Abstract
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory condition that affects millions of people with high morbidity and health care costs. The precise etiology of IBD is unknown, but clear evidence suggests that intestinal inflammation is caused by an excessive immune response to mucosal antigens. Recent studies have shown that activation of the aryl hydrocarbon receptor (AhR) induces regulatory T cells (Tregs) and suppresses autoimmune diseases. In the current study, we investigated if a nontoxic ligand of AhR, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), can attenuate dextran sodium sulfate-induced colitis. Our studies demonstrated that in mice that received ITE treatment in vivo, colitis pathogenesis, including a decrease in body weight, was significantly reversed along with the systemic and intestinal inflammatory cytokines. ITE increased the expression of Tregs in spleen, mesenteric lymph nodes (MLNs), and colon lamina propria lymphocytes (cLPL) of mice with colitis when compared with controls. This induction of Tregs was reversed by AhR antagonist treatment in vitro. ITE treatment also increased dendritic cells (CD11c+) and decreased macrophages (F4/80+) from the spleen, MLNs, and cLPL in mice with colitis. ITE also reversed the systemic and intestinal frequency of CD4+ T cells during colitis and suppressed inflammatory cytokines including IFN-γ, TNF-α, IL-17, IL-6, and IL-1 as well as induced IL-10 levels. These findings suggest that ITE attenuates colitis through induction of Tregs and reduction in inflammatory CD4+ T cells and cytokines. Therefore, our work demonstrates that the nontoxic endogenous AhR ligand ITE may serve as a therapeutic modality to treat IBD.
NEW & NOTEWORTHY We report the novel finding that activation of the aryl hydrocarbon receptor with the nontoxic ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) induces regulatory T cells (Tregs) and suppresses inflammatory bowel disease (IBD). Our data suggest that ITE diminishes colitis pathology through induction of Tregs; reduces inflammatory cytokines, inflammation score, and macrophage frequency; and induces DCs resulting in amelioration of colitis. Therefore, nontoxic endogenous ITE promotes the induction of Tregs and may be useful for the treatment of IBD.
Keywords: Crohn’s disease, inflammatory bowel disease, Th1/Th17, ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC), a chronic disorder of the gastrointestinal tract characterized by remission and relapses. The symptoms of IBD include bodyweight loss, diarrhea, abdominal pain, and rectal bleeding (22). Although the exact pathogenesis of IBD remains unknown, studies suggest that dysregulation in the intestinal immune system associated with genetic and environmental factors are involved with this disease (6, 28). To this end, imprecise T-cell response has been accepted as a key player in IBD development. Recent studies suggest that classic T-cell subsets T helper (Th) 1- and 2-mediated immune responses are responsible for the extensive inflammation and tissue damage that manifests in IBD patients (7, 45). Correspondingly, various experimental models of colitis also show prominent infiltration of various cells, including T lymphocytes, macrophages, and neutrophils, into the colon (37, 38). It is well established that CD4+ regulatory T cells (Tregs) express forkhead box P3 (FOXP3), which plays an important role in the control of intestinal inflammation and is required for effective suppression of colitis (9). To this end, the naturally arising CD4+ CD25+ Tregs have been shown to prevent or even cure colitis in the T-cell transfer model (25, 32). Furthermore, mutations of FOXP3 in mice show uncontrolled T-cell proliferation and increased production of Th1 and Th2 cytokines, suggesting a critical role played by Tregs in immune system homeostasis (14).
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that is activated by small molecules provided by the diet and environmental pollutants that reside in the cytosol. AhR is well conserved throughout vertebrate evolution and is expressed in most cell types (19). AhR was first discovered and characterized as a transcription factor responsible for the activation of genes encoding a number of xenobiotic metabolizing enzymes that mediate the toxicity induced by 2,3,7,8-tetrachlorodibenzodioxin (TCDD). The physiological ligands of AhR have not been identified, and its biological activities have been mainly investigated by using high-affinity ligands. It has been shown that AhR can be activated by environmental contaminants such as TCDD, tryptophan derivatives, flavonoids, and biphenyls (4, 34). Interestingly, studies using AhR knockout mice (AhR−/−) have shown that AhR plays a role in cell proliferation, differentiation, migration, development, cellular function, and cancer (3, 10, 13). Several AhR ligands have received increasing attention, including 6-formylindolo-(3,2-b)-carbazole that enhances the generation of Th17 cells and increases the severity of experimental autoimmune encephalomyelitis (EAE) (8). Furthermore, Quintana et al. (30) confirmed these findings in another study but also demonstrated an opposing effect of TCDD, namely reduction in EAE, apparently because of reduction of Th17 generation along with a concomitant increase in the development of Tregs. The endogenous ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) is an AhR agonist that suppresses tumor growth in uppsala 87 malignant glioma and OVCAR-3 cell xenografts in mice. ITE is a solid yellow compound, and its molecular weight is 286.3. ITE has been shown to act on dendritic cells (DCs) and T cells to suppress EAE (31), inhibit transforming growth factor-β1-induced human myofibroblast differentiation (23), and suppress experimental autoimmune uveitis and T-cell-mediated immunity (27). Therefore, AhR provides a model-signaling pathway to investigate the mechanism through which the environment modulates the immune response in several diseases.
Recent studies suggest that both IBD incidence and prevalence have been increased worldwide in the last fifty years. Despite the increased demand for an improved IBD therapy, a curative therapy for this disease remains absent. Current treatments start with using drugs, including antibiotics to eliminate infections, aminosalicylates and corticosteroids to reduce inflammation, and immune-suppressing drugs against specific targets (anti-TNF-α, etc.). These treatments come with many side effects. Therefore, in the present study, we investigated using a nontoxic endogenous AhR ligand (ITE) to mediate effective suppression of experimental colitis and related immune responses. We found that ITE treatment effectively suppresses colitis symptoms by inducing Tregs as well as mediating cellular immune response possible through induction of DCs.
MATERIALS AND METHODS
Animals.
Female mice of the C57BL/6 background aged 6 to 8 wk were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed and maintained in isolator cages under normal light and dark cycles in conventional housing conditions to minimize animal pain and distress in the University of South Carolina School of Medicine animal facility (Columbia, SC). Experimental groups consisted of six mice and studies were repeated three times. At the end of the experiment, mice were euthanized by Isoflurane gas anesthesia using a two-compartment glass jar, putting mice in the upper chamber to avoid direct contact with anesthesia in the lower chamber. The University of South Carolina’s Institutional Animal Care and Use Committee approved all animal experimentation used in this study. Routine changing of cages, food, and water was done twice weekly by trained personnel.
