Abstract
Liver is the primary source of numerous proteins that are critical for normal function of the blood coagulation cascade. Because of this, diseases of the liver, particularly when affiliated with severe complications like cirrhosis, are associated with abnormalities of blood clotting. Although conventional interpretation has inferred cirrhosis as a disorder of uniform bleeding risk, it is now increasingly appreciated as a disease wherein the coagulation cascade is precariously rebalanced. Moreover, prothrombotic risk factors are also associated with a more rapid progression of fibrosis in humans, suggesting that coagulation proteases participate in disease pathogenesis. Indeed, strong evidence drawn from experimental animal studies indicates that components of the coagulation cascade, particularly coagulation factor Xa and thrombin, drive profibrogenic events, leading to hepatic fibrosis. Here, we concisely review the evidence supporting a pathologic role for coagulation in the development of liver fibrosis and the potential mechanisms involved. Further, we highlight how studies in experimental animals may shed light on emerging clinical evidence, suggesting that beneficial effects of anticoagulation could extend beyond preventing thrombotic complications to include reducing pathologies like fibrosis.
Keywords: anticoagulants, coagulation, fibrosis, liver
HEMOSTATIC SYSTEM FUNCTION IN PATIENTS WITH LIVER DISEASE
Viral hepatitis, alcoholic and nonalcoholic fatty liver disease, and various autoimmune conditions remain major causes of liver-related morbidity and mortality (21). If liver damage driven by these conditions is not met by adequate liver repair, chronic liver injury leads to activation of hepatic stellate cells (and portal fibroblasts), resulting in a replacement of healthy hepatic parenchyma with excess extracellular matrix, including collagen (21). Excessive deposition of extracellular matrix is termed liver fibrosis, which can advance to a severe form termed cirrhosis. Cirrhosis is associated with a loss of hepatic function and can lead to liver failure and liver cancer. Treatment and prevention of cirrhosis in patients with liver disease are essential, because once cirrhosis develops, liver transplantation is often the only life-saving option.
Among its various functions, the liver is tasked with synthesis of numerous proteins involved in the coagulation cascade, the plasma pathway responsible for blood clot formation. The coagulation system is a complex series of serine proteases activated by tissue factor, a transmembrane receptor for coagulation factor VIIa (51, 62). The pinnacle coagulation protease is thrombin (factor IIa), which proteolytically enables conversion of plasma fibrinogen to insoluble fibrin clots. Fibrin clot formation is counterbalanced by a process called fibrinolysis. The tissue- and urokinase- plasminogen activators convert plasminogen to plasmin, the latter capable of degrading fibrin (52). Because the liver is so central in this process, patients with liver fibrosis and cirrhosis display profound changes in the components of the coagulation cascade. Levels of procoagulant proteins, such as factors II, V, VII, X, and others are decreased in patients with hepatic fibrosis and cirrhosis (47, 76). This decrease in procoagulant factors in patients with cirrhosis is counterbalanced by a concomitant decrease in anticoagulant proteins, such as protein C, protein S, and antithrombin. Similarly, thrombocytopenia (i.e., low platelet count) and thrombocytopathy (i.e., platelet dysfunction) in cirrhotic patients are rebalanced by compensatory elevation in the levels of the platelet-adhesive protein von Willebrand factor (VWF) and a decrease in the VWF-cleaving protease, ADAMTS13 (45, 47). Likewise, a decrease in antifibrinolytic factors is rebalanced by the concomitant decrease in profibrinolytic factors (49). The functional consequence of these changes is a rebalanced hemostatic system with normal to increased thrombin generation (49, 76, 77). In patients with compensated cirrhosis, wherein remaining parenchymal cells effectively manage hepatic functions, resulting in largely asymptomatic cirrhosis with few symptoms, the hemostatic system usually remains in this rebalanced state. However, in a decompensated state, wherein concurrent disease challenges cause remaining hepatic function to fail, the hemostatic rebalance can tip easily toward either bleeding or thrombosis (49, 76). Therefore, although patients may suffer from spontaneous or procedural bleeding episodes (58), cirrhosis is also associated with hypercoagulation, and patients are at a higher risk for venous thromboembolism, portal vein thrombosis (PVT), and pulmonary embolism (61, 70, 76).
