Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal fibrotic lung disease associated with aberrant activation and differentiation of fibroblasts, leading to abnormal extracellular matrix production. Currently, it is still an untreatable disease (except for lung transplantation). Here, we demonstrate that the Raf1 inhibitor GW5074 ameliorates lung fibrosis in bleomycin-induced pulmonary fibrosis. Posttreatment with GW5074 reduced fibronectin (FN) expression, collagen deposition, and inflammatory cell infiltration in bleomycin-challenged mice, suggesting an antifibrotic property of GW5074. To determine the molecular mechanisms by which inhibition of Raf1 ameliorates lung fibrosis, we investigated the role of Raf1 in TGF-β1 signaling in human lung fibroblasts. GW5074 or downregulation of Raf1 by siRNAs significantly attenuated TGF-β1-induced smooth muscle actin, FN, and collagen I expression, whereas overexpression of Raf1 promoted the effects of TGF-β1 in lung fibroblasts. Furthermore, we found that Raf1-promoted TGF-β1 signaling was through the Raf1/ERK/Smad pathway and contributed to the cell proliferation and migration in human lung fibroblasts. This study provides preclinical and mechanistic evidence for development of Raf1 inhibitors as potential antifibrotic drugs for the treatment of IPF.
Keywords: lung fibrosis, Raf1, Smad, TGF-β1
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease characterized by aberrant fibroblastic proliferation and accumulation of extracellular matrix (ECM) proteins, with a median survival rate of only 4 years (22). Currently, only two drugs approved by the FDA, pirfenidone and nintedanib, have been shown to slow the progress of IPF (12). Lung transplantation is the only option for patients with IPF for extending life expectancy and enhancing the quality of life. Therefore, it is crucial to better understand the fundamental process involved in its pathogenesis for development of novel antifibrotic therapies.
A wide variety of mediators is involved in IPF. Among them, TGF-β1 is believed to be a key regulator of IPF (20). Inhibition of TGF-β1 or its downstream signaling pathways limits lung fibrosis, whereas overexpression of TGF-β1 triggers lung fibrosis. TGF-β1 controls fibrotic responses through activation of both canonical (Smad based) and noncanonical (non-Smad based) signaling pathways, which result in activation of myofibroblasts and excessive production of ECM (19). Targeting TGF-β1 signaling pathways is an attractive therapeutic strategy to ameliorate lung fibrosis.
Raf1 is a member of Raf serine/threonine kinase family that can be activated by a variety of extracellular stimuli, including TGF-β1 (2, 24). Raf1 phosphorylates and activates the MEK-ERK pathway (1, 15). A rapid activation of Ras in response to TGF-β causes recruitment of Raf and leads to activation of ERK (39), which is involved in cell differentiation (18), proliferation, and migration (4, 7). Raf1 mediates TGF-β1-induced epithelial-mesenchymal transition (EMT) in normal murine mammary gland epithelial cells through activation of ERK (37). Inhibition of ERK has been shown to suppress the fibroblast differentiation (14, 35, 36) and development of lung fibrosis in the bleomycin model (9, 17). However, the roles of Raf1 in the development of lung fibrosis have not been reported.
In the present study, we observed that Raf1 inhibitor GW5074 ameliorates bleomycin-induced pulmonary fibrosis in mice. Furthermore, we revealed that Raf1 regulates ECM production through the Raf1-ERK-Smad pathway in lung fibroblast cells. This study demonstrates that GW5074 may be a candidate for the treatment of IPF through inhibiting TGF-β1-Raf1 signaling.
MATERIALS AND METHODS
Cell culture and reagents.
Human fetal lung fibroblast (Mrc5) cells (ATCC, Manassas, VA) were cultured with EMEM medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FBS and antibiotics at 37°C in a 5% CO2 incubator. Phospho-Smad2, phospho-Smad3, Smad2, Smad3, and Raf1 antibodies were from Cell Signaling Technology (Danvers, MA). Fibronectin (FN) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Human Raf1 siRNA and control siRNA were purchased from Thermo Fisher Scientific. Collagen I, V5 antibody, the mammalian expression plasmid pcDNA3.1/V5-His TOPO, and Escherichia coli Top 10 competent cells were purchased from Invitrogen (Life Technologies, Carlsbad, CA). β-Actin antibody was from Sigma-Aldrich (St. Louis, MO). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Bio-Rad (Hercules, CA). GW5074 was from Cayman Chemical Company (Ann Arbor, MI). PD98059 was from Calbiochem Corp. (La Jolla, CA). Recombinant TGF-β1 was purchased from PeproTech (Rocky Hill, NJ). All materials in highest grades used in the experiments are commercially available.