Induction of colitis by multiple exposures to dextran sodium sulfate.
We have previously described our dextran sodium sulfate (DSS)-induced model of colitis (21, 35). Briefly, 6- to 8-wk-old C57BL/6 mice received either water ad libitum or water containing 1% DSS (molecular weight 36,000–50,000, MP Biomedicals, Solon, OH). DSS was given over three alternate cycles to elicit T-cell response. The first two cycles consisted of 1% DSS in drinking water for 7 days, followed by a 7-day interval of normal drinking water. The third cycle consisted of 2% DSS for 7 days followed by 7 days of normal drinking water (total 42 days). We noticed a significant impact of T cells by multiple exposure of DSS followed by water in this chemically induced model of colitis. The body weight of mice was recorded every two days after the initiation of DSS. Other symptoms of sickness, including diarrhea, stool consistency, and blood in fecal matter, were monitored during this time. At the end of the experimental period, blood was collected, and colon samples were washed with phosphate buffer saline (PBS), cut longitudinally, fixed in formalin, and embedded in paraffin.
ITE treatment.
ITE was purchased from Tocris Bioscience (Minneapolis, MN). The ITE was purified by proprietary chromatographic techniques and was greater than 98% pure. At the start of DSS induction, mice received 100 µl by intraperitoneal injection of vehicle and ITE (10 mg/kg body wt) twice a week on each Monday and Thursday until week 6 at the end point of the experiment. During a pilot study, we used several (5, 10, 20, 40, and 80 mg/kg body wt) doses of ITE and noticed that the 10-mg/kg dose was the lowest dose giving maximum protection. Therefore, we used this dose in our entire study. At the experimental end point blood was collected by tail-vein bleedings and serum was obtained following centrifugation. For comparison, a similar treatment was also given to normal BL/6 mice to see the effect of ITE alone.
Cell isolation.
At the experimental end point, spleens and mesenteric lymph nodes (MLNs) from individual mice from all groups were isolated to make a single-cell suspension. Red blood cells were lysed using lysis buffer (Sigma, St. Louis, MO) for the cells isolated from spleen. After centrifugation, single-cell suspensions of spleen and MLNs were passed through a sterile filter (Sigma to remove any debris. Subsequently, cell suspensions were washed twice in RPMI 1640 (Sigma) and stored in medium containing 5% fetal bovine serum (FBS) on ice or at 4°C until later use on the same day for flow cytometric staining. Cells from the colon lamina propria (cLP) were isolated as described previously (38). In brief, the small intestine/colon (proximal and distal, excluding the cecum) were cut into 1-cm strips and stirred in PBS containing 1 mM EDTA at 37°C for 30 min. The intestinal tissue was digested with collagenase type IV (Sigma) in RPMI 1640 (collagenase solution in complete media) for 45 min at 37°C with moderate stirring. After each 45-min interval, the released cells were centrifuged and stored in complete medium. Intestinal pieces were again treated at least twice with fresh collagenase solution, and cells were then pooled. cLP cells were further purified using a discontinuous Percoll gradient (Pharmacia, Uppsala, Sweden) collecting at the 40–75% interface. Lymphocytes were maintained in complete medium as previously described (35, 36).
Flow cytometry staining and analysis.
Cells from the spleen, MLNs, and cLP for each experimental group were isolated as described above. For three- to four-color cell-surface antigen staining, cells were preblocked with Fc receptors for 15 min at 4°C. The cells were washed with FACS staining buffer containing PBS with 2% FBS then stained with the manufacturer’s suggested concentration (0.2 to 0.5 mg/million cells) for FITC- or allophycocyaninl-conjugated anti-CD4 (GK1.5, Biolegend, San Diego, CA), phycoerythrin-conjugated CD8 (LY-2 53-6.7, BD-PharMingen, San Diego, CA), CD11b (M1/70, BD-PharMingen), FITC-conjugated F4/80 (BM8, Biolegend) PE-conjugated antimouse FOXP3 monoclonal antibody (Ab) (MF-14, Biolegend), FITC-conjugated IFN-γ (XMG-1.2), PE-conjugated anti-IL-17A monoclonal Ab (TC11-18H10.1) and PE-conjugated anti-mouse CD11c (HL3) (BD-PharMingen) for 30 min at 4°C with occasional shaking. The cells were washed two times with FACS staining buffer and thoroughly resuspended in BD Cytofix/Cytoperm (BD-PharMingen) solution for 20 min. The cells were again washed two times with BD perm/wash solution after storage for 10 min at 4°C. Intracellular staining for FOXP3 and analysis was done according to the Biolegend protocol. Cells were then washed thoroughly with FACS staining buffer and analyzed by flow cytometry (FC-500 by Beckman Coulter, Fort Collins, CO).
Systemic cytokine measurement by luminex analysis.
Levels of Th-cell-derived cytokines IL1-α, IL1-β, IL-6, IL-10, IL-17, TNF-α, and IFN-γ in the serum were determined using a luminex ELISA assay kit (Bio-Rad, Hercules, CA). In brief, all analyte beads described above contained in assay buffer were added to prewet vacuum wells followed by 50 µl of assay beads. The buffer was then removed, and the wells underwent a wash cycle. Standard or serum (50 µl) was next added to each well, and the plate was incubated for 1 h and subjected to continuous shaking (at setting No. 3) using a Lab-Line Instrument Titer Plate Shaker (Melrose, IL). The filter bottom plates were then washed and vortexed at 300 g for 30 s. Subsequently, 25 µl of antimouse detection Ab was added to each well and incubated for 30 min at room temperature. Streptavidin-phycoerythrin solution (50 µl) was added, and each plate was again incubated for 10 min at RT with continuous shaking. We then added 125 µl of assay buffer, measured with Bio-Rad readings using a Luminex System (Austin, TX), and calculated the concentration of cytokine (pg/ml) using Bio-Rad software. The Ab Bio-Rad Multiplex assays are capable of detecting >10 pg/ml for each analyte.
Colon anatomy and histology.
The colon was preserved using 10% buffer neutral formalin followed by 4% paraformaldehyde then embedded in paraffin. Fixed tissues were sectioned at 6 µm and stained with hematoxylin-eosin for microscopic examination. Intestinal sections were graded according to the number and severity of lesions as described below.
Quantifying inflammatory score.
