Abstract
The sensory neurotransmitter calcitonin gene-related peptide (CGRP) is associated with vasodilation of systemic arteries through activation of ATP-sensitive K+ (KATP) channels in smooth muscle cells (SMCs); however, its effects on endothelial cell (EC) membrane potential (Vm) are unresolved. In pulmonary arteries (PAs) of C57BL/6J mice, we questioned whether CGRP would hyperpolarize ECs as well as SMCs. Intact PAs were isolated and immunostained for CGRP to confirm sensory innervation; vessel segments (1–2 mm long, ∼150 µm diameter) with intact or denuded endothelium were cannulated and pressurized to 16 cmH2O at 37°C. Increasing concentrations (10−10–10−6 M) of CGRP progressively dilated PAs preconstricted with UTP (10−5 M); SMCs hyperpolarized similarly (ΔVm ∼20 mV) before and after endothelial denudation. To study native intact PA ECs, SMCs were dissociated to isolate endothelial tubes, and their integrity was confirmed by vital dye uptake, nuclear staining, and reproducible electrical and intracellular Ca2+ responses to acetylcholine (10−5 M) over 2 h. Increasing [CGRP] hyperpolarized ECs in a manner similar to SMCs, with each cell layer demonstrating robust immunostaining for CGRP receptor proteins. Increasing concentrations (10−10–10−6 M) of pinacidil, a KATP channel agonist, resulted in progressive hyperpolarization of SMCs of intact PAs (ΔVm ∼30 mV), which was blocked by glibenclamide (10−6 M), as was hyperpolarization of ECs and SMCs to CGRP. Inhibition of protein kinase A with protein kinase inhibitor (10−5 M) also inhibited hyperpolarization to CGRP. We demonstrate [CGRP]-dependent hyperpolarization of ECs for the first time while validating freshly isolated PA endothelial tubes as an experimental model. Redundant electrical signaling to CGRP in ECs and SMCs implies an integral role for KATP channels in PA dilation.
Keywords: hyperpolarization, lung blood flow, protein kinase A, sensory nerves, vasodilation
INTRODUCTION
With lungs receiving the entire cardiac output, the pulmonary circulation is a high-flow system operating at lower pressure than the systemic circulation. Low pulmonary vascular resistance is effected through vasodilators that regulate membrane potential (Vm) through K+ channel activation (1, 14, 24, 64). In such a manner, hyperpolarization of smooth muscle cells (SMCs) decreases voltage-gated Ca2+channel (VGCC) activity, thereby diminishing Ca2+ influx and lowering intracellular Ca2+ concentration ([Ca2+]i) to promote relaxation and vasodilation (43). While pulmonary artery (PA) endothelial cells (ECs) govern smooth muscle function and vasomotor tone (25, 28, 36), little is known with respect to their regulation of Vm.
For the pulmonary vasculature, innervation density is greatest in extrapulmonary and hilar arteries (12, 17, 19, 20). Penetration of sympathetic and parasympathetic nerves along PA branches varies across species. In mice, both sources of innervation stop shortly after the lung hilus (12). In contrast, sensory (peptidergic) axons are found sparingly throughout the pulmonary vasculature (11, 33, 35, 37), where they release multiple neurotransmitters, including calcitonin gene-related peptide (CGRP). CGRP availability is diminished in pulmonary vascular disease (4, 23, 31, 59, 61), implying a compromised functional role for this vasodilator. In PA rings from guinea pigs, CGRP evokes relaxation independent of the endothelium (49), while studies in rats suggest that the endothelial nitric oxide (NO) pathway is required for PA relaxation (36). Thus the role of ECs in vasodilation to CGRP within the lung is unresolved, and this deficit in understanding is exacerbated by the absence of electrophysiological data.
In arteries of the systemic circulation, sensory nerves cause vasodilation via activation of CGRP receptors on both SMCs and ECs (3, 30), with SMCs shown to be hyperpolarized via activation of ATP-sensitive K+ (KATP) channels (8, 42, 51, 62). As with the pulmonary circulation, there is a lack of data for electrophysiological responses to CGRP in ECs of the systemic vasculature. With our focus on the lung, a primary goal of the present study was to determine whether CGRP hyperpolarized PA endothelium independent of the influence of SMCs. For this purpose, we developed the freshly isolated endothelial tube preparation for PAs and validated its integrity. In complementary experiments, we evaluated the effects of CGRP on SMCs in the presence and absence of ECs. Whereas CGRP evokes PA dilation (36, 49), the underlying signaling pathway remains undefined. In SMCs of the aorta and coronary and mesenteric arteries, CGRP can activate KATP channels through cAMP and protein kinase A (PKA) (40, 48, 51, 62). Therefore, we hypothesized that PKA-dependent KATP channel activation mediates hyperpolarization and vasodilation to CGRP in PAs.
Our findings provide the first intracellular recordings from native intact PA endothelium and illustrate that CGRP activates KATP channels through the actions of PKA, with complementary effects in SMCs. We suggest that the intima and media have complementary roles in effecting PA dilation in response to circulating neuropeptide, as well as in response to CGRP released by sensory nerves.
METHODS
Animal Care and Use
All protocols and experimental procedures were reviewed and approved by the Animal Care and Use Committee of the University of Missouri. Experiments were performed on C57BL/6J mice [n = 111 males (27–32 g body wt) and 10 females (24–27 g body wt), 3–4 mo old] purchased from Jackson Laboratories (Bar Harbor, ME). Complementary experiments were performed on PAs from KATP/KIR6.1 knockout (KATP−/−) mice (n = 3) obtained from Washington University (St. Louis, MO) (39). Before they were used in an experiment, mice were housed locally for ≥1 wk on a 12-h:12-h light-dark cycle at ~23°C, with fresh water and food available ad libitum. On the morning of an experiment, a mouse was retrieved from the vivarium and anesthetized with pentobarbital sodium (60 mg/kg ip). The lungs were accessed via a midline sternotomy and excised, and the mouse was euthanized by exsanguination.
Solutions
Freshly isolated arteries and endothelial tubes were superfused with physiological salt solution (PSS, pH 7.4) containing (in mM) 140 NaCl (Fisher Scientific, Pittsburgh, PA), 5 KCl (Fisher Scientific), 2 CaCl2 (Fisher Scientific), 1 MgCl2 (Sigma-Aldrich, St. Louis, MO), 10 HEPES (Sigma-Aldrich), and 10 glucose (Fisher Scientific). During vessel dissection and preparation of endothelial tubes, the PSS contained 0.1% bovine serum albumin (catalog no. 10856, USB, Cleveland, OH). During dissection, CaCl2 was absent and 10−5 M sodium nitroprusside was added to relax SMCs. For dissociation of SMCs, 10−4 M CaCl2 (Sigma) was included (required for enzyme activity) and sodium nitroprusside was omitted.
Staining for CGRP-Positive Nerve Fibers and Receptors
Staining for CGRP was used to identify the presence of perivascular sensory nerves. The PA tree (≥80-μm-diameter arteries dissected from the left lobe of the lung) was transferred to a 12-well plate, each well of which was coated with transparent silicone rubber (Sylgard 184, Dow Corning, Midland, MI). Preparations were pinned to the Sylgard, fixed in 4% paraformaldehyde for 60 min, and washed in phosphate-buffered saline (PBS). Arterial trees were incubated with 10% goat serum and 0.2% Triton X-100 in PBS and then treated for 60 min at room temperature with an antibody for CGRP (1:250 dilution; catalog no. PC205L, EMD Millipore, Billerica, MA) validated with preabsorption in mouse spinal cord sections (22). The arterial trees were then incubated for 60 min at room temperature with an Alexa Fluor 488 secondary antibody (1:500 dilution; Molecular Probes/Life Technologies, Carlsbad, CA) and washed with PBS (9). Preparations were mounted on slides using ProLong Gold (Invitrogen, Carlsbad, CA) and a coverslip and then imaged on a confocal laser-scanning microscope (model SP5, Leica Microsystems, Buffalo Grove, IL). Staining for CGRP was visualized using a ×63 oil-immersion objective [numerical aperture (NA) 1.4; Leica] and 0.5-μm z slices. Sensory nerve density was determined by quantification of fluorescent area of CGRP staining on maximal-intensity z-stack projections of PA segments (9). Background fluorescence (from a region of vessel not containing nerve fibers) was subtracted, and the remaining pixels were counted, compared with the total number of pixels in the vessel area, and expressed as a percentage.