COAGULATION FACTORS CONTRIBUTE TO FIBROSIS
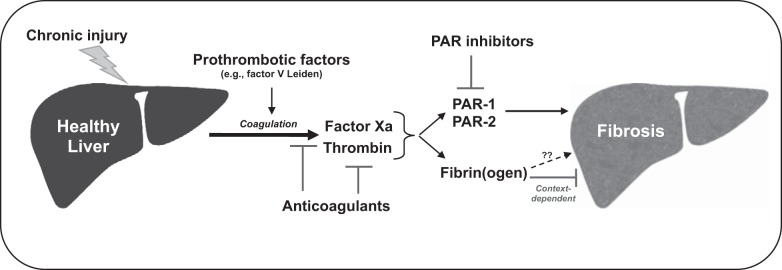
Although changes in coagulation parameters are often observed as a clinical manifestation of liver cirrhosis, studies suggest that coagulation factors themselves, play important roles in the progression of hepatic fibrosis and/or cirrhosis (8). Progression of fibrosis is faster in hepatitis patients who have elevated plasma prothrombin levels, resulting from a single nucleotide polymorphism in the prothrombin gene, and patients who have increased production of thrombin resulting from a single nucleotide polymorphism in the factor V Leiden (53, 63, 80). Similarly, hepatitis C infection-related liver disease and fibrosis is less severe in patients with hemophilia, and elevated plasma VWF levels are associated with poor disease prognosis in patients with hepatic cirrhosis (6, 18, 27, 54). These studies suggest that components of the coagulation cascade, especially the prothrombotic factors, are associated with advanced progression of fibrosis in patients with chronic liver diseases. One of the suggested mechanisms behind this is the formation of intrahepatic microthrombi, which could cause ischemia and disrupt localized blood flow, ultimately leading to replacement of hepatic parenchymal cells with fibrous tissue. This and other mechanisms have been the subject of intense study in experimental settings of liver fibrosis. Thus, while liver disease, particularly cirrhosis, drives marked changes in the coagulation system, there is also evidence that coagulation activity can also worsen the progression of liver fibrosis. In the following section, we discuss examples of how experimental settings of fibrosis, primarily rodent models, have revealed the mechanisms whereby coagulation proteases contribute to liver fibrosis (Fig. 1).
Fig. 1.
Mechanisms of coagulation-directed liver fibrosis discovered in experimental settings of chronic liver injury. Chronic liver injury elicited by various insults is associated with activation of the blood coagulation cascade in experimental settings of liver fibrosis. This leads to generation of the coagulation proteases, coagulation factor Xa, and thrombin. Anticoagulants, including direct inhibitors of factor Xa and thrombin, have been shown to reduce liver fibrosis in animal models. Mice harboring prothrombotic risk factors comparable to those in humans (e.g., factor V Leiden) also develop more severe fibrosis. Studies with mice lacking protease-activated receptors (PARs) strongly suggest that PAR-1 (activated by thrombin) and PAR-2 (activated by factor Xa) contribute to hepatic fibrosis. Therefore, inhibition of PAR-1 and PAR-2 could be a novel strategy to reduce hepatic fibrosis. Fibrin(ogen) and fibrin clots may also contribute to hepatic fibrosis, but published evidence supporting this pathway is very limited. Rather, in specific experimental contexts, such as biliary injury, published studies suggest fibrin(ogen) can inhibit hepatic fibrosis.
ANTICOAGULATION AND ITS EFFECT ON EXPERIMENTAL LIVER FIBROSIS
Liver fibrosis can be induced in rodent models by inducing chronic liver injury through multiple approaches. Hepatotoxic xenobiotics, including carbon tetrachloride (CCl4), thioacetamide, α-naphthylisothiocyanate (ANIT), dimethyl- or diethyl-nitrosamine, and tetrachlorodibenzo-p-dioxin have been used as the source of this chronic insult (35, 44, 55, 59, 60). Additionally, obstructive cholestasis, modeled in animals by bile duct ligation (BDL), also causes liver fibrosis (13, 75). Intrahepatic activation of the coagulation cascade is evident in these experimental settings (41). Moreover, numerous studies have documented a pathologic role for coagulation proteases in the pathogenesis of liver fibrosis. For example, treatment with warfarin, an anticoagulant that blocks the production of coagulation factors by the liver, reduced liver fibrosis in CCl4-challenged mice, as well as in a mouse model of liver injury induced by chronic hepatic congestion (5, 68). Administration of anticoagulant low-molecular weight heparins (LMWHs) reduced hepatic fibrosis in rats induced by chronic CCl4 administration or by BDL (1, 2). Similarly, the thrombin inhibitor SSR182289 reduced liver fibrosis in rats following chronic CCl4 exposure (16). In addition, the direct oral anticoagulants (DOACs) rivaroxaban and dabigatran, novel small-molecule inhibitors of either coagulation factor Xa or thrombin, respectively, have been shown to reduce experimental liver fibrosis in rats (26, 78). Consistent with protective effects of reducing coagulation, mice harboring the procoagulant factor V Leiden mutation, which are genetically prone to increased coagulation activity, developed more fibrosis than wild-type counterparts, mirroring observations in humans (5, 80). Collectively, these studies indicate an important contribution of coagulation protease activity, particularly factor Xa and thrombin, to the pathogenesis of liver fibrosis.
