chemiluminescent microparticle immunoassay (Abbott Diagnostics, sensitivity < 0.3 nmol/l). Testosterone was analyzed using an Immulite 2000 chemiluminescent enzyme immunoassay (Siemens Healthcare Diagnostics, Tarrytown, NY, sensitivity < 0.7 nmol/l). Endogenous sex hormone concentrations are suppressed in women who use OCPs (44).
Hemodynamic Measures
After the blood draw, participants were asked to lie supine on the testing bed for 10 min before hemodynamic and vascular parameters were measured. Resting heart rate was continuously assessed using a single-lead electrocardiogram (AD Instruments, Colorado Springs, CO), a commercial data acquisition tool (PowerLab, AD Instruments), and compatible software (LabChart 7, AD Instruments). Resting brachial artery systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were acquired in the supine position using an automated oscillometric blood pressure device (Dinamap ProSeries, Batesville, IN) according to a standardized protocol (40). A minimum of three blood pressure measurements were obtained at each time point: the first measurement was discarded, and the second and third measurements were averaged. If the second and third SBP measurements were not within 5 mmHg, an additional measurement was collected, and all three measurements were averaged.
Vascular Measures
β-Stiffness index.
Local arterial stiffness of the common carotid artery was assessed as the β-stiffness index with use of simultaneous applanation tonometry and B-mode ultrasonography. To predict local blood pressure, 10 pulse pressure waveforms were collected at the left common carotid artery by means of a handheld tonometer (model SPT-301, Millar Instruments, Houston, TX) applied on the surface of the skin and calibrated to supine brachial artery blood pressure (Dinamap ProSeries). Maximum and minimum carotid artery lumen diameters (LDmax and LDmin, respectively) were assessed using B-mode ultrasound images of the right common carotid artery and a 12-MHz linear-array ultrasound probe attached to a high-resolution ultrasound machine (Vivid Q, GE Medical Systems, Horten, Norway) for 10 consecutive cardiac cycles at 22.5 frames/s. Ultrasound images were stored offline, and lumen diameters were analyzed using semiautomated boundary detection tracking software [Artery Measurement System (AMS), Gothenburg, Sweden]. The β-stiffness index was calculated as follows: β-stiffness index = ln(SBP/DBP)/[(LDmax − LDmin)/LDmin] (17).
Pulse wave velocity.
Central arterial stiffness of the aorta was determined by carotid-femoral pulse wave velocity (cfPWV), according to the most recent published guidelines (53). To assess cfPWV, pulse waves were acquired simultaneously at the left common carotid and femoral arteries by applanation tonometry (model SPT-301, Millar Instruments) and sampled using a commercial data acquisition tool (PowerLab equipped with LabChart 7, AD Instruments). Pulse wave signals were band-pass filtered at 5–30 Hz to detect the foot of each pulse waveform, which was used to calculate pulse transit time. The distance between the carotid and femoral artery measurement sites was determined using an anthropometric measuring tape over the surface of the body. cfPWV was calculated as follows: cfPWV (m/s) = [0.8 × distance]/pulse transit time, where distance is 80% of the distance between the carotid and femoral artery sites and pulse transit time is the foot-to-foot pulse transit time. We report the mean of two sets of 10 continuous cardiac cycles or the median of three sets if the difference between the first and second set was >0.5 m/s.
Repeatability of Vascular Measures
The repeatability of our vascular measures was assessed in all study participants. The repeatability in men was assessed across the three visits 1 wk apart, and the repeatability in NAT and OCP women was assessed in the three visits across a single cycle. Coefficients of variation (CVs) were used to identify repeatability of the β-stiffness index and cfPWV across the three visits in each group. For all groups, low CVs indicated good repeatability for the β-stiffness index (11% for men, 11% for NAT women, and 14% for OCP women) and cfPWV (6% for men, 9% for NAT women, and 5% for OCP women).
Statistical Analysis
Data were assessed for normality using the Kolmogorov-Smirnov test. Parametric statistics were used when data were normally distributed. Differences in age and body mass index between men, NAT women, and OCP women were assessed using one-way ANOVA. Differences in hemodynamic measures between men, NAT women, and OCP women were assessed using a 3 × 3 (group × visit) mixed-model ANOVA, whereas differences in vascular measures between men, NAT women, and OCP women were assessed using a 3 × 3 (group × visit) mixed-model analysis of covariance (ANCOVA) and ∆SBP from visit 1 to visit 3 as a covariate. Significant interactions or main effects were assessed using Tukey’s honestly significant difference post hoc test. In a subanalysis, differences in hemodynamic measures within the OCP group between second-generation (n = 10) and third/fourth (combined)-generation (n = 8) pills were assessed using a 2 × 3 (generation × visit) mixed-model ANOVA, whereas differences in vascular measures within the OCP group between second-generation and third/fourth (combined)-generation pills were assessed using 2 × 3 (generation × visit) mixed-model ANCOVA and ∆SBP from visit 1 to visit 3 as a covariate. Associations between duration of OCP use and local and central arterial stiffness were assessed using Pearson’s bivariate correlations.
