Abstract
Lactobacillus reuteri DSM 17938 (LR 17938) has been shown to reduce the incidence and severity of necrotizing enterocolitis (NEC). It is unclear if preventing NEC by LR 17938 is mediated by Toll-like receptor 2 (TLR2), which is known to mediate proinflammatory responses to bacterial cell wall components. NEC was induced in newborn TLR2−/− or wild-type (WT) mice by the combination of gavage-feeding cow milk-based formula and exposure to hypoxia and cold stress. Treatment groups were administered formula supplemented with LR 17938 or placebo (deMan-Rogosa-Sharpe media). We observed that LR 17938 significantly reduced the incidence of NEC and reduced the percentage of activated effector CD4+T cells, while increasing Foxp3+ regulatory T cells in the intestinal mucosa of WT mice with NEC, but not in TLR2−/− mice. Dendritic cell (DC) activation by LR 17938 was mediated by TLR2. The percentage of tolerogenic DC in the intestine of WT mice was increased by LR 17938 treatment during NEC, a finding not observed in TLR2−/− mice. Furthermore, gut levels of proinflammatory cytokines IL-1β and IFN-γ were decreased after treatment with LR 17938 in WT mice but not in TLR2−/− mice. In conclusion, the combined in vivo and in vitro findings suggest that TLR2 receptors are involved in DC recognition and DC-priming of T cells to protect against NEC after oral administration of LR 17938. Our studies further clarify a major mechanism of probiotic LR 17938 action in preventing NEC by showing that neonatal immune modulation of LR 17938 is mediated by a mechanism requiring TLR2.
NEW & NOTEWORTHY Lactobacillus reuteri DSM 17938 (LR 17938) has been shown to protect against necrotizing enterocolitis (NEC) in neonates and in neonatal animal models. The role of Toll-like receptor 2 (TLR2) as a sensor for gram-positive probiotics, activating downstream anti-inflammatory responses is unclear. Our current studies examined TLR2 −/− mice subjected to experimental NEC and demonstrated that the anti-inflammatory effects of LR 17938 are mediated via a mechanism requiring TLR2.
Keywords: Inflammation, intestinal mucosa, lactobacillus, necrotizing enterocolitis, Toll-like receptor
INTRODUCTION
Necrotizing enterocolitis (NEC) is a devastating disease that results in severe inflammation and necrosis of the bowel walls in premature infants (36). It is one of the leading causes of morbidity and mortality in neonatal intensive care units.
The immature intestinal mucosa is prone to exaggerated responses to proinflammatory stimuli, increasing the risk of inflammatory pathology in the neonatal intestine (35) because relative to the mature intestine, the immature neonatal intestine contains fewer total T cells and innate inflammatory genes, such as nuclear transcription factor-κB (NF-κB), myeloid differentiation primary response 88, Toll-like receptor (TLR) 4, and TNF receptor-associated factors , are overexpressed. At the same time, negative feedback regulatory genes are underexpressed, resulting in the neonatal mucosa being overly sensitive to bacterial infection and food allergy (1, 35).
The pathogenesis of NEC is related to microbial translocation, leading to systemic sepsis and NEC, linked to an overexpression of TLR4 (23). TLR4, expressed on intestinal epithelial cells and lymphocytes, recognizes gram-negative bacteria components such as lipopolysaccharide and activates the inflammatory NF-kB pathway. In addition, TLR 4, when overexpressed on intestinal epithelial cells, attracts inflammatory T helper (TH)17-lymphocytes, which release IL-17, leading to a profound increase of enterocyte apoptosis, a breakdown of the intestinal barrier, microbial translocation, and systemic infection (20). Proliferation of pathogenic microbes continually activates TLR4, which leads to severe NEC.
Evidence of unregulated inflammation in NEC includes increased levels of a wide range of proinflammatory cytokines such as TNF-α, platelet-activating factor, and IL-1β, IL-6, IL-8, IL-12, and IL-18 in plasma and intestinal tissues (seen both in animals and human babies with NEC) (9, 29, 30, 36). In addition, the ratio of regulatory T (Treg) to effector T (Teff) cells, the latter including TH1 and TH17 cells, was found to be significantly decreased in premature infants with NEC (49). The adoptive transfer of Foxp3+Treg cells attenuated experimental NEC (19), indicating a critical role of Tregs in maintaining intestinal homeostasis.
Probiotic supplementation has correlated significantly with a decreased risk of developing NEC in preterm very low birth weight infants (13, 40, 47). Lactobacillus reuteri DSM 17938 (LR 17938) was derived from L. reuteri ATCC 55730, a strain originally cultured from a Peruvian mother’s breast milk, by removal of two plasmids harboring antibiotic resistance genes (39). This gram-positive strain inhibits pathogen growth, modulates the immune system, and has been proven to have strong anti-inflammatory effects when administered to newborn rats and mice, reducing the incidence and severity of NEC (27–29). LR 17938 has been shown to increase the frequency of Tregs in the intestinal mucosa and mesenteric lymph nodes (27, 29). It has been reported recently that LR 17938 may have beneficial effects in preventing NEC in human infants (5, 37).
We postulated that LR 17938 might provide protection via its recognition by a ligand-specific TLR. TLRs, the first line of defense against mucosal pathogens via induction of inflammation, can also provide protective signals that allow the intestine to tolerate commensal organisms that benefit the host. Unlike TLR4, TLR2 recognizes cell wall components of gram-positive bacteria. Previous studies indicated that TLR2 signals to enhance barrier function in the intestine; conversely, mutations in the TLR2 gene appear to predispose to severe inflammatory bowel disease (11). There is evidence for TLR2-mediated benefit in the mouse IL-10−/− model of ulcerative colitis (32). LR 17938 is a gram-positive bacterium, leading us to question whether the mechanism of LR 17938 by its interactions with TLR2 could reduce inflammation during experimental NEC. In our current study, we examined the effects of TLR2 deficiency on the protective effect of LR 17938 during the height of NEC in a well-established neonatal mouse model.
MATERIALS AND METHODS
Experimental NEC model.