The field emphasizes two primary mechanisms, whereby coagulation proteases could contribute to liver fibrosis. Intrahepatic fibrin clot deposition in the form of microthrombi could block blood flow, leading to hypoxia and cellular injury. Coagulation proteases could also promote liver fibrosis by direct activation of hepatic stellate cells, or indirectly by promoting local inflammatory cell activity. We briefly review the evidence supporting each hypothesis below, primarily from experimental studies.
FIBRIN(OGEN) AND HEPATIC FIBROSIS
Hepatic fibrin(ogen) deposits are a conspicuous feature of models of liver fibrosis (41). In principle, fibrin(ogen) deposits could occlude the hepatic sinusoids, disrupting blood flow, serve as a provisional matrix driving fibrosis, or act as a proinflammatory trigger advancing liver disease. The suggestion that fibrin(ogen) deposits drive hepatic fibrosis is anchored in the observed reduction in hepatic fibrin(ogen) upon administration of anticoagulants, which also reduce fibrosis. However, at present, there is a lack of definitive experimental evidence indicating that fibrin(ogen) contributes to liver fibrosis. In fact, the role of fibrin(ogen) in experimental fibrosis may depend heavily on the context of insult driving the chronic liver injury. For example, complete genetic deficiency in fibrin(ogen) did not affect gross liver histopathology in mice chronically exposed to CCl4 (7). Interestingly, in the setting of ANIT-induced biliary injury and fibrosis, several studies have shown that fibrin(ogen) exerts protective effects through activation of integrin receptors on platelets and leukocytes (34–36, 50). Thus, at present, there is no definitive evidence illustrating a unified pathologic role for fibrin(ogen) in liver fibrosis. Fundamental differences in the cause or severity of disease in experimental and clinical settings may ultimately dictate fibrin(ogen) function. Much remains to be learned about the role of fibrin(ogen) in experimental and clinical settings of liver injury, repair, and fibrosis.
PROTEASE-ACTIVATED RECEPTORS SIGNALING AND HEPATIC FIBROSIS
Coagulation proteases trigger cell activation through cleavage of the G protein-coupled protease-activated receptors (PARs), which consist of four family members, PAR-1, PAR-2, PAR-3, and PAR-4 (3, 11, 12, 25, 31). Proteolytic cleavage of an extracellular NH2-terminus of PARs exposes unique tethered ligands, which stimulate distinct intracellular signaling pathways (71, 83). PARs are implicated in a large number of processes and diseases, including hemostasis, thrombosis, inflammation, and cancer progression (3, 25, 83). PAR-1, the primary receptor for thrombin, and PAR-2, a receptor for numerous proteases, including factor Xa, are expressed by hepatic stellate cells (22). Thrombin and factor Xa, as well as selective peptide agonists for these receptors, have been shown to promote a profibrogenic phenotype in cultured stellate cells (19, 22). Liver fibrosis was reduced in PAR-1−/− mice in multiple experimental settings, and administration of a PAR-1 antagonist reduced fibrosis in BDL rats (19, 60, 66, 73). These studies indicate that PAR-1 contributes to experimental liver fibrosis. Although direct activation of PAR-1 or PAR-2 on stellate cells is a plausible mechanism, this has not been unequivocally demonstrated in vivo, and it is possible that PAR-1 expressed by inflammatory cells also contributes to fibrosis by exacerbating inflammation (38). In addition to PAR-1, several studies indicate that PAR-2 also contributes to experimental liver fibrosis (40, 67, 74). Collectively, there is strong experimental evidence to indicate that coagulation proteases can drive hepatic fibrosis through activation of PARs. Although not the primary focus of this review, it is important to note that PARs can be activated by diverse proteases, such as cathepsins and neutrophil elastase (17, 57, 65, 82), widening the pool of potential effectors of fibrosis through PAR activation. The signaling pathways activated by PARs are also heavily dependent on the activating protease, illustrating a need to determine the precise intracellular pathways driven by coagulation protease activation of PARs, particularly as new antagonists are developed (24, 83).
On the basis of ample evidence linking PAR activation by the coagulation cascade to stellate cell activation and fibrosis, there may be therapeutic utility of PAR antagonists in the treatment of liver fibrosis. For example, the U.S. Food and Drug Administration-approved PAR-1 antagonist vorapaxar could be considered, but the risk of bleeding may outweigh potential benefit (10, 20). Unlike in mice, PAR-1 is the primary receptor for thrombin on human platelets (37, 72). However, it is worth noting that beneficial effects of other antiplatelet drugs have been documented in both experimental and clinical settings of liver fibrosis (33, 48, 69, 81). New strategies to target PAR-1, including pepducins and parmodulins, may also offer novel opportunities to treat liver fibrosis (4, 23). On the basis of mechanistic studies, PAR-2 is also an attractive target, particularly because factor Xa activation of PAR-2 could be blocked without affecting hemostasis. Emerging strategies to inhibit PAR-2, including pepducins and small-molecule antagonists are logical candidates to explore as antifibrotic agents (32, 67, 74).