Because sex hormone data were highly nonnormal, nonparametric statistics were used. Differences in sex hormone concentrations between men, NAT women on visit 1 (menstrual), and OCP women on visit 1 (placebo) were assessed using a Kruskal-Wallis test with significant effects followed by a Mann-Whitney U-test with Bonferroni’s correction (P < 0.017). To mimic a 2 × 3 (group × visit) mixed-model ANOVA, differences in sex hormone concentrations across a single cycle between NAT and OCP women were assessed using a Mann-Whitney U-test and Wilcoxon’s signed-rank test with Bonferroni’s corrections (P < 0.0056) for between- and within-group analyses, respectively.
Statistical Package for the Social Sciences (SPSS, version 20.0, Chicago, IL) was used for all analyses. Statistical significance was set at P < 0.05 unless otherwise indicated. Values are means ± SD for parametric statistics and medians ± interquartile ranges for nonparametric statistics.
RESULTS
Participants
Fifty-six participants (20 men, 18 NAT women, and 18 OCP women) were recruited for participation in the study; however, three participants in the NAT group were removed from all analyses because of anovulatory cycles (n = 2) and progesterone concentrations well above reference values in the follicular phase (n = 1). Therefore, 53 participants (20 men, 15 NAT women, and 18 OCP women) were included in all analyses. No group differences were observed in age or body mass index (P > 0.05 for both; Table 1).
Table 1.
Participant characteristics
| Women |
|||
|---|---|---|---|
| Measurement | Men | NAT group | OCP group |
| Age, yr | 21 ± 1 | 22 ± 3 | 22 ± 3 |
| Body mass index, kg/m2 | 24 ± 3 | 22 ± 2 | 23 ± 4 |
| Cycle length, days | 32 ± 4 | 28 ± 0 | |
| OCP duration, mo | 41 ± 34 | ||
| Generation of OCP, n | |||
| Second | 10 | ||
| Third | 3 | ||
| Fourth | 5 | ||
Values are means ± SD; n = 20 men, 15 women in the naturally cycling (NAT) group, and 18 women in the oral contraceptive pill (OCP) group. One-way ANOVA was used for age and body mass index. No differences in age or body mass index between groups were found (P > 0.05 for both).
Sex Hormone Concentrations
Estradiol concentrations were similar between men, women in the NAT menstrual phase, and women in the OCP placebo phase (P = 0.36), whereas progesterone concentrations were lower in men than women in the NAT menstrual phase (P = 0.008) but similar between men and women in the OCP placebo phase (P > 0.017; Table 2). Testosterone concentrations were higher in men than women in the NAT menstrual phase (P < 0.001) and OCP placebo phase (P < 0.001; Table 2).
Table 2.
Sex hormone concentrations
| Women |
|||||||
|---|---|---|---|---|---|---|---|
| NAT group |
OCP group |
||||||
| Men | Menstrual phase | Follicular phase | Luteal phase | Placebo phase | Early active phase | Late active phase | |
| Estradiol, pmol/l | 119 ± 40 | 126 ± 75 | 230 ± 207c,e | 649 ± 490c,d,e | 97 ± 45f | <92g | <92g |
| Progesterone, nmol/l | 0.8 ± 0.4a | 1.1 ± 0.5 | 1.0 ± 0.7 | 43.6 ± 38.3c,d,e | 0.8 ± 0.6 | 0.7 ± 1.0 | 0.7 ± 1.0 |
| Testosterone, nmol/l | 20.1 ± 9.7a,b | 1.0 ± 0.6 | 1.1 ± 0.8 | 0.9 ± 0.8 | 1.2 ± 0.8 | <0.7h | 0.7 ± 0.4 |
Values are medians ± interquartile range; n = 20 men, 15 women in the naturally cycling (NAT) group, and 15 women in the oral contraceptive pill (OCP) group (for the OCP group, n = 15 because of an inability to acquire a blood sample from 3 women at one or two visits). Comparison of men, NAT women during the menstrual phase, and OCP women during the placebo phase: Kruskal-Wallis test with significant effects followed by Mann-Whitney U-test with Bonferroni’s correction (P < 0.017):
vs. NAT menstrual phase and
vs. OCP placebo phase. Within- and between-group comparison of NAT and OCP: Wilcoxon signed-rank test and Mann-Whitney U-test, respectively, with Bonferroni’s corrections (P < 0.0056):
vs. menstrual phase,
vs. follicular phase,
vs. analogous OCP phase, and
vs. late active phase. Endogenous hormones are suppressed in OCP.
Architect estradiol assay has an analytic sensitivity of <92 pmol/l and has no cross-reactivity with synthetic estrogen.
Immulite 2000 testosterone assay has an analytic sensitivity of <0.7 nmol/l.
