Abstract
Paneth cells are a key subset of secretory epithelial cells found at the base of small intestinal crypts. Unlike intestinal goblet cells, which secrete the mucin Muc2, Paneth cells are best known for producing an array of antimicrobial factors. We unexpectedly identified Muc2 staining localized around Paneth cell granules. Electron microscopy (EM) confirmed an electron lucent halo around these granules, which was lost in Paneth cells from Muc2-deficient (−/−) mice. EM and immunostaining for lysozyme revealed that Muc2−/− Paneth cells contained larger, more densely packed granules within their cytoplasm, and we detected defects in the transcription of key antimicrobial genes in the ileal tissues of Muc2−/− mice. Enteroids derived from the small intestine of wild-type and Muc2−/− mice revealed phenotypic differences in Paneth cells similar to those seen in vivo. Moreover, lysozyme-containing granule release from Muc2−/− enteroid Paneth cells was shown to be impaired. Surprisingly, Paneth cells within human ileal and duodenal tissues were found to be Muc2 negative. Thus Muc2 plays an important role in murine Paneth cells, suggesting links in function with goblet cells; however human Paneth cells lack Muc2, highlighting that caution should be applied when linking murine to human Paneth cell functions.
NEW & NOTEWORTHY We demonstrate for the first time that murine Paneth cell granules possess a halo comprised of the mucin Muc2. The presence of Muc2 exerts an impact on Paneth cell granule size and number and facilitates the release and dispersal of antimicrobials into the mucus layer. Interestingly, despite the importance of Muc2 in murine Paneth cell function, our analysis of Muc2 in human intestinal tissues revealed no trace of Muc2 expression by human Paneth cells.
Keywords: enteroids, intestine, mucin 2, Paneth cells
INTRODUCTION
Paneth cells are important secretory epithelial cells that reside within the mammalian small intestine, in clusters at the base of the crypts of Lieberkühn. Paneth cells produce an array of antimicrobial factors, including lysozyme and defensins that are packaged within secretory cytoplasmic granules (7). These granules are easily recognizable in histological sections by their strong eosin staining. Following proper stimulation, Paneth cells secrete these granules apically, into the overlying crypt lumen, where they clear away any potentially harmful bacteria (7). As a result, these specialized cells play a critical role in controlling microbial growth within the small intestine. In addition, Paneth cells produce a number of factors, such as the Wnt protein, which aid in maintaining the nearby intestinal stem cell niche, governing stem cell proliferation and the architecture of the crypt (19).
Paneth cells are derived from the same secretory progenitors as goblet cells; however these two cell types diverge when immature goblet cells migrate up the crypt, away from the Wnt signaling of the stem cell niche (7). As they continue their differentiation, goblet cells specialize in producing large amounts of the mucin Muc2, the main component of the intestinal mucus layer. In contrast to goblet cells, Paneth cells migrate downward, toward the stem cell niche, where, in response to continued Wnt signaling, they specialize in producing antimicrobial peptides. As the antimicrobial-containing granules are released into the crypt lumen, their contents diffuse through the mucus layer and expand outward toward the lumen, protecting the crypts from nearby and potentially dangerous bacteria. The release of antimicrobials by Paneth cells (17) may also have a substantial impact on the microbial population within the small intestine, reducing overall microbial numbers and modulating their composition (18).
Although Paneth cell dysfunction has yet to be shown to directly cause specific disease states, links have been made between Paneth cells and the development of inflammatory bowel disease (IBD) (15, 27). One connection involves the observed reduction in expression of certain Paneth cell-derived defensin proteins in patients with IBD (27). Another link rests largely on associations between mutations in autophagy-related genes and the development of IBD (15). Although defects in autophagy have broad implications for the function of a number of cell types in the gut, defects in genes such as ATG16L1 have been shown to have a particularly drastic impact on Paneth cells, as they develop severe endoplasmic reticulum (ER) stress, disrupting their function and viability (6). Other links between Paneth cells and either IBD or infectious diseases have focused on defects in innate immune receptors, such as Nod2 or Toll-like receptors, which are involved in controlling Paneth cell release of antimicrobial-containing granules (25). Unfortunately at present, our meager understanding of the regulation and/or mechanisms underlying granule exocytosis has limited our ability to define how altered Paneth cell function promotes disease pathogenesis.
We do know, however, that most of the cellular machinery of the Paneth cell is focused on the production of their antimicrobial granules. These granules are known to contain lysozyme, α-defensins, and a number of other antimicrobial peptides that are secreted into the crypt lumen. There is also evidence that Paneth cells produce mucin-like glycoproteins in low quantities (22, 24); however, no research has yet been conducted as to whether these glycoproteins play any role in Paneth cell function or whether they are merely a developmental holdover from its common cellular lineage with the mucin-producing goblet cells. In this study, we determined that the previously identified glycoproteins found in mouse Paneth cells are largely comprised of Muc2, the mucin that is the primary component of the intestinal mucus layer. Furthermore, the Muc2 glycoprotein is specifically localized into a halo around each Paneth cell granule. Notably, studying Paneth cells in mice deficient (−/−) in Muc2 revealed altered granule morphology and function, leading to larger, more densely packed granules within the cytoplasm of the cell, as well as impairment in the production of antimicrobials by these cells. Moreover, assessment of Paneth cells within enteroids derived from Muc2 −/− mice revealed impaired granule release. Interestingly, the expression of Muc2 by Paneth cells is a phenotype found in mice but not in human Paneth cells, highlighting the need for caution when drawing parallels between murine and human Paneth cell function.
MATERIALS AND METHODS
Mouse strains and ethics statement.
The C57BL/6, Muc2−/−, Sigirr−/−, C3Gnt−/−, and C1galt1−/− intestinal epithelial cell (IEC) mice used in this study were all bred in house and kept under specific pathogen-free conditions at the BC Children’s Hospital Research Institute (BCCHRI). All animal experiments were approved by the University of British Columbia’s Animal Care Committee and in direct accordance with the Canadian Council of Animal Care (CCAC) guidelines.
Ethical considerations.
The University of British Columbia Clinical Research Ethics Board approved the clinical study protocol. The study was conducted in accordance with the Panel of Research Ethics and the regulations established in Canada for the Protection of Human Subjects (the Tri-Council Policy Statement: Ethical Conduct for Research Involving Human).
Growth and propagation of small intestinal enteroid cultures.
Small intestinal stem cells from the small intestines of C57BL/6 mice and Muc2−/− mice were isolated in a similar manner to that previously outlined by Sato et al. (20). Briefly, mice were euthanized by isofluorane anesthesia and cervical dislocation; then a 4- to 5-cm segment of the ileum was removed, opened longitudinally, and scraped gently with a pair of tweezers to remove as many of the intestinal villi as possible and immediately submerged in 10 ml of DMEM media (Fisher Scientific, Hampton, NH) containing Penstrep and Gentamicin (Fisher Scientific). The tissue section was washed at least five times in cold PBS and submerged in 5 ml of cell recovery solution (Corning, New York, NY). The tissue was maintained in this solution at 4°C for at least 30 min to weaken the connective tissue attaching the epithelial crypts to the surrounding connective and muscle tissue. Gentle scraping of the epithelium with tweezers detached the crypts from the rest of the tissue. The now detached epithelium was disrupted and resuspended by vigorous pipetting, added to a centrifuge tube, and pelleted by centrifugation at 800 revolutions/min for 5 min. The pellets of crypts were washed once in DMEM; then a small aliquot (0.5–1.5ml depending on the density of suspended crypts) was transferred to a 1.5-ml Eppendorf tube. The suspended crypts were pelleted at 15,000 revolutions/min at 4°C, the supernatant was removed, and the crypts were resuspended in a 1:1 ratio of advanced MEM (Fisher Scientific) and Matrigel (Corning) and then plated as 50-µl drops into a 24-well plate. The plates were placed in a 37°C CO2 incubator for 30 min to allow the Matrigel to solidify completely, after which 0.5 ml of growth media was added (described below). Enteroid cultures were maintained at 37°C and 5% CO2 with growth media changed every 2 days.