Cell lysis and Western blot analysis.
After the indicated treatments, cells were washed with cold PBS and collected in cell lysis buffer, which contains 20 mM Tris·HCl (pH 7.4), 150 mM NaCl, 2 mM EGTA, 5 mM β-glycerophosphate, 1 mM MgCl2, 1% Triton X-100, 1 mM sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. The cell lysates were then sonicated on ice for 12 s, followed by centrifugation at 4°C at 5,000 rotations/min for 10 min. Protein concentrations of the samples were then determined with a Bio-Rad Protein Assay Kit (Bio-Rad). Samples were all equilibrated to 15–20 μg and ran on an SDS-PAGE gel, transferred to a nitrocellulose membrane, and blocked in 5% nonfat biological grade powdered milk dissolved in 25 mM Tris·HCl (pH 7.4), 137 mM NaCl, and 0.1% Tween 20 (TBST) for 30 min. Blots were washed with TBST and incubated with a primary antibody in 5% BSA with TBST for 2 h or overnight. The membranes were then washed three times at 10-min intervals with TBST before the addition of a secondary antibody for 1 h. Blots were developed with an Enhanced Chemiluminescence Detection Kit (Thermo Fisher Scientific), according to the manufacturer’s instructions.
Plasmid and siRNA transfection.
Plasmid pBABEpuro-cRaf was a gift from Matthew Meyerson (Addgene plasmid No. 51124). Human Raf1 cDNA was inserted into pcDNA3.1D/His-V5 TOPO vector. Cells were subcultured on six-well plates to 70%–90% confluence. The cells were then transfected with varying amounts of plasmid using the PolyJet In Vitro DNA Transfection Reagent (SignaGen, Rockville, MD) system based on the manufacturer’s protocol. For siRNA transfection, GenMute Transfection Reagent (SignaGen) was added to the mixture containing varying amounts of siRNA and GenMute transfection buffer working solution and then incubated for 10 min to form transfection reagent-siRNA complexes. The mixture was then added directly to the cells with complete medium.
RNA isolation, reverse transcription, and quantitative PCR.
Total RNA was isolated from cultured Mrc5 cells using the NucleoSpin RNA Extraction Kit (Clontech, Mountain View, CA) according to the manufacturer’s instructions. RNA was quantified by spectrophotometry. cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative PCR was performed to assess expression of FN, collagen I, and α-smooth muscle actin (α-SMA) using primers designed based on human mRNA sequences. FN primers: forward 5′-GGAGAATTCAAGTGTGACCCTCA-3′ and reverse 5′-TGCCACTGTTCTCCTACGTGG-3′. Collagen I primers: forward 5′-CAGCCGCTTCACCTACAGC-3′ and reverse 5′-TTTTGTATTCAATCACTGTCTTGCC-3′. α-SMA primers: forward 5′-CCGACCGAATGCAGAAGGA-3′ and reverse 5′-ACAGAGTATTTGCGCTCCGAA-3′. Real-time PCR was performed using iQ SYBR Green Supermix and the iCycler Real-Time PCR Detection System (Bio-Rad).
Animal model of fibrosis.
C57BL/6 mice with body weight of 20–25 g were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal procedures in this study were performed in adherence with the National Institute of Health Guidelines on the use of Laboratory Animals and have been approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Mice were intranasally challenged with bleomycin (2 U/kg) for 7 days, and the mice were then given intraperitoneal GW5074 (one injection every other day for a total seven injections) for an additional 2 wk. Lung tissues were analyzed at day 21. Partial right lungs were homogenized in cell lysis buffer. Protein levels were analyzed by Western blot analysis with the indicated antibodies. The left lung tissues were fixed with 10% paraformaldehyde for 24 h and embedded in paraffin wax. The lung tissue slides were stained with hematoxylin-eosin and Masson trichrome by the pathology core facility at the University of Pittsburgh. The severity of interstitial fibrosis among the groups was compared using the Ashcroft score.
Statistical analysis.
All results were subjected to statistical analysis using two-way ANOVA and, wherever appropriate, Student’s t-test. Data are expressed as means ± SD of triplicate samples from at least three independent experiments, and P < 0.05 was considered statistically significant.
RESULTS
GW5074 alleviates bleomycin-induced pulmonary fibrosis in mice.