The mouse model we used, which is associated with gut lesions and histology resembling human NEC, includes formula feeding of infant pups with exposure to cold stress and hypoxia, as described from our previous NEC studies (29). Five-day-old C57BL/6J [wild-type (WT)] and B6.129-TLR2tm1kir/J (TLR2−/−) mice pups born from breeding pairs of adult male and female animals (Jackson) were separated from their dams, housed in an incubator, and starved for 12 h before initiation of orogastric feeding with 0.1–0.2 ml of formula using a sterile Solomon 22-gauge 35-mm feeding needle (Instech) 4 times daily for 4 days. To induce NEC, mouse pups were exposed to 5% O2-95% N2 for 10 min in a hypoxia chamber (Billups-Rothenberg, San Diego, CA) and then were subjected to cold stress at 4°C for 5 min 3 times daily for 4 days. The formula consisted of 15 g Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 ml Esbilac canine milk replacement (Pet Ag, Hampshire, IL) amounting to 1.86 kcal/ml. Mice were observed on each day, and pups were euthanized on day 5 after NEC induction for tissue collection. In some cases, pups in the experimental NEC group were euthanized on day 3 or 4 if they were in pain, demonstrated labored respirations, exhibited abdominal distension, or had gastrointestinal bleeding. After euthanization, tissues were collected for histological analysis. Animal studies were approved by the Animal Welfare Committee of The University of Texas Health Science Center at Houston (HSC-AWC-14-056).
Experimental groups.
Mouse pups were randomly divided into three groups for both WT and TLR2−/− mice: dam-fed controls (WT: n = 15; TLR2−/−: n = 23), NEC-induced (WT: n = 23; TLR2−/−: n = 12), and NEC-induced + LR 17938-fed (WT: n = 21; TLR2−/−: n = 12), creating six groups total for comparison. Dam-fed mice were left with their mother to breastfeed, while mice in NEC groups were separated from their mothers, housed in an incubator, fed formula daily by gavage, and exposed to stress. Mice in LR 17938-treated groups were treated as described for the NEC group; however, the formula contained LR 17938, 106 colony-forming units (cfu)·g body wt−1·day−1·mouse−1.
Tissue harvest and NEC evaluation.
An incision into the abdomen was performed to evaluate and collect gastrointestinal tissues. Typical signs of NEC were abdominal distension, bowel necrosis, dilated loops of bowels, or intestinal hemorrhage in the wall of the small intestines. The terminal 5 cm of the small intestine (ileum) of each mouse was collected, and 1 cm of each were fixed with formalin and processed by the Cellular and Molecular Morphology Core Laboratory (Texas Medical Center Digestive Diseases Center, Houston, TX). Sections of ileal tissues were cut longitudinally and stained with hematoxylin and eosin for histological evaluation. Half of the remaining small intestines were used for isolation of lymphocytes, while the other was frozen for processing tissue lysates to examine cytokines. The severity of disease was determined by histological NEC scoring according to methods previously described (29); scores ranged from 0 (normal) to 3 (necrosis of the entire villus). Animals with histological scores ≥ 2 were defined as having NEC.
Preparation of probiotic LR 17938.
LR 17938 was provided by BioGaia (Stockholm, Sweden). LR 17938 was anaerobically cultured in deMan-Rogosa-Sharpe (MRS) medium (Difco, Detroit, MI) at 37°C for 24 h, plated in MRS agar in specific serial dilutions, and grown on agar plates anaerobically at 37°C for 48–72 h. Quantitative analysis of bacteria grown in culture medium was measured as previously described by a standard curve of bacterial cfu per milliliter grown on MRS agar (29). The amount of LR 17938 for daily feeding was calculated as 106 cfu·g body wt−1·day−1.
Preparation of single-cell suspension for flow cytometry.
The ileum and mesenteric lymph nodes were gently fragmented and digested in RPMI 1640 complete media supplemented with collagenase V (0.1 mg/ml) from Clostridium histolyticum (Sigma-Aldrich) at 37°C for 30 min. After the samples were vortexed for 1 min, single-cell suspensions were obtained by filtration through 70 μm cell strainers (BD Biosciences, San Jose, CA) for cell surface and intracellular staining using specific antibodies for flow cytometric analysis.
In vitro experiments for stimulating dendritic cells by LR 17938.
Dendritic cells (DCs) were isolated from WT and TLR2−/− mouse spleens using a negative selective Pan-Dendritic Cell Enrichment Kit (Stemcell Technologies) after careful tissue homogenization and digestion with collagenase-supplemented media. The cells were then seeded onto a 96-well plate (5 × 104 cells/well) in the complete RPMI 1640 media containing L-glutamine, 10% FBS, sodium pyruvate, nonessential amino acids, penicillin/streptomycin, and 50 uM beta-mercaptoethanol and treated with or without LR 17938 (5 cfu to 1 DC) for 16 h. Cells were harvested, stained with DC activation markers (CD80 and CD86), and analyzed by flow cytometry.
Flow cytometric analysis.
Cells were stained with surface and intracellular fluorochrome-conjugated antibodies for mice. For T-cell analysis, we used antibodies such as surface glycoprotein CD4 (GK1.5) conjugated with peridinin-chlorophyll proteins (PerCP/Cy5.5), surface glycoprotein CD44 (IM7) conjugated with fluorescein isothiocyanate (FITC), and intracellular transcription factor FOXP3 (FJK-16a) conjugated with Alexa Fluor 647 (AF647). For DC analysis, we used antibodies to identify tolerogenic DCs including CD11c (N418) conjugated with allophycocyanin, CD11b (M1/70) conjugated with PerCP/Cy5.5, CD103 (2E7) conjugated with phycoerythrin, and MHCII (M5/114.15.2) conjugated with FITC. Isolated and subsequently activated DCs were quantified using CD11c, CD86 (GL-1) conjugated with allophycocyanin-Cy7, and CD80 (16–10A1) conjugated with Pacific Blue. Intracellular staining was performed using a fixation and permeabilization kit according to the manufacturer’s protocol (eBioscience). All conjugated antibodies were purchased from BioLegend (San Diego, CA). Prepared samples were analyzed by flow cytometry using a FACSCalibur (BD Biosciences) and BC Gallios (Beckman Coulter, Brea, CA) for data collection and FlowJo software for data analysis (Tree Star, Ashland, OR).
Cytokine IL-1β and IFN-γ assay.