The histological slides from intestinal tissues (colon) and livers (quality control) were examined and scored by two individuals in a blinded fashion. A score (0 to 8) was given based on previously established criteria (38). The summation of scores provided a total colonic disease score per mouse. Inflammation was graded by extent (focal, multifocal, diffuse, or extensive areas) and depth or penetration of inflammation (into the lamina propria, submucosa, and subserosa) then given a numerical value of 0–8 based on the following criteria: grade 0, no change observed from normal tissue; grades 1–2, 1 to few multifocal mononuclear cell infiltrates in the LP, minimal hyperplasia; grades 3–4, intestinal lesions involved with several multifocal, mild inflammatory cell infiltrates in the LP; grades 5–6, lesions with moderate inflammation and epithelial hyperplasia; grades 7–8, inflammation involves most of the intestinal sections. The summation of these scores provided a total colonic disease score that ranged from 0 to 8, with grade 4 lesions in proximal, middle, and distal colon segments.
In vitro T-cell proliferation and induction of FOXP3 expression.
T cells from MLNs from three naive mice as well as colons from experimental mice were sort-purified using a FACS-Aria (purity >97%, Becton Dickinson, Franklin Lakes, NJ). We used propidium iodide staining gating strategy to sort live T cells. Purified T cells were cultured either alone or cocultured in vitro (quadruplicate) with a 5-, 20-, or 40-μM dose of ITE as well as a 20-μM dose of AhR antagonist (CH-223191, Tocris Bioscience) and then stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml, BD-PharMingen) Abs at 37°C in 5% CO2. After 3 days of culture, the FOXP3 expression was measured by flow cytometry analysis. We also measured the expression of IL-17 and IFN-γ production by intracellular staining from ex vivo isolated cells from colons of mice that received ITE. Proliferation was measured by 5-Bromo-2′-deoxy uridine (BrdU) absorption and detection (Roche Diagnostics, Dusseldörf, Germany). In brief, after 72 h of culture, 10 μl of BrdU labeling solution (10 μM/ml final concentration per well) were added and incubated for 18 h at 37°C with 5% CO2. The cells were then fixed and incubated with 100 µl of nuclease in each well for 30 min at 37°C. Next, cells were pulsed with BrdU labeling solution, and incorporation was detected by ELISA assay (Roche Molecular Biochemical).
Immunocytochemical localization of T cells and FOXP3 in the colon.
Colon tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. Sections were rinsed in Tris-buffered saline (TBS pH 7.4) three times. The section was unmasked with 10 mM sodium citrate buffer (pH 6.0) at 95°C for 5 min and washed with deionized water three times. Sections were incubated in blocking serum (ABC staining kit, SC-2018, Santa Cruz Biotechnology, Dallas, TX) in TBS for 20 min at room temperature. Next, the sections were stained with mouse antihuman CD4 (L3T4, dilution 15 μg/ml) and FOXP3 (259D/C7, dilution 25 μg/ml) (BD-PharMingen), diluted in 1% FBS in PBS for 2 h at 4°C. Sections were rinsed in TBS and stained with biotinylated secondary goat antirabbit diluted in equal amounts of 1% staining solution for 30 min. After washing with PBS, sections were incubated for 30 min with AB enzyme reagent (SC-2018 kit from Santa Cruz Biotechnology). The sections were washed with PBS and incubated with three drops of peroxidase substrate solution. After counter-staining with hematoxylin, coverslips were mounted, and sections viewed under a light microscope. Control slides were performed as follows: 1) use normal rabbit serum or TBS to replace primary antiserum for both CD4 and FOXP3 and 2) use of TBS to replace biotinylated goat antirabbit solution. Nonspecific immunocytochemical reactions were minimal to none in these control stainings.
Statistics.
Differences in parameters were evaluated using factorial ANOVA, Student’s t-test, and XLStat software (Addinosoft, Brooklyn, NY). The results were analyzed using Statview II statistical program (Abacus Concepts, Berkeley, CA) for Macintosh computers. The changes in body weight, colon length, and inflammation score over time were measured longitudinally for the three groups, which depict the standard error of the sample mean (±SE). Comparisons using ANOVA were also performed with respect to all flow cytometry studies. Statistical significance was assessed at the 5% level of significance, when P < 0.05.
RESULTS
Changes in body weight, severity, and colon length after ITE treatment.
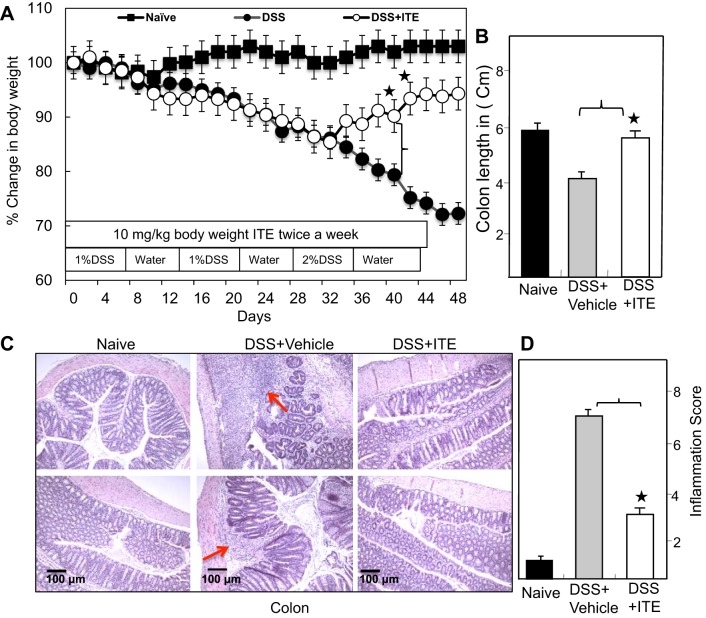
Previous studies have suggested that a reduction in body weight and colon length is the hallmark for monitoring the progression and severity of experimental colitis. Therefore, we examined the effect of ITE on changes in body weight and colon length in naive, ITE alone, DSS-exposed, and DSS-exposed mice treated with ITE. We observed no significant changes in body weight in naive and ITE alone mice in this study; therefore, from this point onward, statistical comparisons were made only between DSS-exposed vehicle-treated mice and mice given ITE treatment except for monitoring the frequency of DCs. In DSS-exposed mice, the development of colitis was shown by ~21% decreases in body weight, which continued to decline throughout the study (Fig. 1A). Furthermore, these mice also had occasional rectal bleeding and diarrhea. In contrast, mice that received ITE had decreases in body weight until the second DSS cycle but soon recovered the weight with little or no evidence of rectal bleeding. The effect of ITE was also assessed by measuring changes in colon length. The mean colon length of mice was significantly shorter in the DSS-exposed group than the colons of colitic mice treated with ITE (Fig. 1B). These results suggest that ITE treatment abrogates colitis-induced weight loss as assessed by recovered body weight in these mouse cohorts as well as having a protective effect in maintaining normal colon length and pathology.