ANTICOAGULATION OF CIRRHOTIC PATIENTS: BENEFITS BEYOND PREVENTION OF THROMBOSIS?
Coagulation disturbances featured in cirrhotic patients, and the potential risk of PVT, have prompted several studies evaluating anticoagulants as a preventative strategy to reduce PVT. This is perhaps the best highlight of how clinical and laboratory evidence has changed the perception of cirrhosis imposing an inherent bleeding risk. Interestingly, there is emerging evidence to suggest that anticoagulation might confer benefits beyond prevention of PVT in cirrhotic patients. For example, anticoagulation with enoxaparin, a LMWH, not only prevented development of PVT in cirrhotic patients, but also delayed hepatic decompensation and improved survival (79). Further, reduction in posttransplant fibrosis was observed in patients who were anticoagulated with warfarin (15). Particularly when viewed in the context of the strong connection between coagulation and fibrosis development in experimental animals, these studies suggest that anticoagulant treatment may, in fact, be a novel approach to reduce the progression of fibrosis or improve prognosis of patients with hepatic cirrhosis. However, much more research is required. Indeed, several clinical trials are addressing this possibility (NCT02643212, NCT02271295).
Emerging questions include which patients should receive anticoagulation, and with the availability of DOACs, which anticoagulant drug is best suited for this patient population (14, 46). Studies on warfarin and LMWHs, like dalteparin and nadroparin, have shown that these drugs have very few severe side effects and low hemorrhagic risk in cirrhotic patients (9, 39, 43). A newer class of DOACs, such as dabigatran and direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban), which have been shown to be safe in cirrhotic patients, could be attractive candidates to study the effects of anticoagulation in fibrosis progression in patients with chronic liver diseases (29, 30, 56). We might also predict a greater efficacy of factor Xa inhibitors in fibrosis, as these drugs would more readily inhibit signaling elicited by PAR-2, as well as a secondary reduction in thrombin activity and downstream PAR-1 activation. However, using these clinically approved drugs in cirrhotic patients is complicated because impaired hepatic function in patients could potentially alter the anticoagulation potency, as well as pharmacokinetic/pharmacodynamic characteristics for these drugs (42, 64). Additionally, cirrhotic patients are frequently excluded from clinical trials investigating anticoagulants because of their precariously rebalanced coagulation status and bleeding risks (28). Therefore, additional studies are required to inform on type, dose, and treatment regimens for the existing anticoagulants in patients with cirrhosis.
CONCLUSIONS
Coagulation abnormalities detected by laboratory tests in patients with cirrhosis do not uniformly predispose them to a bleeding risk. In fact, a normal to hypercoagulable coagulative state is evident in cirrhotic patients. Interestingly, strong experimental evidence from animal models suggests that the coagulation cascade can also promote fibrosis, most likely through activation of PAR signaling and stellate cell activation. These experimental observations are mirrored by observations in humans that prothrombotic risk factors are associated with a more rapid progression of fibrosis, and consistent with emerging observations from clinical studies delineating the effect of anticoagulants on liver disease pathogenesis. Several new options for anticoagulation have emerged, concurrent to a deeper understanding of the complex hemostatic changes observed in cirrhotic patients. This combination of new knowledge and therapeutic options makes this an exciting time to consider the possibility that anticoagulant drugs, or newer agents targeting downstream pathways (e.g., PAR antagonists) discovered in experimental settings, could represent novel strategies to reduce liver fibrosis in patients.
GRANTS
This work was supported by the National Institutes of Health Grants R01-ES-017537 and R01-DK-105099.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P., A.K.K., and J.P.L. prepared figures; A.P., A.K.K., and J.P.L. drafted manuscript; A.P., A.K.K., and J.P.L. edited and revised manuscript; A.P., A.K.K., and J.P.L. approved final version of manuscript.