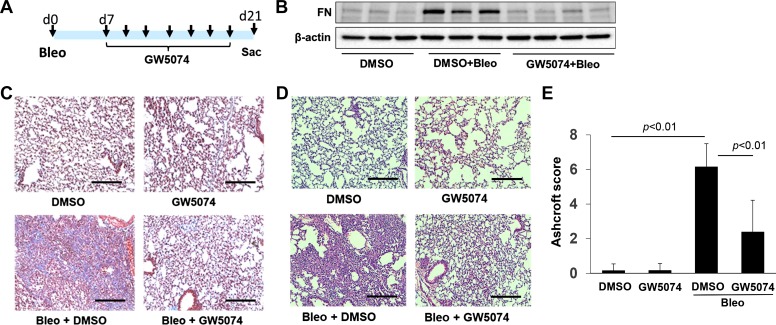
To elucidate the role of Raf1 in the pathogenesis of lung fibrosis, mice were treated with intraperitoneal GW5074 (2 mg/kg) following bleomycin challenge. C57/BL mice were intranasally challenged with bleomycin (2 U/kg, a single instillation) for 7 days, and then the mice were given intraperitoneal GW5074 (1 injection every other day for a total 7 injections) for an additional 2 wk (Fig. 1A). Lung tissues were analyzed at day 21. FN expression was assessed in lung tissues by Western blot analysis. Bleomycin challenge increased FN expression, which was significantly reduced in GW5074-treated mice (Fig. 1B). Collagen deposition in the lungs was examined by trichrome staining. As shown in Fig. 1C, GW5074 posttreatment reduced the deposition of collagen compared with bleomycin + DMSO-challenged mice. Furthermore, reduction of inflammatory cell influx by GW5074 was evaluated by hematoxylin-eosin staining (Fig. 1D). Ashcroft sore analysis indicated that GW5074 reduced the severity of pulmonary fibrosis (Fig. 1E).
Fig. 1.
Raf1 inhibitor protects against lung fibrosis. A: scheme for experiment design. Mice were intranasally instilled with bleomycin (Bleo; 2 U/kg, single injection) for 7 days, and mice were then given intraperitoneal GW5074 as indicated for an additional 2 wk. Lung tissues were analyzed at day 21. B: immunoblotting analysis of fibronectin and β-actin levels in lung lysates from day 21. C: Masson trichrome staining of lung sections (n = 6–7). Scale bar, 200 µm. D: hematoxylin-eosin staining of lung sections (n = 6–7). Scale bar, 200 µm. Magnification in C and D = ×20. E: histopathologic assessment of pulmonary fibrosis by Ashcroft score (n = 3–6). d, day.
Raf1 promotes TGF-β1-induced myofibroblast differentiation and ECM production in human lung fibroblasts.
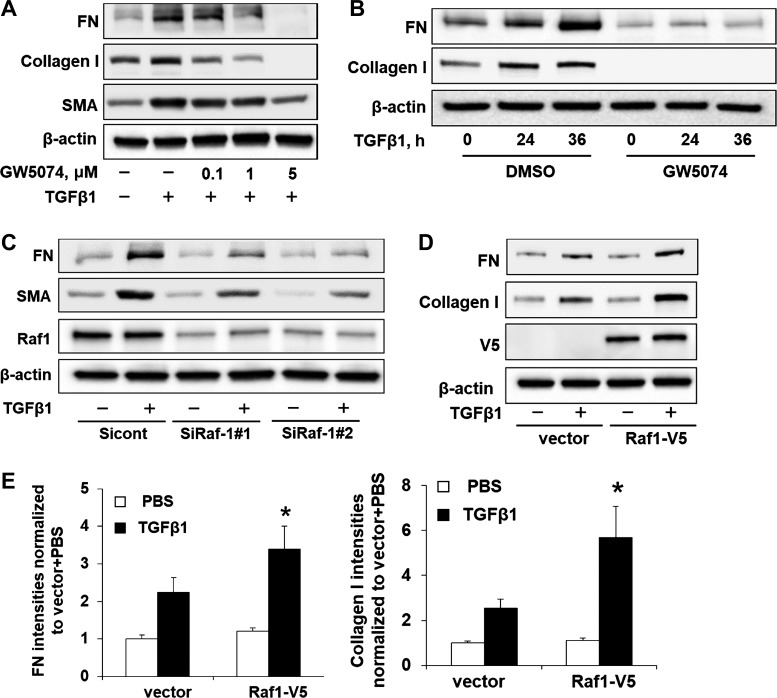
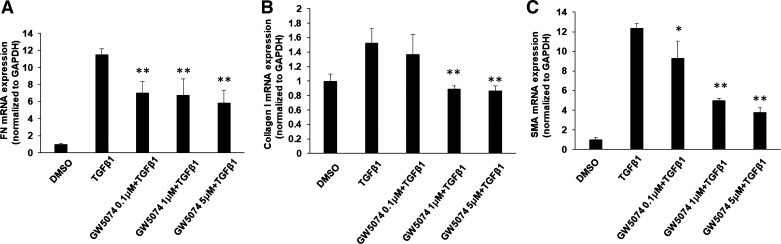
Activation of Raf1 has been shown to be required for TGF-β1-induced EMT in murine mammary gland epithelial cells (37); however, the fibrotic role of Raf1 has not been reported. To investigate whether Raf1 contributes to TGF-β1-induced myofibroblast differentiation and ECM accumulation in lung fibroblasts, Mrc5 was incubated with Raf1 inhibitor GW5074 before TGF-β1 treatment. As shown in Fig. 2A, GW5074 dramatically attenuated TGF-β1-induced FN, collagen I, and SMA in a dose-dependent manner. Furthermore, pretreatment with GW5074 (5 µM) reduced FN and collagen I levels in a time-dependent manner (Fig. 2B), suggesting that GW5074 exhibits an antifibrotic effect through reduction of TGF-β1-induced myofibroblast