Growth media for the mouse and human enteroids were also similar to those previously outlined by Sato et al. (20). Mouse enteroids were grown on ENR media containing conditioned R-spondin and Noggin media, with 50 ng/ml recombinant mouse endothelial growth factor, MEM+++ (advanced MEM, Hepes, Glutamax, and Penstep) (Fisher Scientific), 1.25 mM N-acetyl-cysteine, 1× B27 supplement, and 1× N2 supplement. Cell lines for the production of Noggin- and R-spondin-conditioned media were a generous gift from Dr. Aleixo Muise (Sickkids, University of Toronto, Toronto, Canada).
Enteroid cultures were split every 5–7 days, depending on their size and number. The Matrigel encasing the enteroids was dissolved using cell recovery solution on ice for at least 15 min. Cultures were resuspended and transferred to a 1.5-ml microcentrifuge tube and pelleted at 4°C and 1,500 revolutions/min for 5 min. The cell recovery solution was removed, and the enteroids were washed at least once in MEM+++ and finally resuspended in 0.5 ml of MEM+++. The enteroids were disrupted by pipetting up and down, up to 100 times using a 200-µl pipette, followed by vortexing twice for 5 s. The disrupted enteroids were then pelleted again at 4°C and 1,500 revolutions/min for 5 min. The pellet was resuspended in Matrigel and plated in 24-well plates in the same fashion as the original crypts.
Indicated cultures were treated with 20 µM IFN-γ (Sigma-Aldrich, St. Louis, MO) added directly to the growth medium. Treatment was started 3–4 days after the enteroids were seeded and allowed to continue for 24 h before the cultures were fixed for sectioning and staining.
Immunofluorescence staining.
Mouse ileal sections or human patient biopsies were immediately submerged in 10% formalin and incubated overnight at 4°C. The tissue sections were embedded in paraffin, sectioned, and stained using previously established protocols. Briefly, tissue sections were deparaffinized by heating for 8 min, cleared with xylene, and rehydrated with 100%, 95%, and 70% ethanol, followed by dH2O. Antigen retrieval of the tissue sections was conducted with sodium citrate buffer (pH 6.0) in a steam bath for 30 min. Blocking was done for 1 h using donkey serum blocking buffer (donkey serum in PBS containing 1% BSA, 0.1% Triton X-100, 0.05% Tween 20, and 0.05% sodium azide). Tissue sections were incubated with primary antibodies overnight at 4°C. The antibodies used in this study were goat anti-human lysozyme (Santa Cruz Biotechnology, Dallas, TX), rabbit anti-human Mucin 2 (Santa Cruz Biotechnology), and goat anti-human/mouse actin (Santa Cruz Biotechnology). Each was visualized using the secondary antibodies Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA) or Alexa Fluor 568-conjugated donkey anti-goat IgG (Molecular Probes, Eugene, OR). Negative-isotype control experiments were conducted using rabbit and goat IgG isotype control antibodies (Thermo Fisher Scientific). The tissues were mounted using ProLong Gold antifade reagent containing DAPI (Invitrogen). The stained slides were viewed using a Zeiss Axiomager Z1, photographed using an AxioCam HRm camera with AxioVision software (Jena, Germany). Confocal imaging was conducted with a Leica TCS SP5 system, using the Leica Application suite software (Leica Microsystems, Wetzlar, Germany). Staining of Muc2 in wild-type, Muc2−/−, and human tissues was conducted simultaneously to ensure the success of the antibodies in tissue sections lacking Muc2.
Enteroid cultures were fixed using a slightly different approach. The Matrigel surrounding the enteroids was dissolved using cell recovery solution for 30 min on ice. The enteroids were resuspended, pelleted by centrifugation (1,500 revolutions/min for 5 min at 4°C), and washed once in cold PBS. The enteroids were then resuspended in 10% formalin and incubated at 4°C overnight. The enteroids were once again pelleted with gentle centrifugation (1,500 revolutions/min for 5 min at 4°C), then rapidly resuspended in 100 µl 3% low-melting-point agarose, and quickly placed on ice. The agarose solidified instantly, at which point the agarose block, containing the enteroids, could be paraffin embedded, sectioned, and stained in the same fashion as the mouse tissues described above.
Electron microscopy fixation and imaging.
Samples of ilea from wild-type (C57BL/6) and Muc2−/− mice were excised and fixed by immersion (1.5% paraformaldehyde, 1.5% glutaraldehyde, and 0.1 M sodium cacodylate, pH 7.30) at room temperature for 3 h. The fixative was replaced with buffer (0.1 M sodium cacodylate, pH 7.3), and samples were stored at 4°C until they could be further processed. After storage, samples were washed three times (10 min each wash) in buffer at room temperature and then postfixed in 0.1% OsO4 in 0.1 M sodium cacodylate (pH 7.3) on ice for 1 h. Samples were washed three times (10 min each wash) with ddH20 at room temperature, stained with 0.1% aqueous uranyl acetate for 1 h, washed again three times with ddH20, and then dehydrated through an ascending concentration series of ethyl alcohol solutions (30%, 50%, 70%, 95%, and 100%). The alcohol was replaced with 100% propylene oxide and left for 30 min and then again treated with fresh propylene oxide for another 30 min. The propylene oxide was replaced with a mixture of 1:1 propylene oxide:resin (EMBED 812; EM Sciences, Hatfield, PA) and left overnight. The next day, samples were incubated twice (2 h each) in 100% resin, embedded in 100% resin, and polymerized for 48 h at 60°C. Polymerized blocks were sectioned using a Leica EM UC7 ultramicrotome (Leica Microsystems). Thick (1 μm) sections were mounted on glass slides, stained with toluidine blue, and imaged using a Zeiss Imager.A1 photomicroscope (Zeiss, Oberkochen, Germany). Thin (900 Å) sections were collected on copper grids, stained with uranyl acetate and lead citrate, and imaged using an FEI Tecnai G2 Spirit electron microscope operated at 120 kV.
Paneth cell characterization.
Paneth cell numbers per crypt and the number of granules per cell were assessed using formalin-fixed cross sections, immunofluorescently stained for lysozyme. The number of Paneth cells present at the base of 100 crypts (50 wild-type and 50 Muc2−/−) was counted. Furthermore, with the use of the same slides, the granules in 200 cells (100 wild-type and 100 Muc2−/−) were counted and averaged. Both immunofluorescence and transmission electron microscopy (TEM) images were used for the assessment of Paneth cell granule size. With the use of the TEM images, the diameters of 100 wild-type and 154 Muc2−/− granules from 8 and 11 separate cells, respectively, were measured using Zeiss imaging software. Likewise, 100 wild-type granules (from 13 cells, in 4 separate crypts, from 3 mice) and 146 Muc2−/− granules (from 15 cells, in 4 crypts from 3 mice) immunofluorescently stained for lysozyme were measured. Ten-micron cut slides were imaged with a Zeiss Axiomager Z1 and photographed using an AxioCam HRm camera and measured using AxioVision software. In both cases, statistical differences were determined using a t-test, with significance determined when the P value was <0.05.
Antimicrobial peptide transcription analyses.
Ileal tissue sections were collected in RNALater (Qiagen, Hilden, Germany) and kept stored at −80°C until use. RNA was isolated using the RNeasy kit (Qiagen), and cDNA was prepared from 1 μg of RNA using the Quantitech Reverse Transcription kit (Qiagen). Quantitative PCR reactions were done in an Eppendorf Realplex 2 apparatus using the DyNamo SYBR Green PCR Kit (ThermoFisher, Waltham, MA). The qPCR primers and conditions for the Defa genes have been previously described (13); all other primers are given in Table 1. Relative expression was calculated with respect to wild-type controls using the ΔΔCt method corrected for primer efficiencies according to Pfaffl et al. (16). Statistical analyses were done using Mann-Whitney’s U-test in GraphPad Prism (San Diego, CA). In the case of enteroid cultures, six wells of enteroids were pooled and removed from the Matrigel using four washes in cold PBS. RNA extraction was conducted as described above.
Table 1.