Ileal tissues were homogenized with 0.4 ml of radioimmunoprecipitation assay lysis buffer (Cell Signaling Technology) with an addition of protease inhibitor cocktail (Sigma-Aldrich) to inhibit protein degradation. After homogenization, the tissues were centrifuged at 13,200 g for 10 min at 4°C, and the supernatants were collected. A DC protein assay (Bio-Rad) was performed to measure total protein in the tissue lysates by grams per milliliter using bovine serum albumin as a standard. Mouse Quantikine ELISA cytokine kits (R&D Systems) were used according to manufacturer’s protocol to measure IL-1β and IFN-γ levels in the collected samples. The detection ranges from the standard curve of the assays were 0–800 pg/ml and 0–3,000 pg/ml for IL-1β and IFN-γ, respectively. Cytokine levels measured were normalized by the concentration of total protein measured in homogenized supernatants of ileal tissues and were reported as picograms of IL-1β or IFN-γ per gram total protein.
Statistical analysis.
Experimental results are expressed as means ± SE. Statistical analysis was performed using one-way ANOVA (Prism 4.0, GraphPad Software, San Diego, CA). Tukey’s multiple-comparison tests were used for comparison of multiple groups. A χ2 analysis using contingency tests was performed to compare incidence of NEC between groups. P < 0.05 was considered statistically significant.
RESULTS
Protection from NEC associated with LR 17938 was linked to TLR2.
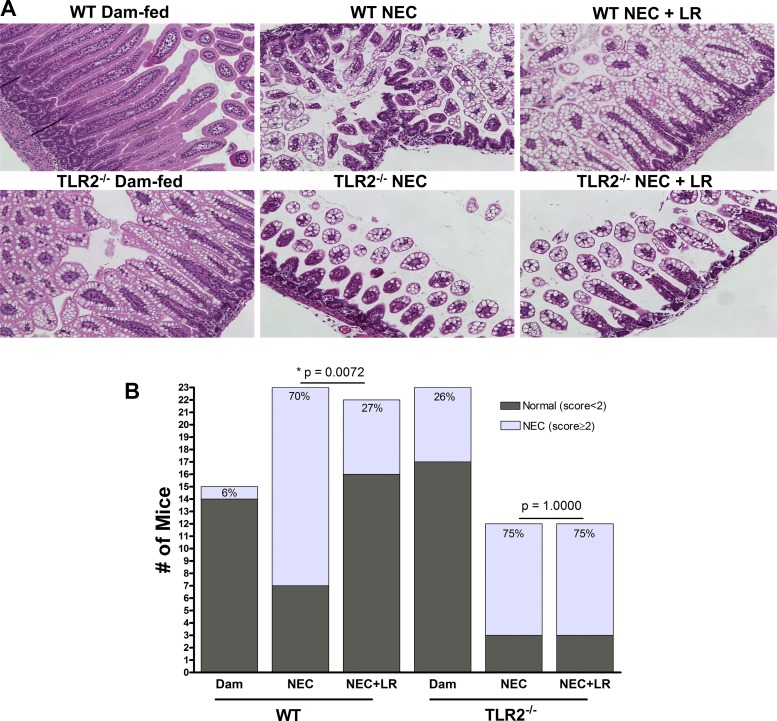
The incidence of NEC was evaluated by histological NEC scoring of ileal tissues on a scale of 1–3 (29). WT mice housed with their dams rarely had any abnormal ileal histological changes, with an incidence of grade 1 NEC of 6% (1/15). Their counterparts with experimental NEC had very noticeable damage of crypt-villus architecture with evidence of necrosis and/or sloughing of epithelial cells (Fig. 1A). The incidence of NEC in this group was 70% (16/23). The incidence of NEC in WT mice that were orally fed LR 17938 was reduced to 27% (6/22), significantly less than that of the non-LR-fed group of mice (P = 0.0072) (Fig. 1B).
Fig. 1.
LR 17938 reduces the incidence of necrotizing enterocolitis (NEC) in wild-type (WT) but not in Toll-like receptor 2 (TLR2)−/− mice. A: hematoxylin-eosin staining representatives of ileal tissue section for histological evaluation and scoring (magnification = 100 × ). B: incidence of NEC in WT and TLR2−/− mice. WT mice fed with LR 17938 showed decreased incidence of NEC, whereas TLR2−/− mice showed no recovery. n = 12–23 in each group. Significant P values indicated in the figure were calculated by χ2.
Breastfed TLR2−/− mouse pups housed with their mothers sometimes demonstrated mild histological NEC-like injury. The incidence of NEC-like changes in gut histology in dam-fed TLR2−/− mice was 26% (6/23), whereas TLR2−/− mice exposed to NEC had an overall NEC incidence of 75% (9/12). Probiotic LR 17938-fed TLR2−/− mice did not have a significant reduction of NEC incidence (75%, 9/12) when compared with TLR2−/− mice that did not receive LR 17938 during NEC induction (Fig. 1, A and B).
During experimental NEC, Teff lymphocyte levels in the intestine were decreased by feeding LR 17938 by a mechanism requiring TLR2.