differentiation and ECM accumulation. To confirm that the effect of GW5074 is due to inhibition of Raf1, we examined whether downregulation of Raf1 exhibits similar effects to GW5074. Mrc5 cells were transfected with siRNAs for Raf1 before TGF-β1 treatment. As shown in Fig. 2C, Raf1 siRNA transfection significantly reduced Raf1 levels as well as TGF-β1-induced FN and SMA expression, whereas overexpression of Raf1 promoted TGF-β1-induced FN and collagen I levels in Mrc5 cells (Fig. 2, D and E). We next tested whether GW5074 regulates TGF-β1-mediated gene transcription. Real-time quantitative PCR studies showed that GW5074 significantly decreased TGF-β1-induced FN, collagen I, and α-SMA mRNA levels in a dose-dependent manner (Fig. 3, A–C).
Fig. 2.
Raf1 enhances TGF-β1-induced myofibroblast differentiation and extracellular matrix production. A: Mrc5 cells were incubated with GW5074 (0–5 µM, 1 h), and cells were then treated with or without TGF-β1 (10 ng/ml, 24 h). Immunoblotting analysis of fibronectin (FN), collagen I, smooth muscle actin (SMA), and β-actin levels. Representative blots from 3 independent experiments are shown. B: Mrc5 cells were incubated with GW5074 (2 µM, 1 h), and then cells were treated with TGF-β1 (10 ng/ml) for 0, 24, and 36 h. Cell lysates were examined by immunoblotting with the indicated antibodies. Representative blots from 3 independent experiments are shown. C: Mrc5 cells were transfected with control siRNA (Sicont) or Raf1 siRNAs (SiRaf) and incubated for 2 days, and then cells were treated with TGF-β1 (10 ng/ml) for 24 h. Cell lysates were examined by immunoblotting with FN, SMA, Raf1, and β-actin antibodies. Representative blots from 2 independent experiments are shown. D: immunoblotting analysis of FN, collagen I, V5, and β-actin levels in TGF-β1-treated (10 ng/ml, 24 h) vector or Raf1-V5 plasmid-transfected Mrc5 cells. Representative blots from 3 independent experiments are shown. E: FN and collagen I levels in D were quantified with ImageJ software (n = 3). *P < 0.05 compared with vector + TGF-β1.
Fig. 3.
Raf1 inhibitor inhibits TGF-β1-mediated gene transcription. Mrc5 cells were incubated with GW5074 (2 µM, 1 h), and cells were then treated with or without TGF-β1 (10 ng/ml, 36 h), total RNA were extracted and analyzed by quantitative real-time PCR with fibronectin (FN), collagen I, and smooth muscle actin (SMA) primers. The relative expression of FN (A), collagen I (B), and SMA (C) were normalized to Gapdh. Data are represented as mean ± SD; n = 3. *P < 0.05, **P < 0.01, compared with TGF-β1 treatment.
GW5074 reduces phosphorylation of Smad2/3 in response to TGF-β1.
