Abstract
Accessory subunits associated with the calcium-sensitive potassium channel (BKCa), a major determinant of vascular tone, confer functional and anatomical diversity. The β1 subunit increases Ca2+ and voltagesensitivity of the BKCa channel and is expressed exclusively in smooth muscle cells. Evidence supporting the physiological significance of the β1 subunit includes the observations that murine models with deletion of the β1 subunit are hypertensive and that humans with a gain-of-function β1 mutation are at a decreased risk of diastolic hypertension. However, whether the β1 subunit of the BKCa channel contributes to the low tone that characterizes the normal pulmonary circulation or modulates the pulmonary vascular response to hypoxia remains unknown. To determine the role of the BKCa channel β1 subunit in the regulation of pulmonary vascular tone and the response to acute and chronic hypoxia, mice with deletion of the Kcnmb1 gene that encodes for the β1 subunit (Kcnmb1−/−) were placed in chronic hypoxia (10% O2) for 21–24 days. In normoxia, right ventricular systolic pressure (RVSP) did not differ between Kcnmb1+/+ (controls) and Kcnmb1−/− mice. After exposure to either acute or chronic hypoxia, RVSP was higher in Kcnmb1−/− mice compared with Kcnmb1+/+ mice, without increased vascular remodeling. β1 subunit expression was predominantly confined to pulmonary artery smooth muscle cells (PASMCs) from vessels ≤ 150 µm. Peripheral PASMCs contracted collagen gels irrespective of β1 expression. Focal adhesion expression and Rho kinase activity were greater in Kcnmb1−/− compared with Kcnmb1+/+ PASMCs. Compromised PASMC β1 function may contribute to the heightened microvascular vasoconstriction that characterizes pulmonary hypertension.
Keywords: focal adhesions, MaxiK channel, microvasculature, pulmonary artery smooth muscle cells, Rho kinase
INTRODUCTION
In utero, pulmonary blood flow is closely circumscribed as pulmonary vascular resistance exceeds systemic resistance, and blood is shunted away from the lungs. At birth, the pulmonary circulation undergoes a marked transition as the lung environment changes from being fluid filled with low oxygen (O2) tension to air filled with relatively high O2 tension (15). The increase in O2 tension normally causes perinatal pulmonary vasodilation, increasing pulmonary blood flow 8–10-fold and inducing a steady decline in pulmonary arterial (PA) pressure over the first several hours of life (35). Work from our laboratory has demonstrated a central role for the large conductance calcium-sensitive potassium channel (BKCa) in regulating fetal pulmonary blood flow and mediating the transition of the pulmonary circulation at birth (12, 42).
Further work has demonstrated that in the neonatal pulmonary circulation, the BKCa channel plays a central role in both O2- and nitric oxide-induced pulmonary vasodilation (37). Specifically, O2 causes localized release of Ca2+, termed Ca2+ sparks, from a ryanodine-sensitive intracellular calcium store in neonatal pulmonary artery smooth muscle cells (PASMCs) (31), which leads to activation of the BKCa channel, generation of spontaneous transient outward potassium currents, membrane hyperpolarization, and vasodilation. In neonatal PASMCs the association of the BKCa channel with the β1 subunit increases the Ca2+ and voltagesensitivity of the BKCa channel (14) to Ca2+ sparks. The β1 subunit is expressed exclusively in smooth muscle cells (SMCs) (6). These data indicate a uniquely important and developmentally conserved role for the BKCa channel in mediating perinatal pulmonary vasodilation, a biologically imperative function.
Evidence in support of the importance of the β1 subunit of the BKCa channel includes the observation that in the absence of the gene encoding the β1 subunit (Kcnmb1), calcium sparks are unable to activate the BKCa channel (6, 16, 20). Murine models with deletion of the β1 subunit are hypertensive, arguing for a role for the subunit in regulating systemic vascular tone (6). In rat models of systemic hypertension, the β1 subunit expression is decreased (10). Estradiol activates the BKCa channel through binding to the β1 subunit (43). From a functional perspective, a genetic modification that affords gain-of-function of the β1 subunit confers protection against hypertension in humans (17). Moreover, a loss-of-function polymorphism in Kcnmb1 is associated with asthma severity (39), lending support to the notion that the β1 subunit plays a more generalized role in modulating SMC tone, including airway. Though prior studies demonstrate a distinct role for the β1 subunit in cerebral compared with the pulmonary vasculature (47), whether and to what extent the β1 subunit plays a role in determining tone and response to hypoxia in the adult pulmonary circulation remains unknown.