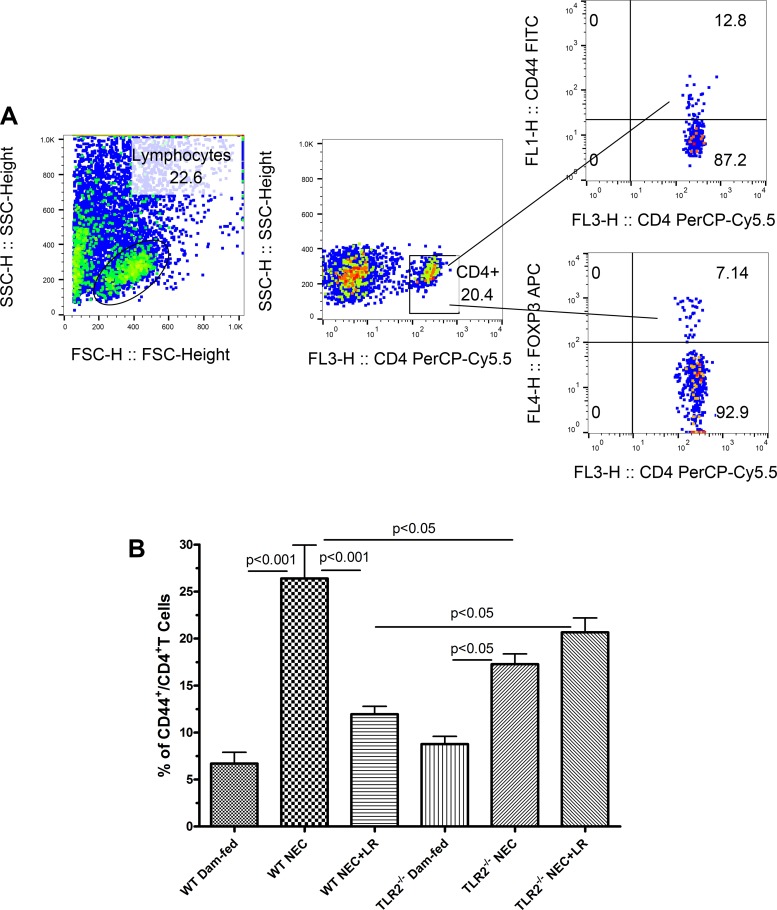
The cell surface adhesion molecule CD44 is required for recruitment of activated Teff lymphocytes to sites of inflammation (6). It has been noted that activation of lymphocytes is associated with increased surface level of CD44 (26). Therefore, we examined the CD44+ expression in TH cells (CD4+) residing in the intestinal tissues using flow cytometric analysis with gating to define the various T cell types and their percentages (Fig. 2A). We found that the percentage of CD44+TH cells in the intestine were significantly increased in WT NEC and TLR2−/− NEC groups compared with dam-fed controls without exposure to NEC (P < 0.001 and P < 0.05, respectively). The increased percentage of CD44+CD4+T cells in the intestine of WT mice was reduced by orally feeding LR 17938. However, the percentage of CD44+CD4+T cells was not changed by orally feeding LR 17938 to TLR2−/− mice (Fig. 2B). In addition, the percentage of CD44+CD4+T cells in the intestine of LR-treated WT mice with NEC was significantly lower than that in LR-treated TLR2−/− mice with NEC (P < 0.05). We also noticed that the percentage of CD44+CD4+T cells in the intestine was significantly higher in WT mice when compared with TLR2−/− mice exposed to the NEC procedure (Fig. 2B). This could indicate that the global knockout of TLR2 reduced the recruitment of CD44+CD4+T cells to sites of inflammation.
Fig. 2.
LR 17938 decreases the frequency of effector T cells in the intestinal mucosa in wild-type (WT) but not Toll-like receptor 2 (TLR2)−/− mice during necrotizing enterocolitis (NEC). A: gating strategy to define percentage of activated T helper lymphocytes (CD44+CD4+T) among lymphocyte population. B: LR 17938 changes the percentages of activated T helper lymphocytes in the intestines of WT and TLR2−/− mice during NEC. n = 6–9 in each group; one-way ANOVA, Tukey’s multiple comparison test.
LR 17938 increased the frequency of CD4+Foxp3+ Treg cells in the intestine of WT mice but not in TLR2−/− mice.
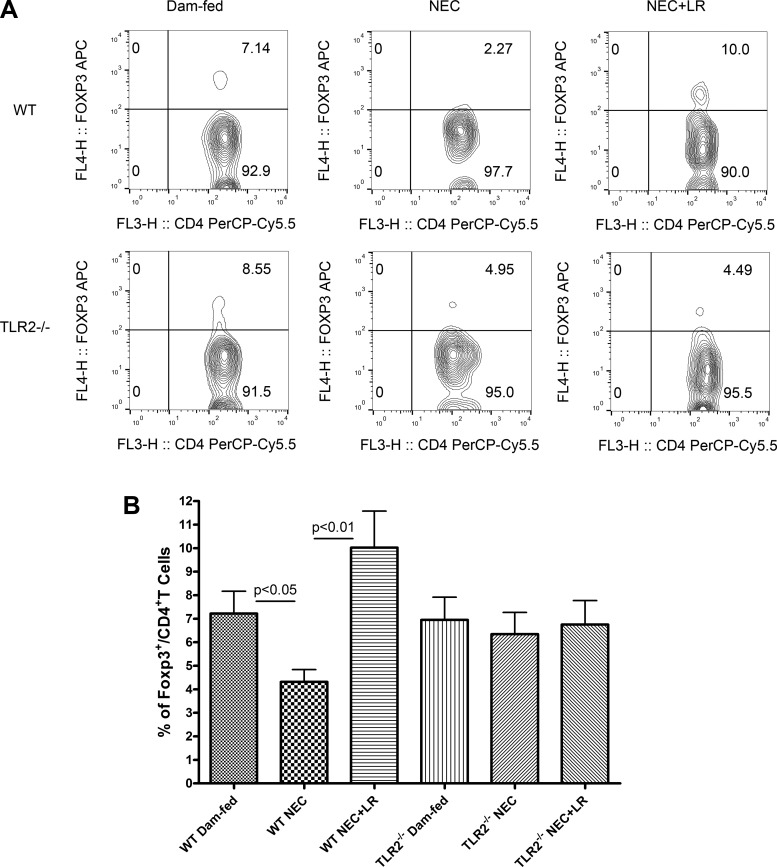
Tregs maintain intestinal homeostasis by controlling inflammation and inducing tolerance (45). We analyzed Tregs by gating CD4+T cells to calculate the frequency of Foxp3+ Tregs within the CD4+ T cell population (Fig. 3A). The percentage of Foxp3+ Tregs significantly decreased in the intestine of WT mice during NEC (P < 0.05), but Foxp3+ Tregs were increased in the intestine of WT mice with LR 17938 feeding (P < 0.01). However, the frequency of Foxp3+ Tregs was not affected in animals with the TLR2−/− phenotype, with or without feeding of LR 17938 during NEC (Fig. 3B).
Fig. 3.
LR 17938 increases the frequency of regulatory T cells in the intestinal mucosa in wild-type (WT) but not Toll-like receptor 2 (TLR2)−/− mice during necrotizing enterocolitis (NEC). A: representative flow cytometry plots in the percentage of intestinal Foxp3+ Tregs among the CD4+ T cell population in the different groups. B: percentages of Fox3+ Tregs in the intestines of dam-fed, NEC, or NEC + LR in WT and TLR2−/− mice. n = 6–9 in each group; one-way ANOVA, Tukey’s multiple comparison test.