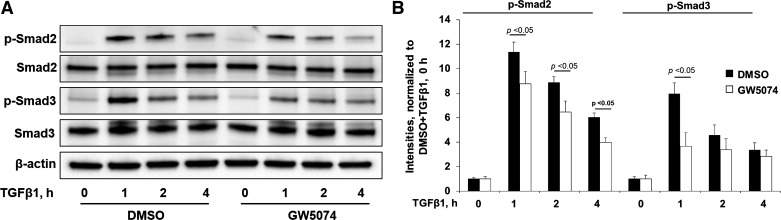
Smad2/3 are downstream signal molecules of TGF-β receptors. TGF-β1 induces myofibroblast differentiation and ECM accumulation through activation of Smad2/3 (5, 31, 33). To determine if Raf1 contributes to the activation of the Smad pathway, Mrc5 cells were treated with GW5074 before TGF-β1 treatment for 1–4 h. TGF-β1 increased phosphorylation of Smad2/3 with a peak at 1 h, and this effect was attenuated by GW5074. GW5074 had no effect on Smad2/3 levels within 4 h (Fig. 4, A and B). This data suggest that Raf1 contributes to TGF-β1-induced Smad2/3 activation in human lung fibroblasts.
Fig. 4.
Raf1 inhibitor inhibits TGF-β1-Smad pathway. A: Mrc5 cells were incubated with DMSO or GW5074 (2 µM, 1 h), and cells were then treated with TGF-β1 (10 ng/ml) for 0, 1, 2, and 4 h. Cell lysates were examined by immunoblotting with phospho (p)-Smad2, total Smad2, p-Smad3, total Smad3, and β-actin levels. Representative blots from 3 independent experiments are shown. B: analysis of p-Smad2 and p-Smad3 by densitometry of the results in A was performed by ImageJ software (n = 3), and statistical analysis was shown.
Raf1 regulates ECM production through ERK-Smad pathway.
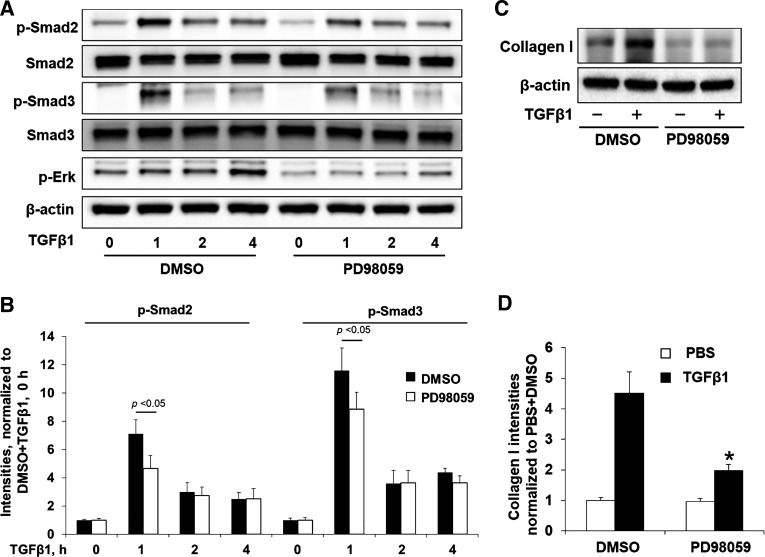
ERK is a downstream target of Raf1, and ERK inhibition has been shown to suppress the development of lung fibrosis following bleomycin injury (9, 17). While cross talk between ERK and Smad2/3 and the role of ERK in collagen expression and EMT have been previously shown (10, 11, 37), the effects of ERK activation on phosphorylation of Smad2/3 and collagen expression in human lung fibroblasts are not well known. We found that an inhibitor of ERK, PD98059, attenuated TGF-β1-induced phosphorylation of Smad 2 and Smad 3 (Fig. 5, A and B) as well as collagen I expression (Fig. 5C and D). Consistent with the above results from Raf1 inhibition, these data suggest that Raf1 regulates ECM production through the ERK-Smad pathway.
Fig. 5.
Raf1 inhibitor attenuates TGF-β1 signaling through inhibition of the Raf1-ERK-Smad pathway. A: Mrc5 cells were incubated with DMSO or PD98059 (10 µM, 1 h), and cells were then treated with TGF-β1 (10 ng/ml) for 0, 1, 2, and 4 h. Cell lysates were examined by immunoblotting with the indicated antibodies. Representative blots from 3 independent experiments are shown. B: analysis of phospho (p)-Smad2 and p-Smad3 by densitometry of the results in A was performed by ImageJ software (n = 3), and statistical analysis was shown. C: immunoblotting analysis of collagen I and β-actin levels in TGF-β1 (10 ng/ml, 36 h) or TGF-β1 + ERK inhibitor (PD98059, 10 µM)-treated Mrc5 cells. Representative blots from 3 independent experiments are shown. D: analysis of collagen I by densitometry of the results in C was performed by ImageJ software (n = 3). *P < 0.01 compared with DMSO + PBS.