The genes analyzed in this study and the sequences of the qPCR primer sets
| Target Gene | ACC No./Product | Primer Set |
|---|---|---|
| Reg3b | NM_011036.1/regenerating islet-derived 3β | GGCTTCATTCTTGTCCTCCA |
| TCCACCTCCATTGGGTTCT | ||
| Reg3g | NM_011260.2/regenerating islet-derived 3γ | AAGCTTCCTTCCTGTCCTCC |
| TCCACCTCTGTTGGGTTCAT | ||
| Lyz1/Lyz2 | NM_013590.4/lysozyme 1 | AGCCGATACTGGTGTAATGATG |
| NM_017372.3/lysozyme 2 | GCACATTGTATGGCTGCAGTG | |
| Mmp7 | NM_010810.4/matrix metallopeptidase 7 | CACTCTAGGTCATGCCTTCGC |
| GGTGGCAGCAAACAGGAAGTTC | ||
| Ang4 | NM_177544.4/angiogenin, ribonuclease A family, member 4 | AACTCTGGCTCAGAATGAAAG |
| GGCGAGGTTAGCTTTCTTTC | ||
| Rplp0 | Ribosomal protein, large, P0 (36B4) | TCTGGAGGGTGTCCGCAAC |
| CTTGACCTTTTCAGTAAGTGG |
The top sequence of each set corresponds to the forward primer, and the bottom corresponds to the reverse.
Western blots were performed by using 200 µl of RIPA lysis buffer (150 mM sodium chloride, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) along with phosphatase inhibitor tablets (Roche, Basel, Switzerland) and a protease inhibitor cocktail for bacterial cell extracts (Sigma-Aldrich); cells were lysed to extract their proteins. Lysates were then resolved by SDS-PAGE and transferred onto 0.2-µm polyvinylidene difluoride membranes (GE, Boston, MA). Membranes were blocked with 5% BSA (Sigma-Aldrich) and probed with anti-lysozyme C and β-actin primary antibodies followed by incubation with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Cell Signaling Technology, Danvers, MA). Protein bands were revealed using SuperSignal West Dura substrate (ThermoFisher) and visualized by the ChemiDocXRS system (Bio-Rad, Hercules, CA). Band intensities were semiquantitatively analyzed by ImageJ software, using β-actin bands as loading controls to normalize values.
RESULTS
Murine Paneth cell granules are surrounded by a Muc2 halo.
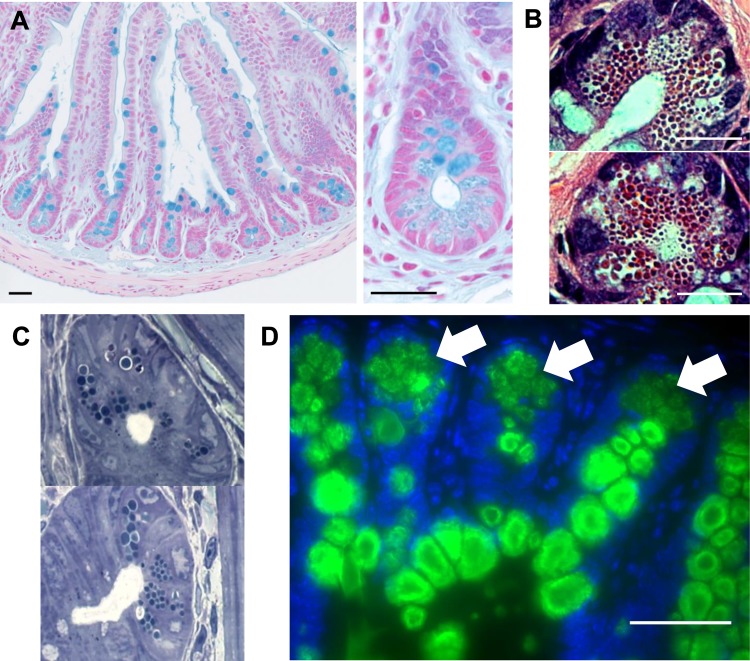
During studies of intestinal goblet cell function in wild-type C57BL/6 mice, ileal tissue sections stained with Alcian blue (a dye used to stain the acid mucopolysaccharides abundant on intestinal mucins) revealed that, in addition to the expected staining of the luminal mucus layer and the secretory mucin granules within goblet cells, other cells at the bottom of small intestinal crypts were consistently and selectively labeled (Fig. 1A). The staining morphology and coloration within these cells were different from the typical staining of the large mucin-filled secretory granules of goblet cells. Instead, it displayed a very distinctive staining pattern outlining multiple independent apical vesicle-like structures, similar to the hematoxylin and eosin (H and E) (Fig. 1B) and toluidine blue (Fig. 1C) staining of Paneth cell granules. Both the localization and the unique pattern of this Alcian blue staining suggested that it was marking the secretory granules within Paneth cells.
Fig. 1.
C57BL/6 mouse ilea stained with Alcian blue (A). Mucins within the goblet cells and secreted mucus layer are clearly visible. At the crypt bases, Alcian blue staining among the Paneth cell granules is also clearly visible under ×100 and ×400 magnification. Paneth cells at the base of the ileal intestinal crypts of a wild-type C57BL/6 mouse are stained with hematoxylin and eosin (B) or toluidine blue (C) (×1,000 magnification). The granules within the cells are easily visible in the cytoplasm of the cell. Wild-type mouse ileal sections were immunofluorescently stained for Muc2 (green) and DAPI (blue) (D). Strong staining for Muc2 within the goblet cells is clearly visible in green, and fainter fluorescence is visible in the Paneth cells at the base of the crypts (indicated by arrows). Scale bars = 50 μm, ×400 magnification.
The presence of mucin-like glycoproteins within murine Paneth cells had been previously observed (12, 22, 24) although neither their identity nor their function was ever resolved. We hypothesized that this granule-associated glycoprotein could be Muc2, which is the main constituent of the intestinal mucus layer. As expected, immunofluorescent staining of ileal sections with an anti-Muc2 antibody revealed strong Muc2 staining within goblet cells (Fig. 1D). Moreover Muc2 staining was also found in each Paneth cell at the base of every crypt, albeit to a lesser degree than typically found in goblet cells (Fig. 1D), indicating much lower Muc2 content per cell than in goblet cells. Moreover, on closer inspection, the Muc2 staining within the Paneth cells was not found in the cytoplasm or within the secretory granules, but rather it formed a small halo around each of the intracellular granules as indicated by costaining for Muc2 and lysozyme (Fig. 2, A and B). This Muc2 layer coincided with a thin, ring-like clear area around most Paneth cell granules that is often visible under H and E, as well as toluidine blue staining (Fig. 2, A and B). These results demonstrate that Paneth cells produce Muc2 and that it is a major component of the glycoprotein shell surrounding their secretory granules. This finding was confirmed in multiple gene-deficient mouse strains by Muc2 staining of ileal tissues from Sigirr−/− (23), Myd88−/−, and Rag1−/− mice (not shown). Furthermore, staining of jejunal and duodenal Paneth cells confirmed the presence of Muc2 in Paneth cells throughout the length of the murine small intestine (not shown). It should be noted that small intestinal crypts also contain small numbers of intermediate cells, unusual cells that express characteristics of both goblet cells and Paneth cells. These cells are usually located midway up the crypt axis and strongly resemble goblet cells but with positive staining for lysozyme in their mucin-filled granules. We observed a number of these intermediate cells in the stained tissue sections as well; however, their appearance and location were quite distinct from the cells we identified at the base of the crypts that expressed Muc2 solely around their granules, rather than within the granules themselves.