Fig. 1.
Change in body weight and colon length of mice after DSS exposure. Normal BL/6 mice were given no treatment (■ naive). Others were given DSS alone (1%) in drinking water for 2 cycles of 7 days and (2%), DSS for third cycle (●), and DSS with ITE (○; 10 mg/kb body wt). After the first 7 days, DSS was replaced with a water cycle (ad libitum) for another 7 days. This was repeated for 2 more cycles of 7 days. The body weight of mice was recorded twice a week (A). The colon length was taken at the experimental end point (B). Histological sections of the colon from the groups of mice are presented. BL/6 mice receiving DSS alone (middle) showed lymphocyte infiltration and distortion of glands, whereas mice that received ITE (C, right) showed decreased lymphocyte infiltration. Naive BL/6 mice showed no cellular infiltration. The pathological changes included diffuse leukocyte infiltrates and thickening of the cLP in the area of distorted crypts in the colon. Two blind investigators unaware of this study evaluated the inflammation score (D). Representative sections from 3 separate experiments (×20 magnification) are shown. Changes in body weight were expressed as the percentage of baseline body weight. ANOVA was used to compare the change in body weight over time, colon length, and inflammation score among the groups. *P < 0.01 compared between DSS + vehicle with DSS + ITE. Data represent the mean of 3 repeated experiments involving 6 mice/group (n = 18). cLP, colon lamina propria; DSS, dextran sodium sulfate; ITE, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester.
Changes in colon pathology and inflammation score were examined in the three groups of mice at the experimental end point. As anticipated, the colons of naive mice exhibited normal epithelial layers at multiple sites with only limited inflammatory infiltrates composed mainly of lymphocytes. The colon histology of mice exposed to DSS showed extensive small to multifocal cellular infiltrates composed mainly of lymphocytes, neutrophils, and macrophages (Fig. 1C, middle). In contrast, ITE treatment reversed and/or reduced the observed increase in cellular infiltrates after DSS treatment (Fig. 1C, right). Similar changes in the inflammatory score of the colon in these groups also occurred (Fig. 1D). These results, together with reduced disease severity and inflammatory infiltrates, support a protective effect of ITE on experimental colitis.
ITE reduces systemic inflammatory cytokines.
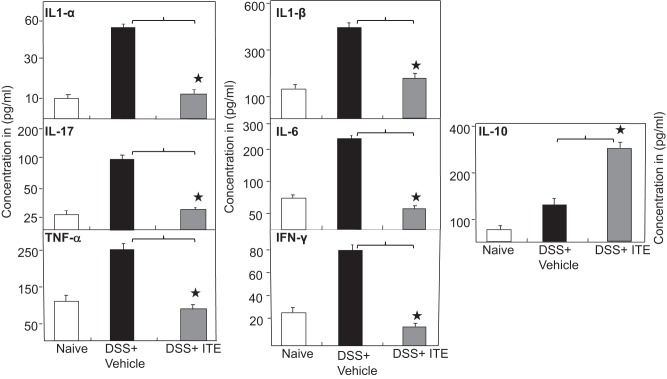
The overproduction of TNF-α, IL-1α, IL-1β, IL-6, and IL-17 in IBD and experimental colitis is well documented (15, 29). Therefore, we next determined whether ITE affects the systemic cytokine concentration that is characteristic of IBD. Our results show that serum IL-6, TNF-α, IFN-γ, IL-1α, IL-1β, and IL-17 levels increased after DSS exposure in wild-type mice as compared with naive mice (Fig. 2). Interestingly, we observed a decrease in serum levels for these cytokines after ITE treatment as compared with DSS-exposed mice (Fig. 2). In contrast, we noticed a significant increase in IL-10 levels after ITE treatment as compared with groups that received DSS alone (Fig. 2). These results indicate that ITE treatment leads to a reduction in systemic inflammatory cytokine levels and an increase in IL-10 levels in an experimental model of colitis and may explain, at least in part, the improved clinical outcomes observed in these mice.
Fig. 2.
Systemic levels of IL 1-α, IL-1-β, IL-6, IL-10, TNF-α, IL-17, and IFN-γ in mice after ITE treatment. After euthanasia, serum levels of IL 1-α, IL-1-β, IL-6, IL-10, TNF-α, IL-17, and IFN-γ were determined by Bio-Rad ELISA multiplex kit, which is capable of detecting >15 pg/ml of these analytes. The data presented are the mean concentrations of IL 1-α, IL-1-β, IL-6, -IL-10, TNF-α, IL-17, and IFN-γ ± SE from 3 separate repeated experiments that include 6 mice each (n = 18). The statistical significance between cytokine levels for various groups was assessed by ANOVA. Statistically significant differences (*P < 0.01) between DSS + vehicle and DSS + ITE groups. DSS, dextran sodium sulfate; ITE, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester.
ITE treatment mediates T-cell responses in DSS induced mice.
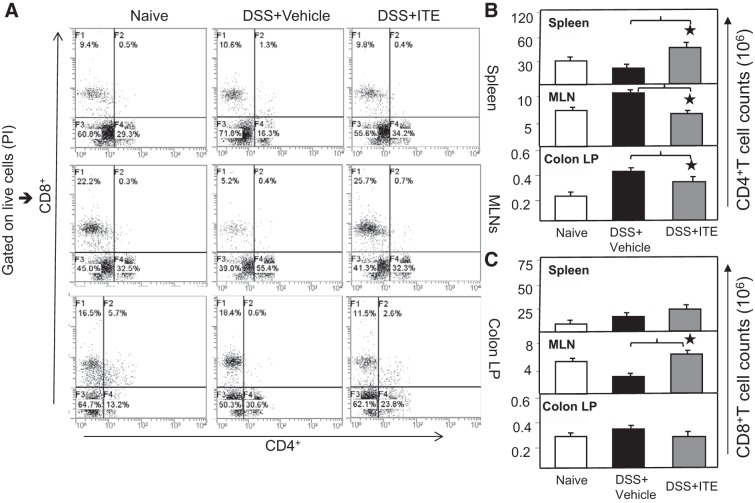
Given the fact that changes in T-cell responses and that Th1 activity are hallmarks of colitis progression, we examined these mice for the frequency and number of T-cell subpopulations in the spleen, MLNs, and colon lamina propria (cLP). After DSS exposure, the frequency of CD4+ T cells increased in the MLNs and cLP of mice as compared with naive mice. In contrast, these cells decreased in the spleen as compared with naive mice (Fig. 3A). Conversely, upon DSS exposure, the percentage of CD8+ T cells declined in MLNs as compared with naive mice, whereas this effect was not evident in the spleen and cLP CD8+ T cells. Splenic CD4+ T cells recovered and decreases in MLNs and cLP CD4+ T cells occurred with ITE treatment similar to that observed in naive mice.