Therefore, to test the overall hypothesis that vascular SMC β1 subunit expression of the BKCa channel modulates pulmonary vascular tone under normoxic conditions and the response to hypoxia, Kcnmb1-deficient (Kcnmb1−/−) and wild-type (WT) (Kcnmb1+/+) mice were placed in either acute or chronic hypoxia, and the pulmonary vascular response was systematically interrogated. Here, we demonstrate that under basal conditions the β1 subunit does not modulate pulmonary vascular tone, but under hypoxic conditions, either acute or chronic, the β1 subunit modulates the pulmonary vascular response to hypoxia by increasing contraction but not vascular remodeling.
MATERIALS AND METHODS
Kcnmb1−/− mice.
The Institutional Animal Care and Use Committee at Stanford University approved all the procedures and protocols governing the care and use of laboratory animals. Kcnmb1−/− mice (kindly provided by Dr. Robert Brenner, University of Texas Health Science Center, San Antonio, TX) (6) were backcrossed repeatedly to C57BL/6 mice to generate Kcnmb1−/− mice with a genetic background nearly identical to C57BL/6 mice. Kcnmb1−/− mice have exon 2 deleted, resulting in the loss of the Kcnmb1 product, the β1 subunit. Age-matched WT Kcnmb1+/+ were used as controls. Animals used were females and males between 3 and 6 mo of age.
Genotyping.
DNA was isolated from the tail clippings of Kcnmb1+/+, Kcnmb1+/−, and Kcnmb1−/− mice, and isolated peripheral PASMCs were evaluated via PCR using the following primer set: forward common primer: 5′-AAA GCT AAC TGT AGG CTG TTA CCA GAG-3′; reverse WT primer: 5′-TGG CAG CAC ACA CTA CCA TTG C-3′; and reverse mutant primer: 5′-CCA GTC ACG ACG TTG TAA AAC GAC-3′. The PCR products were 389 bp for the WT allele and a 440 bp PCR product for the mutant allele.
Lung fixation.
Mice were killed by intraperitoneal injection with an overdose of pentobarbital. A cannula was inserted into the trachea and fixed with a ligature. Lungs were inflation fixed in situ with 10% formalin at a constant fluid pressure of 25 cm for 5 min. After the trachea was tied off, the lungs were placed in 10% formalin for at least 24 h, followed by a 70% ethanol rinse. Each lung was embedded in paraffin and cut in 4-μm sections.
Immunohistochemistry.
To visualize the BK channel β1 subunit, formalin-fixed and paraffin-embedded lung sections from Kcnmb1+/+ and Kcnmb1−/− mice were deparaffinized and rehydrated; incubated with Universal Antigen Retrieval Reagent (R&D Systems, Minneapolis, MN) for 30 min at 95°C; permeabilized with 0.25% Triton X-100/PBS solution for 30 min; incubated with 100 mM glycine solution (pH 7.5) for 20 min to quench auto-fluorescence; blocked with Sea Block Blocking Buffer (Thermo Scientific, Loughborough, UK) for 40 min, Fc Receptor Blocker (Innovex Biosciences, Richmond, CA) for 30 min, and Mouse Detective (Thermo Fisher Scientific, Pacheco, CA) for 30 min; followed by incubation with MaxiKβ (1:100, Santa Cruz sc-14751, Santa Cruz Biotechnology Inc., Dallas, TX) and α-smooth muscle actin (α-SMA, 1:200, Sigma A2547, Sigma-Aldrich, St. Louis, MO) antibodies overnight at 4°C. Sections were then incubated with donkey α-goat Alexa Fluor 488 for 1 h, followed by goat α-mouse Alexa Fluor 568 antibodies (1:200, Invitrogen, Carlsbad, CA) for 1 h, and then incubated with 1 μg/ml Hoechst solution (Sigma-Aldrich) to visualize nuclei and mounted with 70% glycerol solution (magnification ×200, scale bar 100 μm).
To visualize α-SMA and Von Willebrand Factor (VWF), formalin-fixed and paraffin-embedded lung sections from Kcnmb1+/+ and Kcnmb1−/− mice were treated as described above, with the following exceptions: samples were incubated with α-SMA (1:400, Sigma A2547) and VWF (1:100, Abcam ab7356, Abcam, Cambridge, UK) antibodies overnight at 4°C. Sections were then incubated with goat α-mouse Alexa Fluor 488 and donkey α-rabbit Alexa Fluor 568 antibodies (1:200, Invitrogen) for 1 h and then incubated with 1 μg/ml Hoechst solution (Sigma-Aldrich) to visualize nuclei and mounted with 70% glycerol solution (magnification ×200).
