Abstract
Cytosolic phosphoenolpyruvate carboxykinase (PEPCK) is a gluconeogenic enzyme that is highly expressed in the liver and kidney but is also expressed at lower levels in a variety of other tissues where it may play adjunct roles in fatty acid esterification, amino acid metabolism, and/or TCA cycle function. PEPCK is expressed in the enterocytes of the small intestine, but it is unclear whether it supports a gluconeogenic rate sufficient to affect glucose homeostasis. To examine potential roles of intestinal PEPCK, we generated an intestinal PEPCK knockout mouse. Deletion of intestinal PEPCK ablated ex vivo gluconeogenesis but did not significantly affect glycemia in chow, high-fat diet, or streptozotocin-treated mice. In contrast, postprandial triglyceride secretion from the intestine was attenuated in vivo, consistent with a role in fatty acid esterification. Intestinal amino acid profiles and 13C tracer appearance into these pools were significantly altered, indicating abnormal amino acid trafficking through the enterocyte. The data suggest that the predominant role of PEPCK in the small intestine of mice is not gluconeogenesis but rather to support nutrient processing, particularly with regard to lipids and amino acids.
NEW & NOTEWORTHY The small intestine expresses gluconeogenic enzymes for unknown reasons. In addition to glucose synthesis, the nascent steps of this pathway can be used to support amino acid and lipid metabolisms. When phosphoenolpyruvate carboxykinase, an essential gluconeogenic enzyme, is knocked out of the small intestine of mice, glycemia is unaffected, but mice inefficiently absorb dietary lipid, have abnormal amino acid profiles, and inefficiently catabolize glutamine. Therefore, the initial steps of intestinal gluconeogenesis are used for processing dietary triglycerides and metabolizing amino acids but are not essential for maintaining blood glucose levels.
Keywords: amino acids, cataplerosis, gluconeogenesis, glyceroneogenesis, small intestine
INTRODUCTION
Cytosolic phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the decarboxylation of oxaloacetate (OAA) to phosphoenolpyruvate (PEP) and is most notable for its role in gluconeogenesis. More generally, it is a cataplerotic pathway that facilitates the export of organic acids from the TCA cycle. In the liver, PEPCK flux balances pyruvate carboxylation and other pathways of anaplerosis, where it is necessary for gluconeogenesis and normal glycogen storage (4, 14, 41). In adipose tissue and mammary glands, PEPCK supports glyceroneogenesis, a pathway that produces glycerol 3-phosphate for fatty acid esterification (17, 39). In the proximal tubule of the kidney, glutaminolysis generates ammonium and the TCA cycle intermediate α-ketoglutarate (αKG) in response to starvation or acidosis, and PEPCK-mediated cataplerosis prevents the buildup of intermediates by routing them into the gluconeogenic pathway (2). As a cataplerotic pathway of the TCA cycle, PEPCK also interacts with cellular energy metabolism (33). For example, PEPCK deletion leads to an accumulation of TCA cycle intermediates and decreased oxidative metabolism in liver (4, 5), whereas overexpression of PEPCK in muscle endows remarkable exercise stamina (15). In tissues where the function of PEPCK is not explicitly understood, it is presumed to have one of these several roles.
Enterocytes in the small intestine express PEPCK and have an active gluconeogenic pathway (8, 21, 35, 46). The rat small intestine may make glucose as a by-product of glutamine metabolism (46), and it has been suggested that 17% of fasting endogenous glucose production (EGP) originates from the small intestine. During streptozotocin (STZ)-induced diabetes, intestinal glucose production may be as high as 25% of EGP in the rat (8). Yet, the technical challenges of measuring gluconeogenesis across the splanchnic bed in rodents are great; therefore, it is not clear whether intestinal gluconeogenesis could be accurately detected if it exists (27, 35). Nevertheless, a functional cataplerotic pathway may be required in the small intestine for metabolic responsibilities not directly related to gluconeogenesis. Dietary triglyceride is hydrolyzed to free fatty acids (FFAs) and monoacylglyceride in the intestinal lumen by pancreatic lipases (1) but must be reesterified to triglyceride before chylomicron assembly and secretion into circulation. Although the monoglyceride acyltransferase (MGAT) pathway can rebuild triglyceride from existing monoacylglyceride, a significant portion of FFAs are esterified through the glycerol-3-phosphate acyltransferase (GPAT) pathway using newly synthesized glycerol-3-phosphate. If this latter pathway operates analogous to its function in adipose tissue, then PEPCK may be required for absorption, reesterification (20, 32), and secretion of dietary lipids. The small intestine also maintains highly active pathways of amino acid metabolisms, which require cataplerosis. For example, glutamine is avidly used by the small intestine as an energy source (21, 43, 47) or as a substrate for the synthesis of alanine, which is subsequently utilized as a gluconeogenic substrate by the liver. In either case, glutamine must initially be converted to pyruvate through the combined reactions of the TCA cycle and malic enzyme (ME, malate→pyruvate) or PEPCK/pyruvate kinase (OAA→PEP→pyruvate). Therefore, the PEPCK pathway in the small intestine may play multiple roles by facilitating glucose production, lipid absorption/secretion, and amino acid catabolism.
The goal of this study was to examine the function of intestinal PEPCK on intestinal macronutrient metabolism and whole-body energy balance. We hypothesized that in the small intestine, unlike in the liver, the primary role of PEPCK is not to produce glucose but rather to support glyceroneogenesis for triglyceride production and TCA cycle cataplerosis for amino acid catabolism. We tested this hypothesis by generating a small intestine PEPCK knockout (SIPKO) mouse and measured glucose homeostasis, lipid absorption, amino acid metabolism, and enterocyte energetics. SIPKO mice demonstrated that intestinal PEPCK is not essential for normal glycemia or glucose production, nor does its loss protect glycemia during STZ-induced type 1 diabetes or diet-induced obesity. In contrast, SIPKO mice had 1) modest impairments in dietary lipid absorption and secretion, 2) altered amino acid profiles and impaired amino acid catabolism, and 3) elevated TCA cycle intermediates and altered intestinal redox state.
METHODS
Mice.
SIPKO mice were bred by crossing phosphoenolpyruvate carboxykinase 1 floxed (Pck1fl/fl) (41) and mice that express Cre in the intestinal mucosa (colon, cecum, and small intestine) under control of the villin promoter, 12.4KbVilCre (26). The resulting genetic combinations were Pck1fl/fl (controls) and Pck1fl/fl + Villin-Cre+/− (SIPKO). All mice were male and housed at 22°C on a 12-h: 12-h light-dark cycle (6 am to 6 pm). Animal procedures were approved by the University of Texas Southwestern Medical Center at Dallas Animal Care and Use Committee and were conducted in conformity with the Public Health Service Policy on Human Care and Use of Laboratory Animals.
Western blot analysis.
Whole protein extracts were prepared from frozen tissue of fed and fasted mice and homogenized in 1× radioimmunoprecipitation assay buffer by a Powermax AHS homogenizer (VWR International, Radnor, PA). Proteins were separated by SDS-PAGE 10% and transferred to protran nitrocellulose transfer membranes (Whatman plc, Maidstone, UK) by Bio-Rad Trans-Blot Turbo System (Bio-Rad, Hercules, CA). After blocking in 5% wt/vol nonfat dry milk, Tris-buffered saline, and 0.1% Tween 20 for 1 h, the membrane was incubated with polyclonal rabbit primary antibody (PEPCK1, 1:1,000 5% BSA in Tris-buffered saline 0.1% Tween 20) followed by incubation with antirabbit immunoglobulin linked to peroxidase diluted at 1:5,000 (Cell Signaling Technology, Beverly, MA). Detection was performed using SuperSignal West Pico Enhanced Chemiluminescent substrate (Thermo Scientific, Waltham, MA) and exposure to high-sensitivity photographic film (BioMax ML, Kodak, Rochester, NY).