Estradiol concentrations were higher in women in the NAT follicular phase than in the OCP early active phase (P < 0.001) and higher in women in the NAT luteal phase than in the OCP late active phase (P < 0.001; Table 2). Progesterone concentrations were higher in women in the NAT luteal phase than OCP late active phase (P < 0.001; Table 2). No differences were observed in testosterone concentrations between NAT and OCP groups at any visit (P > 0.0056 for all comparisons; Table 2).
Across a NAT cycle, estradiol concentrations were lowest in the menstrual phase (P = 0.001 vs. the follicular phase and P = 0.001 vs. the luteal phase) and higher in the luteal phase than the follicular phase (P = 0.001). Progesterone concentrations were higher in the NAT group during the luteal phase than the menstrual (P < 0.001) and follicular (P < 0.001) phases (Table 2). Across an OCP cycle, endogenous estradiol concentrations were higher during the placebo phase than the late active phase (P = 0.005; Table 2). No differences in testosterone concentration were observed within the NAT and OCP groups (P > 0.0056; Table 2).
Hemodynamics
There were no interactions between group and visit for SBP, DBP, or MAP (P > 0.05 for all; Table 3). SBP was higher in men than NAT and OCP women (main effect of group: P < 0.001; P < 0.001 for men vs. NAT women, P < 0.001 for men vs. OCP women, P = 0.69 for NAT vs. OCP women), and MAP was higher in men than NAT and OCP women (main effect of group: P < 0.001; P < 0.001 for men vs. NAT women, P < 0.001 for men vs. OCP women, P = 0.35 for NAT vs. OCP women; Table 3). No group differences were observed in DBP (main effect of group: P = 0.41; Table 3). SBP was higher at visit 1 than visit 3 (main effect of visit: P = 0.01; Table 3). DBP and MAP were higher at visit 1 than visits 2 and 3 (main effect of visit; P < 0.01 for both; Table 3).
Table 3.
Arterial stiffness and hemodynamic characteristics
| Women |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men |
NAT group |
OCP group |
P Value |
|||||||||
| Visit 1 | Visit 2 | Visit 3 | Menstrual phase | Follicular phase | Luteal phase | Placebo phase | Early active phase | Late active phase | G ×V | Group | Visit | |
| SBP, mmHg | 122 ± 8 | 120 ± 6 | 119 ± 7 | 103 ± 9 | 101 ± 8 | 100 ± 10 | 105 ± 6 | 103 ± 7 | 102 ± 5 | 0.98 | <0.001 | 0.01 |
| DBP, mmHg | 67 ± 4 | 65 ± 4 | 66 ± 5 | 68 ± 5 | 65 ± 3 | 65 ± 4 | 68 ± 5 | 68 ± 5 | 67 ± 5 | 0.62 | 0.41 | <0.01 |
| MAP, mmHg | 89 ± 3 | 87 ± 3 | 87 ± 4 | 82 ± 5 | 80 ± 5 | 80 ± 5 | 83 ± 3 | 82 ± 4 | 82 ± 3 | 0.91 | <0.001 | <0.01 |
| β-Stiffness, AU | 4.77 ± 0.56 | 4.88 ± 0.83 | 4.59 ± 1.06 | 3.44 ± 0.82 | 3.42 ± 0.73 | 3.58 ± 0.76 | 3.65 ± 0.80 | 3.61 ± 0.89 | 3.68 ± 0.86 | 0.47 | <0.001 | 0.86 |
| cfPWV, m/s | 6.29 ± 0.52 | 6.25 ± 0.77 | 6.27 ± 0.46 | 6.01 ± 0.60 | 5.86 ± 0.60 | 5.91 ± 0.53 | 6.29 ± 0.54 | 6.14 ± 0.65 | 5.94 ± 0.53 | 0.52 | 0.09 | 0.41 |
Values are means ± SD; n = 20 men, 15 women in the naturally cycling (NAT) group, and 18 women in the oral contraceptive pill (OCP) group. 3 × 3 (group × visit) mixed-model ANOVA for hemodynamic measures or 3 × 3 (group × visit) mixed-model analysis of covariance with change in systolic blood pressure (SBP) from visit 1 to visit 3 as covariate for arterial stiffness measures was used. No significant group × visit interactions were found. There was a main effect of visit for SBP (visit 1 > visit 3), diastolic blood pressure (DBP; visit 1 > visit 2 and visit 3), and mean arterial pressure (MAP; visit 1 > visit 2 and visit 3). There was a main effect of group for SBP [men > NAT and OCP women], MAP (men > NAT and OCP women), and β-stiffness index (men > NAT and OCP women). AU, arbitrary units; cfPWV, cardiac-femoral pulse wave velocity.