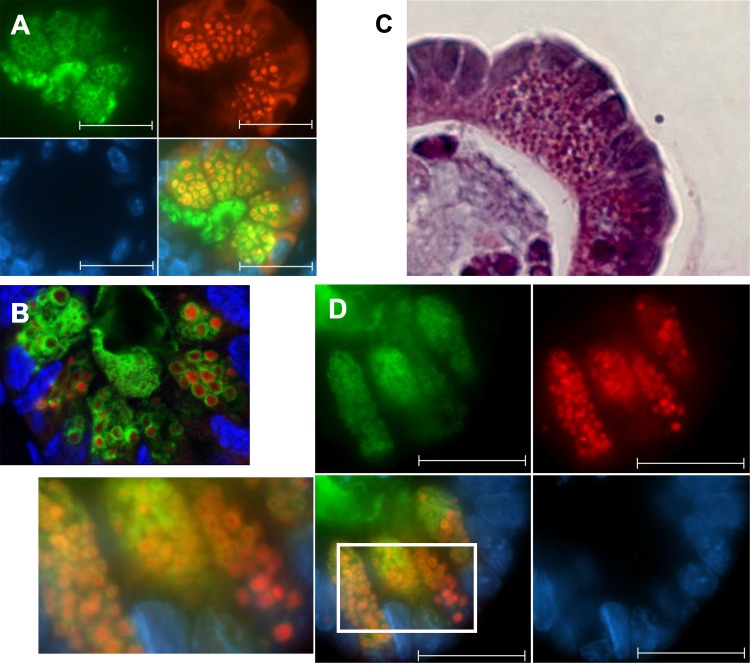
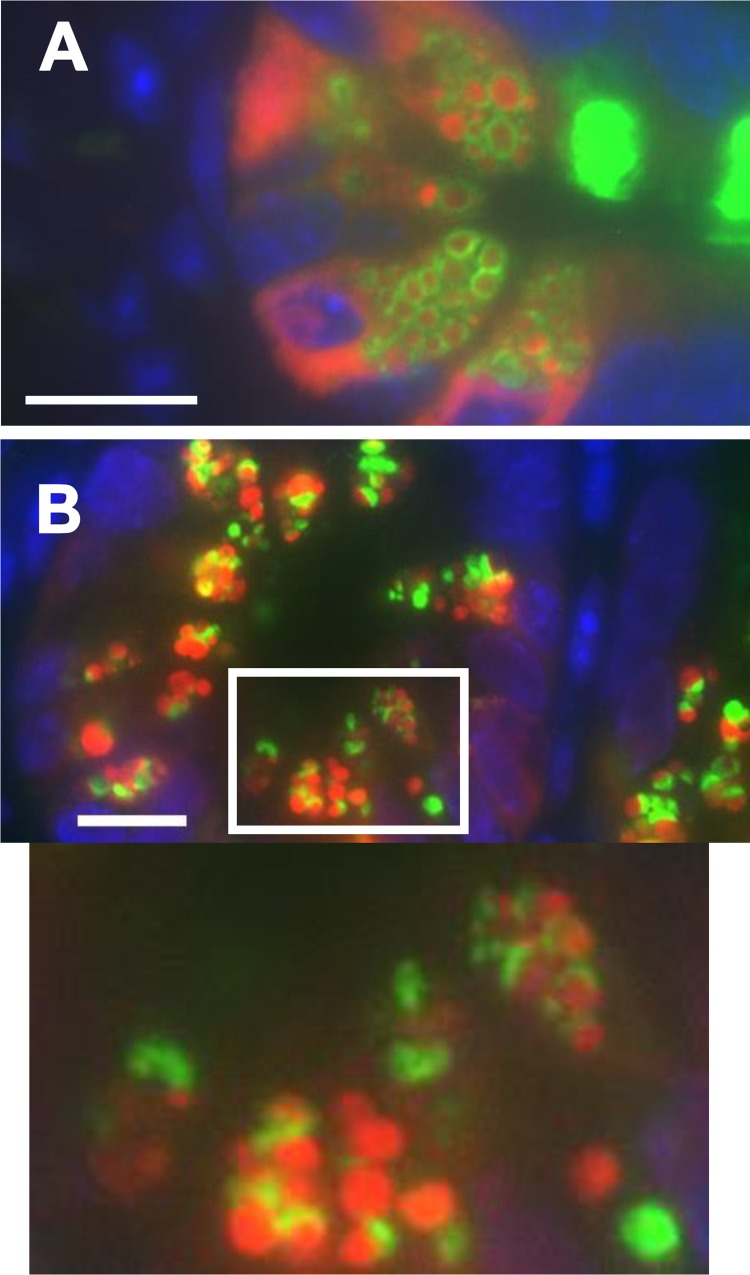
Fig. 2.
Paneth cells from formalin-fixed wild-type mouse ileal sections were immunofluorescently stained for Muc2 (green), lysozyme (red), and DAPI (blue). With the use of both an epifluorescent (A) and a confocal microscope (B), the granules are visible in red, and each is surrounded with a halo of Muc2 (green). Paneth cells from wild-type murine ileal enteroids were stained with hematoxylin and eosin (C) or for Muc2, lysozyme, and DAPI (as described above) (D). Individual granules are on average smaller than those seen in tissue sections, but each is once again surrounded by a layer of Muc2. Scale bars = 10 μM, ×630 magnification.
Muc2 expression in the professional mucus-producing goblet cells is regulated by a number of mechanisms, including microbial signaling (5, 26). However, the regulation of Muc2 expression may be different in Paneth cells given the differences in expression levels and localization between the two cell types. To determine whether expression of Muc2 by Paneth cells required stimulation from the microbiota or from cells of the intestinal stroma, we analyzed its expression in cultured cells. Because no immortalized cell line accurately reflects Paneth cell structure or function, these in vitro studies were done using enteroids derived from small intestinal stem cells.
H and E staining easily identified Paneth cells at or near the base of the crypts of each ileal enteroid (Fig. 2C). Although the structure of enteroids is not as rigidly organized as that seen in crypts in the intestine, most Paneth cells were located at the base of crypts, with goblet cells and mature enterocytes at more central locations, although a few identifiable Paneth cells were found throughout the enteroids. Similar to their morphology in intestinal tissue, Paneth cells in cultured enteroids contained clear areas among their granules. Immunofluorescent staining of enteroid Paneth cells for Muc2 and lysozyme displayed a pattern similar to that seen in the Paneth cells in tissue sections (Fig. 2D). Paneth cells contained numerous lysozyme-positive granules that were consistently and uniformly surrounded by Muc2, showing that their coating with Muc2 is a process that takes place independent of signaling from the stroma or the luminal microbiota.
Muc2 deficiency alters Paneth cell granule structure.
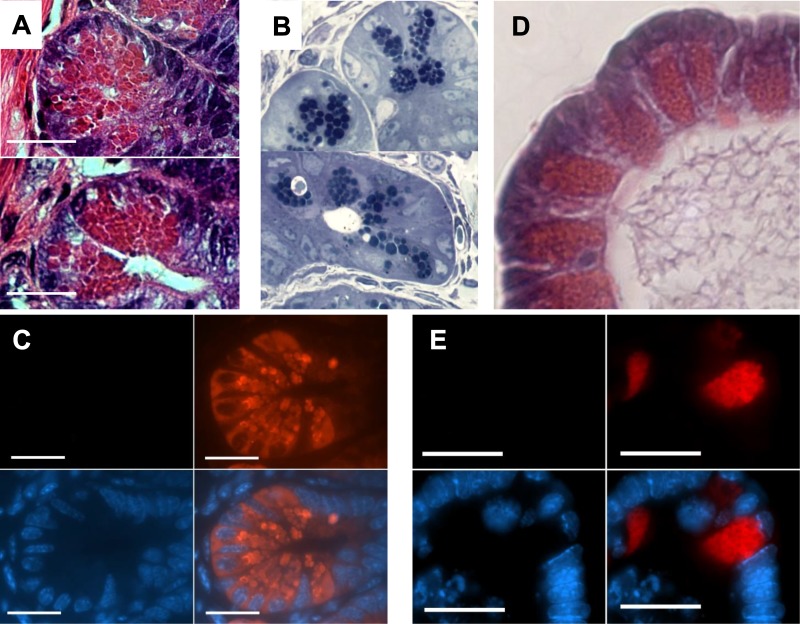
Muc2−/− mice were next used to investigate the potential contribution of Muc2 to the structure of Paneth cell granules. Ileal tissue sections collected from Muc2−/− mice were stained for several markers and evaluated by H and E and immunofluorescent microscopy. As expected, no positive staining for Muc2 was seen in Paneth cells or goblet cells from Muc2−/− mice. A comparison of the Paneth cell granules of Muc2−/− mice to those of wild-type mice under both H and E and toluidine blue staining revealed a shift in granule size and number. Whereas individual granules were readily visible in the wild-type mice, granules in the Muc2−/− mice were more closely packed, often without any halo or clear space visible between granules (Fig. 3, A and B). This difference with wild-type mice was also observed in enteroids from Muc2−/− mice using both light (H and E and toluidine blue) and immunofluorescent (lysozyme) microscopy (Fig. 3, A–C) with tightly packed granules observed in the Paneth cells in Muc2−/− ileal enteroids (Fig. 3, D and E).