LR 17938 increased tolerogenic DCs in the intestine during NEC and stimulated DC activation in culture that were mediated by TLR2.
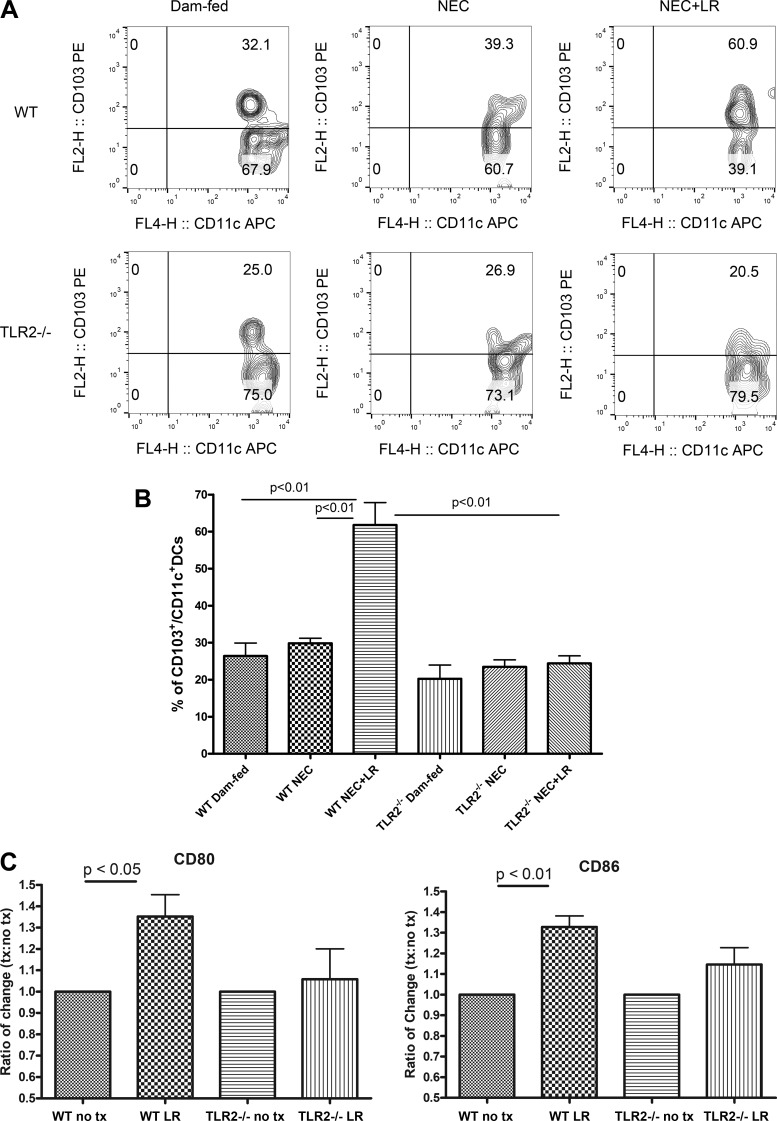
Intestinal DCs are central to maintaining immune activation or, alternatively, promoting immune tolerance in the gut, the latter by generation of tolerogenic T-cell responses. Tolerogenic T cells protect against immune activation by food antigens and the commensal microbiota (31). CD103 (α integrin)-expressing CD11c+ DCs in the small intestine orchestrate immune tolerance in the gut (17). Mice lacking this DC subset have fewer Tregs in the colon and are predisposed to colitis (3). We examined the frequency of CD103+ cells in CD11c+ DC populations (Fig. 4A) in the intestine of mice to compare WT and TLR2−/− mice with respect to their response to LR 17938 treatment during NEC. We found that in the intestine of WT and TLR2−/− mice, ~20%–30% of intestinal DCs expressed CD103, regardless of their exposure to NEC. However, LR 17938 powerfully increased the percentage of CD103+ DCs in the intestine of mice during NEC (~60%) in WT mice (P < 0.01), whereas we saw no increase in tolerogenic intestinal DCs in TLR2−/− mice that were fed LR 17938 during experimental NEC (Fig. 4B).
Fig. 4.
LR 17938 significantly increases the frequency of intestinal tolerogenic dendritic cells (DCs) in wild-type (WT) mice but not Toll-like receptor 2 (TLR2)−/− mice. A: representative flow cytometry plots in the percentage of intestinal CD103+ cells among the CD11c+ DC population, defined as tolerogenic DCs, in the different groups. B: percentages of CD103+CD11c+ DCs in the intestines of dam-fed, necrotizing enterocolitis (NEC), or NEC + LR in WT and TLR2−/− mice. n = 3–9 in each group; one-way ANOVA, Tukey’s multiple comparison test. C: ratios of CD80 and CD86 expression in isolated splenic DCs from WT and TLR2−/− following LR treatment. no tx, cells were treated with deMan-Rogosa-Sharpe (MRS) medium. n = 5 for each group; one-way ANOVA, Tukey’s multiple comparison test.
It has been known that DCs, when activated, have the ability to modulate T-cell activity through the expression of costimulatory molecules CD80 and CD86, which ligate to surface markers of CD28 or cytotoxic T-lymphocyte-associated protein 4 on T cells, or specifically interaction with Treg cells (25). To examine further whether LR 17938 stimulates DC activation that is mediated by TLR2, DCs were isolated from WT or TLR2−/− splenocytes, treated by LR 17938, and analyzed for the presence of DC activation markers of CD80 and CD86. We found that LR 17938 treatment of WT DCs significantly increased the expression of CD80 and CD86 when compared with the nontreatment group (P < 0.05 and P < 0.01, respectively) (Fig. 4C). However, there was no significant change in the activation of DCs from TLR2−/− animals in response to LR 17938, indicating LR 17938-stimulated DC activation was mediated by TLR2.