Fig. 3.
Changes in T-cell responses after DSS exposure. Spleen cells, MLNs, and LP lymphocytes were isolated from 3 groups of 6 mice each and stained for CD4 and CD8 T-cell markers, analyzed by flow cytometry. The numbers in the bottom right quadrant indicate the total percentage of CD4 T cells and top left quadrant indicates CD8 T cells (A). The changes in the number of CD4 and CD8 T cells are shown (B and C). Representative data from three independent repeated experiment involving six mice per group (n = 18) are shown. The statistical significance between flow cytometry data for various groups was assessed by ANOVA. *Statistically significant differences between DSS + vehicle and DSS + ITE groups; *P < 0.01. Representative data of 1 of at least 3 experiments that produced similar results are shown. DSS, dextran sodium sulfate; ITE, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester; LP, lamina propria.
We next examined the numbers of CD4+ and CD8+ T cells in these mice after ITE treatment. As shown, the number of CD4+ T cells was recovered in the ITE treated group as compared with DSS-exposed vehicle-treated mice (Fig. 3B). In contrast, CD8+T cells significantly increased in MLNs of ITE treated mice as compared with DSS-induced vehicle-treated mice (Fig. 3C). These findings clearly indicate that DSS-induced colitis leads to a considerable increase in the Th1 response, and the percentages of Th lymphocytes in the MLNs and cLP, but decreases the frequency of CD4+ T cells in the spleens. However, the DSS-induced increase in CD4+ T lymphocytes in the MLNs and cLP, as well as the decrease in the spleen, was reversed by ITE treatment.
ITE alters DCs and macrophage responses.
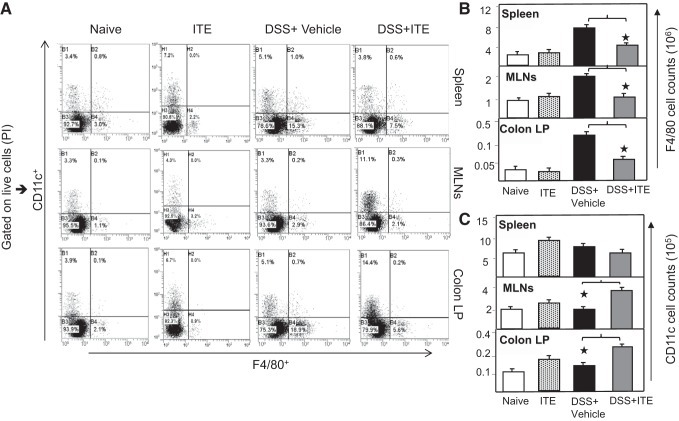
DCs control the activation and polarization of T cells (40), and it has been shown that DCs express functional AhR receptors (18). Based on this, we next examined the changes in numbers and frequency of DCs (CD11c) and macrophages (F4/80) in mice after DSS-induced colitis treated with ITE. ITE treated mice, as compared with naive and DSS-exposed mice, showed an increased frequency of DCs in MLNs and cLP (Fig. 4A). We also noticed a modest increase in CD11c population in naive mice that received ITE alone as compared with naive control mice (Fig. 4A). Furthermore, we observed a decreased frequency of F4/80 in the spleen, MLNs, and cLP of ITE-treated mice as compared with DSS-induced mice. This corresponds with similar decreases in the number of F4/80 in the spleen, MLNs, and cLP (Fig. 4B). In contrast, we noticed an increase in the number of CD11c cells in MLNs and cLP (Fig. 4C) after ITE treatment compared with vehicle. These findings indicate that ITE-treated mice experience an induction in their DC populations after colitis induction by DSS, suggesting a mechanism that possibly contributes to the observed changes in the T-cell population in these mice.
Fig. 4.
ITE treatment differentially mediates macrophages and dendritic cells (DCs). Spleen, MLNs, and cLP lymphocytes were isolated from all 4 groups of 6 mice each in 3-repeated experiments (n = 18) at the experimental end point. In this experiment, we added naive-treated with ITE alone without any exposure to determine the independent effect of ITE without colitis. Changes in the frequency and expression of macrophages (F4/80) and DCs (CD11c) from spleens, MLNs, and cLP are shown (A). Changes in the number of macrophages (F4/80) and DCs (CD11c) are shown (B and C, respectively). The statistical significance in flow cytometry data among 4 groups was assessed by ANOVA. *Statistically significant differences between DSS + vehicle and DSS + ITE groups; i.e.; *P < 0.01. Representative data of one of at least 3 experiments that produced similar results are shown. cLP, colon lamina propria; DSS, dextran sodium sulfate; ITE, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester; MLN, mesenteric lymph node.
ITE induced various immune cell populations during experimental colitis.
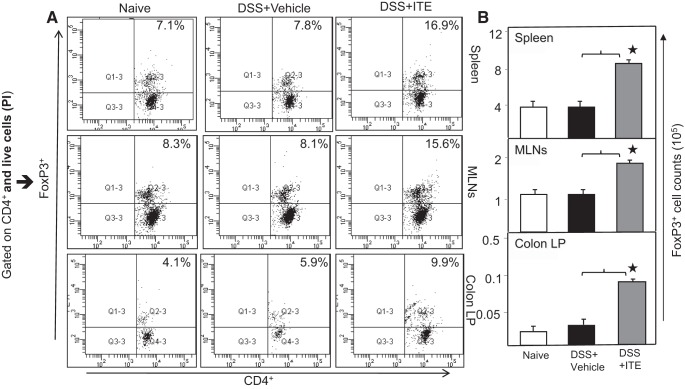
Tregs play an important role in the control of intestinal inflammation (9), and naturally arising Tregs cure colitis in a T-cell transfer model (25). Based on this, we next determined whether ITE treatment influences Treg frequency and numbers during multiple DSS-exposure-induced colitis. We determined the frequency of Tregs at the experimental end point in the spleen, MLNs, and cLP by flow cytometry. Tregs (gated on CD4+ T cells) in the spleen, MLNs, and cLP after DSS-induced colitis were similar to naive mice (Fig. 5A). The frequency and numbers of Tregs in the spleen, MLNs and cLP were significantly increased after ITE treatment as compared with vehicle as well as in naive mice (Fig. 5B). These data clearly indicate that the endogenous AhR ligand, ITE, induces Tregs that may be in part to suppress intestinal inflammation.