Gene expression.
Mice were fed ad libitum or fasted for 24 h on grates before euthanasia under isoflurane anesthesia. Intestinal tissue was quickly rinsed with ice-cold saline, trimmed of adipose, and segmented into duodena (first 6 cm), jejuna, and ilea (last 10 cm). Intestinal segments were frozen in liquid nitrogen and pulverized by a large Bessman Tissue Pulverizer (SpectrumLabs, USA). RNA was extracted by STAT60 (Tel-Test, Friendswood, TX) and then DNase treated with RNase-Free DNase Set (Qiagen, Valencia, CA). cDNA was synthesized from 1 µg DNase-treated RNA by High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was run in triplicate using SYBR GreenERTM qPCR SuperMix for ABI PRISM instrument (Invitrogen, Carlsbad, CA) and ABI PRISM 7900HT Fast Real-Time PCR System (Applied Biosystems). Gene expression was normalized to cyclophilin b (PpiB).
Pck1del - For - 5′-GCTGGAGGCCCTGGGAGATGGGGAG-3′
Pck1del - Rev - 5′-CTCTCTGCGGTCCGGGAGGTGGGCG-3′
Ppib - For - 5′-GGAGATGGCACAGGAGGAA-3′
Ppib - Rev - 5′-GCCCGTAGTGCTTCAGCTT-3′
High-fat diet.
Mice were fed a standard chow diet (2016 Teklad Global Rodent Diet, kcal%: 22% protein, 12% fat, and 66% carbohydrate; Envigo, Huntingdon, UK) or a 60% calorie-from-fat high-fat diet (HFD; D12492, kcal%: 20% protein, 60% fat, and 20% carbohydrate; Research Laboratories Inc., Fort Wayne, IA) from the time of weaning. Body weights were monitored biweekly with exceptions at 8, 18, 20, and 24 wk. Plasma glucose was measured in 12-h fasted mice maintained on the diets for 16 wk.
STZ-induced type 1 diabetes mellitus.
On days 1 through 4, control and knockout mice were either treated with 200 mg/kg body wt STZ (S-0130 Sigma S-0130-5G) or vehicle alone as described previously (48). Fed ad libitum, 10-h fasted, and 24-h fasted glucose concentrations were measured on day 21.
Blood glucose concentrations.
Except where noted otherwise, mice were fed ad libitum or fasted for 24 h (2 pm to 2 pm) on grates, and blood glucose was measured by Aviva Accu-Chek (Roche, Indianapolis, IN). An oral glucose tolerance test (200 µl/mouse of 250 mg/m glucose solution) was performed on 24-h fasted mice. Tail vein blood glucose was measured during the test by Aviva Accu-Chek glucometer (Roche) at 0, 30, 60, 90, and 120 min.
Everted small intestine perfusion.
Mice were fasted for 24 h on metal grates. Mice were anesthetized with intramuscular ketamine/xylazine (100 µl/20 g body wt, 87.5 mg/kg ketamine, 12.5 g/kg xylazine) and heparin (100 μl of 1,000 United States Pharmacopeia units/ml) as an anticoagulant. The small intestine was excised and placed in ice-cold phosphate-buffered saline (PBS; pH 7.2) containing calcium and magnesium. The intestine was treated as previously described (16), with some modifications. The everted intestine was tied to a 15-gauge gavage needle at the duodenum and a 20-gauge gavage needle at the ileum. The intestine was placed into a beaker containing PBS with calcium and magnesium (pH 7.2, at 37°C). The everted intestine was then connected to a peristaltic pump flowing at 0.5 ml/min a nonrecirculating perfusion medium made of a custom 1:1 DMEM/F-12 containing no glucose, no pyruvate, and no glutamine but supplemented with 1 mM [U-13C5]l-glutamine (605166, Sigma-Aldrich) for 70 min. The last 60 min of effluent perfusate was collected on ice.
Endogenous glucose production.
Mice were surgically fit with jugular vein catheters 5 days before glucose infusion as previously described (6). After fasting for 24 h (2 pm to 2 pm), mice were infused with 3.75 mg/ml d-glucose-13C6 (389374, Sigma-Aldrich) in normal saline at a priming rate of 0.30 ml/h for 10 min, followed by 80 min at 0.06 ml/h to maintain a steady state. Mice were then anesthetized with isoflurane, and blood was rapidly collected via cardiac puncture. Plasma glucose enrichment was determined as M+6 glucose detected by GC-MS, and EGP was calculated as the fold-dilution of the glucose tracer times the infusion rate (40).
Jejunum glycogen content.
Frozen jejuna (~50 mg) were homogenized in 300 µl of 10% perchloric acid. Homogenates were centrifuged at 4°C at 2,300 relative centrifugal force (rcf) for 10 min. Supernatants were collected and 1 ml of 95% ethanol was added to precipitate glycogen. The suspension was centrifuged at 4°C at 23,000 rcf for 20 min, and the resulting glycogen pellet was washed two more times with 95% ethanol. Ethanol was removed by suction and allowed to dry. Glycogen was hydrolyzed by 1 mg/ml amyloglucosidase (A-7095, Sigma-Aldrich) in 50 mM sodium acetate at 55°C for 1 h. Glucose units were measured by GC-MS. A d-Glucose-13C6, 1, 2, 3, 4, 5, 6, 6-d7 (552151 Sigma-Aldrich) internal standard was added to each sample.
Oral triglyceride tolerance test.
Triglyceride tolerance tests were performed as previously described (44). After an overnight fast (6 pm to 9 am), 20 µl of blood was collected from the submandibular vein and used to determine baseline plasma triglyceride concentration. Immediately following an initial blood collection, mice were intragastrically administered 200 µl of olive oil. Blood samples were collected at 1, 2, 3, and 4 h. Plasma triglyceride concentration was assayed by Wako L-Type Triglyceride determination kit (Wako Chemical USA, Richmond, VA).
Postprandial intestine triglyceride secretion.
Triglyceride secretion was performed as previously described (44). Briefly, after an overnight fast (6 pm to 9 am) mice were injected intraperitoneally with 500 mg/kg Tyloxapol (T-0307 Sigma-Aldrich), an inhibitor of capillary lipases. After 30 min, mice were intragastrically administered 200 µl of olive oil. Submandibular blood was collected at 0, 2, and 4 h. Plasma triglyceride concentration was assayed by Wako L-Type Triglyceride determination kit (Wako Chemical USA).
Total fat absorption.
Dietary fat absorption was measured as described previously (18). Briefly, a semisynthetic 30% butter fat diet containing 5% sucrose polybehenate, a nonabsorbable fecal marker, was fed to the mice for 3 days. On the third and fourth days, three fecal pellets were collected. These pellets were pulverized and saponified in 0.5 N methanolic sodium hydroxide at 80°C. After cooling, the solution was treated with trifluoroborane. The resulting sample contained methyl esters of all fecal FFAs (and saponified triglycerides). Food and fecal fatty acid methyl esters were analyzed by GC-MS. Fat absorption was calculated by the ratios of behenic acid to other dietary fatty acids from the diet and in the feces. Sample analysis, materials, and services were provided by the University of Cincinnati, Mouse Metabolic Phenotyping Center.