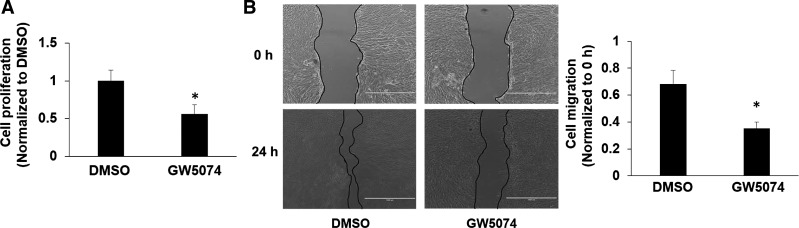
GW5074 inhibits cell proliferation and migration in human lung fibroblasts.
The proliferation and migration of fibroblasts play important roles in the pathogenesis of pulmonary fibrosis (21, 29). Furthermore, we investigated the effect of GW5074 on the proliferation of lung fibroblasts. As shown in Fig. 6A, GW5074 suppressed cell growth in Mrc5 cells. Furthermore, a scratch assay was used to determine cell migration. GW5074 significantly reduced fibroblast migration (Fig. 6B), suggesting that promotion of lung fibroblast cell proliferation and migration by Raf1 contributes to the pathogenesis of lung fibrosis.
Fig. 6.
Raf1 inhibitor inhibits lung fibroblast cell proliferation and migration. A: Mrc5 cells were incubated with DMSO or GW5074 (5 µM) in media containing 1% FBS. After 2 days, cell proliferation was determined by cell counting. *P < 0.05, compared with DMSO-treated cells. B: scratch assay for cell migration. Mrc5 cells were scratched and treated with or without GW5074 (2 µM) for 24 h. Migration area was quantified. *P < 0.05, compared with DMSO-treated cells.
DISCUSSION
IPF is a chronic, irreversible, and ultimately fatal lung disease. The prognosis of IPF has been estimated to be worse than some cancers. Fibroblasts maintain normal lung architecture. However, following lung repair and modeling phase after injury, fibroblasts differentiate to myofibroblasts by increasing α-SMA expression in response to TGF-β1, which is locally activated by a variety of mechanisms (32, 38). Myofibroblasts produce various ECM components, including FN and collagens (13). Fibroblast-to-myofibroblast differentiation, ECM accumulation, and myofibroblast growth and migration play central roles in the pathogenesis of IPF. The rapidly expanding fields of cellular and molecular biology continue to explore the fundamental process involved in its initiation and progression. However, the role of Raf1 in pulmonary fibrosis is still unclear. In the present study, we demonstrate that Raf1 promotes TGF-β1 signaling and exhibits a profibrotic property. We show that 1) Raf1 inhibitor GW5074 ameliorates bleomycin-induced pulmonary fibrosis in mice, 2) Raf1 regulates myofibroblast differentiation and ECM production via ERK-dependent activation of Smads, and 3) Raf1 promotes human lung fibroblast cell proliferation and migration. This study reveals a new signaling pathway by which the TGF-β1/Raf1-ERK/Smad pathway contributes to the activation of lung fibroblast and pulmonary fibrosis.