Fig. 5.
ITE induces the number and frequency of Tregs. Spleen, MLNs, and LP lymphocytes were isolated from all 3 groups of 6 mice each at the experimental end point. Changes in the frequency and expression of FOXP3-expressing CD4 T cells from spleen lymphocyte, MLNs, and cLP are shown (left). The numbers in the top right quadrant indicate the total percentage of Tregs (A). Changes in the number of CD4 T cells expressing FOXP3 are shown (right) (B). Experiments involved 6 mice per group (n = 18). The statistical significance between frequencies of Tregs for each group was assessed by ANOVA. *Statistically significant differences between DSS vehicle and DSS + ITE groups; i.e.; *P < 0.01. Representative data of one of at least 3 experiments that produced similar results are shown. cLP, colon lamina propria; DSS, dextran sodium sulfate; FOXP3, forkhead box 3; ITE, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester; MLN, mesenteric lymph node; Tregs, regulatory T cells.
ITE induces Tregs through AhR interactions.
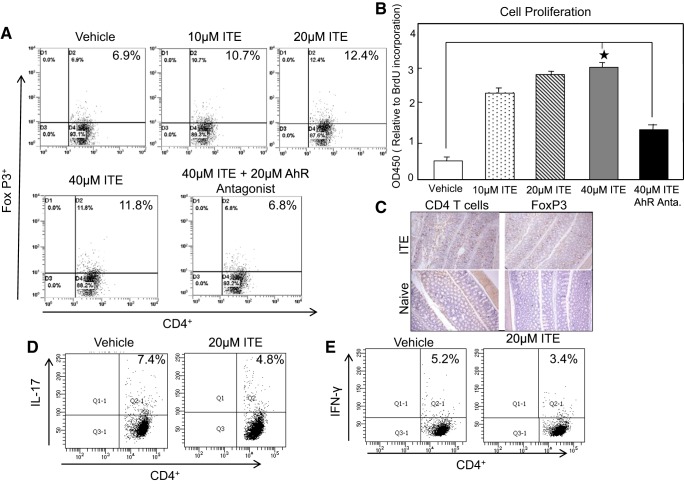
TCDD is a valuable tool used to study the immunological effects of AhR activation. TCDD is not a natural AhR ligand and is toxic in nature, which rules out its clinical therapeutic use. Therefore, we examined whether induction of Tregs by natural nontoxic ITE is mediated through AhR interactions. We tested this by in vitro analysis using purified T cells from MLNs. T cells at a density of 106 cells/ml in complete medium were activated using CD3 and CD28 monoclonal antibody in the presence or absence of ITE (10–40 μM) (448906-42-1, Tocris Bioscience) as well as AhR antagonist (CH-223191, Tocris Bioscience) for 48 h. The proliferation is reduced after the AhR antagonist as compared with vehicle treatment. The results indicate that ITE enhances Tregs frequency as compared with vehicle alone (Fig. 6A). We found that ITE caused a significant increase in T-cell proliferation as compared with stimulated or vehicle control (Fig. 6B). Interestingly, the increase of Tregs by ITE declined after using AhR antagonist as compared with vehicle control. We next examined whether ITE affects IL-17 and IFN-γ production in cLP ex vivo isolated cells treated with ITE. We noticed that both IL-17 and IFN-γ levels decreased after ITE treatment (Fig. 6, D and E). Furthermore, we also confirmed the expression of CD4 T cells and FOXP3 in the colon after ITE treatment (Fig. 6C). These data clearly suggest that ITE induces Tregs in the colon through the interaction with AhR.
Fig. 6.
ITE treatment induces T-cells proliferation and FOXP3 expression in vitro. Lymphocytes obtained from mesenteric lymph nodes (MLNs) and cLP were further sort-purified by using a FACS-Aria (Becton Dickinson) (purity >97%) from 3 normal BL/6 mice (n = 9 in quadruplicate each time) and were cultured with anti-CD3/CD28 Abs activation in the presence of either vehicle or ITE (0, 10, 20, or 40 μm). Some cultures were also received ITE + 20 and 40 μm of AhR antagonist. The induction of in vitro CD4 + FOXP3+ (A), ex vivo IL-17 (D), and IFN-γ (E) expression was measured by flow cytometry analysis. The in vivo induction of CDT cells and FOXP3 expression was also confirmed by immunocytochemistry in the colon (C). B, top right, shows BrdU cell proliferation assay using lymphocytes as described above after 72 h of culture. Right data presented are the mean optical density (OD)450 for proliferation ± SE of quadruplicate cultures. The statistical significance between various concentration of ITE and its antagonist group was assessed using Student’s t-test. The statistical significance between in vitro flow cytometry data was assessed by ANOVA. Cell proliferation data were analyzed by using Student’s t-test. Data represent the mean of 3 independent experiments. *Statistically significant difference (*P < 0.01) between vehicle and ITE- and ITE antagonist-treated groups. Representative data of one of at least 3 experiments (quadruplicate) (n = 12) that produced similar results are shown. Abs, antibodies; AhR, aryl hydrocarbon receptor; BrdU, 5-bromo-2′-deoxy uridine; cLP, colon lamina propria; DSS, dextran sodium sulfate; FOXP3, forkhead box 3; ITE, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester.
DISCUSSION
In the present study, we investigated the protective role of the AhR ligand, ITE, in a chemically induced experimental model of colitis. We found that nontoxic endogenous AhR ligand ITE, a potentially unique compound, promotes the induction of regulatory T cells (Tregs) and DCs to ameliorate experimental colitis. ITE reduced in vivo colitis pathogenesis, reversed the decrease in body weight, reduced systemic and mucosal cytokines, increased DCs, and decreased in macrophages from systemic and intestinal sites. Furthermore, ITE reversed the systemic and intestinal frequency of activated T cells during colitis.