Glutamine tolerance test.
Mice were fasted for 24 h (2 pm to 2 pm) and gavaged with 200 μl of 240 mM U-13C5-l-glutamine. Precisely 10 min post gavage, mice were anesthetized, blood was collected, and intestines immediately rinsed in ice-cold PBS. Samples were frozen in liquid nitrogen and stored at −80°C until processing.
Glucose concentrations and enrichment analyses.
Plasma samples (50–100 µl) were deproteinized by addition of cold acetone and centrifuged at 21,000 rcf for 10 min. Tissue samples (50–100 mg) were homogenized in 450 µl of 4% perchloric acid and centrifuged, and the supernatant was applied to a cation exchange resin (Dowex 50-WX-8 hydrogen form, ~3-ml column size, Sigma-Aldrich) and eluted with 4 ml of deionized water. Upon elution, the sample was lyophilized, and glucose was derivatized to di-O-isopropylidene acetate for GC-MS (GC 7890-A and MSD 5975-C, Agilent Technologies) as previously described (13). Mass isotopomers were corrected for natural abundance as previously described (11). In some samples, a known amount of d-Glucose-13C6, 1, 2, 3, 4, 5, 6, 6-d7 (552151 Sigma-Aldrich) was spiked into each sample before processing to determine glucose concentration.
Amino acid concentrations and enrichment analyses.
Tissue samples were handled as described above, but amino acids were eluted from the ion exchange resin with 4 ml of 2.0 M NH4OH. The sample was lyophilized and derivatized for liquid chromatography tandem mass spectrometry (LC-20-AD, Shimadzu, and MDS API-3200, Applied Biosystems) as previously described (7). In some cases, a known amount of isotopically labeled amino acids was added to samples before processing to determine concentrations.
Organic acid concentrations.
Approximately 100 mg of jejunum was analyzed by GC-MS for organic acids, including TCA cycle intermediates αKG, citrate, fumarate, lactate, malate, OAA, pyruvate, and succinate, as described previously (10).
Enterocyte oxygen consumption.
Mice fed ad libitum were euthanized, and the small intestine was immediately rinsed in ice-cold PBS. Intestines were cut longitudinally to expose the enterocytes. On a cold glass mirror, intestine mucosa were scraped free of the lamina propria with a microscope slide, placed in an 1.5-ml tube, rinsed with PBS, vortexed for ~20 s at 7/10 vortex, centrifuged at 4°C at 50 rcf for 3 min, and enterocytes were resuspended in 300 μl of PBS per 750 μl cells. Oxygen consumption was measured in a Hansatech Instruments, Oxygraph Plus System at 37°C. Reaction media was similar to the everted intestine perfusion, 1:1 DMEM/F-12 without glucose, without glutamine, and without pyruvate, but glucose (5 mM) and glutamine (5 mM) were supplemented. The 2.0-ml reaction volume was allowed to equilibrate with air. Then 50 μl of enterocyte suspension was added to the media alone, and the lid was sealed. After oxygen consumption reached linearity for 60 s, an anaplerotic challenge was induced by ~400 mM sodium propionate. Oxygen consumption was allowed to stabilize, and a 60-s average was recorded. Oxygen consumption was normalized to protein content.
Statistical analyses.
All data are presented as means ± SE. P < 0.05 was considered statistically significant. For comparisons between groups, Student’s t-tests were used. To determine the main effects of, and interactions between, time and genotype, a two-way analysis of variance (ANOVA) with repeated measures, followed by either Sidak’s multiple comparisons or Student’s t-test, when appropriate. Analyses were conducted using GraphPad Prism statistical analysis software (version 6.03, GraphPad Software, La Jolla, CA).
RESULTS
Authentication of intestinal Pck1 knockout.
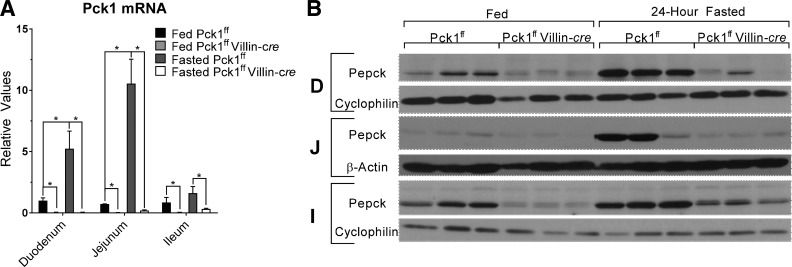
Analysis of mRNA by quantitative real-time PCR was performed to examine Pck1 transcript in the small intestine. Pck1 mRNA was induced during fasting by 12-fold in the jejuna, fivefold in the duodena, and twofold in the ilea of control mice (Fig. 1A). PEPCK protein levels displayed a similar regional response to fasting (Fig. 1B). The loss of Pck1 in SIPKO mice was confirmed by mRNA and Western blotting in the duodena, jejuna, and ilea (Fig. 1, A and B).
Fig. 1.
Intestinal cytosolic phosphoenolpyruvate carboxykinase (Pck1) is responsive to feeding and fasting and is lost in small intestine PEPCK knockout (SIPKO) mice. A: quantitative real-time PCR of Pck1 mRNA in the duodenum (D), jejunum (J), and ileum (I) from fed and 24-h fasted control and SIPKO mice. B: Western blot analysis of PEPCK protein content in response to feeding and fasting. *P < 0.05 by t-test, n = 3 mice. PEPCK; phosphoenolpyruvate carboxykinase.
Deletion of Pck1 impaired intestinal gluconeogenesis but did not affect systemic glycemia.