REFERENCES
- 1.Abdel-Salam OM, Baiuomy AR, Ameen A, Hassan NS. A study of unfractionated and low-molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol Res 51: 59–67, 2005. doi: 10.1016/j.phrs.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Abe W, Ikejima K, Lang T, Okumura K, Enomoto N, Kitamura T, Takei Y, Sato N. Low-molecular-weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J Hepatol 46: 286–294, 2007. doi: 10.1016/j.jhep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 130: 248–282, 2011. doi: 10.1016/j.pharmthera.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Aisiku O, Peters CG, De Ceunynck K, Ghosh CC, Dilks JR, Fustolo-Gunnink SF, Huang M, Dockendorff C, Parikh SM, Flaumenhaft R. Parmodulins inhibit thrombus formation without inducing endothelial injury caused by vorapaxar. Blood 125: 1976–1985, 2015. doi: 10.1182/blood-2014-09-599910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstee QM, Goldin RD, Wright M, Martinelli A, Cox R, Thursz MR. Coagulation status modulates murine hepatic fibrogenesis: implications for the development of novel therapies. J Thromb Haemost 6: 1336–1343, 2008. doi: 10.1111/j.1538-7836.2008.03015.x. [DOI] [PubMed] [Google Scholar]
- 6.Assy N, Pettigrew N, Lee SS, Chaudhary RK, Johnston J, Minuk GY. Are chronic hepatitis C viral infections more benign in patients with hemophilia? Am J Gastroenterol 102: 1672–1676, 2007. doi: 10.1111/j.1572-0241.2007.01223.x. [DOI] [PubMed] [Google Scholar]
- 7.Bezerra JA, Bugge TH, Melin-Aldana H, Sabla G, Kombrinck KW, Witte DP, Degen JL. Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver. Proc Natl Acad Sci USA 96: 15143–15148, 1999. doi: 10.1073/pnas.96.26.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borensztajn K, von der Thüsen JH, Peppelenbosch MP, Spek CA. The coagulation factor Xa/protease activated receptor-2 axis in the progression of liver fibrosis: a multifaceted paradigm. J Cell Mol Med 14: 143–153, 2010. doi: 10.1111/j.1582-4934.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerini F, Gonzalez JM, Torres F, Puente Á, Casas M, Vinaixa C, Berenguer M, Ardevol A, Augustin S, Llop E, Senosiaín M, Villanueva C, de la Peña J, Bañares R, Genescá J, Sopeña J, Albillos A, Bosch J, Hernández-Gea V, Garcia-Pagán JC. Impact of anticoagulation on upper-gastrointestinal bleeding in cirrhosis. A retrospective multicenter study. Hepatology 62: 575–583, 2015. doi: 10.1002/hep.27783. [DOI] [PubMed] [Google Scholar]
- 10.Chackalamannil S, Wang Y, Greenlee WJ, Hu Z, Xia Y, Ahn HS, Boykow G, Hsieh Y, Palamanda J, Agans-Fantuzzi J, Kurowski S, Graziano M, Chintala M. Discovery of a novel, orally active himbacine-based thrombin receptor antagonist (SCH 530348) with potent antiplatelet activity. J Med Chem 51: 3061–3064, 2008. doi: 10.1021/jm800180e. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3: 1800–1814, 2005. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 407: 258–264, 2000. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 13.Desmoulière A, Darby I, Costa AM, Raccurt M, Tuchweber B, Sommer P, Gabbiani G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest 76: 765–778, 1997. [PubMed] [Google Scholar]
- 14.Dhar A, Mullish BH, Thursz MR. Anticoagulation in chronic liver disease. J Hepatol 66: 1313–1326, 2017. doi: 10.1016/j.jhep.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Dhar A, Tschotazis E, Brown R, Manousou P, Millson C, Aldersley M, Forbes S, Aggarwal K, Gera A, Cramp M, Holt A, Mutimer D, McPherson S, Burroughs A, Anstee Q, Goldin R, Thursz MR. Warfarin anticoagulation for liver fibrosis in patients transplanted for hepatitis C (Waft-C): results at one year. J Hepatol 62: S268–S269, 2015. doi: 10.1016/S0168-8278(15)30165-3. [DOI] [Google Scholar]
- 16.Duplantier JG, Dubuisson L, Senant N, Freyburger G, Laurendeau I, Herbert JM, Desmoulière A, Rosenbaum J. A role for thrombin in liver fibrosis. Gut 53: 1682–1687, 2004. doi: 10.1136/gut.2003.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmariah SB, Reddy VB, Lerner EA. Cathepsin S signals via PAR2 and generates a novel tethered ligand receptor agonist. PLoS One 9: e99702, 2014. doi: 10.1371/journal.pone.0099702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, Payer BA, Trauner M, Peck-Radosavljevic M, Ferlitsch A. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 56: 1439–1447, 2012. doi: 10.1002/hep.25806. [DOI] [PubMed] [Google Scholar]
- 19.Fiorucci S, Antonelli E, Distrutti E, Severino B, Fiorentina R, Baldoni M, Caliendo G, Santagada V, Morelli A, Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology 39: 365–375, 2004. doi: 10.1002/hep.20054. [DOI] [PubMed] [Google Scholar]