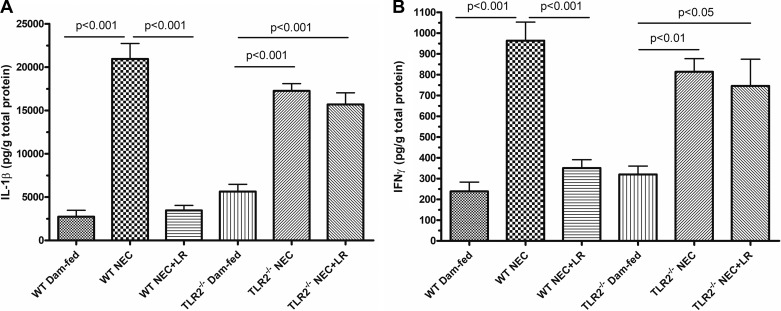
LR 17938 reduced IL-1β and IFN-γ levels in WT mice, but not in TLR2−/−, mice during NEC.
Cytokines IL-1β and IFN-γ are key mediators of the proinflammatory response. High levels of these markers in the intestinal mucosa indicate unregulated inflammation during NEC (30). In WT mice experiencing NEC, levels of IL-1β and IFN-γ were significantly higher than those without NEC (P < 0.001). Treatment with LR 17938 decreased the amounts of these cytokines measured in the ileal tissues (P < 0.001). TLR2−/− mice also showed increased levels of IL-1β and IFN-γ during experimental NEC (P < 0.001). However, treatment of TLR−/− mice with LR 17938 resulted in no significant reduction of these proinflammatory cytokines (Fig. 5, A and B).
Fig. 5.
LR 17938 decreases proinflammatory cytokines in ileal tissue lysates of wild-type (WT) but not Toll-like receptor 2 (TLR2)−/− mice during necrotizing enterocolitis (NEC). A: levels of IL-1β in the intestinal tissue lysates of WT and TLR2−/− mice in each group. B: levels of IFN-γ in the intestinal tissue lysates of WT and TLR2−/− mice in each group. n = 4–8 in each group; one-way ANOVA, Tukey’s multiple comparison test.
DISCUSSION
NEC is the most common gastrointestinal emergency of very low birth weight premature infants, accounting for 0.3–2.4 cases per 1,000 live births in the United States. NEC has an overall disease-specific mortality rate of > 15%, and its occurrence results in an increase in hospital costs of $100,000/case (14). There is strong evidence from meta-analyses suggesting that a wide variety of probiotics can prevent NEC in human infants (2, 13, 40, 47). LR 17938 has been also been shown to prevent NEC in experimental rodent models (27, 29). Recent evidence also suggests that L. reuteri (strain 17938) has the potential to reduce the risk of NEC in humans. It is manufactured in sunflower oil, which is easy to administer to babies (5). In the current study, we further probed the mechanism of protective effects by LR 17938 against NEC, including microbial recognition.
TLRs are pattern recognition receptors that signal intracellularly to modulate the release of biological signals that are mandatory to initiate and sustain effective immunologic responses (10, 46). TLR activation by commensal bacteria plays an essential role in intestinal epithelial homeostasis. We have shown that gut epithelial TLRs are upregulated in NEC (30). We have observed that LR 17938 can reduce the incidence and severity of NEC via dampening TLR4-associated NF-κB signaling in the intestine (28). TLR2 is known to be essential for probiotic modulation of cytokine production in vitro (4, 15, 43, 48). Recent studies reported that certain lactobacilli (for example Lactobacillus rhamnosus GG) can protect from experimental colitis via mechanisms involving TLR2 and cyclo-oxygenase-2 (15). TLR2-dependent induction of Tregs (Lactobacillus casei Lbs2) (44) and TLR2-dependent inhibition of NF-κB signaling by macrophages have been shown in studies examining the mechanism of Lactobacillus paracasei (43). Our findings suggest that L. reuteri DSM 17938 attenuates experimental NEC by induction of Tregs and tolerogenic DCs, which in turn reduce the proliferation of proinflammatory lymphocytes and the elaboration of their associated cytokines via a mechanism also dependent on TLR2.
Foxp3+ Treg cells are known to be essential for intestinal immune homeostasis via suppression of innate and adaptive host responses. Effector CD4+ T cells express high levels of CD44, which is a driver of TH1-driven inflammation, including recirculation of T cells and chemotaxis to sites of inflammation (16, 34). CD4+CD44+ T cells were found to have an effector phenotype, demonstrated by enhanced reactivity to CD44 ligand, production of inflammatory cytokines, and participation in the induction of ileitis (16). The inhibition of inflammation in response to LR 17938 in our model by increasing Foxp3+Tregs and reducing CD4+CD44+ Teffs, which was not seen in TLR2−/− mice, strongly suggests that the mediation of this effect occurred via a TLR2 signal.
Intestinal DCs are central to maintaining immune tolerance in the gut, ultimately by generation of tolerogenic T-cell responses toward food antigens and the commensal microbiota (31). Direct DC recognition of viral and microbial products is also mediated by TLRs. CD103-expressing DCs confer the ability to synthesize retinoic acid, express thymic stromal lymphopoietin, and induce Foxp3+ Tregs (8, 33, 38). Several studies identified a key role of DCs in probiotic functionality by using either bone marrow-derived DCs or human monocyte-derived DCs in vitro. A probiotic was taken up by bone marrow-derived DCs, facilitated their maturation, and was linked to the induction of the anti-inflammatory cytokine IL-10, as well as an increase in Treg levels (7, 22, 24, 41, 42). Adoptive transfer of Lactobacillus-primed DCs attenuated Citrobacter rodentium colitis and 2, 4, and 6-trinitrobenzenesulfonic acid-induced colitis (12, 18, 21). In our current studies, we showed that the percentage of gut-derived CD103+ DCs and Foxp3+ Tregs of WT mice were significantly increased by LR 17938 feeding during NEC. Absence of CD103+ DC induction in TLR2−/− mice indicates that oral LR 17938 protection is linked to TLR2. The activation of DCs by LR 17938 was also associated with the expression of antigen presenting cell co-stimulatory markers CD80 and CD86. The upregulation of these markers on DCs by other probiotics has been shown to significantly attenuate bacterial colitis, associated with CD80 and CD86 activation, increased CD4+ T cell expression of IL-10, and increased levels of Treg cells (12).