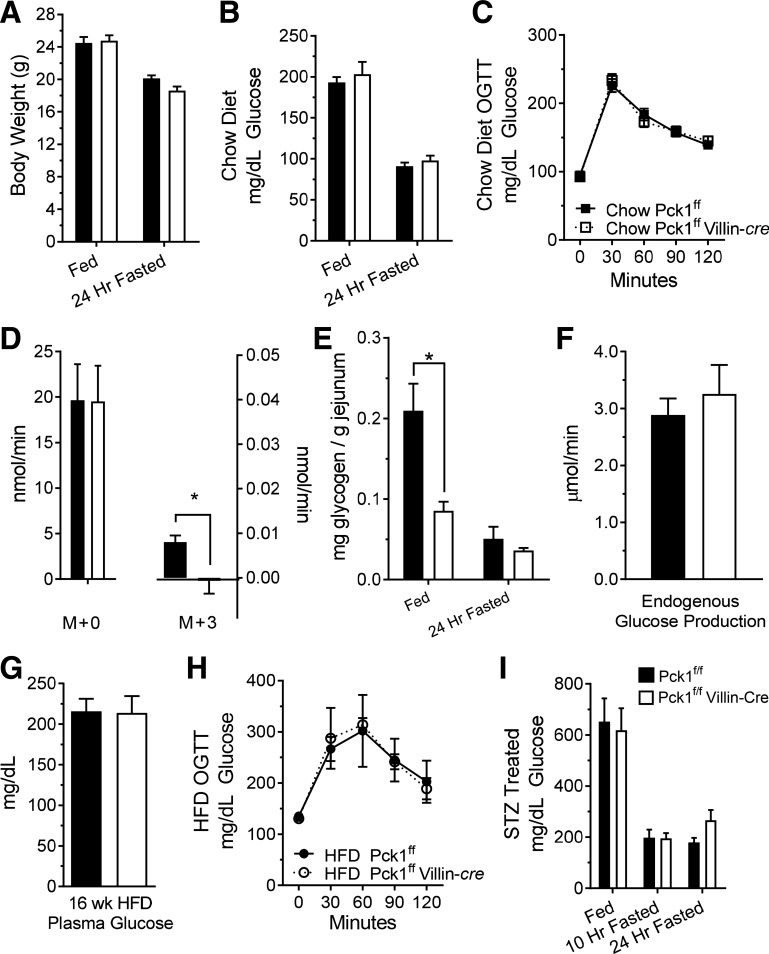
The small intestine expresses all of the enzymes necessary for gluconeogenesis, and some studies have indicated that it may play a role in glucose homeostasis (8, 36, 37). Therefore, we investigated whether Pck1 deletion altered small intestine gluconeogenesis and/or systemic glycemia. SIPKO mice had normal body weights (Fig. 2A), plasma glucose levels (Fig. 2B), and glucose tolerance (Fig. 2C). To evaluate the gluconeogenic potential of the small intestine and to confirm that Pck1 deletion ablates gluconeogenesis, we perfused everted intestine with media containing [U-13C]glutamine. The conversion of glutamine to glucose could be detected as the appearance of glucose M + 1, M + 2, and M + 3 in the effluent of control intestines but was not detected in the effluent of SIPKO intestines (Fig. 2D). Both control and SIPKO small intestines released unlabeled glucose (M + 0) at a rate much higher than the labeled glucose. The appearance of unlabeled glucose is expected based on the utilization of other unlabeled substrates, the dilution of label in the TCA cycle, and/or glycogenolysis. Inasmuch as unlabeled glucose was produced by the SIPKO intestine, we suspected that a substantial portion originated from glycogen. The jejuna of fed mice indeed stored glycogen, which was reduced by fasting (Fig. 2E). The jejuna of SIPKO mice stored only about half as much glycogen as control mice, but this was still likely sufficient to account for unlabeled glucose production detected in the tracer experiment. To determine whether intestinal Pck1 deletion had an effect on EGP, we performed in vivo glucose tracer infusions in conscious, unrestrained mice. EGP was not different between control and SIPKO mice and was notably 100-fold higher than glucose production from the ex vivo small intestine (Fig. 2F).
Fig. 2.
Effect of small intestine PEPCK knockout (SIPKO) on glycemia. A: body weight on chow diet; n = 8. B: fed/fasted plasma glucose concentration on chow diet; n = 8. C: oral glucose tolerance test; n = 10. D: mass-enriched glucose production by everted small intestine perfused with [U-13C5]glutamine; n = 3. E: glycogen content of small intestine; n = 6. F: endogenous glucose production determined by dilution of infused [U-13C6]glucose; n = 7. G: fasting plasma glucose concentration after 16 wk of a 60% high-fat diet (HFD); n = 6. H: oral glucose tolerance test after 16 wk of a 60% HFD; n = 3. I: plasma glucose concentration after streptozotocin (STZ) treatment; n = 7 mice. *P < 0.05 by two-tailed t-test. n = no. of mice. OGTT, Oral Glucose Tolerance Test.
Gluconeogenesis in the small intestine may play a signaling role in the portal system that impacts glycemic regulation (9, 34), especially during diabetes (29, 37). However, neither fasting plasma glucose (Fig. 2G) nor glucose tolerance (Fig. 2H) in SIPKO mice on a 60% HFD for 16 wk was different from control mice on the same diet. To examine a more severe model of insulinopenic diabetes, we treated the mice with STZ and examined plasma glucose levels. There was no difference in plasma glucose levels of STZ-treated SIPKO mice versus controls following feeding, a 10-h fast, or a 24-h fast (Fig. 2I). Overall, we were able to confirm that isolated small intestines produce glucose and perform gluconeogenesis from glutamine, and although PEPCK is essential for this latter pathway, it was not essential for the regulation of glycemia or the development of hyperglycemia during diet-induced or insulinopenic diabetes.
Pck1 deletion impairs triglyceride absorption.
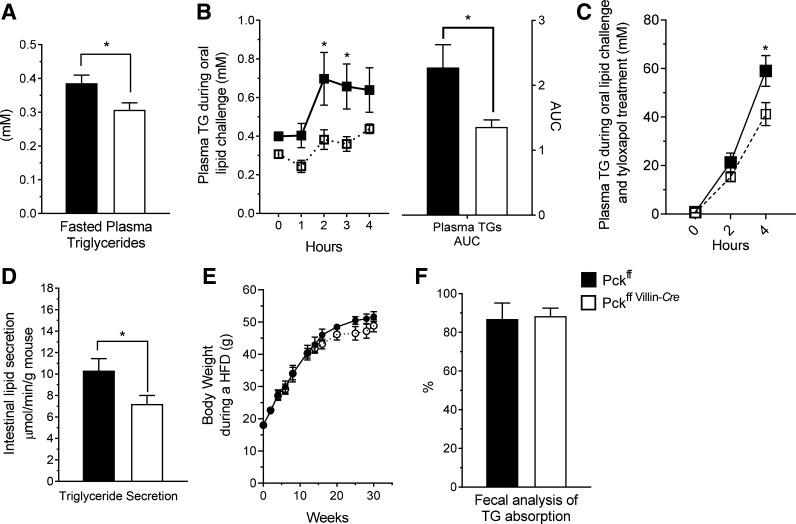
Glyceroneogenesis utilizes the seminal pathways of gluconeogenesis, including PEPCK, to produce glycerol-3P, which is important for the esterification of FFAs (22, 39). Because intestinal absorption of triglyceride involves its hydrolysis and reesterification, we tested whether intestinal PEPCK is important for absorption and secretion of dietary lipids. After an overnight fast, SIPKO mice had lower circulating triglycerides (Fig. 3A) and were remarkably unresponsive to a triglyceride challenge (Fig. 3B). To control for peripheral utilization, the experiment was repeated while blocking triglyceride clearance with Tyloxapol. Under these conditions, SIPKO mice had decreased intestinal triglyceride secretion as indicated by lower plasma triglyceride concentrations over the 4-h time course (Fig. 3C) and by lower secretion rates calculated from that data (Fig. 3D). Despite reduced lipid absorption rate, SIPKO mice were not protected from weight gain during an HFD (Fig. 3E). Total lipid absorption, as indicated by fecal analysis of a nonabsorbable lipid marker, was not substantially reduced (Fig. 3F). Hence, lipid absorption in SIPKO mice appears to be slower than normal but not otherwise incomplete.
Fig. 3.
Cystolic phosphoenolpyruvate carboxykinase (Pck1) deletion impairs small intestine triglyceride metabolism. A: plasma triglyceride concentration; n = 10. B: plasma triglyceride concentration and area under the curve (AUC) following an oral dose of 200 µl of olive oil; n = 10. C: plasma triglyceride concentration and AUC after 500 mg/kg Tyloxapol; n = 14. D: total triglyceride secretion following an oral dose of olive oil; n = 14. E: body weights throughout 32 wk of a 60% high-fat diet (HFD); n = 6. F: total dietary lipid absorption; n = 4. *P < 0.05 by two-way ANOVA or two-tailed t-test. TG, triglycerides. n = no. of mice.