Recent and previous studies clearly suggest that CD4+ T cells play a major role in the induction of colitis. CD8+ T cells also play a role in autoimmunity and the activation and function of these cells in colitis is an under-studied area of research. Furthermore, it has been shown that intestinal damage associated with colitis is primarily due to a consequence of T-cell-mediated injury (12). To this end, results from our laboratory and others support that adoptive transfer of Th1-biased CD4+ T cells results in induction of colitis (39). In the present study, upon multiple DSS exposures, we noticed a significant decrease in CD4+ T cells in the spleen as compared with naive and ITE-treated mice. In contrast, we observed a significant induction of CD4+ T cells in the MLNs and cLP in the DSS-exposed mice as compared with naive mice. Interestingly, we noticed a reduction of these cells after ITE treatment both in MLNs and cLP. We also noticed an increased number of CD8+ T cells in MLNs of mice that received ITE as compared with DSS alone. It has been shown that AhR signaling also has significant effects on CD8+ T cells, and activation by TCDD results in indirect suppression of primary CD8+ T response to influenza virus through modulation of DC function (44). Our findings suggest that multiple DSS exposures lead to systemic reduction of CD4+ T cells but induction in MLNs (inductive site) as well as the colon (effector site) that results in elevation of colitis; however, ITE treatment brings a reduction in the differentiation of these cells at the MLNs as well as the colon that leads to protection from advanced development of experimental colitis. A more in-depth study is needed for any conclusion on the role of CD8+ T cells in the DSS-induced model of colitis.
It has been well established that in colitis, intestinal macrophage populations play an important role in disease progression as compared with normal mucosa (24, 41). In this study, we noticed a significant increase of macrophages in both spleen and cLP in DSS-exposed mice as compared with naive mice. The results are in agreement with our study and other studies using the DSS-induced model of colitis, which showed increased macrophages during colitis (33, 35). ITE treatment significantly decreases both number and percentage of macrophage in systemic and intestinal organs as compared with DSS exposure alone.
It is well known that mucosal DCs can take up antigens from the gut and present them to T cells (5). It has been shown that activation of DCs is crucial for colitis development and reportedly enhanced in the colons of patients with CD (42). In this study, we observed an increase in the frequency of DCs in the spleen and cLP, whereas we did not notice any difference in MLNs of DSS-exposed mice compared with naive mice. Furthermore, we noticed an increase in DCs in MLNs and cLP in the group of mice that received ITE as compared with DSS and naive mice alone. These results suggest a differential induction of DCs in DSS-exposed mice that received ITE as compared with naive mice. Dendritic cells have a central role in the control of T-cell response and polarization of T cells in regulating immune tolerance (40). Furthermore, ITE induces the tolerogenic DCs to support FOXP3 and suppress EAE (31). It has been shown that ITE treatment increased the ability of DCs to expand FOXP3+ Treg cells and IL-10 Tr1 cells and suppress EAE (31). Our data support a function of ITE in reducing macrophages, which are related to protection against colitis, whereas induction of DCs suggests an indirect effect on T cells. Therefore, the suppression of colitis by ITE administration likely involves multiple AhR-dependent mechanisms, such as suppression of effector T-cell function by Tregs, inducing IL-10 cytokine, reducing macrophages, and modulating DC function. However, a more detailed study on the precise mechanism by which ITE mediates these effects on DCs is necessary for a prudent conclusion.
It has been well established that systemic levels of many cytokines and chemokines like TNF-α and IL-6 increase in several models of colitis and in patients with CD (1, 38). Furthermore, IL-12 drives Th1 differentiation and IFN-γ production. Now, evidence supports that these factors play a central role in the progression of colitis (11, 26, 43). Here, we demonstrate decreased secretion of TNF-α, IL-6, IL 1-α, IL 1-β, IL-17, and IFN-γ levels and increase in IL-10 levels in the serum of DSS-exposed mice that received ITE, thus indicating the involvement of ITE in decreasing Th1 responses and reducing IFN-γ and IL-17 levels.
It is well established that Tregs play a crucial role in maintaining immune homeostasis and limiting autoimmune responses by modulating cells of both the innate and adaptive immune systems. Furthermore, there is evidence that mice simply lacking Tregs leads to IBD and genetic mutations in FOXP3, or absent Tregs have severe intestinal inflammation associated with lymphocytic infiltration in the intestinal mucosa (2). AhR has significant effects in the control of adaptive immunity modulating T-cell differentiation and function through its effects on antigen-presenting cells. It has been shown that activation of AhR by high affinity ligand TCDD in vivo results in expansion of functional Tregs and suppresses EAE (30). In the present study, we saw that ITE, an endogenous AhR ligand, induces Treg number and frequency in both systemic and mucosal organs as compared with naive and DSS alone exposed mice. To confirm whether activation of AhR plays a role, we used an AhR ligand antagonist and noticed a significant reduction of Tregs in vitro as compared with ITE alone. To this end, it has been shown that ITE efficiently suppresses uveitis as well as T-cell-mediated immunity (27). Furthermore, it has been shown that ITE administration ameliorated colitis in humanized mice by increasing CD39 and IL-10-secreting human Tregs in a trinitrobenzene sulfonic acid-induced model of colitis (16). The model we used presents a low dose with multiple exposures of DSS to induce T-cell response, suggesting it is suitable to study T-cell-mediated effects. The protecting role of AhR agonist in the control of functional FOXP3 has also been shown in diabetes (20). This study clearly demonstrates that endogenous AhR ligand acts on T cells and induces the number and frequency of Tregs that suppress experimental colitis.
In summary, we have demonstrated that the endogenous AhR ligand ITE protects from DSS-induced colitis development. It is likely that ITE mediates this protection through multiple pathways, including by reducing activated and effector T cells, a Th1-biased phenotype, inducing Tregs’ number and frequency, inducing frequency of DCs, and reducing systemic cytokine production, including IL-17, presumably as a consequence of the downregulation of macrophages (both frequency and number). These findings are in agreement with other studies on regulation of immune response by AhR (17). These combined effects are likely to be responsible for the reduced severity of colitis in mice that received ITE. In conclusion, a nontoxic endogenous AhR ligand ITE is a potential new compound for the treatment of autoimmune disease and colitis. However, a more detailed study is needed before drawing any firm conclusions regarding how induction of DCs facilitates the expansion of functional Tregs to mediate experimental colitis.
GRANTS
This work was supported in part by National Institutes of Health grants R-56-DK-087836 (to U. Singh) and P01-AT-003961 (to M. Nagarkatti and P. Nagarkatti).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.P.S. and U.P.S. conceived and designed research; J.D.A. performed experiments; N.P.S., M.K.M., and U.P.S. analyzed data; N.P.S., M.K.M., and M.N. interpreted results of experiments; J.D.A. and U.P.S. prepared figures; U.P.S. drafted manuscript; N.P.S., M.K.M., R.L.P., M.N., and P.S.N. edited and revised manuscript.