Quantification of pulmonary arterioles and arteries positive for β1 expression.
Lung tissue sections were processed for detection of the β1 subunit (MaxiKβ, 1:100, Santa Cruz sc-14751) and α-SMA (1:200, Sigma A2547) as described above. To assess the number of pulmonary arterioles (1–150 μm in diameter) and arteries (≥150 μm in diameter) positive for β1 and α-SMA expression, a minimum of 10 fields at ×200 magnification were examined per condition (normoxia and chronic hypoxia with n = 6 per group) for Kcnmb1+/+ mice. α-SMA positive vessels were localized throughout the lung, and the largest diameter of the vessel wall, as visualized by α-SMA staining, was measured using Metamorph software (Molecular Devices, San Jose, CA). Over 100 α-SMA-positive vessels were examined per group with 60 ± 12.6% of Kcnmb1+/+ under normoxic conditions and 59.4 ± 10.7% of Kcnmb1+/+ under chronic hypoxic conditions positive for β1 expression. Results are shown as the percentage of β1 positive vessels as sorted by diameter range (1–150, 151–300, and 301–500 μm) (27).
Primary mouse peripheral PASMC isolation.
Peripheral (distal) PASMCs were isolated from the tissue obtained from the peripheral lobes of Kcnmb1+/+ and Kcnmb1−/− mice. The pleura, large airways, and large blood vessels were flushed repeatedly with sterile ice-cold PBS to remove contaminating macrophages and neutrophils. Peripheral lung tissues included the most peripheral 2–5 mm from each lobe of the lungs (n = 5 for each group), minced in small pieces, and digested in dispersion medium containing 40 μmol/l CaCl2, 0.5 mg/ml elastase (Worthington Biochemical Corporation, Lakewood, NJ), 0.5 mg/ml collagenase (Worthington Biochemical Corporation), 0.2 mg/ml soybean trypsin inhibitor (Worthington Biochemical Corporation), and 2 mg/ml albumin (Sigma-Aldrich) in Hanks’ balanced salt solution for 30 min at 37°C. Cells were filtered from the undigested debris with 100 μm pore nylon mesh cell strainers (Fisher Scientific, Hampton, NH) and rinsed with D10F + A/A (DMEM containing 10% FBS, 1% l-glutamine, 1% penicillin-streptomycin solution, and 1% antibiotic-antimycotic solution) (Invitrogen; Gibco, Gaithersburg, MD). Cells were then incubated with Dynabeads (Invitrogen) coated with CD31 antibody (BD Biosciences, San Jose, CA) for 30 min to deplete endothelial cells expressing CD31. Remaining cells were collected through centrifugation at 250 g for 5 min at 4°C and cultured in D10F + A/A. Cells were incubated at 37°C, 5% CO2 with 90% humidity followed by media changes every 24 h for up to 2 wk. Starting with the first media change (24 h postseeding), cells were rinsed with ice-cold PBS to remove nonadherent cells and then cultured SMC medium (Smooth Muscle Growth Medium-2, Lonza, Walkersville, MD), which supports the maintenance and expansion of SMCs, containing smooth muscle basic medium plus supplements [5% FBS with 0.1% insulin, 0.2% basic human fibrolastic growth factor, 0.1% human epidermal growth factor, and 0.1% gentamicin/amphotericin (GA-1000)] (23). Cultured SMCs obtained by this method routinely stain positive for α-SMA (Sigma A2547) with 84.1 ± 3.9% Kcnmb1+/+ and 87.5 ± 2.9% Kcnmb1−/− cells staining positive for α-SMA. Peripheral PASMCs from passages 2–5 were used for all experiments.
The proximal PASMCs were isolated from the main pulmonary artery from WT (C57BL/6) mice (n = 5) using a modified elastase/collagenase digestion protocol as previously described (23). Proximal PASMCs from passages 2–5 were used for all experiments.
Immunocytochemistry.
To visualize the β1 subunit, subconfluent cultures of peripheral PASMCs isolated from Kcnmb1+/+ and Kcnmb1−/− mice were fixed in 3% paraformaldehyde in PBS for 30 min, blocked and permeabilized in blocking solution (0.1% Triton X-100, 15 mg/ml glycine, and 2.5% FBS in PBS) for 1 h, and then incubated with MaxiKβ (1:200, Santa Cruz sc-14751) and α-SMA (1:200, Sigma A2547) antibodies overnight at 4°C. Cells were then incubated with rabbit α-goat Alexa Fluor 568 antibody (1:200, Invitrogen) for 1 h, followed by goat α-mouse Alexa Fluor 488 antibody (1:200, Invitrogen) for 1 h, and then mounted with Vectashield Mounting Medium with DAPI (Vector, Burlingame, CA). The number of isolated peripheral PASMCs expressing the β1 subunit was assessed as a percentage of total cells per high-powered field (magnification ×200; 10 fields counted). In addition, isolated cells were examined for α-SMA (1:200, Sigma A2547) expression, a marker of vascular SMCs, with 84.1 ± 3.9% Kcnmb1+/+ and 87.5 ± 2.9% Kcnmb1−/− cells staining positive for α-SMA expression (magnification ×100; 40 fields counted).