Proinflammatory cytokines IFN-γ and IL-1β are excellent biomarkers of inflammation, which are produced by proliferating TH1 cells leading to downstream activation of the NF-κB pathway (30). Previous studies showed that activation of TLR2 with gram-positive Lactobacilli initiates an alternative, anti-inflammatory route of phosphorylation producing negative regulators of the NF-κB pathway (43). These negative regulators include the deubiquitinating enzyme A20, IRAK3, toll-interacting proteins, and suppressors of cytokine signaling (SOCS1 and SOCS3), which reduce the induced expression of proinflammatory cytokines by inhibiting translocation of NF-κB and signal transduction of the JAK/STAT pathway. Although the mechanism of action is not completely understood, future studies of these signaling pathways may shed additional light on the anti-inflammatory activities by probiotic LR 17938.
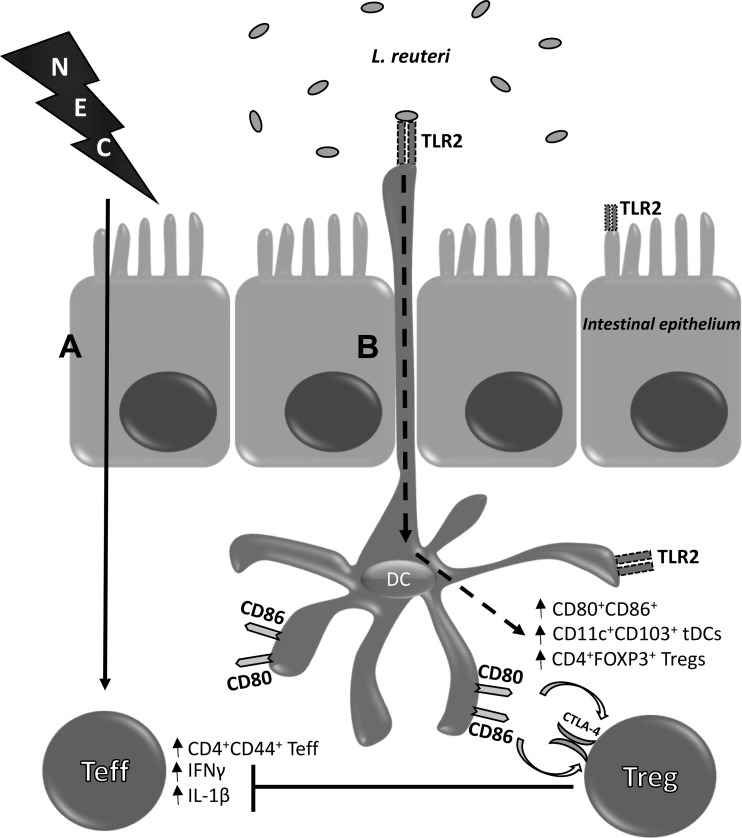
In summary, our data support our hypothesis on the mechanism of LR 17938 in regulating intestinal inflammation during NEC with a proposed mechanism that is summarized in Fig. 6. The interaction between LR 17938 and TLR2 on DCs in the lamina propria promotes the priming of T cells to a regulatory phenotype that reduces the severity of NEC by halting the proliferation of Teff cells and the expression of proinflammatory cytokines. As shown, LR 17938 activates and increases the number of mucosal tolerogenic DCs, which prime T cells to an immunotolerant phenotype, as evidenced by increased Tregs in the face of decreased inflammatory Teff cells (such as TH1 cells), and finally reduces levels of IFNγ and IL-1β in the intestinal mucosa of WT mice but not in the TLR2−/− mice. The direct and/or indirect interactions of LR with DCs, Tregs, and enterocytes within the intestinal mucosa, as well as the intracellular signaling pathways, warrant further exploration.
Fig. 6.
The protective effect of LR 17938 is mediated by a mechanism requiring Toll-like receptor 2 (TLR2). A: induction of necrotizing enterocolitis (NEC) increases CD4+CD44+ effector T (Teff) cells in the intestines, as well as the levels of proinflammatory cytokines IFNγ and IL-1β. B: LR 17938 interacts with TLR2 on the intestinal epithelial cells and dendritic cells (DCs) extending their dendrites at the luminal surface, increasing DC activation to prime CD4+Foxp3+ regulatory T (Treg) cells through CD80 and CD86 within the lamina propria. An increase in CD11c+CD103+ tolerogenic DCs was evident in the intestine after probiotic treatment. L. reuteri, Lactobacillus reuteri.
GRANTS
These studies were supported by National Center for Complementary and Integrative Health Grant R01-AT-007083 (to J. M. Rhoads and Y. Liu).
DISCLOSURES
We acknowledge support of BioGaia AB in other research projects, but this project was not supported by BioGaia AB.
AUTHOR CONTRIBUTIONS
D.Q.T., J.M.R., and Y.L. conceived and designed research; T.K.H., B.H., T.W., and Y.L. performed experiments; T.K.H., B.H., T.W., and Y.L. analyzed data; T.K.H., B.H., D.Q.T., J.M.R., and Y.L. interpreted results of experiments; T.K.H. prepared figures; T.K.H. drafted manuscript; T.K.H., D.Q.T., J.M.R., and Y.L. edited and revised manuscript; T.K.H., B.H., T.W., D.Q.T., J.M.R., and Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Pamela Parson of the Cellular and Molecular Morphology Core of the Texas Medical Center Digestive Disease Center at Houston (supported by National Institute of Diabetes and Digestive and Kidney Diseases Public Health Service Grant DK56338) for performing histological preparations, Dr. Amy Hazen of the IMM Flow Cytometry Core at The University of Texas Health Science Center for assisting flow cytometry analysis, and Dr. Eamonn Connolly at BioGaia AB (Stockholm, Sweden) for providing Lactobacillus reuteri strain DSM 17938. The probiotic Lactobacillus reuteri DSM 17938 was obtained as a gift from BioGaia AB.