Fig. 3.
Paneth cells in the ileal crypts of a Muc2−/− mouse. The Paneth cells and their granules are still clearly visible under hematoxylin and eosin (H and E) (A) and toluidine blue (B) staining; however, the granules are consistently larger and more tightly packed, with no clear areas between granules. Immunofluorescent staining for Muc2 (green) (C), lysozyme (red), and DAPI (blue) confirms the shift in granule shape and size and confirms the complete absence of Muc2 staining from the Muc2−/− mice. Paneth cells from wild-type murine ileal enteroids were stained with H and E (D) or for Muc2, lysozyme, and DAPI (as described above) (E). Granules are more densely packed compared with wild-type Paneth cells, with no positive Muc2 staining. Scale bars = 10 μM, ×630 magnification.
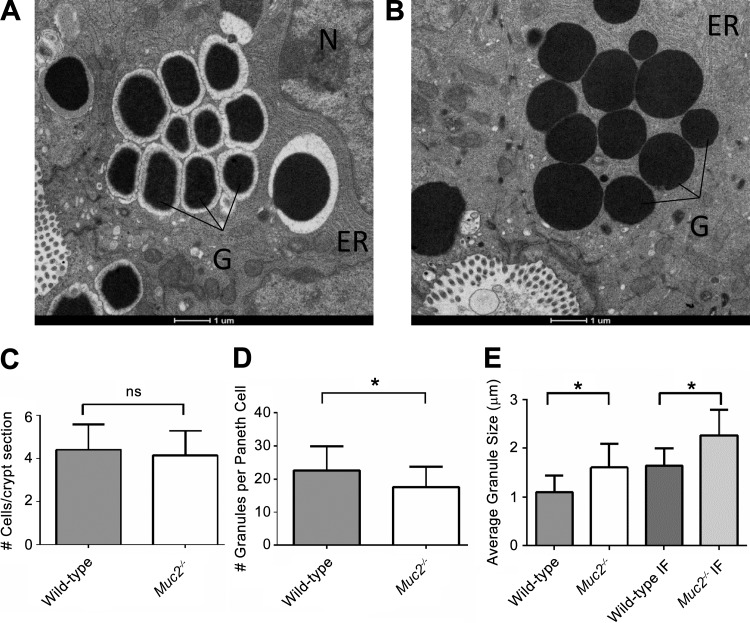
Using TEM, we further examined the Paneth cells of both wild-type and Muc2−/− mice (Fig. 4, A and B). As previously reported, Paneth cell granules appeared as dark, electron-dense bodies within the cytoplasm of the cell (21, 24). These granules predominantly resided within the apical region of the cell nearest the crypt lumen, whereas the nucleus was found at the basal region of each cell. Consistent with their role as secretory cells, the stacked folds of the ER covered much of the remaining space within the cytoplasm of the cell. A further characteristic of murine Paneth cells that has been previously described is an electron-lucent layer surrounding each of the granules (21). The appearance of this electron-lucent layer is consistent with the presence of large glycoproteins, and we found that it correlated with the location of our Muc2 immunofluorescent staining around each granule.
Fig. 4.
Electron micrograph of wild-type (A) and Muc2−/− (B) Paneth cells taken from fixed ileal samples of 6-wk-old mice. The granules (G) appear as dark electron-dense circles. The nucleus (N) and the endoplasmic reticulum (ER) can be seen in the surrounding cell. The clearest difference was the absence of the electron-lucent halo around each of the electron-dense granules in the Muc2−/− mice. The absence of Muc2 does not impact the number of Paneth cells per crypt (C), whereas granules were significantly fewer in number per cell (D) (*P < 0.001, Mann-Whitney test). Moreover, the granules in the Muc2−/− Paneth cells were on average of a larger diameter (E), as measured in both transmission electron microscopy (TEM) images (left) and immunofluorescent images (IF) (right) (*P < 0.0001, Mann-Whitney test). Error bars represent standard deviation.
Similar to our previous imaging of Muc2−/− Paneth cells, the granules in the Muc2−/− tissues appeared larger and more disordered in their arrangement compared with wild-type cells. Notably, the characteristic electron-lucent layer was missing from around the Muc2−/− granules, indicating that this layer is largely comprised of Muc2. Other aspects of the Muc2−/− Paneth cells appeared normal, including the ER, which suggests that Muc2−/− Paneth cells are not undergoing any substantial ER stress, such as that previously observed in ATG16L1−/− Paneth cells (6). Interestingly, Muc2 deficiency did not overtly change the number of Paneth cells per crypt (Fig. 4C); however, by comparing both the size of each granule (as measured using electron microscopy images) and the number of granules per cell (using toluidine blue-stained sections), we found that Muc2−/− Paneth cells contained fewer granules than those in wild-type mice (Fig. 4D) and that the granules were on average ~0.5μM larger in the Muc2−/− cells, as measured in both TEM micrographs and immunofluorescent images (Fig. 4E). To determine whether the differences in granule appearance could reflect differences in the microbiota (and hence bacterial signaling) between mouse strains, as opposed to a direct effect of the loss of Muc2, we crossed homozygous Muc2−/− mice with heterozygous Muc2-/+ mice and compared the Paneth cell morphology of heterozygous wild-type and Muc2−/− littermates. Such cross breeding minimizes differences in baseline gut microbiota although differences in microbial makeup attributable to the loss of Muc2 should remain. Much like the results outlined above, the Muc2−/− mice showed significant differences in granule size and density compared with their Muc2-expressing littermates, demonstrating that the observed defects in Paneth cell function are due to the loss of Muc2 (not shown).
Muc2 is a heavily O-glycosylated protein, with its glycosylation playing a key role in its ability to generate a functional mucus layer (28). To test whether the Muc2 within Paneth cells was glycosylated and whether such glycosylation was required for it to form the shell around Paneth cell granules, we stained for lysozyme and Muc2 in ileal tissues of mice lacking Core-3 glycosylation (C3Gnt−/−) and in mice that lack Core-1 glycosylation in villin-expressing IECs (IEC-C1galt1−/−), which includes Paneth cells. Not surprisingly, because murine Muc2 only undergoes modest levels of Core-3 glycosylation, staining for lysozyme and Muc2 in the Paneth cells of C3Gnt−/− mice revealed a full mucin shell and no morphological difference to wild-type cells (Fig. 5A). In contrast, murine Muc2 undergoes extensive Core-1 glycosylation, so IEC-C1galt1−/− mice lack most O-glycosylation on their mucins and thus express a much thinner mucus barrier than normal. Staining of their Paneth cells revealed an overt depletion of the Muc2 content (Fig. 5B), with the mucin no longer surrounding the granules but rather localized to one pole, indicating that properly glycosylated Muc2 is required to form the outer mucin shell of Paneth cell secretory granules. Interestingly, unlike in the Muc2−/− Paneth cells, there was no noticeable difference in granule size or number in IEC-C1galt1−/− mice, suggesting that it is the loss of the Muc2 protein, rather than its glycosylation, that leads to the aberrant Paneth cell phenotype in Muc2−/− mice.
Fig. 5.
Paneth cells from Core-3-deficient (C3Gnt−/−) (A) and Core-1-deficient (C1galt1−/−intestinal epithelial cell) (B) mice, stained for Muc2 (green), lysozyme (red), and DAPI (blue). C3Gnt−/− Paneth cells were indistinguishable from wild-type Paneth cells; however, the C1galt1−/− Paneth cells were lacking most of their Muc2 halo and instead displayed only a patch of Muc2 at 1 pole of each granule (arrows). Granule structure and function were not otherwise affected. Scale bars = 10 μM, ×1,000 magnification.
Muc2 deficiency leads to altered Paneth cell function in the mouse ileum.