Additional experiments stained for two of the three CGRP receptor components: receptor activity-modifying protein 1 (RAMP1) and calcitonin receptor-like receptor (CRLR). Thus PAs were prepared as described above, cut along one edge to open the vessel for en face preparation, and treated with primary antibodies for RAMP1 (1:250 dilution; catalog no. sc-11379, Santa Cruz Biotechnology, Dallas, TX; excludes RAMP2/3 isoforms) and CRLR (1:250 dilution; catalog no. sc-18007, Santa Cruz Biotechnology) overnight (9). Specificity of these antibodies for their antigens has been demonstrated in transfected COS-7 cells (67), and we previously established their lack of nonspecific binding in mouse mesenteric arteries (9). After the samples were incubated with Alexa Fluor secondary antibodies [1:500 dilution; RAMP1 (488-nm excitation) and CRLR (546-nm excitation)] and mounted, slides were imaged on a Leica SP8 confocal laser-scanning microscope using a ×63 glycerol-immersion objective (NA 1.3; Leica) with ×2.5 digital zoom. Image stacks of SMCs and ECs were acquired in this manner from the same preparations.
Microdissection, Arterial Isolation, Endothelial Denudation, and Endothelial Tube Production
Under stereomicroscopic observation, individual PAs [100–150 µm inner diameter (ID)] were dissected from surrounding tissue and placed in a tissue chamber (model RC-27N, Warner Instruments, Hamden, CT) secured on a rectangular (24 × 14.5 cm) aluminum platform (4 mm thick). An isolated PA was cannulated onto heat-polished micropipettes (100 μm outer diameter) and tied in place with a strand of silk suture. Cannulation pipettes were pulled (model P-97, Sutter Instruments, Novato, CA) from glass capillaries (0.94 mm ID, 1.2 mm outer diameter; Warner Instruments). Each pipette was secured in a holder (Warner Instruments) held in a three-axis micromanipulator (model no. DT3-100, Siskiyou, Grants Pass, OR) mounted on the aluminum platform at each end of the tissue chamber. Intact PAs were pressurized to 16 cmH2O during experiments (45).
Endothelial denudation.
To ascertain SMC responses independent of EC influences (and to determine the role of ECs during responses of intact PAs), the endothelium was disrupted: a tungsten wire (50 µm diameter; Goodfellow, Huntington, UK) was rubbed three times through the lumen of the vessel before cannulation. Once the vessel was cannulated and pressurized, selective damage of ECs was confirmed by ~50% constriction to uridine triphosphate (UTP; EC50 = 5 × 10−6 M) to verify SMC integrity with loss of dilation to maximal acetylcholine (ACh) concentration ([ACh], 10−5 M).
Endothelial tube isolation.
To obtain intact endothelium that was devoid of SMCs, isolated PAs were placed into PSS containing 0.62 mg/ml papain (catalog no. P4762, Sigma), 1.0 mg/ml dithioerythritol (catalog no. D8255, Sigma), 1.5 mg/ml collagenase (catalog no. C8051, Sigma), and 30 µg/ml elastase (catalog no. LS002290, Worthington Biochemical, Lakewood, NJ) and incubated for 25 min at 32°C. After incubation, the enzyme solution was replaced with dissociation solution and arterial segments were transferred to the tissue chamber. During visual observation at ×200 magnification, segments were gently triturated to remove SMCs. For this purpose, trituration pipettes were pulled from borosilicate glass capillary tubes [stock no. 1B100-4, World Precision Instruments (WPI), Sarasota, FL], heat-polished to a tip ID of 120–150 µm, and connected to a Nanoliter injector (WPI) for controlled aspiration and ejection of the arterial segment. After removal of SMCs, an intact endothelial tube (0.5–1 mm long) was secured at each end against the bottom of the tissue chamber (a 24 × 54 mm coverslip) and extended to approximate in situ length by means of glass pinning pipettes (heat-blunted tips, ~80 μm diameter) secured in micromanipulators, as described above (57).
Completed preparations of PAs or endothelial tubes were placed on an inverted microscope (Eclipse TS100, Nikon, Tokyo, Japan) mounted on a vibration-isolated table (Technical Manufacturing, Peabody, MA) and superfused at 4 ml/min with PSS. An in-line heater (model SH-27B, Warner) and heating platform (model PH6, Warner) coupled to a temperature controller (model TC-344B, Warner) maintained the tissue chamber at 37°C. Once pressurized, vessels with leaks were discarded. Endothelial tubes from PAs were unpressurized and studied at 32°C to maintain stability for the duration (≤2 h) of experiments (57). Pharmacological agents were added to the superfusion solution to achieve final concentrations, thereby exposing the entire preparation to the designated treatment. Each of the protocols was performed on independent preparations from separate mice.
To evaluate cell viability, preparations were subjected to live/dead viability/cytotoxicity assay (catalog no. 300002, Biotium, Fremont, CA). After equilibration in control PSS, endothelial tubes were incubated in PSS containing calcein-AM (2 × 10−6 M), which hydrolyzes and is retained in the cytosol of viable cells, and ethidium bromide (4 × 10−6 M), which stains nuclei of membrane-permeant dead cells, for 30 min and then washed for 2 min in control PSS (all at 32°C). To determine whether isolation of endothelial tubes effectively removed SMCs, completed preparations were labeled with the nuclear stain Hoechst 33342 (10−6 M; 40-min incubation + 20-min wash) to distinguish between SMCs and ECs. These preparations were imaged with a ×10 (NA 0.3; Nikon) or ×40 (NA 0.8; Nikon) objective coupled to a Nikon DS-Qi2 camera mounted on a Nikon Eclipse 800 microscope. Images were acquired using Nikon Elements software (version 4.51) on a personal computer.
Intracellular Recording
The Vm of SMCs within intact and denuded PAs and of ECs within endothelial tubes was recorded with an amplifier (AxoClamp 2B, Molecular Devices, Sunnyvale, CA) using sharp microelectrodes pulled (P-97, Sutter Instruments) from glass capillary tubes (stock no. GC100F-10, Warner) and backfilled with 2 M KCl (~150-MΩ tip resistance). An Ag/AgCl pellet in the effluent PSS served as a reference electrode. The output of the amplifier was connected to a data acquisition system (Digidata 1322A, Molecular Devices) and an audible baseline monitor (model ABM-3, WPI). Successful impalements were indicated by sharp negative deflection of Vm, stable Vm for >1 min, depolarization (≥20 mV) to 10−1 M KCl (SMCs) or hyperpolarization (≥20 mV) to 10−5 M ACh (endothelial tubes) (6), recovery to resting Vm after KCl or ACh washout, and prompt return to ~0 mV upon electrode withdrawal. Data were acquired at 1 kHz on a personal computer using AxoScope 10.1 software (Molecular Devices). After cell impalement, baseline Vm was recorded for 10 min to ensure stable electrode placement. Increasing [CGRP] (10−10−10−6 M; AnaSpec, Fremont, CA) was added cumulatively in half-log molar steps, with each equilibrated for 5 min. For analyses, data were averaged for 30 s during a stable Vm at each [CGRP]. Concentration response to the KATP channel agonist pinacidil (10−9−10−5 M; catalog no. P154, Sigma) was evaluated in the same manner as responses to CGRP. Protocols were routinely completed within 2 h.
Additional experiments were performed in the presence of protein kinase inhibitor [PKI-(14–22) amide; Tocris Bioscience, Bristol, UK] or the KATP channel inhibitor glibenclamide (catalog no. G2539, Sigma) dissolved in DMSO (final concentration, 0.1%; vehicle = DMSO alone). Preparations were equilibrated for 20 min with respective agents before evaluation of electrophysiological responses to CGRP. To determine an effective concentration of glibenclamide, SMCs and ECs from PAs were first hyperpolarized with maximal [pinacidil] (10−5 M) to evaluate the effect of cumulative increases in [glibenclamide] (10−10−10−6 M). For analyses, data were sampled as described above.
Vasomotor Responses
During pressure myography, the agonist UTP was chosen for its stable pressor effect in PAs (27), while vasodilation to [ACh] (10−5 M) confirmed functional integrity of the endothelium (and loss thereof following denudation). Using bright-field illumination, images of a PA were acquired with a ×20 objective (NA 0.45; Nikon Fluor 20) and a charge-coupled device camera (MyoCam-S, IonOptix, Milford, MA). During focus on the widest section of the vessel, ID was recorded during the 5th min at each concentration of agonist using IonWizard software (version 6.3, IonOptix) on a personal computer. The concentration-response curve to UTP (10−9−10−3 M) provided an EC50 (5 × 10−6 M) from which to preconstrict vessels to examine dilation to CGRP. A concentration-response curve to CGRP (10−10−10−6 M) defined EC50 and maximal concentrations for simultaneous evaluation of changes in ID and vessel wall [Ca2+] (see Ca2+ Signaling) at resting baseline, during constriction to UTP, and during dilations to EC50 and maximal [CGRP]. Experiments were also performed in PAs pretreated with glibenclamide, PKI, or the NO synthase inhibitor l-NAME (10−4 M; catalog no. N5751, Sigma). In each case, the vessel was equilibrated for 20 min before responses to CGRP were evaluated. Respective treatments were each studied in a separate PA.