Arterial Stiffness
As a result of the changes in blood pressure (see above), ∆SBP from visit 1 to visit 3 was used as a covariate in the statistical model for measurements of arterial stiffness. There were no interactions between group and visit for the β-stiffness index or cfPWV (P > 0.05 for both). The β-stiffness index was higher in men than NAT and OCP women (main effect of group: P < 0.001; P < 0.05 for men vs. NAT women, P < 0.05 for men vs. OCP women, P > 0.05 for NAT vs. OCP women). No group differences were observed in cfPWV (main effect of group: P = 0.09). There were no main effects of visit for the β-stiffness index or cfPWV (P > 0.05 for both). A visual representation of these arterial stiffness data is shown in Fig. 1, and all numeric data and P values for these data are shown in Table 3.
Fig. 1.
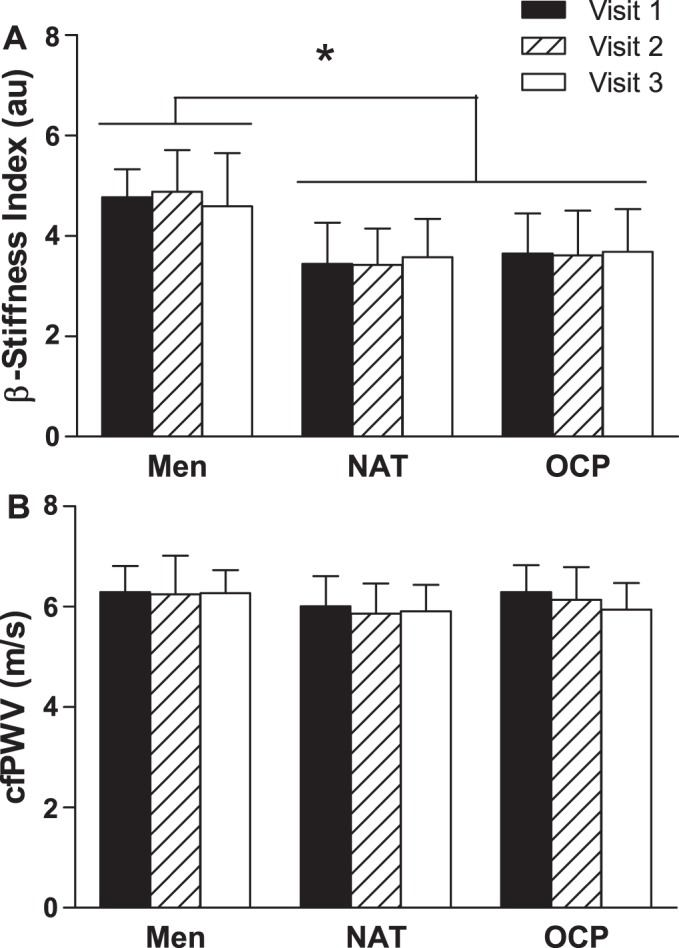
β-Stiffness index (A) and carotid-femoral pulse wave velocity (cfPWV; B) in men, women with natural menstrual cycles (NAT), and women who use oral contraceptive pills (OCPs). Visits 1, 2, and 3 were scheduled weekly in men and during different phases across a single cycle (menstrual, follicular, and luteal phases in NAT women; placebo, early active, and late active phases in OCP women) in women. au, Arbitrary units. Values are means ± SD. No interactions between group and visit or main effects of visit for β-stiffness index or cfPWV were found (P > 0.05 for all). There was a main effect of group for β-stiffness index [*P < 0.001 (men > NAT and OCP)]. There was no main effect of group for cfPWV (P = 0.09).
In a subanalysis comparing differences between second-generation and third/fourth (combined)-generation pills, there were no interactions between pill generation and visit, no main effects of pill generation, and no main effects of visit for SBP (interaction: P = 0.47; pill generation: P = 0.18; visit: P = 0.23), DBP (interaction: P = 0.85; pill generation: P = 0.31; visit: P = 0.21), MAP (interaction: P = 0.53; pill generation: P = 0.62; visit: P = 0.27), β-stiffness index (interaction: P = 0.88; pill generation: P = 0.29; visit: P = 0.67), or cfPWV (interaction: P = 0.70; pill generation: P = 0.98; visit: P = 0.06).
OCP Duration
The range of duration of OCP use within participants was 5–144 mo. No significant associations were observed between duration of OCP use and β-stiffness index (r = 0.003, P = 0.99) or between duration of OCP use and cfPWV (r = −0.261, P = 0.30; Fig. 2).
Fig. 2.