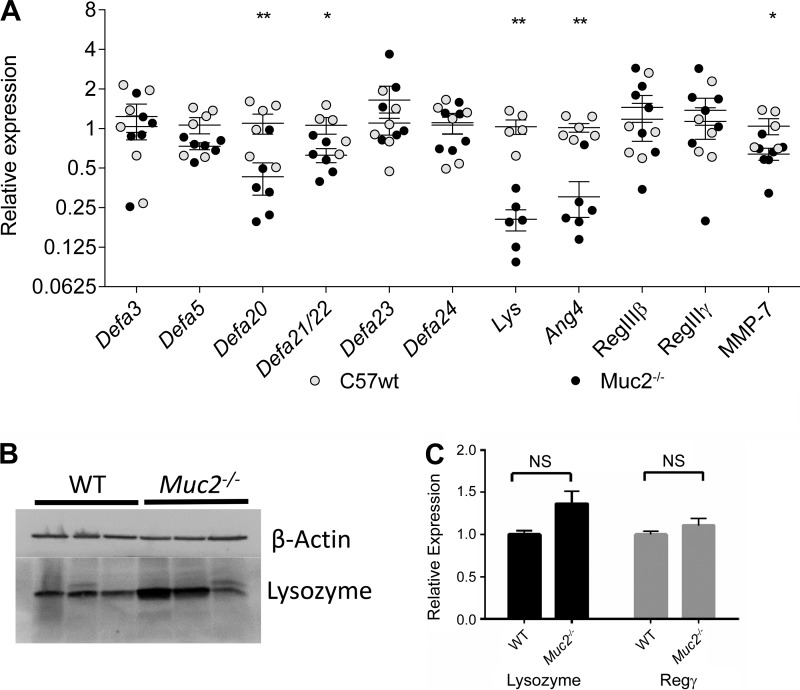
On the basis of the morphological differences observed in the Muc2−/− Paneth cells, we hypothesized that they might suffer altered functional characteristics. As Paneth cells are best known for their production of antimicrobial peptides and proteins (7), we measured the transcription of an array of antimicrobial factors expressed by Paneth cells and normally packaged within their granules. Despite carrying larger secretory granules, which would suggest overproduction of granule proteins, the transcription of several key antimicrobial peptides was reduced in the Muc2−/− mice. These included the antimicrobial enzyme lysozyme, reduced by a striking 80%, angiogenin 4 (70%), α-defensins 20 (57%), 21, and 22 (37%), and the α-defensin-maturing enzyme MMP7 (36%). Other α-defensin genes, as well as RegIIIβ and RegIIIγ, were not significantly altered in their transcription levels (Fig. 6A). Despite the transcriptional downregulation of lysozyme expression, Western blots showed that lysozyme protein levels were significantly higher in total ileal lysates from Muc2−/− mice, in keeping with their larger Paneth cell granules and their stronger lysozyme immunostaining, suggesting intracellular lysozyme accumulation (Fig. 6B). Together with the lower number and larger size of granules in Muc2−/− mice, these results point to a secretory defect characterized by intracellular retention and expansion of secretory granules in Muc2−/− Paneth cells.
Fig. 6.
A: relative expression of key antimicrobial peptides in the ilea of wild-type (WT) (○) and Muc2−/− mice (●). No significant difference was detected in the transcription of Defa3, 5, 23, or 24; however, a significant drop was measured in the transcription of Defa20, 21/22, lysozyme (Lys), and angiogenin 4 (Ang4) as determined by a Mann-Whitney U-test (*P < 0.05; **P < 0.005). B: Western blot of lysozyme from protein extracted from 3 representative WT and 3 representative Muc2−/− mice. Two of 3 Muc2−/− mice exhibited increased lysozyme content in their ilea, relative to WT despite reduced mRNA levels. C: relative expression of lysozyme and RegIIIy in WT and Muc2−/− small intestinal enteroids. No statistically significant difference was detected based on a Mann-Whitney U-test (P < 0.05), despite the Muc2−/− Paneth cell exhibiting similar morphological differences both in vivo and in enteroids.
Surprisingly, in contrast to our in vivo expression data, there was little difference in lysozyme expression between wild-type and Muc2−/− enteroids, despite the Muc2−/− Paneth cells exhibiting similar morphological differences both in vivo and in enteroids (Fig. 6C). This may reflect that baseline lysozyme expression is very low in the sterile environment of the enteroid culture, and thus our observed functional differences in Paneth cells are not wholly intrinsic to the cell but may be linked to microbial and/or immunological stimulation as well.
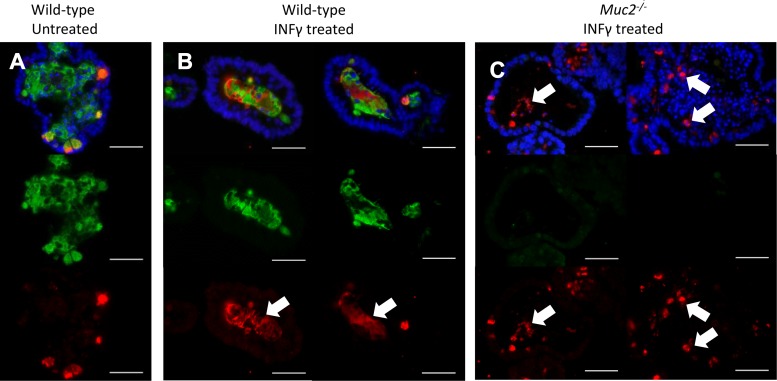
On the basis of our in vivo findings, we hypothesized that the Muc2 coating on secretory granules plays a role in facilitating granule secretion and/or dispersal into the intestinal lumen. To test this hypothesis, we treated ileal enteroids overnight with 20 µM of IFN-γ to induce granule exocytosis, as previously described (10). Staining of wild-type enteroids revealed that, although untreated enteroids maintained the majority of their lysozyme within Paneth cells (Fig. 7A), IFN)-γ -treated enteroids had released the majority of their lysozyme content into the enteroid lumen, where it was well intermixed with the large quantities of Muc2 found therein (Fig. 7B). In contrast, although Paneth cells in the Muc2−/− enteroids also expelled their contents following IFN-γ treatment, the released granules often remained intact within the enteroid lumen (Fig. 7C). These results suggest that the Muc2 shell facilitates the release of Paneth cell granules into the intestinal lumen, as well as the dispersal of their contents into the mucus layer.
Fig. 7.
Murine small intestinal enteroids were treated with 20 µM IFN-γ and 24 h later fixed and immunofluorescently labeled for Muc2 (green), lysozyme (red), and with DAPI (blue). Untreated enteroids (A) contained only minimal lysozyme in the lumen. Following treatment with IFN-γ, both lysozyme and mucus are exocytosed from the Paneth cells and evenly mixed within the lumen of wild-type enteroids (indicated by arrows) (B). In contrast, Muc2−/− enteroids treated with IFN-γ did not exhibit an even distribution of the released lysozyme (C), with much of the released lysozyme remaining in clumps and granules (indicated by arrows). Scale bars = 10 μM, ×400 magnification.
Muc2-coated granules are not found in human Paneth cells.
Given the lack of in vitro Paneth cell models and the difficulty in obtaining regular access to human tissues, most Paneth cell research has been conducted using mouse models. However, as informative as those models can be, there is always the question as to how fully mouse Paneth cell biology replicates that of human cells. To address this, we obtained formalin-fixed and sectioned intestinal biopsy samples from 10 pediatric patients. These patients were undergoing diagnostic biopsies and were subsequently diagnosed with Crohn’s disease (4 patients) or ulcerative colitis (4 patients) or were found to be negative for IBD (2 patients).