Ca2+ Signaling
Vessel wall (primarily SMC) or endothelial tube [Ca2+]i was measured using fura 2-AM dye (Invitrogen) fluorescence by alternative excitation (10 Hz) at 340 and 380 nm and recording of emission at 510 nm using an IonOptix system (57). PAs were cannulated and pressurized (see above) and then incubated at 37°C for 40 min with 10−6 M fura 2 dye in the bath (for preferential loading of SMCs); then the vessels were washed for 20 min to allow deesterification of fura 2 within cells and to remove excess dye. Alternatively, endothelial tubes were loaded with fura 2 dye for 30 min at 32°C and then washed for 15 min. Values are expressed as fluorescence (F) ratios [510-nm fluorescence at 340-nm excitation relative to 510-nm fluorescence at 380-nm excitation (F340/F380)] after subtraction of autofluorescence recorded before dye loading.
Data Presentation and Analysis
Electrophysiological analyses included resting Vm (mV) under control conditions; peak Vm (mV), i.e., the maximal response to pharmacological intervention; and change in Vm (ΔVm), i.e., peak-response Vm – preceding baseline Vm. Vasomotor responses are presented as percent constriction or dilation calculated as follows: Vasoconstriction (%) = [(IDbase – IDresp)/IDbase] × 100, and Vasodilation (%) = [(IDresp – IDUTP) (IDbase – IDUTP)] × 100, where IDbase is baseline ID under control conditions before constriction with UTP, IDresp is response ID during a given agonist concentration, and IDUTP is ID of vessel preconstricted with UTP (EC50) before dilator application. Because of the absence of basal vasomotor tone during pressure myography, IDbase also represented maximal ID. For summary data, responses were normalized across experiments to account for differences in absolute size between arteries.
Data were analyzed using Student’s t-tests or ANOVA (Prism 5, GraphPad Software, La Jolla, CA), as appropriate. When significant main effects were detected with ANOVA, post hoc comparisons were performed using Bonferroni’s test. P ≤ 0.05 was considered statistically significant. Summary data are presented as means ± SE; n refers to the number of vessels (each from a different mouse) in a given experimental group. Findings are from male mice, unless stated otherwise.
RESULTS
PAs Are Innervated by Sensory Nerves That Contain CGRP
Immunofluorescence labeling for CGRP confirmed the presence of sensory nerves along the adventitia of isolated PAs (Fig. 1). In larger (≥150 µm diameter) PAs, 5 ± 3% of the vessel wall area was associated with sensory nerves. In small (80 to 150 µm diameter) PAs (as studied for electrophysiology and vasoreactivity), 6 ± 3% of the vessel wall was associated with sensory nerves (area of arteries imaged = 9,012 ± 1,797 μm2, n = 8 images from PAs of 4 mice).
Fig. 1.
Mouse pulmonary arteries are innervated by sensory nerves. Representative calcitonin-gene-related peptide (CGRP) staining (maximum z projections) confirms sensory innervation in larger (~300 μm diameter, A) and smaller (~150 μm diameter, B) pulmonary arteries. Arrows indicate nerve fibers; dotted lines in B indicate vessel edge. Scale bars = 20 µm.
CGRP Dilates PAs
Given the absence of spontaneous vasomotor tone during pressure myography, PAs were preconstricted to characterize vasodilation to CGRP. For this purpose, we first defined the EC50 of UTP. Cumulative addition of UTP produced concentration-dependent vasoconstriction of intact PAs (Fig. 2A) with an EC50 of 5 × 10−6 M. Constriction to UTP was maintained following endothelial denudation (Fig. 2B). Whereas addition of ACh (10−5 M) evoked robust vasodilation of preconstricted intact PAs (81 ± 8%; Fig. 2C), this response was abolished following endothelial denudation. For intact PAs preconstricted with 5 × 10−6 M UTP, CGRP evoked dilation in a concentration-dependent manner, with EC50 = 10−8 M (Fig. 2D). Peak dilation at 10−6 M CGRP was 89 ± 4%.
Fig. 2.
Calcitonin-gene-related peptide (CGRP) dilates pulmonary arteries (PAs) preconstricted with UTP. A: concentration response for vasoconstriction to UTP (10−9–10−3 M) in isolated, pressurized (16 cmH2O), intact PAs. B: UTP (5 × 10−6 M) constricted intact PAs, and this effect was not different for endothelium-denuded PAs. C: vasodilation of intact PAs preconstricted with UTP (5 × 10−6 M) to maximal [ACh] (10−5 M) was abolished following endothelial denudation. D: concentration response for vasodilation to CGRP (10−10–10−6 M) of intact PAs preconstricted with UTP (5 × 10−6 M). Values are means ± SE; n = 4–5 vessels per group. *P < 0.05 vs. intact. Details: UTP constricted arteries from 126 ± 12 µm inner diameter (ID) at rest to 19 ± 3 µm ID (maximal response). To preconstrict arteries for dilator studies, 5 × 10−6 M (EC50) UTP constricted intact PAs from 128 ± 8 to 68 ± 6 µm ID. In the presence of UTP, CGRP dilated intact PAs to maximal ID of 119 ± 7 µm.
Characterization of PA Endothelial Tubes
We developed the endothelial tube preparation based on methods originally developed for resistance arteries of skeletal muscle (6, 57, 58). After removal of SMCs and adventitia, only the cylindrical monolayer of endothelial cells remained. A live/dead assay (Fig. 3, A–C) illustrates that cells in the midregion (~300–500 µm long) of the tube used for data acquisition remain viable. In contrast, cells at each end of the tube that were crushed under the pinning pipettes are no longer viable. Bright-field imaging illustrates a cobblestone appearance of the ECs within the intact endothelium (Fig. 3D), a morphology consistent with previous reports (15). Staining for nuclei verifies the presence of SMCs for intact PAs and their absence from endothelial tubes (Fig. 3, E and F, respectively). These experiments illustrate that the endothelial tube preparation provides a pure population of viable PA ECs that maintain their physical association with each other. We then characterized the stability of their responses by stimulating endothelial tubes repeatedly with a maximal [ACh] (10−5 M) (Fig. 4). In each case, addition of ACh led to an increase in Ca2+ (ΔF340/F380 = 0.33 ± 0.07) and hyperpolarization (ΔVm = 33 ± 3 mV) that was reversible upon washout. Responses were consistent and reproducible for ≥2 h, which encompasses our longest experimental protocol.
Fig. 3.
Live/dead assay and cell morphology of pulmonary artery (PA) endothelial tubes. A: calcein staining of cytoplasm of live cells. B: ethidium bromide staining of dead cells beneath pinning pipettes (located at each end of the endothelial tube in respective panels). C: overlay image of A and B. D: bright-field image of boxed region in C shows endothelial cell (EC) orientation and intact morphology of endothelial tube secured between pinning pipettes. E: staining with Hoechst 33342 illustrates smooth muscle cell nuclei (thin, vertical) and EC nuclei (oval, horizontal) of an intact pressurized PA. F: nuclei staining showing purity for EC composition of endothelial tube. Apparent increase in EC nuclei compared with E reflects “flattened” tube with top and bottom sides within the same focal plane. Scale bars = 50 µm.
Fig. 4.
Stability of electrical and Ca2+ signaling in pulmonary artery (PA) endothelial tubes. A: representative continuous recording for membrane potential (Vm) and intracellular Ca2+ concentration ([Ca2+]i) responses (see methods) to intermittent exposure to ACh (10−5 M) during a 2-h protocol. Horizontal bars indicate periods of ACh superfusion. B: summary data. Pre, before ACh; ACh, peak hyperpolarization; Post, at 5-min washout. Values are means ± SE; n = 5 per group.