REFERENCES
- 1.Autenrieth IB, Bucheler N, Bohn E, Heinze G, Horak I. Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation. Gut 41: 793–800, 1997. doi: 10.1136/gut.41.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 116: 1713–1722, 2006. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett 581: 3608–3615, 2007. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ; NIDDK IBD Genetics Consortium; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium . Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 40: 955–962, 2008. doi: 10.1038/ng.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanas E, Davey GM, Carbone FR, Heath WR. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J Immunol 164: 2890–2896, 2000. doi: 10.4049/jimmunol.164.6.2890. [DOI] [PubMed] [Google Scholar]
- 6.Braegger CP, MacDonald TT. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy 72: 135–141, 1994. [PubMed] [Google Scholar]
- 7.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 58: 1152–1167, 2009. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 8.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164: 1079–1106, 2011. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev 204: 184–194, 2005. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 10.Corchero J, Martín-Partido G, Dallas SL, Fernández-Salguero PM. Liver portal fibrosis in dioxin receptor-null mice that overexpress the latent transforming growth factor-beta-binding protein-1. Int J Exp Pathol 85: 295–302, 2004. doi: 10.1111/j.0959-9673.2004.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrhardt RO, Lúdvíksson BR, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol 158: 566–573, 1997. [PubMed] [Google Scholar]
- 12.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol 157: 2174–2185, 1996. [PubMed] [Google Scholar]
- 13.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268: 722–726, 1995. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336, 2003. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52: 65–70, 2003. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goettel JA, Gandhi R, Kenison JE, Yeste A, Murugaiyan G, Sambanthamoorthy S, Griffith AE, Patel B, Shouval DS, Weiner HL, Snapper SB, Quintana FJ. AHR activation is protective against colitis driven by T cells in humanized mice. Cell Reports 17: 1318–1329, 2016. doi: 10.1016/j.celrep.2016.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48: 19–33, 2018. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschläger M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood 112: 1214–1222, 2008. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 19.Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys 450: 67–77, 2006. doi: 10.1016/j.abb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Kerkvliet NI, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, Pham D, Mourich DV. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy 1: 539–547, 2009. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotakadi VS, Jin Y, Hofseth AB, Ying L, Cui X, Volate S, Chumanevich A, Wood PA, Price RL, McNeal A, Singh UP, Singh NP, Nagarkatti M, Nagarkatti PS, Matesic LE, Auclair K, Wargovich MJ, Hofseth LJ. Ginkgo biloba extract EGb 761 has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis. Carcinogenesis 29: 1799–1806, 2008. doi: 10.1093/carcin/bgn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kugathasan S, Judd RH, Hoffmann RG, Heikenen J, Telega G, Khan F, Weisdorf-Schindele S, San Pablo W JR, Perrault J, Park R, Yaffe M, Brown C, Rivera-Bennett MT, Halabi I, Martinez A, Blank E, Werlin SL, Rudolph CD, Binion DG; Wisconsin Pediatric Inflammatory Bowel Disease Alliance . Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr 143: 525–531, 2003. doi: 10.1067/S0022-3476(03)00444-X. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann GM, Xi X, Kulkarni AA, Olsen KC, Pollock SJ, Baglole CJ, Gupta S, Casey AE, Huxlin KR, Sime PJ, Feldon SE, Phipps RP. The aryl hydrocarbon receptor ligand ITE inhibits TGFβ1-induced human myofibroblast differentiation. Am J Pathol 178: 1556–1567, 2011. doi: 10.1016/j.ajpath.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mee AS, Jewell DP. Monocytes in inflammatory bowel disease: monocyte and serum lysosomal enzyme activity. Clin Sci (Lond) 58: 295–300, 1980. doi: 10.1042/cs0580295. [DOI] [PubMed] [Google Scholar]
- 25.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170: 3939–3943, 2003. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 26.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 182: 1281–1290, 1995. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugent LF, Shi G, Vistica BP, Ogbeifun O, Hinshaw SJ, Gery I. ITE, a novel endogenous nontoxic aryl hydrocarbon receptor ligand, efficiently suppresses EAU and T-cell-mediated immunity. Invest Ophthalmol Vis Sci 54: 7463–7469, 2013. doi: 10.1167/iovs.12-11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podolsky DK. Inflammatory bowel disease (1). N Engl J Med 325: 928–937, 1991. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 29.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1: 553–562, 1994. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 30.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71, 2008. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 31.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 107: 20768–20773, 2010. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192: 295–302, 2000. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omata M. Cutting edge: The IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol 179: 2681–2685, 2007. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 34.Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor-kappaB motifs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated regulation of Fas and Fas ligand expression. Mol Pharmacol 71: 145–157, 2007. doi: 10.1124/mol.106.028365. [DOI] [PubMed] [Google Scholar]
- 35.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-κΒ activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther 332: 829–839, 2010. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain Behav Immun 26: 72–82, 2012. doi: 10.1016/j.bbi.2011.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW JR. CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(−/−) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res 28: 31–43, 2008. doi: 10.1089/jir.2007.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh UP, Singh S, Taub DD, Lillard JW JR. Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol 171: 1401–1406, 2003. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 39.Singh UP, Singh S, Iqbal N, Weaver CT, McGhee JR, Lillard JW JR. IFN-gamma-inducible chemokines enhance adaptive immunity and colitis. J Interferon Cytokine Res 23: 591–600, 2003. doi: 10.1089/107999003322485099. [DOI] [PubMed] [Google Scholar]
- 40.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 449: 419–426, 2007. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 41.Strober W, James SP. The immunologic basis of inflammatory bowel disease. J Clin Immunol 6: 415–432, 1986. doi: 10.1007/BF00915248. [DOI] [PubMed] [Google Scholar]
- 42.te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12-IL-18- dendritic cell populations in the colonic mucosa of patients with Crohn’s disease. Eur J Immunol 33: 143–151, 2003. doi: 10.1002/immu.200390017. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84: 4008–4027, 1994. [PubMed] [Google Scholar]
- 44.Vorderstrasse BA, Cundiff JA, Lawrence BP. A dose-response study of the effects of prenatal and lactational exposure to TCDD on the immune response to influenza a virus. J Toxicol Environ Health A 69: 445–463, 2006. doi: 10.1080/15287390500246985. [DOI] [PubMed] [Google Scholar]
- 45.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434, 2007. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]