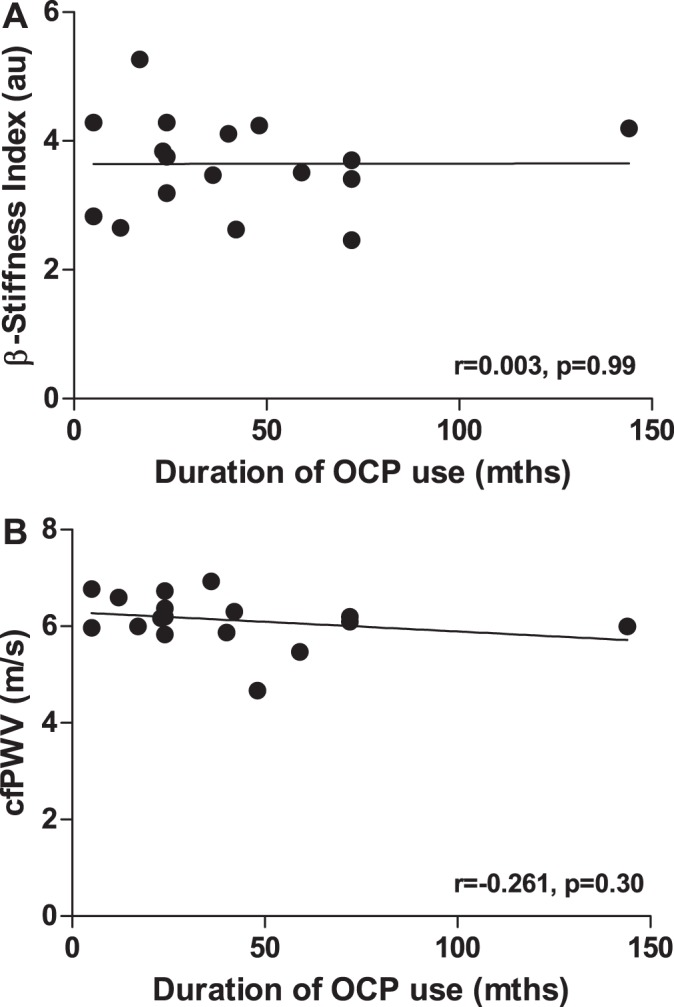
Associations between duration of oral contraceptive pill (OCP) use and β-stiffness index (A) and carotid-femoral pulse wave velocity (cfPWV; B). au, Arbitrary units. No significant correlations between duration of OCP use and local or central arterial stiffness were found (P > 0.05 for both).
DISCUSSION
In this study, we make three observations that substantially advance the knowledge of the impact of sex hormones on arterial stiffness. We found higher local arterial stiffness in men than women but no differences in central arterial stiffness while controlling for menstrual cycle phase and OCP cycle phase in women. We observed no differences in local or central arterial stiffness in NAT or OCP women between groups or across a cycle within a group. Moreover, we found no relationship between local or central arterial stiffness and duration of OCP use.
Sex Differences
In support of our hypothesis, we found higher local arterial stiffness, as measured by carotid artery β-stiffness index, in men than NAT and OCP women but no differences in central arterial stiffness as measured by cfPWV. Previous studies of local arterial stiffness have reported less stiff carotid arteries in naturally cycling women than age-matched men (8, 24), although this is not always the case (53a). However, no study to date has controlled for menstrual cycle phase. To address this important gap in the previous literature, we controlled for both menstrual cycle phase and OCP phase.
The known beneficial effects of the sex hormone estrogen on vascular structure and function (27, 29, 33, 37) are a likely explanation for lower local arterial stiffness in women than men. Despite the fluctuations in endogenous and synthetic estrogen across a natural menstrual cycle or an OCP cycle, over the long term, circulating estrogen concentrations are higher in women than in men (46, 48). As arterial structural changes do not occur rapidly and, likely, evolve over many months or years, it is probable that the higher concentrations of estrogen throughout the majority of a cycle each month in women might elicit long-term intrinsic changes in arterial wall properties that are not substantially impacted by a 1-wk reduction in circulating estrogen each month (52). It is plausible that nongenomic effects of estrogen would be altered by cyclical fluctuations of estrogen each month, but genomic effects would persist, as they are longer-lasting. Therefore, it is likely that, regardless of the timing of carotid artery stiffness assessment in women, arterial stiffness would still be higher in men.
The higher local arterial stiffness observed in men than in women in the present study could also be related to the sex difference in blood pressure (55). In agreement with the literature, we found higher SBP and MAP in men than in both groups of women. Testosterone is a likely explanation for this sexual dimorphism, as shown in a causal study in rats, in which the androgen receptor was blocked and blood pressure was reduced (42). Testosterone has been shown to increase both sympathetic nervous system activation and RAS activation, both of which can result in increased blood pressure (23). Specifically, testosterone has been shown to increase plasma renin activity, angiotensin II, oxidative stress, aldosterone, and sodium reabsorption (16, 32, 41). Additionally, estrogen has been shown to have a protective effect on blood pressure, as it exerts opposite effects on the RAS, including a decrease in expression of angiotensin-converting enzyme inhibitor and a decrease in angiotensin II, both of which can yield lower blood pressure (15, 36). Although the β-stiffness index is partly independent of blood pressure, it is likely that chronic elevations in blood pressure in men may alter the structure and vascular tone of the carotid artery, leading to higher local arterial stiffness.