Jejunum amino acid catabolism and TCA cycle function are impaired by Pck1 deletion.
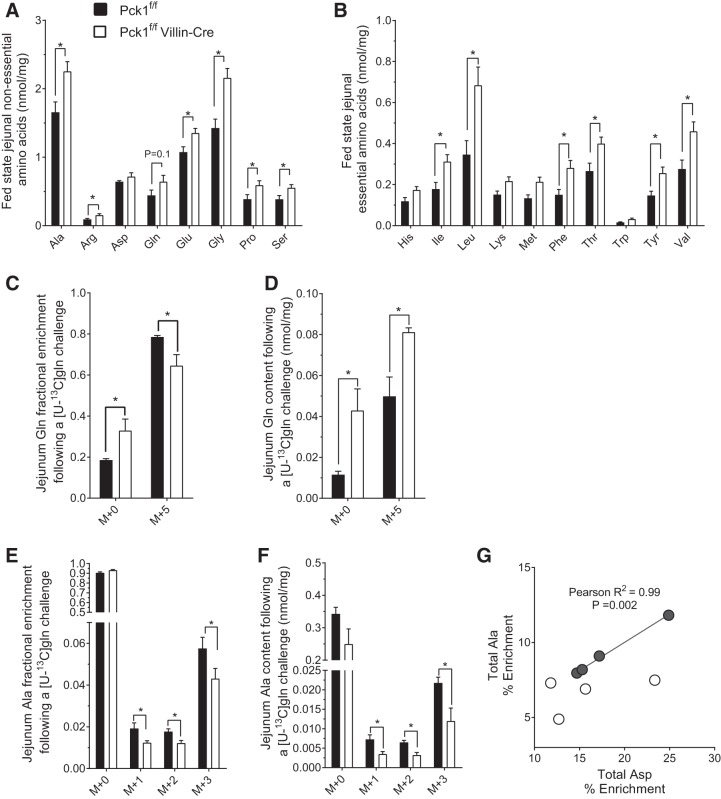
The enterocyte relies heavily on amino acid oxidation for cellular energy and may also convert certain amino acids to alanine for gluconeogenesis in liver (28). Cataplerosis is required, for example, to convert glutamine to pyruvate, whether it is to be oxidized or transaminated to alanine (Gln→Glu→αKG→→malate/OAA→→pyruvate→acetyl-CoA or Ala). To determine if intestinal amino acid metabolisms are broadly affected by PEPCK, we examined steady-state amino acid concentrations. SIPKO jejuna had a remarkably altered amino acid profile in the fed state (Fig. 4, A and B) but not in the fasted state (data not shown). Nonessential amino acids were generally increased (Fig. 4A). Many essential amino acids were also markedly higher in the SIPKO jejuna (Fig. 4B). His, Lys, Met, and Trp were exceptions and were not elevated in the SIPKO jejuna. SIPKO plasma amino acid concentrations were normal except for a few essential amino acids (Ile, Trp, and Val) that were slightly increased in the fed state (not shown).
Fig. 4.
Cystolic phosphoenolpyruvate carboxykinase (Pck1) deletion impairs small intestine amino acid metabolism. Nonessential (A) and essential (B) amino acids from jejuna of fed mice; n = 8. Jejunum glutamine (C) enrichment and (D) content, and alanine (E) enrichment, (F) content, and (G) correlation between aspartate and alanine percent enrichment 10 min after a labeled [U-13C5]glutamine oral challenge; n = 4. *P < 0.05 by two-tailed t-test or Pearson correlation. n = no. of mice.
To examine if intestinal PEPCK is necessary for glutamine metabolism, we performed an oral [U-13C5]glutamine challenge. Fractional glutamine enrichment (M + 5) was slightly lower in SIPKO jejuna (Fig. 4C) but total [U-13C5]glutamine accumulated in SIPKO jejuna (Fig. 4D). Because the small intestine converts glutamine into alanine (28), we examined the incorporation of glutamine tracer into tissue alanine. Alanine M + 3, M + 2, and M + 1 fractional enrichments were modestly reduced (Fig. 4E), and their absolute concentrations were decreased by almost half in the jejuna of SIPKO mice (Fig. 4F). We also compared alanine enrichment to aspartate enrichment, which equilibrates with OAA and is only one reaction removed from PEPCK. Aspartate and alanine enrichments were tightly correlated in control intestine as expected, but SIPKO alanine enrichments were not correlated with asparte enrichments (Fig. 4G). These data indicate that PEPCK is important for facilitating intestinal amino acid metabolism, in particular the catabolism of glutamine.
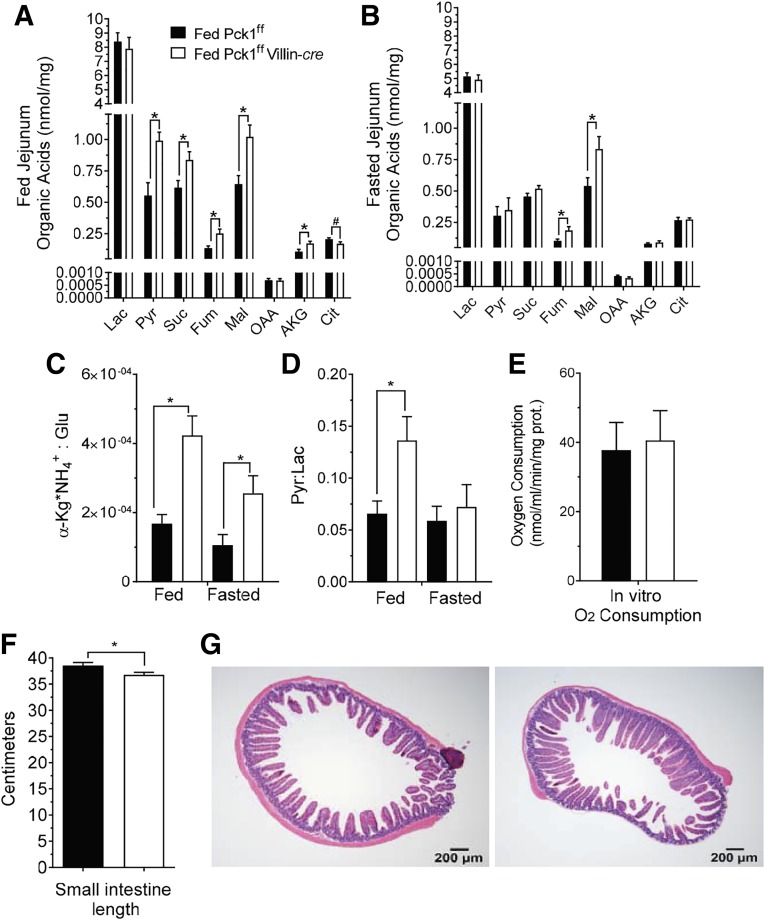
In the liver, cataplerotic flux affects TCA cycle function by altering intermediate pool sizes and redox state (4, 14). Therefore, we examined organic acid concentrations of the jejunum in the fed and fasted state (Fig. 5, A and B). As expected, loss of PEPCK impaired cataplerosis, resulting in elevated pyruvate, succinate, fumarate, and malate in the fed state (Fig. 5A). Similar to amino acid profiles, the effects in the fasted state were blunted but still observable in fumarate and malate (Fig. 5B). To determine if decreased cataplerosis was accompanied by a change in redox state, the lactate/pyruvate and αKG x NH4+Glu ratios were determined as indicators of cytosolic and mitochondria redox states, respectively (24). The cytosolic redox state was more oxidized (higher NAD+/NADH) in the SIPKO intestine in the fed state, whereas the mitochondrial redox state was more oxidized in both the fed and fasted state (Fig. 5C and D). This might occur because the enterocyte cannot accommodate anaplerotic flux through critical oxidative steps of the TCA cycle (i.e., αKG→succinyl-CoA→fumarate/malate→OAA), or cataplerotic flux out of the cycle (i.e., malate/OAA→→pyruvate). Despite defects in enterocyte amino acid catabolism and TCA cycle intermediates, oxygen consumption by isolated enterocytes incubated with a modified DMEM/F-12 media containing 5 mM glutamine and 5 mM glucose was not different from control (Fig. 5E). Although loss of PEPCK caused detectable effects on enterocyte nutrient and energy metabolism, loss of PEPCK did not substantially impact intestine length (Fig. 5F) or cause frank defects in the hematoxylin-eosin staining of the villi (Fig. 5G).