- 20.Flaumenhaft R, De Ceunynck K. Targeting PAR1: Now what? Trends Pharmacol Sci 38: 701–716, 2017. doi: 10.1016/j.tips.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275: 2247–2250, 2000. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 22.Gaça MD, Zhou X, Benyon RC. Regulation of hepatic stellate cell proliferation and collagen synthesis by proteinase-activated receptors. J Hepatol 36: 362–369, 2002. doi: 10.1016/S0168-8278(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 23.Gurbel PA, Bliden KP, Turner SE, Tantry US, Gesheff MG, Barr TP, Covic L, Kuliopulos A. Cell-penetrating pepducin therapy targeting PAR1 in subjects with coronary artery disease. Arterioscler Thromb Vasc Biol 36: 189–197, 2016. doi: 10.1161/ATVBAHA.115.306777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton JR, Trejo J. Challenges and opportunities in protease-activated receptor drug development. Annu Rev Pharmacol Toxicol 57: 349–373, 2017. doi: 10.1146/annurev-pharmtox-011613-140016. [DOI] [PubMed] [Google Scholar]
- 25.Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev 54: 203–217, 2002. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh YC, Lee KC, Yang YY, Huang YH, Hsu WF, Lin HC. Dabigatran reduces liver fibrosis in thioacetamide-injured rats. J Hepatol 66: S657–S657, 2017. doi: 10.1016/S0168-8278(17)31780-4. [DOI] [PubMed] [Google Scholar]
- 27.Hugenholtz GC, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT, Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology 58: 752–761, 2013. doi: 10.1002/hep.26372. [DOI] [PubMed] [Google Scholar]
- 28.Intagliata NM, Caldwell SH. Management of disordered hemostasis and coagulation in patients with cirrhosis. Clin Liver Dis 3: 114–117, 2014. doi: 10.1002/cld.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intagliata NM, Henry ZH, Maitland H, Shah NL, Argo CK, Northup PG, Caldwell SH. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci 61: 1721–1727, 2016. doi: 10.1007/s10620-015-4012-2. [DOI] [PubMed] [Google Scholar]
- 30.Intagliata NM, Maitland H, Northup PG, Caldwell SH. Treating thrombosis in cirrhosis patients with new oral agents: ready or not? Hepatology 61: 738–739, 2015. doi: 10.1002/hep.27225. [DOI] [PubMed] [Google Scholar]
- 31.Isermann B. Homeostatic effects of coagulation protease-dependent signaling and protease activated receptors. J Thromb Haemost 15: 1273–1284, 2017. doi: 10.1111/jth.13721. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Yau MK, Lim J, Wu KC, Xu W, Suen JY, Fairlie DP. A potent antagonist of protease-activated receptor 2 that inhibits multiple signaling functions in human cancer cells. J Pharmacol Exp Ther 364: 246–257, 2018. doi: 10.1124/jpet.117.245027. [DOI] [PubMed] [Google Scholar]
- 33.Jiang ZG, Feldbrügge L, Tapper EB, Popov Y, Ghaziani T, Afdhal N, Robson SC, Mukamal KJ. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther 43: 734–743, 2016. doi: 10.1111/apt.13515. [DOI] [PubMed] [Google Scholar]
- 34.Joshi N, Kopec AK, O’Brien KM, Towery KL, Cline-Fedewa H, Williams KJ, Copple BL, Flick MJ, Luyendyk JP. Coagulation-driven platelet activation reduces cholestatic liver injury and fibrosis in mice. J Thromb Haemost 13: 57–71, 2015. doi: 10.1111/jth.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi N, Kopec AK, Ray JL, Cline-Fedewa H, Nawabi A, Schmitt T, Nault R, Zacharewski TR, Rockwell CE, Flick MJ, Luyendyk JP. Fibrin deposition following bile duct injury limits fibrosis through an αMβ2-dependent mechanism. Blood 127: 2751–2762, 2016. doi: 10.1182/blood-2015-09-670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi N, Kopec AK, Towery K, Williams KJ, Luyendyk JP. The antifibrinolytic drug tranexamic acid reduces liver injury and fibrosis in a mouse model of chronic bile duct injury. J Pharmacol Exp Ther 349: 383–392, 2014. doi: 10.1124/jpet.113.210880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 103: 879–887, 1999. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kallis YN, Scotton CJ, Mackinnon AC, Goldin RD, Wright NA, Iredale JP, Chambers RC, Forbes SJ. Proteinase activated receptor 1-mediated fibrosis in a mouse model of liver injury: a role for bone marrow derived macrophages. PLoS One 9: e86241, 2014. doi: 10.1371/journal.pone.0086241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoury T, Ayman AR, Cohen J, Daher S, Shmuel C, Mizrahi M. The complex role of anticoagulation in cirrhosis: an updated review of where we are and where we are going. Digestion 93: 149–159, 2016. doi: 10.1159/000442877. [DOI] [PubMed] [Google Scholar]
- 40.Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology 55: 879–887, 2012. doi: 10.1002/hep.24784. [DOI] [PubMed] [Google Scholar]