PA Endothelium and Smooth Muscle Are Hyperpolarized by CGRP
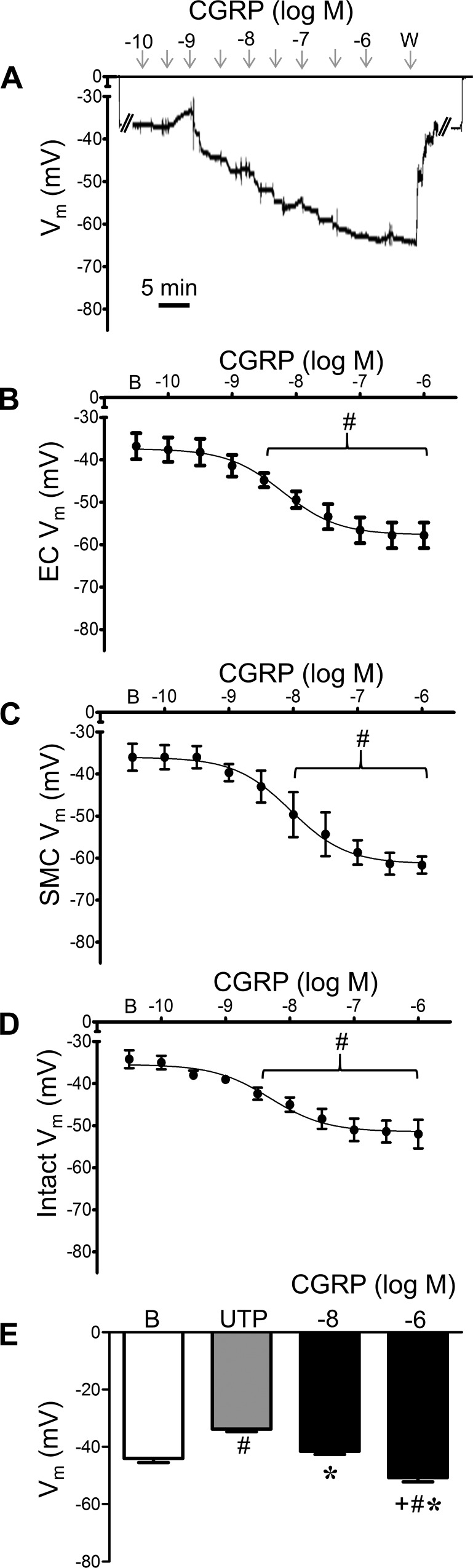
Given the absence of electrophysiological data addressing the effect of CGRP on ECs, we questioned whether CGRP could hyperpolarize the endothelium of PAs. During continuous recording of Vm, CGRP hyperpolarized ECs in a concentration-dependent manner (Fig. 5). In denuded PAs, SMCs were hyperpolarized by CGRP as well, and baseline Vm was similar between cell types in respective preparations. Remarkably, SMCs hyperpolarized to similar levels (by ~20 mV) whether or not the endothelium was present (Table 1), and respective EC50 values were not significantly different between cell types. Responses to CGRP in preparations from female mice were similar to those from male mice (Table 1). To relate electrophysiology with vascular reactivity (Fig. 2), Vm was recorded from intact PAs during exposure to UTP before and during addition of CGRP (Fig. 5E).
Fig. 5.
Pulmonary artery (PA) smooth muscle cells (SMCs) and endothelial cells (ECs) are hyperpolarized by calcitonin gene-related peptide (CGRP). A: representative continuous recording of membrane potential (Vm) illustrates concentration-dependent hyperpolarization of SMCs by CGRP in a denuded PA. From baseline (zero), note abrupt change in Vm upon entry and exit from cell. W, washout. B: summary data for Vm of PA endothelial tubes to CGRP. B, baseline. C: summary data for Vm of SMCs of denuded PAs to CGRP. D: summary data for Vm of SMCs of intact PAs to CGRP. E: summary data for Vm during resting baseline, with 5 × 10−6 M UTP alone and during addition of 10−8 or 10−6 M CGRP. Values are means ± SE; n = 5 per group. #P < 0.05 vs. baseline Vm. *P < 0.05 vs. UTP. +P < 0.05 vs. 10−8 M CGRP. UTP depolarized PAs 10 ± 1 mV, from which 10−6 M CGRP hyperpolarized PAs 17 ± 2 mV.
Table 1.
Vm of ECs and SMCs in PAs of male and female mice at rest and in response to CGRP
| Vm, mV |
||||
|---|---|---|---|---|
| Rest | Max | Δ | EC50, nM | |
| Males | ||||
| ECs | −37 ± 3 | −58 ± 3 | −21 ± 1 | 6 |
| SMCs | ||||
| Denuded | −36 ± 3 | −61 ± 2 | −25 ± 1 | 9 |
| Intact | −34 ± 2 | −52 ± 3 | −18 ± 2 | 5 |
| Females | ||||
| ECs | −44 ± 2 | −65 ± 4 | −21 ± 2 | 5 |
| SMCs | ||||
| Intact | −45 ± 2* | −69 ± 2* | −24 ± 3 | 13 |
Values are means ± SE; n = 4–5 per group. Vm, membrane potential; Rest, resting Vm; Max, Vm at maximum response; Δ, Max – Rest; ECs, endothelial tubes; SMCs, smooth muscle cells; PAs, pulmonary arteries.
P < 0.05 vs. respective value in males. EC50 values pertain to respective [CGRP] – response relationship.
CGRP Receptor Proteins Are Expressed on PA ECs and SMCs
With both ECs and SMCs of PAs demonstrating hyperpolarization to CGRP, we tested for the expression of CGRP receptors. Immunostaining confirmed that the CGRP receptor protein components RAMP1 and CRLR are expressed in respective cell layers, with overlap of images consistent with belonging to the same receptor complexes (Fig. 6). These findings substantiate the ability of both cell types to respond to CGRP.
Fig. 6.
Immunostaining for calcitonin receptor proteins in pulmonary arteries (PAs). Representative dual staining of PAs for receptor activity-modifying protein 1 (RAMP1, left, green) and calcitonin receptor-like receptor (CRLR; middle, red) confirms CRLR expression in endothelial cells (ECs) and smooth muscle cells (SMCs). Overlay images (right) illustrate corresponding localization (yellow) of respective proteins. Scale bar = 20 µm.
PA ECs and SMCs Are Hyperpolarized by KATP Channel Activation
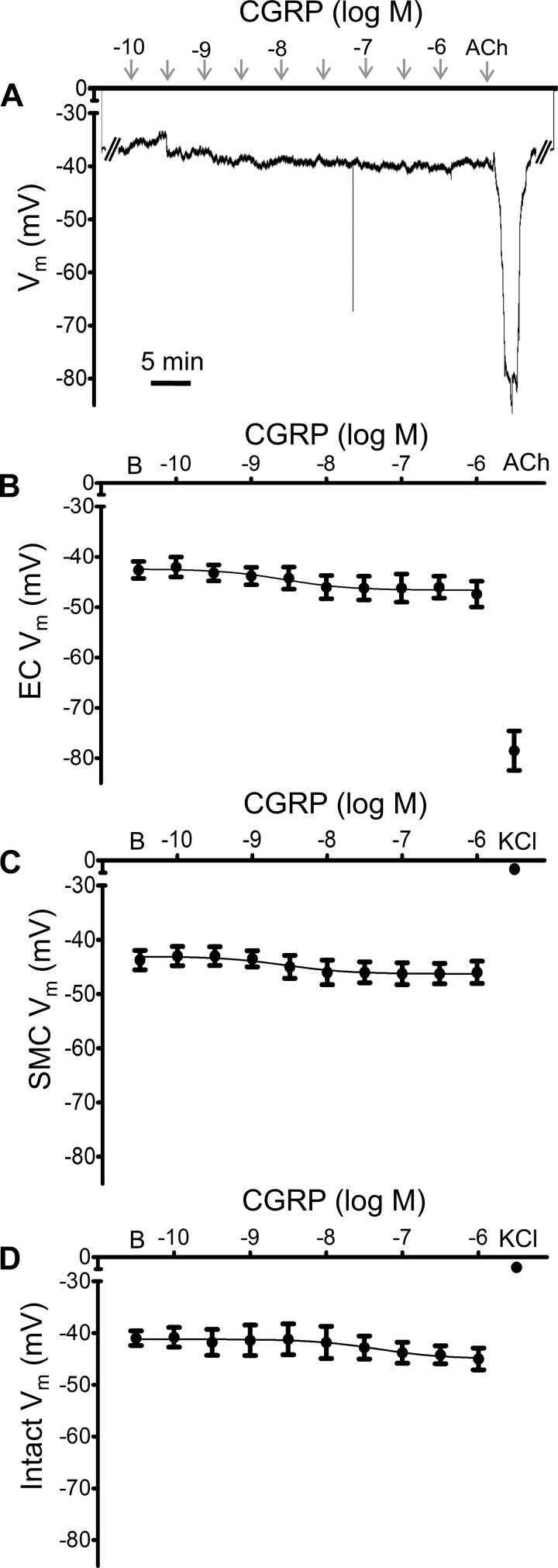
To determine if KATP channel activation could hyperpolarize ECs and SMCs, we evaluated the effect of a KATP channel agonist on Vm. Pinacidil evoked concentration-dependent hyperpolarization in endothelial tubes (Fig. 7, A and B), as well as SMCs (Fig. 7C), of intact PAs. In the presence of 10−5 M pinacidil, increasing concentrations of the KATP channel antagonist glibenclamide progressively reversed hyperpolarization of endothelial tubes (Fig. 8, A and B) and of SMCs within intact PAs (Fig. 8C). At 10−6 M, glibenclamide restored Vm to resting baseline in the presence of 10−5 M pinacidil, confirming inhibition of KATP channels. Under the same respective conditions, hyperpolarization to CGRP was nearly abolished (Fig. 9), with maximal ΔVm ≤5 mV (compare with Fig. 5). Consistent with the effect of glibenclamide, hyperpolarization to CGRP was nearly eliminated in PAs from mice lacking KATP channels (Fig. 10). To confirm the integrity of microelectrode placement in these experiments, ACh (10−5 M for ECs; ~35-mV hyperpolarization) or KCl (10−1 M for SMCs; ~30-mV depolarization) was added at the end of each recording period. Electrode placement within SMCs was further confirmed with the sympathetic neurotransmitter norepinephrine (10−6 M), which depolarized SMCs from −47 ± 1 mV at rest to −39 ± 2 mV (n = 3).