NAT Versus OCP Cycle
To our knowledge, this is the first study to examine local arterial stiffness in women who use OCPs and to compare NAT women at multiple time points across a single cycle. A previous study assessed local arterial stiffness across a natural menstrual cycle and observed a lower β-stiffness index during ovulation than in the luteal phase (11). Contrary to the findings from that study and to our hypothesis, we did not observe fluctuations in carotid artery β-stiffness across the menstrual cycle. However, our findings are in agreement with another previous study that also did not observe changes in carotid artery stiffness across a menstrual cycle (56). We also did not observe differences in local arterial stiffness between NAT and OCP groups. These findings suggest that cyclical monthly fluctuations of sex hormones likely do not greatly influence arterial wall properties. The premise that arterial structural changes occur over a longer time frame coupled with the long-term genomic effects of estrogen is evidence to support our observations in this study (28, 29, 37, 52).
In support of our hypothesis, we also did not observe any differences in central arterial stiffness between NAT and OCP groups across a single cycle. This agrees with the study of Yu et al. (58), the only other study to compare cfPWV between NAT women across a menstrual cycle and women who use OCPs across a pill cycle, and several other studies that assessed cfPWV across the natural menstrual cycle (1, 38, 57). In a cross-sectional study, Hickson et al. (13) compared central arterial stiffness between women who use OCPs and women who do not at only one time point and found higher central arterial stiffness in women who use OCPs than in naturally cycling women, but they did not control for menstrual cycle phase or OCP phase. Additionally, the reported PWV is only 0.1 m/s higher in OCP women than in NAT women (13). A 1 m/s increase in PWV has been shown to be associated with a 7% increase in CVD risk (2). Therefore, a 0.1 m/s increase in PWV, as observed in the study by Hickson et al. between naturally cycling women and women who use OCPs, would correspond to only a 0.7% increase in CVD risk, which is likely not clinically meaningful, especially since both group values were below the identified reference values for risk (25).
Previous studies have observed a difference in blood pressure between NAT women and women who use OCPs, whereby blood pressure has been elevated in women who use OCPs (10, 13, 58); however, we did not observe a group difference in the present study. It has been shown that blood pressure is more likely to be elevated in women who use OCPs if they smoke, are obese, or are already hypertensive (47). Participants included in our study were nonsmokers, of healthy weight, and had normal blood pressure. Furthermore, newer generations of OCPs have been shown to have a more favorable effect on blood pressure, especially fourth-generation pills, which have even been shown to reduce blood pressure (46). All participants in the OCP group in our study were using second-, third-, or fourth-generation pills.
Duration of OCP Use
This was the first study to examine the association between duration of OCP use and both local and central arterial stiffness, and we observed no significant relationships. A previous study that investigated the relationship between duration of OCP use and alternate measures of arterial structure and function, including common carotid artery intima-media thickness and endothelial function using a flow-mediated dilation test, also did not observe any association (12). The mean duration of OCP use was 54 ± 27 mo (12). Among the participants included in our study, the mean duration of use was 41 ± 34 mo (range 5–144 mo); however, the duration of use was >72 mo for only one participant. Subsequent work with the inclusion of more participants with an extended duration of use is required to confirm our findings regarding duration of OCP use and its relationship with local and central arterial stiffness.
Strengths and Limitations
A major strength of this study was that we compared age-matched men, NAT women, and women who use OCPs across a single cycle and confirmed cycle phases in both groups of women by measuring sex hormone concentrations. We also recruited only women who were using monophasic OCPs for the OCP group and were able to control for the timing of hormone dosing. Additionally, we stratified women using different generations [second vs. third/fourth (combined)] of OCPs into different groups in a subanalysis and compared local and central arterial stiffness between these groups and found no differences in both stiffness indexes. However, we were unable to further stratify women into third- and fourth-generation pill groups and compare differences in arterial stiffness between the most recent generations of OCPs. Additionally, although we recruited recreationally active participants, we did not confirm the physical fitness of our participants by maximal O2 uptake test to ensure similar fitness levels between groups, nor did we document modes of exercise participation (i.e., aerobic or resistance) among our participants, and this could potentially cause differences in arterial stiffness between groups. We also did not control for the hydration status of our participants, and this could potentially impact arterial stiffness measurements.
Conclusions
In our study, we observed higher local arterial stiffness, as measured by the carotid artery β-stiffness index, in men than in NAT women and women who use OCPs but no differences in central arterial stiffness as measured by cfPWV. We also demonstrated that there are no differences in local or central arterial stiffness between women who use newer-generation monophasic OCPs and women who have natural menstrual cycles and that the length of OCP use does not influence local or central arterial stiffness. The findings from this study suggest that future investigations may need to assess β-stiffness and cfPWV during only one cycle phase in healthy premenopausal women, such as always during the menstrual or placebo phase in NAT women and women using OCPs, respectively. This has important implications for the experimental design of future research studies related to assessment of arterial stiffness using these indexes, as it makes it more feasible for the inclusion of women as study participants.