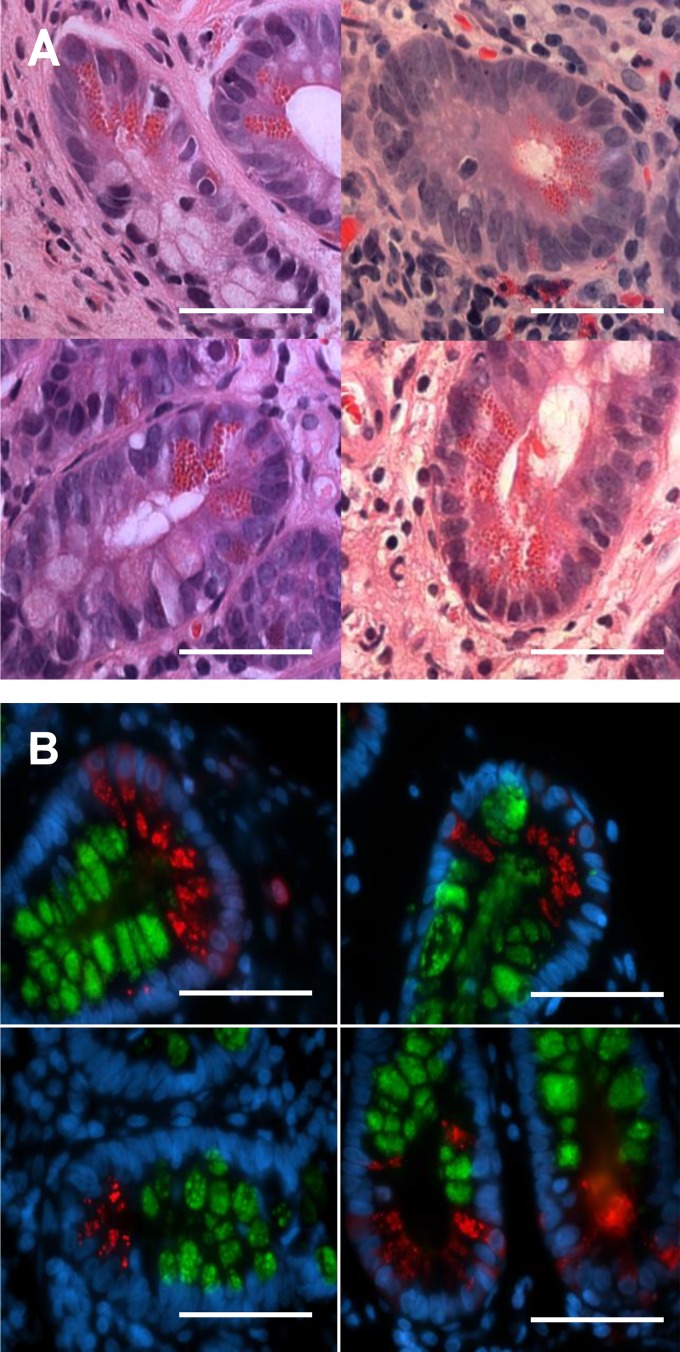
Intestinal sections were stained with H and E (Fig. 8A) or immunofluorescently stained for lysozyme and MUC2 (Fig. 8B). H and E stains did not reveal any noticeable abnormalities in Paneth cell granules among any of the patient tissues, regardless of disease type, and their morphology was not noticeably different from wild-type mouse Paneth cells. However, immunofluorescent staining for MUC2 and lysozyme on both ileal and duodenal sections revealed that, in contrast to mouse Paneth cells, human Paneth cells did not express any MUC2. These results are consistent with previous electron micrograph studies, suggesting that human Paneth cell granules do not possess the electron-lucent layer seen surrounding granules in mouse Paneth cells and that significant differences in granule structure and function exist between human and murine Paneth cells.
Fig. 8.
Four representative examples of formalin-fixed, hematoxylin and eosin-stained human ileal crypts containing Paneth cells (A). Left: ulcerative colitis. Top, right: patient without inflammatory bowel disease. Bottom, right: patient with Crohn’s disease. No significant differences in Paneth cell morphology were observed between patients, regardless of disease state. Immunofluorescent staining of formalin-fixed human ileal biopsies is shown (B). MUC2 is represented in green, lysozyme in red, and the nuclei are stained for DAPI in blue. The 4 panels are at ×1,000 magnification of the base of the crypts, where the Paneth cells reside. MUC2 staining within the epithelium is largely limited to the goblet cells lining the villi and crypts and is also visible within the thick mucus layer lining the tissue. The lysozyme is largely limited to the Paneth cells as the base of the crypts, and, unlike the murine Paneth cells, there were no signs of positive MUC2 staining within human Paneth cells. Scale bars = 20 μM, ×630 magnification.
DISCUSSION
A number of previous studies have noted the presence of mucin-like glycoproteins within murine Paneth cells (12, 22, 24) based on histological staining with periodic acid-Schiff stain (or other stains) for mucins or large glycoproteins. Despite these observations, no previous research has explored the identity of these glycoproteins or whether they play a role in the function of Paneth cells. In this study, we compared Paneth cells from wild-type C57BL/6 mice, Muc2−/− mice, and human patients and found that the previously observed glycoproteins within murine Paneth cells are primarily composed of the mucin Muc2 and that Muc2 is present as a thin layer that independently coats each of the Paneth cell granules. Surprisingly, this expression of Muc2 is not found in human Paneth cells.
Muc2 expression within Paneth cells is modest compared with the expression of Muc2 within goblet cells or in the intestinal mucus layer, which is perhaps why its presence has been generally overlooked. Nonetheless, every Paneth cell within wild-type mice was found to contain Muc2, which formed a very well-defined halo or shell surrounding each granule. Presumably, this shell corresponds to the electron-lucent layer seen around each granule when observed using electron microscopy. Accordingly, in Muc2−/− mice, the electron-lucent layer surrounding each granule was completely absent, and no Muc2 immunofluorescent staining was visible inside the Paneth cells, confirming that the perigranular staining is indeed Muc2. These findings support our conclusion that the shell is comprised primarily, if not entirely, of the mucin protein Muc2. Paneth cells grown in small intestinal enteroids in vitro largely reflected the granular morphology observed in their in vivo counterparts. This demonstrates that differences in granule appearance between wild-type and Muc2−/− Paneth cells in vivo are intrinsic to the intestinal epithelium or the Paneth cells and do not require nonepithelial cell types or the gut microbiome to manifest.
It has been previously reported that Muc2 deficiency negatively impacts the expression of the colonic β-defensins 4 and 14 (the orthologs of human β-defensins 2 and 3) and CRAMP (the ortholog of the human cathelicidin LL-37) (8, 9). In the ileum, lack of Muc2 was previously shown to induce the expression of Reg3b, Reg3g, and angiogenin 4 (4). However, we obtained different results showing that Muc2 deficiency does not affect ileal Reg3b and Reg3g expression while negatively impacting the transcriptional regulation of angiogenin 4. The reasons for these differences are unclear, but they might reflect differences in the age of the Muc2−/− mice tested, as well as their propensity to develop spontaneous intestinal inflammation. In our hands, lack of Muc2 also affected the synthesis of lysozyme and several α-defensins, exclusively produced by Paneth cells. Interestingly, only α-defensins 20, 21, and 22 were sensitive to the lack of Muc2, and these three are the highest expressed α-defensins in C57/BL6 mice (13). Differences in lysozyme expression, however, were not reproduced in the enteroid cultures, suggesting that other factors may play a role in antimicrobial expression beyond simple Muc2 deficiency.
Muc2−/− mice are highly susceptible to enteric bacterial infections and develop spontaneous colitis (2, 14, 28). Interestingly, this susceptibility to colitis is also found in mice that express the Muc2 protein but lack core-3 and/or core-1 O-glycans (1, 11). The intestinal epithelium of Muc2−/− mice is more exposed to, and thus more affected by, both pathogens and commensal bacteria. Moreover, the makeup of the commensal microbes found in Muc2−/− mice has been shown to be substantially different from wild-type mice, likely attributable to differences in nutrient (i.e., mucin) availability, as well as the altered immune responses in these mice (2). This in turn further disrupts the homeostasis of the gut and the maturation of the immune system. Although these issues are a clear reflection of the loss of the intestinal mucus layer, our results suggest that the observed impairment of secretory function in Paneth cells in Muc2−/− mice (namely, a defect in antimicrobial synthesis and secretion) may have a direct impact on the susceptibility phenotypes of these mice.