Raf1 participates in multiple cellular processes, including cell proliferation, differentiation, cell cycle progression, senescence, and apoptosis (8, 23, 34). Raf kinase activity can be targeted with small molecule inhibitors, reducing Raf expression by RNA interference or antisense RNA molecules and interruption of Ras-Raf interaction (16, 26–28). Chen et al. (6) had shown that sorafenib, which is a dual-action inhibitor for Raf1 and vascular endothelial growth factor/platelet-derived growth factor receptor pathway, ameliorated bleomycin-induced pulmonary fibrosis through inhibition of EMT and fibroblast activation. However, in their study, they did not reveal whether the effect of sorafenib was through inhibition of Raf1 or not. Inhibition of vascular endothelial growth factor/platelet-derived growth factor receptor is well known to reduce fibrotic responses via the inhibition of fibrocyte activity (25), and thus the anti-fibrotic effect of sorafenib may also be due to inhibition of receptor tyrosine kinases. GW5074 has a high specificity for Raf kinases (3). In the current study, we provide evidence showing that in addition to inhibition of Raf1 by GW5074, knockdown of Raf1 exhibits a significant reduction of TGF-β1-mediated fibrotic responses in human lung fibroblast, indicating that the activation of Raf1 contributes to the pathogenesis of lung fibrosis. Consistent with this conclusion, inhibition of the Raf1 downstream signal molecule, ERK, attenuated TGF-β1-induced phosphorylation of Smad2 and Smad3 and ECM production. We conclude that GW5074 attenuates TGF-β1 signaling at least through inhibition of Raf1/ERK/Smad pathway. It has been shown that Rho signaling is important for Raf1-mediated cell migration (7). Future studies will focus on the role of Rho signaling in Raf1-mediated lung fibroblast cell proliferation and migration. Tsai et al. (30) have found a novel combination therapy with sorafenib and GW5074 for cancer therapy. These findings indicate that sorafenib and GW5074 may be a promising new approach for the treatment of pulmonary fibrosis.
In conclusion, we identified a new molecular target for the development of medicine for treatment of IPF. In our cellular model system, Raf1 promotes TGF-β1 signaling through the ERK/Smad pathway, thereby promoting myofibroblast differentiation, ECM production, cell proliferation, and migration. This study provides preclinical and molecular mechanistic evidence showing that GW5074 is a potential small molecule for treating IPF.
GRANTS
This work was supported by NIH Grants R01-HL-112791, HL-131665, and HL-136294 (to Y. Zhao), NIH Grant R01-GM-115389 (to J. Zhao), and the American Heart Association GIA award (to Y. Zhao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.K., D.S., and Y.Z. conceived and designed research; S.L., J.L., J.T., L.L., M.J.K., and J.Z. performed experiments; S.L. analyzed data; S.L. and D.S. interpreted results of experiments; S.L. prepared figures; S.L. drafted manuscript; J.Z., D.J.K., and Y.Z. edited and revised manuscript; D.J.K., D.S., and Y.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
Present addresses for J. Zhao and Y. Zhao: Dept. of Physiology and Cell Biology, Ohio State Univ., 333 W. 10th Ave., Graves Hall, Columbus, OH 43210.
REFERENCES
- 1.Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J 13: 1610–1619, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axmann A, Seidel D, Reimann T, Hempel U, Wenzel KW. Transforming growth factor-beta1-induced activation of the Raf-MEK-MAPK signaling pathway in rat lung fibroblasts via a PKC-dependent mechanism. Biochem Biophys Res Commun 249: 456–460, 1998. doi: 10.1006/bbrc.1998.9188. [DOI] [PubMed] [Google Scholar]
- 3.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beith JL, Alejandro EU, Johnson JD. Insulin stimulates primary beta-cell proliferation via Raf-1 kinase. Endocrinology 149: 2251–2260, 2008. doi: 10.1210/en.2007-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camoretti-Mercado B, Solway J. Transforming growth factor-beta1 and disorders of the lung. Cell Biochem Biophys 43: 131–148, 2005. doi: 10.1385/CBB:43:1:131. [DOI] [PubMed] [Google Scholar]
- 6.Chen YL, Zhang X, Bai J, Gai L, Ye XL, Zhang L, Xu Q, Zhang YX, Xu L, Li HP, Ding X. Sorafenib ameliorates bleomycin-induced pulmonary fibrosis: potential roles in the inhibition of epithelial-mesenchymal transition and fibroblast activation. Cell Death Dis 4: e665, 2013. doi: 10.1038/cddis.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenreiter K, Piazzolla D, Velamoor V, Sobczak I, Small JV, Takeda J, Leung T, Baccarini M. Raf-1 regulates Rho signaling and cell migration. J Cell Biol 168: 955–964, 2005. doi: 10.1083/jcb.200409162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer A, Baljuls A, Reinders J, Nekhoroshkova E, Sibilski C, Metz R, Albert S, Rajalingam K, Hekman M, Rapp UR. Regulation of RAF activity by 14-3-3 proteins: RAF kinases associate functionally with both homo- and heterodimeric forms of 14-3-3 proteins. J Biol Chem 284: 3183–3194, 2009. doi: 10.1074/jbc.M804795200. [DOI] [PubMed] [Google Scholar]