Fig. 7.
ATP-sensitive K+ channel activation hyperpolarizes pulmonary artery (PA) endothelial cells (ECs) and smooth muscle cells (SMCs). A: representative continuous recording of membrane potential (Vm) illustrates concentration-dependent hyperpolarization of ECs to pinacidil (10−9−10−5 M) in a PA endothelial tube. W, washout. B: summary data for hyperpolarization to pinacidil in endothelial tubes. C: summary data for hyperpolarization to pinacidil in SMCs of intact PAs. Values are means ± SE; n = 4 per group. B, baseline Vm. #P < 0.05 vs. baseline Vm. EC50 values for pinacidil were 7 × 10−8 M in ECs and 4 × 10−8 M in SMCs.
Fig. 8.
Glibenclamide reverses ATP-sensitive K+ channel-dependent hyperpolarization of pulmonary artery (PA) endothelial cells (ECs) and smooth muscle cells (SMCs). A: representative continuous recording of membrane potential (Vm) from an endothelial tube illustrates concentration-dependent reversal of hyperpolarization to pinacidil (P; 10−5 M, maintained throughout) by glibenclamide. B: summary data for reversal of hyperpolarization to pinacidil in endothelial tubes with glibenclamide (10−10−10−6 M). C: summary data for reversal of hyperpolarization to pinacidil in SMCs of intact PAs with glibenclamide (10−10−10−6 M). Values are means ± SE; n = 4 per group. B, baseline Vm; P, Vm during 10−5 M pinacidil. #P < 0.05 vs. baseline Vm. EC50 values for glibenclamide were 6 × 10−9 M in ECs and 2 × 10−8 M in SMCs.
Fig. 9.
Hyperpolarization to calcitonin gene-related peptide (CGRP) in pulmonary artery (PA) endothelial cells (ECs) and smooth muscle cells (SMCs) requires ATP-sensitive K+ (KATP) channels. A: representative continuous recording of membrane potential (Vm) from an endothelial tube in the presence of glibenclamide (10−6 M) illustrates lack of responses to CGRP (10−10−10−6 M) with maintenance of hyperpolarization to ACh (10−5 M; 5 min). B: summary data for loss of hyperpolarization to CGRP (but not to ACh) for endothelial tubes in the presence of glibenclamide (10−6 M). C: summary data for loss of hyperpolarization to CGRP for SMCs of denuded PAs; note final depolarization with KCl (10−1 M). D: summary data for loss of hyperpolarization to CGRP (but not depolarization to KCl) for SMCs of intact PAs. Values are means ± SE; n = 4–5 per group. B, baseline Vm. Details: inhibition of KATP channels also attenuated hyperpolarization in female mice: ΔVm = 3 ± 1 mV for ECs and 4 ± 2 mV for SMCs from intact PAs (n = 3 per group). To confirm electrode placement, from a resting Vm of −43 ± 2 mV, ACh hyperpolarized ECs to −79 ± 4 mV (n = 4), while KCl depolarized SMCs from a resting Vm of −41 ± 3 to −9 ± 3 mV in intact PAs (n = 4) and to −7 ± 2 mV in denuded PAs (n = 2).
Fig. 10.
Genetic deletion of ATP-sensitive K+ (KATP) channels inhibits hyperpolarization to calcitonin gene-related peptide (CGRP). A: representative continuous recording of membrane potential (Vm) from endothelial tube of a mouse lacking KATP channels illustrates absence of responses to CGRP (10−10−10−6 M) or pinacidil (P; 10−5 M) followed by hyperpolarization to ACh (10−5 M; 5 min). B: summary data for responses to CGRP in smooth muscle cells of intact pulmonary arteries from KATP−/− mice. Values are means ± SE; n = 3 per group. B, baseline Vm. #P < 0.05 vs. baseline Vm.
Inhibition of PKA with PKI, an inhibitor chosen for its selectively for PKA over PKG (41), nearly eliminated hyperpolarization to CGRP for both ECs and SMCs (Fig. 11). In the presence of PKI, maximal ΔVm in response to CGRP was ~5 mV; electrode placement was confirmed as described above.
Fig. 11.
Calcitonin gene-related peptide (CGRP)-dependent hyperpolarization in endothelial cells (ECs) and smooth muscle cells (SMCs) requires PKA. A: representative continuous recording of membrane potential (Vm) from a SMC of an intact pulmonary artery (PA) in the presence of PKI (10−5 M) illustrates lack of response to CGRP (10−10−10−6 M) followed by depolarization to KCl (10−1 M; 5 min). B: summary data for Vm during exposure of endothelial tubes to CGRP in the presence of PKI (10−5 M) followed by hyperpolarization to ACh (10−5 M). C: summary data for Vm during exposure of SMCs of denuded PAs to CGRP followed by depolarization to KCl (10−1 M). D: summary data for Vm during exposure of intact PAs to CGRP followed by depolarization to KCl (10−1 M). ACh hyperpolarized ECs by ∼36 mV, and KCl depolarized SMCs by ∼30 mV. Values are means ± SE; n = 4 per group. B, baseline Vm. #P < 0.05 vs. baseline Vm.
CGRP Dilates PAs via KATP Channel Activation by PKA
For intact PAs preconstricted with UTP, addition of CGRP produced dilation in association with a reduction in vessel wall [Ca2+]i, and these effects were attenuated significantly following addition of glibenclamide or PKI (Fig. 12). In the presence of PKI, vasodilation (Fig. 12C) and vessel wall Ca2+ (Fig. 12D) responses to CGRP were diminished in a manner similar to the effect of KATP channel inhibition with glibenclamide.
Fig. 12.
Roles for ATP-sensitive K+ channels and PKA in pulmonary artery (PA) dilation to calcitonin gene-related peptide (CGRP). A: vasodilation during EC50 (10−8 M) and maximal (10−6 M) [CGRP] for intact PAs preconstricted with UTP (5 × 10−6 M) in the absence (vehicle) and presence of glibenclamide (10−6 M). B: changes in vessel wall [Ca2+]i (ΔF340/F380; see methods) of intact PAs corresponding with A. C: vasodilation to CGRP in the absence and presence of PKI (10−5 M). D: changes in vessel wall [Ca2+]i (ΔF340/F380) corresponding with C. Values are means ± SE; n = 4–5 per group. #P < 0.05 vs. vehicle. +P < 0.05 vs. 10−8 M CGRP. Details: in A and B, baseline diameters [137 ± 9 and 134 ± 8 µm in the absence (vehicle) and presence of glibenclamide, respectively] and resting [Ca2+]i (F340/F380 = 0.51 ± 0.05 and 0.57 ± 0.06, respectively) were similar between groups. Responses to UTP were not significantly different between PAs during superfusion with respective treatments [inner diameter (ID) = 72 ± 5 µm, constriction = 46 ± 4%, and ΔF340/F380 = 0.24 ± 0.05 for vehicle; ID = 71 ± 10 µm, constriction = 47 ± 6%, and ΔF340/F380 = 0.21 ± 0.04 for glibenclamide]. C and D: baseline diameters (134 ± 4 and 124 ± 11 µm for vehicle and PKI, respectively), Ca2+ (F340/F380 = 0.63 ± 0.06 and 0.60 ± 0.08 for vehicle and PKI, respectively), and responses to UTP (ID = 75 ± 5 µm, constriction = 43 ± 3%, and ΔF340/F380 = 0.17 ± 0.02 for vehicle; ID = 64 ± 8 µm, constriction = 47 ± 2%, and ΔF340/F380 = 0.20 ± 0.06 for PKI) were similar between conditions.
To address the role of the endothelium in vasodilation to CGRP, diameter and vessel wall Ca2+ were measured in intact and denuded PAs preconstricted with UTP. Vasodilation to CGRP in denuded arteries (using EC50 and maximal concentration defined for intact PAs) was reduced by ~20% (Fig. 13A), suggesting a minor EC component. Neither baseline F340/F380 nor reductions in vessel wall [Ca2+]i were significantly different between intact and denuded PAs (Fig. 13B), indicating that fura 2 signals originated from SMCs and that ECs do not affect the SMC Ca2+ response to CGRP. To determine if the endothelial contribution is mediated by NO, responses were evaluated for intact PAs in the presence of l-NAME (10−4 M). Inhibition of NO synthase attenuated vasodilation ~20% (Fig. 13A) and did so without altering the [Ca2+]i response (Fig. 13B). In PA endothelial tubes, CGRP (10−6 M) increased [Ca2+]i significantly (ΔF340/F380 = 0.13 ± 0.03, n = 4, P < 0.05). Vm and Ca2+ responses to CGRP peaked in ~5 min, whereas responses to ACh peaked in ~1 min (Fig. 4). Collectively, these findings indicate that endothelial-derived NO mediates a relatively small component of PA dilation to CGRP.
Fig. 13.