Fig. 5.
Cystolic phosphoenolpyruvate carboxykinase (Pck1) deletion causes a buildup of jejunum TCA cycle metabolites and an oxidized redox state. Jejunum organic acids in fed (A) and 24-h fasted (B) mice; n = 8. Mitochondrial redox state represented by the αKG x NH4/Glu ratio; n = 7 (C). Cytosolic redox state represented by the lactate/pyruvate ratio; n = 8 (D). Oxygen consumption by isolated villus cells incubated in modified DMEM/F-12 containing 5 mM glutamine and 5 mM glucose; n = 5 (E). Total intestine length; n = 15 (F). Hematoxylin-eosin (H&E) staining of jejunum slices (G). *P < 0.05 by two-tailed t-test. n = no. of mice. αKG, α-ketoglutarate; Cit, citrate; Fum, fumarate; Lac, lactate; OAA, oxaloacetate; Pyr, pyruvate; Succ, succinate.
DISCUSSION
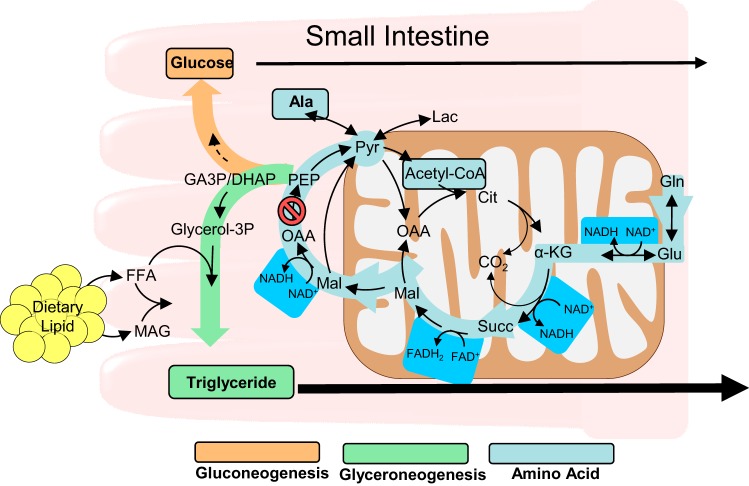
The enterocytes of the small intestine are necessary for nutrient absorption and may also contribute intestinal metabolic pathways essential to systemic nutrient metabolism. Cytosolic PEPCK catalyzes the conversion of OAA to PEP and is therefore critical in the synthesis of glycerol-3-phosphate for triglyceride formation, the de novo synthesis of glycogen or glucose, and the utilization of anaplerotic amino acids such as glutamine (Fig. 6). The SIPKO (Pck1fl/fl + Villin-cre+/−) mouse was generated to test the role of intestinal PEPCK in these pathways. SIPKO mice were largely normal with regard to bodyweight, intestinal histology, and glycemia but had abnormal intestinal lipids and amino acid metabolisms.
Fig. 6.
Metabolic pathways impacted by PEPCK in the small intestine of mice. αKG, α-ketoglutarate; DHAP, dihydroxyacetone phosphate; FFA, free fatty acid; GA3P, glyceraldehyde 3-phosphate; Lac, lactate; MAG, monoacylglyceride; Mal, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; PEPCK, phosphoenolpyruvate carboxykinase; Succ, succinate.
The mucosa of the small intestine contains all of the enzymes necessary to produce glucose (3, 46). The rat small intestine was found to contribute significantly to endogenous gluconeogenesis in some studies (8) but not others (27, 35). More recently, the small intestine was found to produce glucose from fructose when small amounts of this sugar are ingested (19). Although fructose is converted to gluconeogenic intermediates (glyceraldehyde-3 phosphate and dihydroxyacetone phosphate) before synthesis of glucose, this pathway does not require PEPCK. We were able to confirm that the everted perfused intestine of mice produces glucose and that PEPCK was required for gluconeogenesis from [U-13C]glutamine. However, most of the glucose released was unlabeled and presumably originated from glycogen because it was not affected by PEPCK deletion. Although intestinal gluconeogenesis has been noted as a significant contributor to hyperglycemia during diabetes (29, 37), loss of PEPCK did not alter plasma glucose in the chow, HFD, or STZ-induced diabetic mice. These findings are different than when glucose 6-phosphatase was deleted from the intestine of mice (30, 34). In those studies, small amounts of glucose released by the small intestine, into the portal vein, were reported to regulate hepatic glucose production. Inasmuch as loss of glucose 6-phosphatase would result in total loss of intestinal glucose production (glycogenolysis and gluconeogenesis), PEPCK deletion may not have been sufficient to recapitulate this mechanism. It is possible that intestinal glycogen has an incompletely understood role in systemic metabolism, but it is worth noting that EGP was roughly 100-fold greater than total glucose production by the ex vivo intestine.
In some tissue, PEPCK supports the production of glycerol 3-phosphate from pyruvate or amino acids, a pathway known as glyceroneogenesis (38). This pathway is necessary for the reesterification of FFAs and, therefore, normal triglyceride storage in adipose (32) and lipid for milk production by mammary tissue (17). Dietary triglycerides are initially hydrolyzed to FFAs and a monacylglycerol in the lumen of the small intestine and must be reesterified in the enterocyte before chylomicron formation and secretion. Enterocytes use the MGAT and diglyceride acyltransferase 1/2 (DGAT) pathways to directly reassemble FFA and monoacylglycerol into triglyceride (49), without the need for glyceroneogenesis. However, intestinal knockout of MGAT decreased plasma triglyceride by only ~50% and did not eliminate secretion following an oral triglyceride bolus (31), suggesting that an independent pathway also contributes to intestinal triglyceride synthesis. Therefore, PEPCK-mediated glyceraldehyde 3-phosphate synthesis and the consecutive actions of GPAT, 1-acylglycerol-3-phosphate-O-acyltransferase (AGPAT), and phosphatidic acid phosphatase may supplement the MGAT pathway in the enterocyte. Indeed, GPAT3−/− mice had an attenuated response during an oral triglyceride bolus (23), similar to the SIPKO mice. Despite a lower rate of triglyceride secretion, total lipid absorption appears to be complete in the SIPKO mice. They were not protected from weight gain during an HFD and did not have lipid malabsorption evident on fecal analysis. In contrast, loss of MGAT2 from the small intestine resulted in increased energy expenditure, less weight gain, and improved glycemia during an HFD (31). Therefore, the PEPCK-mediated GPAT pathway is likely a less-substantial pathway for intestinal lipid absorption and secretion than MGAT, but it may be required as a housekeeping mechanism to maintain the rate of fatty acid esterification as monoacylglycerol concentration is diminished during reesterification.