- 41.Kopec AK, Luyendyk JP. Role of fibrin(ogen) in progression of liver disease: guilt by association? Semin Thromb Hemost 42: 397–407, 2016. doi: 10.1055/s-0036-1579655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubitza D, Roth A, Becka M, Alatrach A, Halabi A, Hinrichsen H, Mueck W. Effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of a single dose of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 76: 89–98, 2013. doi: 10.1111/bcp.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol 23: 13–21, 2017. doi: 10.3350/cmh.2016.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair 6: 19, 2013. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 44: 53–61, 2006. doi: 10.1002/hep.21231. [DOI] [PubMed] [Google Scholar]
- 46.Lisman T, Kamphuisen PW, Northup PG, Porte RJ. Established and new-generation antithrombotic drugs in patients with cirrhosis—possibilities and caveats. J Hepatol 59: 358–366, 2013. doi: 10.1016/j.jhep.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Lisman T, Leebeek FW, de Groot PG. Haemostatic abnormalities in patients with liver disease. J Hepatol 37: 280–287, 2002. doi: 10.1016/S0168-8278(02)00199-X. [DOI] [PubMed] [Google Scholar]
- 48.Lisman T, Luyendyk JP. Platelets as modulators of liver diseases. Semin Thromb Hemost 44: 114–125, 2018. doi: 10.1055/s-0037-1604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood 116: 878–885, 2010. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 50.Luyendyk JP, Kassel KM, Allen K, Guo GL, Li G, Cantor GH, Copple BL. Fibrinogen deficiency increases liver injury and early growth response-1 (Egr-1) expression in a model of chronic xenobiotic-induced cholestasis. Am J Pathol 178: 1117–1125, 2011. doi: 10.1016/j.ajpath.2010.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol 24: 1015–1022, 2004. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 52.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol 25: 2273–2281, 2005. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 53.Maharshak N, Halfon P, Deutsch V, Peretz H, Berliner S, Fishman S, Zelber-Sagi S, Rozovski U, Leshno M, Oren R. Increased fibrosis progression rates in hepatitis C patients carrying the prothrombin G20210A mutation. World J Gastroenterol 17: 5007–5013, 2011. doi: 10.3748/wjg.v17.i45.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maieron A, Salzl P, Peck-Radosavljevic M, Trauner M, Hametner S, Schöfl R, Ferenci P, Ferlitsch M. Von Willebrand factor as a new marker for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther 39: 331–338, 2014. doi: 10.1111/apt.12564. [DOI] [PubMed] [Google Scholar]
- 55.Martínez AK, Maroni L, Marzioni M, Ahmed ST, Milad M, Ray D, Alpini G, Glaser SS. Mouse models of liver fibrosis mimic human liver fibrosis of different etiologies. Curr Pathobiol Rep 2: 143–153, 2014. doi: 10.1007/s40139-014-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez M, Tandra A, Vuppalanchi R. Treatment of acute portal vein thrombosis by nontraditional anticoagulation. Hepatology 60: 425–426, 2014. doi: 10.1002/hep.26998. [DOI] [PubMed] [Google Scholar]
- 57.Mihara K, Ramachandran R, Renaux B, Saifeddine M, Hollenberg MD. Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1). J Biol Chem 288: 32979–32990, 2013. doi: 10.1074/jbc.M113.483123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol 12: 713–724, 2013. [PubMed] [Google Scholar]
- 59.Müller A, Machnik F, Zimmermann T, Schubert H. Thioacetamide-induced cirrhosis-like liver lesions in rats—usefulness and reliability of this animal model. Exp Pathol 34: 229–236, 1988. doi: 10.1016/S0232-1513(88)80155-5. [DOI] [PubMed] [Google Scholar]
- 60.Nault R, Fader KA, Kopec AK, Harkema JR, Zacharewski TR, Luyendyk JP. From the cover: coagulation-driven hepatic fibrosis requires protease activated receptor-1 (PAR-1) in a mouse model of TCDD-elicited steatohepatitis. Toxicol Sci 154: 381–391, 2016. doi: 10.1093/toxsci/kfw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Northup PG, Sundaram V, Fallon MB, Reddy KR, Balogun RA, Sanyal AJ, Anstee QM, Hoffman MR, Ikura Y, Caldwell SH; Coagulation in Liver Disease Group . Hypercoagulation and thrombophilia in liver disease. J Thromb Haemost 6: 2–9, 2008. doi: 10.1111/j.1538-7836.2007.02772.x. [DOI] [PubMed] [Google Scholar]