GRANTS
This work was supported by a Natural Sciences and Engineering Research Council Discovery Grant (to M. J. MacDonald).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.P., N.S., and M.J.M. conceived and designed research; S.E.P. and N.S. performed experiments; S.E.P. analyzed data; S.E.P. and M.J.M. interpreted results of experiments; S.E.P. prepared figures; S.E.P. and M.J.M. drafted manuscript; S.E.P., N.S., and M.J.M. edited and revised manuscript; S.E.P., N.S., and M.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Vanessa Rizzuto, Srikesh Rudrapatna, and Josie Jakubowski for assistance with data collection. We acknowledge members of the EMRG laboratory for help with blood collection.
REFERENCES
- 1.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 235: 111–118, 2010. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C-H, Cruickshank JK, Hwang S-J, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang K-L, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black A, Francoeur D, Rowe T, Collins J, Miller D, Brown T, David M, Dunn S, Fisher WA, Fleming N, Fortin CA, Guilbert E, Hanvey L, Lalonde A, Miller R, Morris M, O’Grady T, Pymar H, Smith T, Henneberg E; Society of Obstetricians and Gynaecologists of Canada . SOGC clinical practice guidelines: Canadian contraception consensus. J Obstet Gynaecol Can 26: 219–296, 2004. doi: 10.1016/S1701-2163(16)30260-2. [DOI] [PubMed] [Google Scholar]
- 4.Bourassa P-AK, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA 93: 10022–10027, 1996. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor-α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103: 401–406, 1999. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J 10: 615–624, 1996. doi: 10.1096/fasebj.10.5.8621060. [DOI] [PubMed] [Google Scholar]
- 7.Fleenor BS, Berrones AJ. Arterial Stiffness Implications and Interventions. New York: Springer, 2015. doi: 10.1007/978-3-319-24844-8. [DOI] [Google Scholar]
- 8.Hansen F, Mangell P, Sonesson B, Länne T. Diameter and compliance in the human common carotid artery—variations with age and sex. Ultrasound Med Biol 21: 1–9, 1995. doi: 10.1016/0301-5629(94)00090-5. [DOI] [PubMed] [Google Scholar]
- 10.Harvey RE, Hart EC, Charkoudian N, Curry TB, Carter JR, Fu Q, Minson CT, Joyner MJ, Barnes JN. Oral contraceptive use, muscle sympathetic nerve activity, and systemic hemodynamics in young women. Hypertension 66: 590–597, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi K, Miyachi M, Seno N, Takahashi K, Yamazaki K, Sugawara J, Yokoi T, Onodera S, Mesaki N. Variations in carotid arterial compliance during the menstrual cycle in young women. Exp Physiol 91: 465–472, 2006. doi: 10.1113/expphysiol.2005.032011. [DOI] [PubMed] [Google Scholar]
- 12.Heidarzadeh Z, Asadi B, Saadatnia M, Ghorbani A, Fatehi F. The effect of low-dose combined oral contraceptive pills on brachial artery endothelial function and common carotid artery intima-media thickness. J Stroke Cerebrovasc Dis 23: 675–680, 2014. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Hickson SS, Miles KL, McDonnell BJ, Yasmin, Cockcroft JR, Wilkinson IB, McEniery CM; ENIGMA Study Investigators . Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: the ENIGMA study. J Hypertens 29: 1155–1159, 2011. doi: 10.1097/HJH.0b013e328346a5af. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman BL, Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham G. Williams Gynecology (2nd ed.). Dallas: McGraw Hill, 2012. [Google Scholar]
- 15.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1–7) in 17β-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol 93: 648–657, 2008. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 16.Katz FH, Roper EF. Testosterone effect on renin system in rats. Proc Soc Exp Biol Med 155: 330–333, 1977. doi: 10.3181/00379727-155-39800. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res 21: 678–687, 1987. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- 18.Latham RD, Westerhof N, Sipkema P, Rubal BJ, Reuderink P, Murgo JP. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation 72: 1257–1269, 1985. doi: 10.1161/01.CIR.72.6.1257. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 21.Lidegaard Ø, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception 65: 187–196, 2002. doi: 10.1016/S0010-7824(01)00307-9. [DOI] [PubMed] [Google Scholar]
- 22.Lim J, Pearman M, Park W, Alkatan M, Tanaka H. Interrelationships among various measures of central artery stiffness. Am J Hypertens 29: 1024–1028, 2016. doi: 10.1093/ajh/hpw045. [DOI] [PubMed] [Google Scholar]
- 23.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 125: 311–318, 2013. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marlatt KL, Kelly AS, Steinberger J, Dengel DR. The influence of gender on carotid artery compliance and distensibility in children and adults. J Clin Ultrasound 41: 340–346, 2013. doi: 10.1002/jcu.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattace-Raso F, Hofman A, Verwoert GC, Wittemana JC, Wilkinson I, Cockroft J, McEniery C; Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J 31: 2338–2350, 2010. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease: the Rotterdam Study. Circulation 113: 657–663, 2006. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 27.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol 89, 12A: 12E–17E, 2002. doi: 10.1016/S0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- 28.