The finding that expression of Muc2 was not consistent between murine and human Paneth cells was unexpected. Patient tissues clearly showed MUC2-positive immunofluorescent staining within goblet cell granules, as well as lysozyme-positive staining in Paneth cells, but no trace of MUC2 was observed in the Paneth cells, leading us to conclude that, unlike their murine counterparts, human Paneth cells do not produce MUC2. Our findings are supported by a previous electron microscopy study (21) that showed that, when murine Paneth cell granules were observed at the ultrastructural level, they contained an electron-lucent layer, whereas this layer was not observed in human Paneth cells. This presence of a mucin halo/shell in mouse Paneth cells and its absence in human cells add to other known functional differences between Paneth cells from these two species, regarding the synthesis and secretion of antimicrobial peptides (by far, the best known function of Paneth cells). Mice possess more than 20 genes for Paneth cell α-defensins, whereas humans only have two (3). Moreover, in mice, Paneth cell α-defensins are processed to their mature form by MMP7 within the secretory granules before secretion, whereas in humans the proteolytic processing is mediated by trypsin and occurs within the intestinal lumen, following their secretion (3). These differences are indicative of a clear developmental and functional divergence between human and murine Paneth cells.
Given that Muc2−/− murine Paneth cells showed overt structural and functional defects, the question remains as to what the role is of Muc2 in mouse Paneth cells. In the gastrointestinal system, Muc2 has been traditionally viewed as a goblet cell-secreted product whose function centers on the formation of the intestinal mucus layer. However, our data suggest that the outer Muc2 shell of mouse Paneth cell granules facilitates the rapid dispersal of the antimicrobial peptides of the granules into the mucus layer, and it is intriguing that human Paneth cells do not utilize this mechanism. Perhaps the physically deeper crypts of the human ileum provide more time for effective dispersal of antimicrobial proteins, thereby making Muc2 expression unnecessary in human Paneth cells. Conversely, increased efficiency in antimicrobial dispersion within the mucus layer might be more beneficial in mice, given the shorter distance between their Paneth cells and the bacteria-filled lumen. Additional studies of the detailed mechanisms and dynamics of granule and mucus release may be necessary to completely elucidate the full role Muc2 plays in the mouse Paneth cells and exactly how human Paneth cells have developed to function in its absence. With this study, we have confirmed the location and identity of a Muc2 layer around murine Paneth cells granules and have shown that its loss leads to a shift in granule size and quantity of the Paneth cell granules, with implications for the functional efficiency of these important innate immune cells.
GRANTS
Funding for Bruce A. Vallance was provided by the Canadian Institutes of Health Research (CIHR) (project grant PJT-148846), Crohn’s and Colitis Canada, and the National Science and Engineering Research Council (NSERC) (discovery grant RGPIN 436233-13). Funding for Alfredo Menendez was provided by an NSERC discovery grant (401949-2011), and he is a member of (and supported by) the Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke (CRCHUS). Martin Stahl was supported by a Michael Smith Foundation for Health Research Postdoctoral Fellowship, and Sarah Tremblay was supported by a Fonds de recherche du Québec - Nature et technologies (FRQNT) doctoral scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S. and S.T. conceived and designed research; M.S., S.T., M.M., and W.V. performed experiments; M.S., S.T., M.M., W.V., K.J., and A.M. analyzed data; M.S., W.V., K.J., A.M., and B.A.V. interpreted results of experiments; M.S. and A.M. prepared figures; M.S. drafted manuscript; M.S., S.T., M.M., W.V., L.X., K.J., A.M., and B.A.V. edited and revised manuscript; M.S., S.T., M.M., W.V., L.X., K.J., A.M., and B.A.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We Caixia Ma and Tina Huang for technical assistance.
Present address for M. Montero: Department of Biochemistry and Molecular Medicine, Université de Montréal, Montreal, Quebec, Canada.
REFERENCES
- 1.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204: 1417–1429, 2007. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6: e1000902, 2010. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9: 356–368, 2011. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 4.Burger-van Paassen N, Loonen LM, Witte-Bouma J, Korteland-van Male AM, de Bruijn AC, van der Sluis M, Lu P, Van Goudoever JB, Wells JM, Dekker J, Van Seuningen I, Renes IB. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3β, Reg3γ and angiogenin-4. PLoS One 7: e38798, 2012. doi: 10.1371/journal.pone.0038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 420: 211–219, 2009. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 6.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW IV. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263, 2008. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75: 289–311, 2013. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 8.Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2. Mucosal Immunol 8: 1360–1372, 2015. doi: 10.1038/mi.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobo ER, Kissoon-Singh V, Moreau F, Holani R, Chadee K. MUC2 mucin and butyrate contribute to the synthesis of the antimicrobial peptide cathelicidin in response to Entamoeba histolytica- and dextran sodium sulfate-induced colitis. Infect Immun 85: 85, 2017. doi: 10.1128/IAI.00905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, Kalkhoven E, Nieuwenhuis EE, Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-γ. J Exp Med 211: 1393–1405, 2014. doi: 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, McDaniel JM, Sferra TJ, Turner JR, Chen H, Hansson GC, Braun J, Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest 121: 1657–1666, 2011. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge YB, Yang DH, Ohmori J, Tsuyama S, Kim BS, Kim JB, Murata F. Cationic colloidal gold staining of acidic glycoconjugates in mouse Paneth cells. Arch Histol Cytol 60: 133–142, 1997. doi: 10.1679/aohc.60.133. [DOI] [PubMed] [Google Scholar]
- 13.Menendez A, Willing BP, Montero M, Wlodarska M, So CC, Bhinder G, Vallance BA, Finlay BB. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J Innate Immun 5: 39–49, 2013. doi: 10.1159/000341630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morampudi V, Dalwadi U, Bhinder G, Sham HP, Gill SK, Chan J, Bergstrom KS, Huang T, Ma C, Jacobson K, Gibson DL, Vallance BA. The goblet cell-derived mediator RELM-β drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol 9: 1218–1233, 2016. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 15.Parkes M. Evidence from genetics for a role of autophagy and innate immunity in IBD pathogenesis. Dig Dis 30: 330–333, 2012. doi: 10.1159/000338119. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito H, Kasajima T, Masuda A, Imai Y, Ishikawa M. Lysozyme localization in human gastric and duodenal epithelium. An immunocytochemical study. Cell Tissue Res 251: 307–313, 1988. doi: 10.1007/BF00215838. [DOI] [PubMed] [Google Scholar]
- 18.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 19: 70–83, 2007. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 21.Satoh Y, Yamano M, Matsuda M, Ono K. Ultrastructure of Paneth cells in the intestine of various mammals. J Electron Microsc Tech 16: 69–80, 1990. doi: 10.1002/jemt.1060160109. [DOI] [PubMed] [Google Scholar]
- 22.Selzman HM, Liebelt RA. Paneth cell granule of mouse intestine. J Cell Biol 15: 136–139, 1962. doi: 10.1083/jcb.15.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sham HP, Yu EY, Gulen MF, Bhinder G, Stahl M, Chan JM, Brewster L, Morampudi V, Gibson DL, Hughes MR, McNagny KM, Li X, Vallance BA. SIGIRR, a negative regulator of TLR/IL-1R signalling promotes microbiota dependent resistance to colonization by enteric bacterial pathogens. PLoS Pathog 9: e1003539, 2013. doi: 10.1371/journal.ppat.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spicer SS, Staley MW, Wetzel MG, Wetzel BK. Acid mucosubstance and basic protein in mouse Paneth cells. J Histochem Cytochem 15: 225–242, 1967. doi: 10.1177/15.4.225. [DOI] [PubMed] [Google Scholar]
- 25.Van Limbergen J, Geddes K, Henderson P, Russell RK, Drummond HE, Satsangi J, Griffiths AM, Philpott DJ, Wilson DC. Paneth cell marker CD24 in NOD2 knockout organoids and in inflammatory bowel disease (IBD). Gut 64: 353–354, 2015. doi: 10.1136/gutjnl-2013-305077. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Kim JJ, Denou E, Gallagher A, Thornton DJ, Shajib MS, Xia L, Schertzer JD, Grencis RK, Philpott DJ, Khan WI. New role of Nod proteins in regulation of intestinal goblet cell response in the context of innate host defense in an enteric parasite infection. Infect Immun 84: 275–285, 2015. doi: 10.1128/IAI.01187-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA 102: 18129–18134, 2005. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarepour M, Bhullar K, Montero M, Ma C, Huang T, Velcich A, Xia L, Vallance BA. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun 81: 3672–3683, 2013. doi: 10.1128/IAI.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]