Pulmonary artery (PA) dilation to calcitonin gene-related peptide (CGRP) has both endothelial and smooth muscle components. A: vasodilation to CGRP of UTP-preconstricted (5 × 10−6 M) intact (±l-NAME, 10−4 M) and endothelium-denuded PAs during EC50 (10−8 M) and maximal (10−6 M) [CGRP]. B: changes in vessel wall [Ca2+]i (ΔF340/F380; see methods) in response to CGRP were similar for intact (±l-NAME) and denuded arteries. Values are means ± SE; n = 4 per group. #P < 0.05 vs. intact. +P < 0.05 vs. 10−8 M CGRP. Details: baseline diameter = 132 ± 11, 133 ± 6, and 128 ± 4 µm for intact, denuded, and l-NAME, respectively; resting intracellular [Ca2+] (F340/F380) = 0.62 ± 0.04, 0.58 ± 0.04, and 0.57 ± 0.02 for intact, denuded, and l-NAME, respectively. Responses to 5 × 10−6 M UTP (ID = 76 ± 11 µm, constriction = 44 ± 5%, and ΔF340/F380 = 0.19 ± 0.03 (intact); ID = 66 ± 10 µm, constriction = 47 ± 7%, and ΔF340/F380 = 0.20 ± 0.02 (denuded); and ID = 68 ± 3 µm, constriction = 47 ± 3%, and ΔF340/F380 = 0.15 ± 0.04 (l-NAME)] were not significantly different between groups.
DISCUSSION
CGRP has been associated with PA dilation, but the effect of this sensory neurotransmitter on Vm of PA ECs and SMCs was unknown, particularly in the context of vasomotor control. As ECs are important regulators of pulmonary SMC function (25, 28, 36), we sought to examine how these cells respond independently and when associated with each other. To achieve this end, we developed and validated the endothelial tube preparation of mouse PAs for analysis of Vm and [Ca2+]i and, for the first time, demonstrated CGRP-dependent hyperpolarization in ECs. Using pressure myography, we evaluated electrophysiological and vasomotor responses of SMCs to CGRP in the presence and absence of the endothelium in cannulated PAs. Our electrophysiological data are unique in terms of the stability of Vm recordings, which have enabled complete continuous and uninterrupted evaluation of concentration-response curves to CGRP. The major finding of this study is that CGRP hyperpolarizes both SMCs and ECs via activation of KATP channels through PKA, leading to a fall in vessel wall [Ca2+]i and PA dilation in a concentration-dependent manner. These data indicate independent, yet complementary, roles of the smooth muscle and endothelium to effect dilation through hyperpolarization in response to CGRP released abluminally by sensory nerves or when presented via the bloodstream.
Sensory Innervation of the Vessel Wall
Sensory nerves produce biologically active peptides, including substance P, neurokinins A and B, and CGRP (34). Our focus on CGRP stems from its role as a neurotransmitter as well as a powerful vasodilator (10). The presence of sensory nerves in murine PAs was confirmed by CGRP immunostaining on isolated PAs (Fig. 1) and is consistent with findings in pigs, rats, and guinea pigs (11, 35, 37). Sensory nerve density of mouse PAs was sparse in the main PA and its smaller branches, covering ~6% of the vessel wall. In contrast, sensory innervation of mesenteric arteries in the mouse encompasses ~25% of the vessel wall (9). One explanation for the lower density of sensory innervation in PAs may be that mesenteric arteries are also innervated by sympathetic nerves, which negatively inhibit sensory nerves through presynaptic inhibition via α2-adrenoreceptors (30). As smaller pulmonary vessels lack sympathetic innervation (12, 17, 19, 20), CGRP release is not inhibited by this means. Regional differences between the systemic and pulmonary circulation may also be attributed to the lungs receiving the entire cardiac output, whereas blood flow to respective beds of the systemic circulation can be redistributed via sympathetic nerve activity, e.g., from the gut to skeletal muscle during exercise, particularly in hot environments (54).
Functional CGRP receptors comprise CRLR, RAMP1, and receptor component protein subunits (38). In light of our findings that both ECs and SMCs hyperpolarize in response to CGRP (Fig. 5), we determined whether receptors for CGRP were expressed on both cell types. Immunostaining with confocal imaging verified that both RAMP1 and CRLR are expressed on SMCs and ECs (Fig. 6) and that the overlap in staining of receptor components is consistent with the presence of functional CGRP receptors. As the endothelium would most likely be activated by circulating (luminal) CGRP (4) and vascular smooth muscle by nerves running along the adventitia (Fig. 1), we evaluated how each cell layer responds to CGRP.
Cell-Type-Specific Hyperpolarization Through KATP Channels
To study the properties of native intact endothelium independent of the influence of SMCs, nerves, or blood flow and circulating agents, our laboratory developed the endothelial tube preparation in resistance arteries of skeletal muscle (6, 57, 58). For the present study we found it necessary to modify the isolation procedure to include elastase during digestion. The viability of these preparations was verified using a live/dead assay, while staining of nuclei confirmed the absence of SMCs (Fig. 3). The cobblestone appearance of ECs within the tube is consistent with previous descriptions of pulmonary endothelium (15). In response to ACh, hyperpolarization of endothelial tubes was concomitant with a rise in [Ca2+]i. These are the first data in native intact PA endothelium to assess Vm and Ca2+ simultaneously, and repeated stimulation with ACh confirmed the reproducibility of responses for ≥2 h, i.e., the duration of our longest experimental protocols. In addition to maintaining integrity of the endothelium (which is lost with isolated cells), the ability to measure responses in freshly isolated preparations avoids changes in phenotype that occur when cells are cultured (55).
Our findings demonstrate that CGRP not only hyperpolarizes ECs, but that CGRP hyperpolarizes EC and SMC layers of PAs independently. Thus, ECs are not required for SMC hyperpolarization, nor are SMCs required for EC hyperpolarization (Fig. 5). The EC50 values for CGRP (Table 1) are consistent with those reported for pulmonary endothelium of the rat (4 × 10−9 M) (21). However, the signaling events were undefined. KATP channel activation in response to CGRP is well documented in SMCs from the systemic vasculature, including the aorta (48) and coronary (40, 62), renal (53), and mesenteric (8, 42, 51) arteries. These earlier studies further suggest that KATP channels are activated by PKA (40, 48, 51, 62). We therefore questioned whether this signaling pathway prevails in the pulmonary circulation.
KATP channels are octameric protein complexes composed of four inward rectifying subunits [KIR 6.1 and 6.2, each associated with a larger sulfonylurea receptor (SUR)] (2). In human PAs, KATP channels are comprised of KIR6.1 and SUR2B channels (16), which are expressed in both ECs and SMCs (14, 29, 44, 56, 60). PKA can phosphorylate the KIR6.1/SUR2B channels at three residues (S385 for KIR6.1 and T633 and S1465 for SUR2B) (52). KATP channel activation with pinacidil evoked hyperpolarization of pulmonary ECs and SMCs (Fig. 7), and this effect was reversed in a concentration-dependent manner upon administration of the KATP channel blocker glibenclamide (Fig. 8). Our EC50 for glibenclamide in ECs (6 × 10−9 M) is lower than that reported for SMCs in the portal vein of rabbits (2.5 × 10−8 M) (5), while our EC50 value for PA SMCs (2 × 10−8 M) is similar, suggesting greater sensitivity to this inhibitor in ECs vs. SMCs. The concentration of glibenclamide used to inhibit KATP channels in our experiments (10−6 M; Fig. 9) is selective for KATP over other K+ channels, including voltage-gated (KV), Ca2+-activated (KCa), and KIR channels (43). A role for the KATP channel in mediating CGRP-dependent hyperpolarization is further supported by results from PAs of mice lacking KATP channels (Fig. 10). Similar to the actions of glibenclamide, PKA inhibition with the selective inhibitor PKI (41) effectively prevented hyperpolarization to CGRP in both SMCs and ECs of PAs (Fig. 11). It is possible that the residual hyperpolarization to CGRP in the presence of PKI was mediated by alternative pathways for KATP channel activation; however, KATP channel activation through PKA appears to be the primary mechanism of hyperpolarization to CGRP within mouse PAs. While ECs and SMCs in PAs hyperpolarize to CGRP in a concentration-dependent manner with similar EC50 values (Table 1), pinacidil hyperpolarized both cell types to a greater extent than did CGRP (compare Figs. 7 and 5), indicating that CGRP signaling does not maximally activate KATP channels within the pulmonary circulation.
Pulmonary SMCs hyperpolarized to CGRP in the absence of ECs. These results raise the following question. Why are ECs hyperpolarizing to CGRP? One possibility is that electrical coupling between respective cell layers through myoendothelial gap junctions helps coordinate hyperpolarization and SMC relaxation along the vessel wall. While electrical coupling between ECs and SMCs has been demonstrated in resistance arteries of the systemic circulation (18), electrical coupling between cells comprising the wall of PAs remains to be characterized. Nevertheless, a role for myoendothelial coupling through gap junctions has been implicated during the sustained phase of hypoxic pulmonary vasoconstriction (32) and may serve to ensure that CGRP elicits vasodilation whether presented to SMCs from perivascular nerves or to ECs via the circulation.