With regard to the role of PEPCK in lipid secretion, it is notable that this is most important in the fed state, when PEPCK expression is typically suppressed. Most studies that examined PEPCK in the small intestine considered the effect of fasting but not the effect of feeding. The distinction is important, because PEPCK expression is high in the intestine after fasting but may linger transiently after feeding begins. Findings vary, but in one study 48-h starved rats maintained elevated duodenal PEPCK activity for up to 7 h following refeeding, whereas jejunal PEPCK activity fell to 30% of the starved state (37). In another study, starved jejunal PEPCK activity remained elevated for up to 6 h following refeeding (12). PEPCK activity is not modulated by posttranscriptional mechanisms, so it is likely that its activity persists for at least several hours after feeding.
Nearly 80% of substrate oxidation in the TCA cycle of the small intestine has been attributed to amino acids (21), especially glutamine (46). Glutamine oxidation requires a cataplerotic pathway (33). For example, after conversion of the 5 carbons of glutamine to αKG, one carbon is oxidized to CO2 by the α-ketoglutarate dehydrogenase reaction to produce succinate. No carbons are lost when succinate produces fumarate/malate/OAA in the TCA cycle, but a second carbon is oxidized to CO2 when PEPCK converts OAA to PEP, or the ME converts malate to pyruvate. Subsequent reactions through pyruvate kinase/pyruvate dehydrogenase oxidize a third carbon to CO2 during the conversion of pyruvate to acetyl-CoA. The final two carbons are oxidized to CO2 when acetyl-CoA enters the TCA cycle. Alternatively, the small intestine converts some glutamine to alanine and releases it into the portal track to support hepatic gluconeogenesis (28). This conversion requires the same pathways as glutamine oxidation, except that pyruvate is converted to alanine instead of being oxidized. Indeed, the elevation of nonessential amino acids suggests that PEPCK is required for normal amino acid catabolism by the small intestine. Furthermore, after a [U-13C5]l-glutamine tolerance test, higher concentrations of tracer and 50% lower 13C-alanine in SIPKO mice suggest a role for PEPCK in the intestine’s ability to metabolize glutamine. Inasmuch as the conversion of glutamine to alanine was not completely eliminated, there is likely an active ME pathway. However, we noted that malic enzyme expression (data not shown) was actually reduced in the SIPKO mice and there were no changes in the pyruvate/malate ratio.
Similar to nonessential amino acids, most essential amino acids were increased in SIPKO intestine. We presume that the loss of PEPCK either decreased utilization, increased delivery from the lumen, or decreased secretion. Essential amino acids are extensively catabolized by the intestinal mucosa (42), and this could be altered by the absence of PEPCK, though many can form acetyl-CoA without requiring cataplerosis. In addition to decreased utilization, it is also possible that elevated ketoacids (pyruvate, OAA, and αKG) changed absorption and release through effects on transamination. For example, αKG supplementation was found to increase the net release of branched chain amino acids by the small intestine (25). However, Ile and Trp were the only essential amino acids that were effected (modestly increased) in plasma, suggesting a limited effect on systemic essential amino acid metabolism.
Loss of PEPCK not only suppressed glutamine catabolism but it also caused a build-up of TCA cycle intermediates. In the liver, the loss of PEPCK and accumulation of TCA cycle intermediates were associated with a dramatic decrease in oxidative metabolism (4, 5, 14). This could be explained by the impairment of downstream gluconeogenesis leading to an energy excess and feedback inhibition by reduced redox state (4, 40). In contrast, PEPCK deletion from the small intestine caused a more oxidized redox state in both cytosolic and mitochondrial compartments. This likely occurs because rather than feeding substrate into an endergonic pathway (e.g., gluconeogenesis) as in the hepatocyte, intestinal PEPCK supports the exergonic metabolism of glutamine (45) in the enterocyte. Consequently, SIPKO intestines produce less NADH through the dehydrogenase reactions associated with glutaminecatabolism, and therefore develop an oxidized redox state. We expected to observe impaired oxygen consumption in isolated enterocytes because of inhibition of glutamine catabolism, but it was normal. This may have been the result of substrate switching because other substrates were present in the media, or as a result of feedback stimulation of the TCA cycle by a more oxidized mitochondria. Whatever the case, we did not observe histological evidence of cell damage that one might expect with a significant mitochondrial dysfunction.
In conclusion, in the small intestine PEPCK fulfills a broad range of cataplerotic functions, including gluconeogenesis to a minor extent, but more importantly, it supports glyceroneogenesis and TCA cycle-mediated amino acid catabolism.
GRANTS
This work was supported by National Institutes of Health Grants RO1-DK-078184 (to S. Burgess), P41-EB-015908 (to S. Burgess and S. Deja), and P01-DK-058398 (to S. Burgess), and Robert A. Welch Foundation Grant I-1804 (to S. Burgess and S. Deja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P., A.U., E.D.B., J.A.D., J.D.B., M.A.M., and S.C.B. conceived and designed research; A.P., A.U., S.D., B.K., and X.F. performed experiments; A.P., A.U., S.D., B.K., J.A.D., X.F., and S.C.B. analyzed data; A.P., A.U., S.D., and S.C.B. interpreted results of experiments; A.P. prepared figures; A.P. drafted manuscript; A.U., S.D., and S.C.B. edited and revised manuscript; A.P., A.U., S.D., E.D.B., B.K., J.A.D., X.F., J.D.B., M.A.M., and S.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Joyce Repa and Ben Tu, at the University of Texas Southwestern Medical Center, for invaluable insight.