REFERENCES
- 1.Abrahamsson TR, Sandberg Abelius M, Forsberg A, Björkstén B, Jenmalm MC. A Th1/Th2-associated chemokine imbalance during infancy in children developing eczema, wheeze and sensitization. Clin Exp Allergy 41: 1729–1739, 2011. doi: 10.1111/j.1365-2222.2011.03827.x. [DOI] [PubMed] [Google Scholar]
- 2.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev (3): CD005496, 2011 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med 202: 1051–1061, 2005. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aprahamian CJ, Lorenz RG, Harmon CM, Dimmit RA. Toll-like receptor 2 is protective of ischemia-reperfusion-mediated small-bowel injury in a murine model. Pediatr Crit Care Med 9: 105–109, 2008. doi: 10.1097/01.PCC.0000288717.44702.C0. [DOI] [PubMed] [Google Scholar]
- 5.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: A strain-specific systematic review. JPEN J Parenter Enteral Nutr 40: 783–794, 2016. doi: 10.1177/0148607115588113. [DOI] [PubMed] [Google Scholar]
- 6.Baaten BJ, Li CR, Bradley LM. Multifaceted regulation of T cells by CD44. Commun Integr Biol 3: 508–512, 2010. doi: 10.4161/cib.3.6.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol 84: 468–476, 2008. doi: 10.1189/jlb.0108017. [DOI] [PubMed] [Google Scholar]
- 8.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 204: 1765–1774, 2007. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J Pediatr 116: 960–964, 1990. doi: 10.1016/S0022-3476(05)80661-4. [DOI] [PubMed] [Google Scholar]
- 10.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 54: 1182–1193, 2005. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Chiu CH, Lin TY, Shi HN, Walker WA. Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells. Pediatr Res 65: 169–175, 2009. doi: 10.1203/PDR.0b013e31818d5a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury T, Ali MM, Hossain MM, Singh J, Yousuf AN, Yasmin F, Chowdhury FR. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak 26: 770–774, 2016. [PubMed] [Google Scholar]
- 14.Christensen RD, Gordon PV, Besner GE. Can we cut the incidence of necrotizing enterocolitis in half–today? Fetal Pediatr Pathol 29: 185–198, 2010. doi: 10.3109/15513815.2010.483874. [DOI] [PubMed] [Google Scholar]
- 15.Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, Walker MR, Marinshaw JM, Stappenbeck TS, Stenson WF. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 61: 829–838, 2012. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins CB, Ho J, Wilson TE, Wermers JD, Tlaxca JL, Lawrence MB, Solga M, Lannigan J, Rivera-Nieves J. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology 135: 1993–2002, 2008. doi: 10.1053/j.gastro.2008.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764, 2007. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 174: 3237–3246, 2005. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 19.Dingle BM, Liu Y, Fatheree NY, Min J, Rhoads JM, Tran DQ. FoxP3+ regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS One 8: e82963, 2013. doi: 10.1371/journal.pone.0082963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Niño DF, Prindle T Jr, Ozolek JA, Hackam DJ. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126: 495–508, 2016. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol 13: 236–243, 2007. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foligne B, Zoumpopoulou G, Dewulf J, Ben Younes A, Chareyre F, Sirard JC, Pot B, Grangette C. A key role of dendritic cells in probiotic functionality. PLoS One 2: e313, 2007. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hackam DJ, Good M, Sodhi CP. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg 22: 76–82, 2013. doi: 10.1053/j.sempedsurg.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J Allergy Clin Immunol 117: 696–702, 2006. doi: 10.1016/j.jaci.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Kornete M, Piccirillo CA. Functional crosstalk between dendritic cells and Foxp3(+) regulatory T cells in the maintenance of immune tolerance. Front Immunol 3: 165, 2012. doi: 10.3389/fimmu.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WT, Vitetta ES. The differential expression of homing and adhesion molecules on virgin and memory T cells in the mouse. Cell Immunol 132: 215–222, 1991. doi: 10.1016/0008-8749(91)90020-C. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads JM. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 8: e56547, 2013. doi: 10.1371/journal.pone.0056547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol 302: G608–G617, 2012. doi: 10.1152/ajpgi.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Tran DQ, Fatheree NY, Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 307: G177–G186, 2014. doi: 10.1152/ajpgi.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G442–G450, 2009. doi: 10.1152/ajpgi.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann ER, Landy JD, Bernardo D, Peake ST, Hart AL, Al-Hassi HO, Knight SC. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett 150: 30–40, 2013. doi: 10.1016/j.imlet.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Messlik A, Schmechel S, Kisling S, Bereswill S, Heimesaat MM, Fischer A, Göbel U, Haller D. Loss of Toll-like receptor 2 and 4 leads to differential induction of endoplasmic reticulum stress and proapoptotic responses in the intestinal epithelium under conditions of chronic inflammation. J Proteome Res 8: 4406–4417, 2009. doi: 10.1021/pr9000465. [DOI] [PubMed] [Google Scholar]
- 33.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260, 2007. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 34.Nácher M, Blázquez AB, Shao B, Matesanz A, Prophete C, Berin MC, Frenette PS, Hidalgo A. Physiological contribution of CD44 as a ligand for E-Selectin during inflammatory T-cell recruitment. Am J Pathol 178: 2437–2446, 2011. doi: 10.1016/j.ajpath.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 6: e17776, 2011. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, Oguz SS, Dilmen U. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 99: F110–F115, 2014. doi: 10.1136/archdischild-2013-304745. [DOI] [PubMed] [Google Scholar]
- 38.Reardon C, Lechmann M, Brüstle A, Gareau MG, Shuman N, Philpott D, Ziegler SF, Mak TW. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity 35: 223–235, 2011. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol 74: 6032–6040, 2008. doi: 10.1128/AEM.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawh SC, Deshpande S, Jansen S, Reynaert CJ, Jones PM. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ 4: e2429, 2016. doi: 10.7717/peerj.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol 115: 1260–1267, 2005. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 42.Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol 34: 1371–1380, 2004. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 43.Sun KY, Xu DH, Xie C, Plummer S, Tang J, Yang XF, Ji XH. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine 92: 1–11, 2017. doi: 10.1016/j.cyto.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Thakur BK, Saha P, Banik G, Saha DR, Grover S, Batish VK, Das S. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int Immunopharmacol 36: 39–50, 2016. doi: 10.1016/j.intimp.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 45.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110: 2983–2990, 2007. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 7: 179–190, 2007. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 47: 241–248, 2012. doi: 10.1016/j.jpedsurg.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 48.Weiss G, Rasmussen S, Zeuthen LH, Nielsen BN, Jarmer H, Jespersen L, Frøkiaer H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 131: 268–281, 2010. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, Polk DB. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62: 73–82, 2013. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]