For the present experiments we elected to use sharp microelectrodes because of their ability to provide long recordings from intact preparations without dialyzing the interior of cells. Our priority was to study intact vessel and endothelial tube preparations over isolated cells to more closely approximate how cells are signaling and functioning in vivo. Patch-clamp studies measuring Vm of isolated SMCs or ECs are a viable alternative to the data presented here and may be used in future studies to characterize the biophysical properties of channel regulation.
During preconstriction with UTP, CGRP dilated PAs, with an EC50 (10−8 M; Fig. 2) slightly greater than that for hyperpolarization of respective cell layers (Table 1). This subtle difference in sensitivity to CGRP between respective conditions can be attributed to the effect of UTP, which was not present during intracellular recording but was requisite to develop tone from which to study vasodilation. The associated fall in vessel wall [Ca2+]i (Fig. 12) is consistent with CGRP-dependent hyperpolarization minimizing the activity of VGCC (43). Therefore, inhibition of KATP channels or PKA, which we identified as mediators of CGRP-dependent hyperpolarization, also prevented reductions in vessel wall [Ca2+]i and vasodilation.
In PA rings from guinea pigs, relaxation to CGRP was independent of the endothelium (49), while studies in rats suggested that the endothelial NO pathway is required for relaxation of the pulmonary vasculature to CGRP (36, 63). Such differences may be due to variability in vessel size or species studied (46). In denuded PAs from this study, vasodilation to CGRP was attenuated by ~20% compared with intact PAs (Fig. 13A), with no apparent difference in the vessel wall Ca2+ response (Fig. 13B). These findings indicate that the endothelium contributes to, but is not essential for, smooth muscle relaxation to CGRP and suggest that the vasodilator role of ECs may be mediated through Ca2+ desensitization in SMCs (26). In endothelial tubes, [Ca2+]i increased during exposure to CGRP (10−6 M), consistent with stimulation of endothelial NO synthase activity and NO production. The finding that endothelial NO synthase inhibition attenuated vasodilation to CGRP by ~20% is consistent with the effect of denudation. Thus our results suggest that a relatively small component (~20%) of PA dilation to CGRP entails endothelial-derived NO, while the electrophysiological actions of CGRP on SMCs are independent of the endothelium. Nevertheless, in the intact pulmonary circulation, circulating CGRP may act on ECs to contribute to basal NO production and, thereby, assist in maintaining low pulmonary vascular resistance. Through modulation of Vm and [Ca2+]i, CGRP receptor activation may also affect dilation to other endothelium-dependent vasodilators. Together, our findings indicate that CGRP leads to PKA-dependent activation of KATP channels in respective cell layers of PAs, resulting in hyperpolarization and vasodilation. While previous reports in systemic arteries have demonstrated this signaling pathway in vascular smooth muscle (42, 51, 65), the present findings are the first to identify this response in any source of ECs while confirming that it occurs in SMCs of the pulmonary vasculature.
Summary and Conclusion
We have investigated the effect of CGRP on the Vm of ECs and SMCs of PAs from C57BL/6 mice. To study native, intact PA endothelium, we developed freshly isolated endothelial tubes as a preparation and validated its application as an effective model. Our data from male (and confirmed in female) mice uniquely illustrate that both the intima and media of PAs hyperpolarize directly in response to CGRP and do so in a concentration-dependent manner through activation of KATP channels via PKA. For PAs preconstricted with UTP, CGRP evoked concentration-dependent dilation with a fall in vessel wall [Ca2+]i; a minor (~20%) role for endothelial-derived NO was in accord with a subtle increase in [Ca2+]i of endothelial tubes. The redundancy in hyperpolarization for ECs and SMCs may underscore a central role for KATP channels in promoting pulmonary perfusion. As CGRP can attenuate pulmonary hypertension (7, 13, 50, 66) and SMCs are depolarized in this setting (45, 47), gaining new insight into how CGRP signaling is regulated within respective layers of the vessel wall may enable more precise treatment of pulmonary vascular disease.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant R37-HL-041026.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
C.E.N. and S.S.S. conceived and designed research; C.E.N. performed experiments; C.E.N. and S.S.S. analyzed data; C.E.N. and S.S.S. interpreted results of experiments; C.E.N. prepared figures; C.E.N. drafted manuscript; C.E.N. and S.S.S. edited and revised manuscript; C.E.N. and S.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Drs. Erik Behringer, Erika Boerman, and Jorge Castorena-Gonzalez provided valuable technical assistance.
REFERENCES
- 1.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA 91: 7583–7587, 1994. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60: 667–687, 1998. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 3.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bartosik I, Eskilsson J, Ekman R, Akesson A, Scheja A. Correlation between plasma concentrations of calcitonin gene related peptide and pulmonary pressure in patients with systemic sclerosis. Ann Rheum Dis 61: 261–263, 2002. doi: 10.1136/ard.61.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol 110: 573–582, 1993. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol 166: 774–787, 2012. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bivalacqua TJ, Hyman AL, Kadowitz PJ, Paolocci N, Kass DA, Champion HC. Role of calcitonin gene-related peptide (CGRP) in chronic hypoxia-induced pulmonary hypertension in the mouse. Influence of gene transfer in vivo. Regul Pept 108: 129–133, 2002. doi: 10.1016/S0167-0115(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 8.Blanco-Rivero J, Márquez-Rodas I, Sastre E, Cogolludo A, Pérez-Vizcaíno F, del Campo L, Nava MP, Balfagón G. Cirrhosis decreases vasoconstrictor response to electrical field stimulation in rat mesenteric artery: role of calcitonin gene-related peptide. Exp Physiol 96: 275–286, 2011. doi: 10.1113/expphysiol.2010.055822. [DOI] [PubMed] [Google Scholar]
- 9.Boerman EM, Segal SS. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J Physiol 594: 2323–2338, 2016. doi: 10.1113/JP270710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature 313: 54–56, 1985. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 11.Cadieux A, Springall DR, Mulderry PK, Rodrigo J, Ghatei MA, Terenghi G, Bloom SR, Polak JM. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: effect of capsaicin treatment and surgical denervations. Neuroscience 19: 605–627, 1986. doi: 10.1016/0306-4522(86)90285-X. [DOI] [PubMed] [Google Scholar]
- 12.Ĉech S. Cholinesterase-containing nerve fibres on blood vessels in lungs of some laboratory mammals. Z Zellforsch Mikrosk Anat 140: 91–100, 1973. doi: 10.1007/BF00307060. [DOI] [PubMed] [Google Scholar]
- 13.Champion HC, Bivalacqua TJ, Toyoda K, Heistad DD, Hyman AL, Kadowitz PJ. In vivo gene transfer of prepro-calcitonin gene-related peptide to the lung attenuates chronic hypoxia-induced pulmonary hypertension in the mouse. Circulation 101: 923–930, 2000. doi: 10.1161/01.CIR.101.8.923. [DOI] [PubMed] [Google Scholar]
- 14.Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 262: H916–H920, 1992. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- 15.Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res 55: 65–76, 1998. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of KATP channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol 26: 135–143, 2002. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- 17.El-Bermani AW, Bloomquist EI, Montvilo JA. Distribution of pulmonary cholinergic nerves in the rabbit. Thorax 37: 703–710, 1982. doi: 10.1136/thx.37.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000. doi: 10.1161/01.RES.87.6.474. [DOI] [PubMed] [Google Scholar]
- 19.Fisher AW. The intrinsic innervation of the pulmonary vessels. Acta Anat (Basel) 60: 481–496, 1965. doi: 10.1159/000142658. [DOI] [PubMed] [Google Scholar]
- 20.Haberberger R, Schemann M, Sann H, Kummer W. Innervation pattern of guinea pig pulmonary vasculature depends on vascular diameter. J Appl Physiol (1985) 82: 426–434, 1997. doi: 10.1152/jappl.1997.82.2.426. [DOI] [PubMed] [Google Scholar]
- 21.Han ZQ, Coppock HA, Smith DM, Van Noorden S, Makgoba MW, Nicholl CG, Legon S. The interaction of CGRP and adrenomedullin with a receptor expressed in the rat pulmonary vascular endothelium. J Mol Endocrinol 18: 267–272, 1997. doi: 10.1677/jme.0.0180267. [DOI] [PubMed] [Google Scholar]