- 9.Galuppo M, Esposito E, Mazzon E, Di Paola R, Paterniti I, Impellizzeri D, Cuzzocrea S. MEK inhibition suppresses the development of lung fibrosis in the bleomycin model. Naunyn Schmiedebergs Arch Pharmacol 384: 21–37, 2011. doi: 10.1007/s00210-011-0637-7. [DOI] [PubMed] [Google Scholar]
- 10.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17: 1576–1578, 2003. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 11.Hough C, Radu M, Doré JJ. Tgf-beta induced Erk phosphorylation of smad linker region regulates smad signaling. PLoS One 7: e42513, 2012. doi: 10.1371/journal.pone.0042513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis–FDA review of pirfenidone and nintedanib. N Engl J Med 372: 1189–1191, 2015. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 13.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 229: 298–309, 2013. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J 24: 4503–4512, 2010. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J. Raf-1 activates MAP kinase-kinase. Nature 358: 417–421, 1992. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 16.Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta 1773: 1196–1212, 2007. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol 46: 380–388, 2012. doi: 10.1165/rcmb.2011-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36: 320–328, 2011. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12: 325–338, 2016. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 20.Nan L, Jacko AM, Tan J, Wang D, Zhao J, Kass DJ, Ma H, Zhao Y. Ubiquitin carboxyl-terminal hydrolase-L5 promotes TGFβ-1 signaling by de-ubiquitinating and stabilizing Smad2/Smad3 in pulmonary fibrosis. Sci Rep 6: 33116, 2016. doi: 10.1038/srep33116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Evanoff H, Flaherty KR, Martinez FJ, Toews GB, Hogaboam CM. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur Respir J 29: 1082–1093, 2007. doi: 10.1183/09031936.00122806. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapp UR, Götz R, Albert S. BuCy RAFs drive cells into MEK addiction. Cancer Cell 9: 9–12, 2006. doi: 10.1016/j.ccr.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Reimann T, Hempel U, Krautwald S, Axmann A, Scheibe R, Seidel D, Wenzel KW. Transforming growth factor-beta1 induces activation of Ras, Raf-1, MEK and MAPK in rat hepatic stellate cells. FEBS Lett 403: 57–60, 1997. doi: 10.1016/S0014-5793(97)00024-0. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Shinohara S, Hayashi S, Morizumi S, Abe S, Okazaki H, Chen Y, Goto H, Aono Y, Ogawa H, Koyama K, Nishimura H, Kawano H, Toyoda Y, Uehara H, Nishioka Y. Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the inhibition of fibrocyte activity. Respir Res 18: 172, 2017. doi: 10.1186/s12931-017-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreck R, Rapp UR. Raf kinases: oncogenesis and drug discovery. Int J Cancer 119: 2261–2271, 2006. doi: 10.1002/ijc.22144. [DOI] [PubMed] [Google Scholar]
- 27.Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther 4: 677–685, 2005. doi: 10.1158/1535-7163.MCT-04-0297. [DOI] [PubMed] [Google Scholar]
- 28.Strumberg D, Seeber S. Raf kinase inhibitors in oncology. Onkologie 28: 101–107, 2005. doi: 10.1159/000083373. [DOI] [PubMed] [Google Scholar]
- 29.Suganuma H, Sato A, Tamura R, Chida K. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 50: 984–989, 1995. doi: 10.1136/thx.50.9.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai YT, Chuang MJ, Tang SH, Wu ST, Chen YC, Sun GH, Hsiao PW, Huang SM, Lee HJ, Yu CP, Ho JY, Lin HK, Chen MR, Lin CC, Chang SY, Lin VC, Yu DS, Cha TL. Novel cancer therapeutics with allosteric modulation of the mitochondrial C-Raf-DAPK complex by Raf inhibitor combination therapy. Cancer Res 75: 3568–3582, 2015. doi: 10.1158/0008-5472.CAN-14-3264. [DOI] [PubMed] [Google Scholar]
- 31.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matri11x gene expression and regulation. J Invest Dermatol 118: 211–215, 2002. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 32.Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci 593, 1 Transforming: 188–196, 1990. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- 33.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol 8: 461, 2017. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol 5: 875–885, 2004. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Li GN, Xie J, Li R, Chen QH, Chen JZ, Wei ZH, Kang LN, Xu B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc Disord 16: 5, 2016. doi: 10.1186/s12872-015-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao L, Du Y, Shen Y, He Y, Zhao H, Li Z. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front Biosci 17: 2667–2674, 2012. doi: 10.2741/4077. [DOI] [PubMed] [Google Scholar]
- 37.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 6: 603–610, 2004. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu YD, Hua J, Mui A, O’Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 285: L527–L539, 2003. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 19: 128–139, 2009. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]