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90, 1A: 3F–6F, 2002. doi: 10.1016/S0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall. Curr Opin Cardiol 9: 619–626, 1994. doi: 10.1097/00001573-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 32.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int 55: 278–285, 1999. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 33.do Nascimento GRA, Barros YV, Wells AK, Khalil RA. Research into specific modulators of vascular sex hormone receptors in the management of postmenopausal cardiovascular disease. Curr Hypertens Rev 5: 283–306, 2009. doi: 10.2174/157340209789587717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 46: 1129–1134, 2005. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 35.Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles (5th ed.). Oxford, UK: Oxford Univ. Press, 2005. [Google Scholar]
- 36.Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 97: 2197–2201, 1998. doi: 10.1161/01.CIR.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 37.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 38.Ounis-Skali N, Mitchell GF, Solomon CG, Solomon SD, Seely EW. Changes in central arterial pressure waveforms during the normal menstrual cycle. J Investig Med 54: 321–326, 2006. doi: 10.2310/6650.2006.05055. [DOI] [PubMed] [Google Scholar]
- 39.Park H, Kim K. Associations between oral contraceptive use and risks of hypertension and prehypertension in a cross-sectional study of Korean women. BMC Womens Health 13: 39, 2013. doi: 10.1186/1472-6874-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals. I. Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 41.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 34: 920–923, 1999. doi: 10.1161/01.HYP.34.4.920. [DOI] [PubMed] [Google Scholar]
- 43.Reymond P, Merenda F, Perren F, Rüfenacht D, Stergiopulos N. Validation of a one-dimensional model of the systemic arterial tree. Am J Physiol Heart Circ Physiol 297: H208–H222, 2009. doi: 10.1152/ajpheart.00037.2009. [DOI] [PubMed] [Google Scholar]
- 44.Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol 181: 1263–1269, 1999. doi: 10.1016/S0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- 45.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol 280: H1699–H1705, 2001. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- 45a.Shenouda N, Priest SE, Rizztuo VI, MacDonald MJ. Brachial artery endothelial function is stable across a menstrual and oral contraceptive pill cycle , but lower in premenopausal women than age-matched men. Am J Physiol Heart Circ Physiol In press. doi: 10.1152/ajpheart.00102.2018. [DOI] [PubMed] [Google Scholar]
- 46.Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 53: 221–231, 2009. doi: 10.1016/j.jacc.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sitruk-Ware R, Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract Res Clin Endocrinol Metab 27: 13–24, 2013. doi: 10.1016/j.beem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Stanczyk FZ, Archer DF, Bhavnani BR. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 87: 706–727, 2013. doi: 10.1016/j.contraception.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Suissa S, Blais L, Spitzer WO, Cusson J, Lewis M, Heinemann L. First-time use of newer oral contraceptives and the risk of venous thromboembolism. Contraception 56: 141–146, 1997. doi: 10.1016/S0010-7824(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 50.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study . Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka H. Various indices of arterial stiffness: are they closely related or distinctly different? Pulse (Basel) 5: 1–6, 2018. doi: 10.1159/000461594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thijssen DHJ, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond) 122: 311–322, 2012. doi: 10.1042/CS20110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FUS, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30: 445–448, 2012. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 53a.Van Merode T, Hick PJ, Hoeks AP, Smeets FA, Reneman RS. Differences in carotid artery wall properties between presumed-healthy men and women. Ultrasound Med Biol 14: 571–574, 1988. doi: 10.1016/0301-5629(88)90123-8. [DOI] [PubMed] [Google Scholar]
- 54.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens 26: 1411–1419, 2008. doi: 10.1097/HJH.0b013e3282ffac00. [DOI] [PubMed] [Google Scholar]
- 55.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 8: 978–986, 1995. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 56.Willekes C, Hoogland HJ, Keizer HA, Hoeks AP, Reneman RS. Female sex hormones do not influence arterial wall properties during the normal menstrual cycle. Clin Sci (Lond) 92: 487–491, 1997. doi: 10.1042/cs0920487. [DOI] [PubMed] [Google Scholar]
- 57.Williams MRI, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86: 5389–5395, 2001. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- 57a.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113: 664–670, 2006. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 58.Yu A, Giannone T, Scheffler P, Doonan RJ, Egiziano G, Gomez Y-H, Papaioannou TG, Daskalopoulou SS. The effect of oral contraceptive pills and the natural menstrual cYCLe on arterial stiffness and hemodynamICs (CYCLIC). J Hypertens 32: 100–107, 2014. doi: 10.1097/HJH.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 59.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]