- 22.Harigai Y, Natsume M, Li F, Ohtani A, Senzaki K, Shiga T. Differential roles of calcitonin family peptides in the dendrite formation and spinogenesis of the cerebral cortex in vitro. Neuropeptides 45: 263–272, 2011. doi: 10.1016/j.npep.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Helset E, Kjaeve J, Bjertnaes L, Lundberg JM. Acute alveolar hypoxia increases endothelin-1 release but decreases release of calcitonin gene-related peptide in isolated perfused rat lungs. Scand J Clin Lab Invest 55: 369–376, 1995. doi: 10.3109/00365519509104975. [DOI] [PubMed] [Google Scholar]
- 24.Higashi T, Ishizaki T, Shigemori K, Yamamura T, Nakai T. Pharmacological characterization of endothelin-induced rat pulmonary arterial dilatation. Br J Pharmacol 121: 782–786, 1997. doi: 10.1038/sj.bjp.0701177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jernigan NL, Resta TC, Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 286: L947–L955, 2004. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- 26.Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol 287: L1220–L1229, 2004. doi: 10.1152/ajplung.00196.2004. [DOI] [PubMed] [Google Scholar]
- 27.Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004. doi: 10.1152/ajplung.00443.2003. [DOI] [PubMed] [Google Scholar]
- 28.Jernigan NL, Walker BR, Resta TC. The pulmonary endothelium and vasomotor control. In: The Pulmonary Endothelium: Function in Health and Disease, edited by Voelkel NF, Rounds S. Hoboken, NJ: Wiley-Blackwell, 2009. doi: 10.1002/9780470747490.ch12 [DOI] [Google Scholar]
- 29.Katnik C, Adams DJ. An ATP-sensitive potassium conductance in rabbit arterial endothelial cells. J Physiol 485: 595–606, 1995. doi: 10.1113/jphysiol.1995.sp020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawasaki H. Regulation of vascular function by perivascular calcitonin gene-related peptide-containing nerves. Jpn J Pharmacol 88: 39–43, 2002. doi: 10.1254/jjp.88.39. [DOI] [PubMed] [Google Scholar]
- 31.Keith IM, Tjen-A-Looi S, Kraiczi H, Ekman R. Three-week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 279: H1571–H1578, 2000. doi: 10.1152/ajpheart.2000.279.4.H1571. [DOI] [PubMed] [Google Scholar]
- 32.Kizub IV, Strielkov IV, Shaifta Y, Becker S, Prieto-Lloret J, Snetkov VA, Soloviev AI, Aaronson PI, Ward JP. Gap junctions support the sustained phase of hypoxic pulmonary vasoconstriction by facilitating calcium sensitization. Cardiovasc Res 99: 404–411, 2013. doi: 10.1093/cvr/cvt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu T, Yamamoto M, Shimokata K, Nagura H. Distribution of substance P-immunoreactive and calcitonin gene-related peptide-immunoreactive nerves in normal human lungs. Int Arch Allergy Appl Immunol 95: 23–28, 1991. doi: 10.1159/000235449. [DOI] [PubMed] [Google Scholar]
- 34.Kummer W. Pulmonary vascular innervation and its role in responses to hypoxia: size matters! Proc Am Thorac Soc 8: 471–476, 2011. doi: 10.1513/pats.201101-013MW. [DOI] [PubMed] [Google Scholar]
- 35.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737, 1992. doi: 10.1016/0306-4522(92)90239-X. [DOI] [PubMed] [Google Scholar]
- 36.Mannan MM, Springall DR, Enard C, Moradoghli-Haftvani A, Eddahibi S, Adnot S, Polak JM. Decreased endothelium-dependent pulmonary vasodilator effect of calcitonin gene-related peptide in hypoxic rats contrasts with increased binding sites. Eur Respir J 8: 2029–2037, 1995. doi: 10.1183/09031936.95.08122029. [DOI] [PubMed] [Google Scholar]
- 37.Martling CR, Matran R, Alving K, Hökfelt T, Lundberg JM. Innervation of lower airways and neuropeptide effects on bronchial and vascular tone in the pig. Cell Tissue Res 260: 223–233, 1990. doi: 10.1007/BF00318626. [DOI] [PubMed] [Google Scholar]
- 38.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333–339, 1998. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 39.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 8: 466–472, 2002. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 40.Miyoshi H, Nakaya Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res Cardiol 90: 332–336, 1995. doi: 10.1007/BF00797911. [DOI] [PubMed] [Google Scholar]
- 41.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 1: re4, 2008. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 42.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 344: 770–773, 1990. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 43.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 44.Noel J, Wang H, Hong N, Tao JQ, Yu K, Sorokina EM, Debolt K, Heayn M, Rizzo V, Delisser H, Fisher AB, Chatterjee S. PECAM-1 and caveolae form the mechanosensing complex necessary for NOX2 activation and angiogenic signaling with stopped flow in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 305: L805–L818, 2013. doi: 10.1152/ajplung.00123.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal 18: 1777–1788, 2013. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nossaman BD, Feng CJ, Kaye AD, DeWitt B, Coy DH, Murphy WA, Kadowitz PJ. Pulmonary vasodilator responses to adrenomedullin are reduced by NOS inhibitors in rats but not in cats. Am J Physiol Lung Cell Mol Physiol 270: L782–L789, 1996. doi: 10.1152/ajplung.1996.270.5.L782. [DOI] [PubMed] [Google Scholar]
- 47.Olschewski A, Hong Z, Nelson DP, Weir EK. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 283: L1143–L1150, 2002. doi: 10.1152/ajplung.00104.2002. [DOI] [PubMed] [Google Scholar]
- 48.Park WS, Hong DH, Son YK, Kim MH, Jeong SH, Kim HK, Kim N, Han J. Alteration of ATP-sensitive K+ channels in rabbit aortic smooth muscle during left ventricular hypertrophy. Am J Physiol Cell Physiol 303: C170–C178, 2012. doi: 10.1152/ajpcell.00041.2012. [DOI] [PubMed] [Google Scholar]
- 49.Pinto A, Sekizawa K, Yamaya M, Ohrui T, Jia YX, Sasaki H. Effects of adrenomedullin and calcitonin gene-related peptide on airway and pulmonary vascular smooth muscle in guinea-pigs. Br J Pharmacol 119: 1477–1483, 1996. doi: 10.1111/j.1476-5381.1996.tb16061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qing X, Keith IM. Targeted blocking of gene expression for CGRP receptors elevates pulmonary artery pressure in hypoxic rats. Am J Physiol Lung Cell Mol Physiol 285: L86–L96, 2003. doi: 10.1152/ajplung.00356.2002. [DOI] [PubMed] [Google Scholar]
- 51.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol 475: 9–13, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res 94: 1359–1366, 2004. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 53.Reslerova M, Loutzenhiser R. Renal microvascular actions of calcitonin gene-related peptide. Am J Physiol Renal Physiol 274: F1078–F1085, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 55.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297: H1–H7, 2009. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- 56.Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- 57.Socha MJ, Hakim CH, Jackson WF, Segal SS. Temperature effects on morphological integrity and Ca2+ signaling in freshly isolated murine feed artery endothelial cell tubes. Am J Physiol Heart Circ Physiol 301: H773–H783, 2011. doi: 10.1152/ajpheart.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Socha MJ, Segal SS. Isolation of microvascular endothelial tubes from mouse resistance arteries. J Vis Exp 81: e50759, 2013. doi: 10.3791/50759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sørhaug S, Steinshamn S, Munkvold B, Waldum HL. Release of neuroendocrine products in the pulmonary circulation during intermittent hypoxia in isolated rat lung. Respir Physiol Neurobiol 162: 1–7, 2008. doi: 10.1016/j.resp.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Theis JGW, Liu Y, Coceani F. ATP-gated potassium channel activity of pulmonary resistance vessels in the lamb. Can J Physiol Pharmacol 75: 1241–1248, 1997. doi: 10.1139/y97-155. [DOI] [PubMed] [Google Scholar]
- 61.Tjen-A-Looi S, Ekman R, Lippton H, Cary J, Keith I. CGRP and somatostatin modulate chronic hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 263: H681–H690, 1992. doi: 10.1152/ajpheart.1992.263.3.H681. [DOI] [PubMed] [Google Scholar]
- 62.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol 507: 117–129, 1998. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wisskirchen FM, Burt RP, Marshall I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br J Pharmacol 123: 1673–1683, 1998. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc Natl Acad Sci USA 93: 10489–10494, 1996. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Bonev AD, Nelson MT, Mawe GM. Activation of ATP-sensitive potassium currents in guinea-pig gall-bladder smooth muscle by the neuropeptide CGRP. J Physiol 478: 483–491, 1994. doi: 10.1113/jphysiol.1994.sp020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Q, Liu Z, Wang Z, Yang C, Liu J, Lu J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg 84: 544–552, 2007. doi: 10.1016/j.athoracsur.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Bell D, Smith LR, Zhao L, Devine AB, McHenry EM, Nicholls DP, McDermott BJ. Differential expression of components of the cardiomyocyte adrenomedullin/intermedin receptor system following blood pressure reduction in nitric oxide-deficient hypertension. J Pharmacol Exp Ther 316: 1269–1281, 2006. doi: 10.1124/jpet.105.092783. [DOI] [PubMed] [Google Scholar]