REFERENCES
- 1.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev 92: 1061–1085, 2012. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alleyne GA, Flores H, Roobol A. The interrelationship of the concentration of hydrogen ions, bicarbonate ions, carbon dioxide and calcium ions in the regulation of renal gluconeogenesis in the rat. Biochem J 136: 445–453, 1973. doi: 10.1042/bj1360445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JW. Pyruvate carboxylase and phosphoenolpyruvate carboxykinase in rat intestinal mucosa. Biochim Biophys Acta 208: 165–167, 1970. doi: 10.1016/0304-4165(70)90066-8. [DOI] [PubMed] [Google Scholar]
- 4.Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem 279: 48941–48949, 2004. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- 5.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 5: 313–320, 2007. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, Mulder H, Holm C, Sherry AD, Malloy CR. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab 289: E53–E61, 2005. doi: 10.1152/ajpendo.00601.2004. [DOI] [PubMed] [Google Scholar]
- 7.Casetta B, Tagliacozzi D, Shushan B, Federici G. Development of a method for rapid quantitation of amino acids by liquid chromatography-tandem mass spectrometry (LC-MSMS) in plasma. Clin Chem Lab Med 38: 391–401, 2000. doi: 10.1515/CCLM.2000.057. [DOI] [PubMed] [Google Scholar]
- 8.Croset M, Rajas F, Zitoun C, Hurot J-M, Montano S, Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes 50: 740–746, 2001. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 9.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 24: 151–157, 2016. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Des Rosiers C, Fernandez CA, David F, Brunengraber H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. Evidence from isotopomer analysis of citric acid cycle intermediates. J Biol Chem 269: 27179–27182, 1994. [PubMed] [Google Scholar]
- 11.Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom 31: 255–262, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 12.Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH, Oudart H. Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol 566: 575–586, 2005. doi: 10.1113/jphysiol.2005.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hachey DL, Parsons WR, McKay S, Haymond MW. Quantitation of monosaccharide isotopic enrichment in physiologic fluids by electron ionization or negative chemical ionization GC/MS using di-O-isopropylidene derivatives. Anal Chem 71: 4734–4739, 1999. doi: 10.1021/ac990724x. [DOI] [PubMed] [Google Scholar]
- 14.Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, Reshef L, Tilghman SM, Hanson RW. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond) 2: 33, 2005. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem 282: 32844–32855, 2007. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton KL, Butt AG. Glucose transport into everted sacs of the small intestine of mice. Adv Physiol Educ 37: 415–426, 2013. doi: 10.1152/advan.00017.2013. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh CW, Huang C, Bederman I, Yang J, Beidelschies M, Hatzoglou M, Puchowicz M, Croniger CM. Function of phosphoenolpyruvate carboxykinase in mammary gland epithelial cells. J Lipid Res 52: 1352–1362, 2011. doi: 10.1194/jlr.M012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, Liu W, Tesz GJ, Birnbaum MJ, Rabinowitz JD. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab 27: 351–361.e3, 2018. doi: 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA 102: 6207–6212, 2005. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungas RL, Halperin ML, Brosnan JT. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol Rev 72: 419–448, 1992. doi: 10.1152/physrev.1992.72.2.419. [DOI] [PubMed] [Google Scholar]
- 22.Kalhan SC, Mahajan S, Burkett E, Reshef L, Hanson RW. Glyceroneogenesis and the source of glycerol for hepatic triacylglycerol synthesis in humans. J Biol Chem 276: 12928–12931, 2001. doi: 10.1074/jbc.M006186200. [DOI] [PubMed] [Google Scholar]
- 23.Khatun I, Clark RW, Vera NB, Kou K, Erion DM, Coskran T, Bobrowski WF, Okerberg C, Goodwin B. Characterization of a novel intestinal glycerol-3-phosphate acyltransferase pathway and its role in lipid homeostasis. J Biol Chem 291: 2602–2615, 2016. doi: 10.1074/jbc.M115.683359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs HA, Veech RL. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv Enzyme Regul 7: 397–413, 1969. doi: 10.1016/0065-2571(69)90030-2. [DOI] [PubMed] [Google Scholar]
- 25.Lambert BD, Filip R, Stoll B, Junghans P, Derno M, Hennig U, Souffrant WB, Pierzynowski S, Burrin DG. First-pass metabolism limits the intestinal absorption of enteral alpha-ketoglutarate in young pigs. J Nutr 136: 2779–2784, 2006. doi: 10.1093/jn/136.11.2779. [DOI] [PubMed] [Google Scholar]
- 26.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–33283, 2002. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 27.Martin G, Ferrier B, Conjard A, Martin M, Nazaret R, Boghossian M, Saadé F, Mancuso C, Durozard D, Baverel G. Glutamine gluconeogenesis in the small intestine of 72 h-fasted adult rats is undetectable. Biochem J 401: 465–473, 2007. doi: 10.1042/BJ20061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mithieux G. New data and concepts on glutamine and glucose metabolism in the gut. Curr Opin Clin Nutr Metab Care 4: 267–271, 2001. doi: 10.1097/00075197-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Mithieux G, Bady I, Gautier A, Croset M, Rajas F, Zitoun C. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 286: E370–E375, 2004. doi: 10.1152/ajpendo.00299.2003. [DOI] [PubMed] [Google Scholar]
- 30.Mutel E, Abdul-Wahed A, Ramamonjisoa N, Stefanutti A, Houberdon I, Cavassila S, Pilleul F, Beuf O, Gautier-Stein A, Penhoat A, Mithieux G, Rajas F. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J Hepatol 54: 529–537, 2011. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Nelson DW, Gao Y, Yen MI, Yen CL. Intestine-specific deletion of acyl-CoA: monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem 289: 17338–17349, 2014. doi: 10.1074/jbc.M114.555961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olswang Y, Cohen H, Papo O, Cassuto H, Croniger CM, Hakimi P, Tilghman SM, Hanson RW, Reshef L. A mutation in the peroxisome proliferator-activated receptor γ-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci USA 99: 625–630, 2002. doi: 10.1073/pnas.022616299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277: 30409–30412, 2002. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 34.Penhoat A, Fayard L, Stefanutti A, Mithieux G, Rajas F. Intestinal gluconeogenesis is crucial to maintain a physiological fasting glycemia in the absence of hepatic glucose production in mice. Metabolism 63: 104–111, 2014. doi: 10.1016/j.metabol.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Previs SF, Brunengraber DZ, Brunengraber H. Is there glucose production outside of the liver and kidney? Annu Rev Nutr 29: 43–57, 2009. doi: 10.1146/annurev-nutr-080508-141134. [DOI] [PubMed] [Google Scholar]
- 36.Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 117: 132–139, 1999. doi: 10.1016/S0016-5085(99)70559-7. [DOI] [PubMed] [Google Scholar]
- 37.Rajas F, Croset M, Zitoun C, Montano S, Mithieux G. Induction of PEPCK gene expression in insulinopenia in rat small intestine. Diabetes 49: 1165–1168, 2000. doi: 10.2337/diabetes.49.7.1165. [DOI] [PubMed] [Google Scholar]
- 38.Reshef L, Hanson RW, Ballard FJ. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem 245: 5979–5984, 1970. [PubMed] [Google Scholar]
- 39.Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem 278: 30413–30416, 2003. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 40.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 125: 4447–4462, 2015. [Erratum in J Clin Invest, 2016.] doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol 20: 6508–6517, 2000. doi: 10.1128/MCB.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, Burrin DG. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr 128: 606–614, 1998. doi: 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- 43.Thibault R, Welch S, Mauras N, Sager B, Altomare A, Haymond M, Darmaun D. Corticosteroids increase glutamine utilization in human splanchnic bed. Am J Physiol Gastrointest Liver Physiol 294: G548–G553, 2008. doi: 10.1152/ajpgi.00461.2007. [DOI] [PubMed] [Google Scholar]
- 44.Uchida A, Slipchenko MN, Cheng JX, Buhman KK. Fenofibrate, a peroxisome proliferator-activated receptor α agonist, alters triglyceride metabolism in enterocytes of mice. Biochim Biophys Acta 1811: 170–176, 2011. doi: 10.1016/j.bbalip.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinay P, Mapes JP, Krebs HA. Fate of glutamine carbon in renal metabolism. Am J Physiol 234: F123–F129, 1978. doi: 10.1152/ajprenal.1978.234.2.F123. [DOI] [PubMed] [Google Scholar]
- 46.Windmueller HG, Spaeth AE. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem 253: 69–76, 1978. [PubMed] [Google Scholar]
- 47.Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem 255: 107–112, 1980. [PubMed] [Google Scholar]
- 48.Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 5.47.1–5.47.14, 2008. doi: 10.1002/0471141755.ph0547s40. [DOI] [PubMed] [Google Scholar]
- 49.Yen CL, Nelson DW, Yen MI. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J Lipid Res 56: 489–501, 2015. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]