Focal adhesions and focal complexes were assessed as previously described (5). In brief, cells were fixed and permeabilized, incubated with Alexa Fluor 488 phalloidin (1:40, Invitrogen) and vinculin (1:200, Millipore-Sigma MAB3574, Millipore-Sigma, St. Louis, MO) antibodies for 1 h, followed by incubation with Alexa Fluor 568 α-mouse antibody (1:200, Invitrogen) for 30 min, then mounted with Vectashield mounting medium with DAPI. Results shown represent the quantification of the number of focal adhesions formed per cell or the percentage of cells with focal complexes as demonstrated by vinculin staining with a minimum of 200 cells counted per sample (n = 3 for Kcnmb1+/+ and n = 3 for Kcnmb1−/−) (magnification ×200; 10 fields counted).
To visualize myosin light chain phosphorylation (pMLC) expression, subconfluent cultures of peripheral PASMCs isolated from Kcnmb1+/+ and Kcnmb1−/− mice (3 h and 24 h postseeding) were fixed and permeabilized as described above, then incubated with pMLC (1:50, Cell Signaling 3671) and α-actinin (1:50, Millipore/Upstate 05–384) antibodies for 1 h. Cells were then incubated with donkey α-rabbit Alexa Fluor 568 (1:200, Invitrogen) and goat α-mouse Alexa Fluor 488 (1:200, Invitrogen) antibodies for 1 h and then mounted with Vectashield Mounting Medium with DAPI (Vector) (magnification ×400).
Western immunoblotting.
For Western blot analysis, protein content was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Fifteen micrograms of protein/sample were subjected to SDS-PAGE analysis. Immobilon-P (MilliporeSigma) membranes were incubated with primary antibodies to detect β1 (KCNMB1, GeneTex GTX105666, Irvine, CA), β-actin (Sigma A5441), α-tubulin (Sigma T6074), pMLC (Abcam ab2480), myosin light chain (MLC) (Sigma M4401), Rho kinase (Cell Signaling 9029), and vinculin (Millipore-Sigma MAB3574), and then incubated with horseradish peroxidase-conjugated secondary antibodies, followed by detection with ECL reagents (GE Healthcare, Chicago, IL). Graph represents quantification of β1 expression by densitometry with results represented relative to β-actin expression. Graph represents quantification of Rho kinase, phospho-vinculin, and pMLC expression by densitometry with results represented relative to respective controls (β-actin for Rho kinase, vinculin for phospho-vinculin, and MLC for pMLC).
RT-qPCR.
To assay for Kcnmb1 mRNA expression, total RNA was isolated from cultured PASMCs using the RNeasy Mini Kit (Qiagen Sciences, Germantown, MD) and reverse-transcribed using Superscript III Reverse Transcriptase (Invitrogen) per the manufacturer’s instructions. cDNA (50 ng) was used per PCR with each sample analyzed in triplicate. PCRs were performed using the TaqMan Gene Expression Assays (Thermo Fisher Scientific) probe spanning the exon 2–3 junction of Kcnmb1. PCR conditions were as follows: 3 min at 95°C for one cycle, followed by 40 cycles at 95°C for 15 s, 60°C for 60 s, and 72°C for 30 s. Target gene mRNA was normalized to 18S rRNA using the comparative CT method.
Hemodynamic assessments.
Adult littermates (3–6 mo) were used in each group. Right ventricular systolic pressures (RVSPs) were measured as previously described (23). In brief, mice were anesthetized with 1.5–2.0% isofluorane, and RVSP measurements were obtained using a 1.4-Fr Millar catheter (Millar, Houston, TX) at baseline (21% O2; males: n = 5 per group; females: n = 6 per group), after exposure to chronic hypoxia (10% O2 for 3 wk; males: n = 5 per group; females: n = 10–11 per group), and after exposure to acute hypoxia (10% O2 for 5 min; n = 5–6 per group). Blood was collected by direct heart venipuncture under either normoxic conditions or hypoxic conditions and assayed for hematocrit levels (n = 11–15 per group). Body weights of age-matched mice were measured under normoxic (21% O2; males: n = 4–5 per group; females: n = 3–6 per group) or after chronic hypoxic exposure (10% O2; males: n = 7–8 per group; females: n = 7–8 per group). Percent body weight (BW) gain was determined using the following formula: BW gain (%) = [(BWfinal − BWinitial)/BWinitial] × 100 (46).