- 62.Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth 58: 515–523, 2014. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plompen EP, Darwish Murad S, Hansen BE, Loth DW, Schouten JN, Taimr P, Hofman A, Uitterlinden AG, Stricker BH, Janssen HL, Leebeek FW. Prothrombotic genetic risk factors are associated with an increased risk of liver fibrosis in the general population: The Rotterdam Study. J Hepatol 63: 1459–1465, 2015. doi: 10.1016/j.jhep.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Potze W, Arshad F, Adelmeijer J, Blokzijl H, van den Berg AP, Meijers JC, Porte RJ, Lisman T. Differential in vitro inhibition of thrombin generation by anticoagulant drugs in plasma from patients with cirrhosis. PLoS One 9: e88390, 2014. doi: 10.1371/journal.pone.0088390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J Biol Chem 286: 24638–24648, 2011. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rullier A, Gillibert-Duplantier J, Costet P, Cubel G, Haurie V, Petibois C, Taras D, Dugot-Senant N, Deleris G, Bioulac-Sage P, Rosenbaum J. Protease-activated receptor 1 knockout reduces experimentally induced liver fibrosis. Am J Physiol Gastrointest Liver Physiol 294: G226–G235, 2008. doi: 10.1152/ajpgi.00444.2007. [DOI] [PubMed] [Google Scholar]
- 67.Shearer AM, Rana R, Austin K, Baleja JD, Nguyen N, Bohm A, Covic L, Kuliopulos A. Targeting liver fibrosis with a cell-penetrating protease-activated receptor-2 (PAR2) pepducin. J Biol Chem 291: 23,188–23,198, 2016. doi: 10.1074/jbc.M116.732743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simonetto DA, Yang HY, Yin M, de Assuncao TM, Kwon JH, Hilscher M, Pan S, Yang L, Bi Y, Beyder A, Cao S, Simari RD, Ehman R, Kamath PS, Shah VH. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology 61: 648–659, 2015. doi: 10.1002/hep.27387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, Esposito A, Ruggeri ZM, Chisari FV, Iannacone M, Guidotti LG. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA 109: E2165–E2172, 2012. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Søgaard KK, Horváth-Puhó E, Grønbaek H, Jepsen P, Vilstrup H, Sørensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol 104: 96–101, 2009. doi: 10.1038/ajg.2008.34. [DOI] [PubMed] [Google Scholar]
- 71.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol 160: 191–203, 2010. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stalker TJ, Newman DK, Ma P, Wannemacher KM, Brass LF. Platelet signaling. In: Antiplatelet Agents, edited by Gresele P, Born GVR, Patrono C, Page CP. Berlin: Springer, 2012, p. 59–85. doi: 10.1007/978-3-642-29423-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sullivan BP, Weinreb PH, Violette SM, Luyendyk JP. The coagulation system contributes to αVβ6 integrin expression and liver fibrosis induced by cholestasis. Am J Pathol 177: 2837–2849, 2010. doi: 10.2353/ajpath.2010.100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Q, Wang Y, Zhang J, Lu J. ENMD-1068 inhibits liver fibrosis through attenuation of TGF-β1/Smad2/3 signaling in mice. Sci Rep 7: 5498, 2017. doi: 10.1038/s41598-017-05190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp 96: e52438, 2015. doi: 10.3791/52438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost 9: 1713–1723, 2011. doi: 10.1111/j.1538-7836.2011.04429.x. [DOI] [PubMed] [Google Scholar]
- 77.Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 41: 553–558, 2005. doi: 10.1002/hep.20569. [DOI] [PubMed] [Google Scholar]
- 78.Vilaseca M, García-Calderó H, Lafoz E, García-Irigoyen O, Avila MA, Reverter JC, Bosch J, Hernández-Gea V, Gracia-Sancho J, García-Pagán JC. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology 65: 2031–2044, 2017. doi: 10.1002/hep.29084. [DOI] [PubMed] [Google Scholar]
- 79.Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S, Lei B, Bernabucci V, Vukotic R, De Maria N, Schepis F, Karampatou A, Caporali C, Simoni L, Del Buono M, Zambotto B, Turola E, Fornaciari G, Schianchi S, Ferrari A, Valla D. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 143: 1253–1260.e4, 2012. doi: 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 80.Wright M, Goldin R, Hellier S, Knapp S, Frodsham A, Hennig B, Hill A, Apple R, Cheng S, Thomas H, Thursz M. Factor V Leiden polymorphism and the rate of fibrosis development in chronic hepatitis C virus infection. Gut 52: 1206–1210, 2003. doi: 10.1136/gut.52.8.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida S, Ikenaga N, Liu SB, Peng ZW, Chung J, Sverdlov DY, Miyamoto M, Kim YO, Ogawa S, Arch RH, Schuppan D, Popov Y. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 147: 1378–1392, 2014. doi: 10.1053/j.gastro.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 82.Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, Kocan M, Sostegni S, Haerteis S, Baraznenok V, Henderson I, Lindström E, Guerrero-Alba R, Valdez-Morales EE, Liedtke W, McIntyre P, Vanner SJ, Korbmacher C, Bunnett NW. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem 289: 27215–27234, 2014. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Front Endocrinol (Lausanne) 5: 67, 2014. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]