Blood pressure and heart rate measurements.
Blood pressure and heart rate measurements were made using a BP-2000 blood pressure analysis system (Visitech Systems, Apex, NC) with all mice undergoing 2–5 training sessions to acclimate the animal and prevent stress induced fluctuations in blood pressure. Reported blood pressure and heart rate represent the average of the first and last measurements. Blood pressure and heart rate measurements were recorded the week before (normoxic) and during the last week of chronic hypoxic exposure (n = 15 per group).
Morphometric analysis.
Assessment of PA muscularization was performed as previously described (22, 23). In brief, formalin-fixed and paraffin-embedded lung sections from mice exposed to normoxia or chronic hypoxia were examined for peripheral PA wall thickness by measuring Movat-stained vessels less than 100 μm in diameter using ImageJ software (magnification ×200, scale bar 25 μm). Quantification of PA muscularization is expressed using the following equation: Medial thickness index = [(areaext − areaint)/areaext], where areaext and areaint represent the areas within the external and internal boundaries of the elastic fibers as detected by Movat stain (n = 5 per group with 10 fields assessed per mouse).
PAs were categorized into nonmuscularized (NM), partially muscularized (PM), or fully muscularized (FM) using ImageJ software to measure α-SMA staining within the medial layer of each PA (19). In brief, vessels up to 150 μm in external diameter were counted in 12 fields from each mouse at ×200 magnification and scale bar 25 μm. The counted vessels were categorized as FM (70–100% of medial layer positive for α-SMA staining), PM (1–69% of medial layer positive for α-SMA staining), or NM (vessels positive for VWF staining). The percentage of pulmonary vessels in each category was calculated by dividing the number of vessels in the category by the total number of counted vessels. α-SMA wall thickness was determined by using the following equation: α-SMA relative area = [(diameterext − diameterint)/diameterext], where diameterext and diameterint represent the diameters within the external and internal boundaries of the α-SMA stained medial layer (n = 3–6 per group).
Distal arteries.
To quantify the number of distal PAs, mean vessel density (number of α-SMA positive distal arteries ≤ 150 μm in diameter per high-powered field) was assessed for lungs from mice exposed to either normoxia or chronic hypoxia with 12 fields per mouse counted at ×200 magnification (n = 5–6 per group).
Collagen gel contraction assay.
Collagen gel contraction assays were performed as previously described (5, 45). In brief, 75 μl of collagen (PureCol EZ Gel Bovine Collagen Solution Type 1 in DMEM-F12, 5 mg/ml, Advanced BioMatrix, Carlsbad, CA) was aliquotted per well of a 48-well plate. After 1 h of polymerization, 1 × 105 PASMCs were added per well in 200 μl of smooth muscle basic medium plus supplements [5% FBS with 0.1% insulin, 0.2% basic human fibrolastic growth factor, 0.1% human epidermal growth factor, and 0.1% gentamicin/amphotericin (GA-1000)] (smooth muscle growth medium-2, Lonza). Cells were seeded in triplicate. Cells were allowed to attach for 1 h before incubation at 5% O2 (hypoxia) or 21% O2 (normoxia). After 18 h, the contracted collagen gels were fixed with 3% paraformaldehyde in PBS for 30 min. The degree of contraction was assessed by measuring the area of the gels with ImageJ software. Results shown are represented as a percentage relative to the area of no cell control (set at 100%).
Drug treatment.
Isolated peripheral PASMCs from Kcnmb1+/+ and Kcnmb1−/− mice were treated with either an agonist of the β1 subunit of the BKCa channel, lithocholic acid (LCA; Sigma-Aldrich) (7) at 45 μM for 30 min, a selective BKCa channel antagonist, iberiotoxin (Alomone, Jerusalem, Israel) (18) at 55 nM for 1 h, or hypoxia (5% O2) for 1 h, 3 h postseeding.
Focal complex intensity.
For focal complex quantification of Kcnmb1+/+ and Kcnmb1−/− cells incubated under normoxic and acute hypoxic conditions (5% O2 for 1 h), the maximum intensity of cell images stained for vinculin expression was measured. In brief, 30 cells per group were outlined using ImageJ software. Background fluorescence was measured and substracted from cell values. To calculate the Corrected Total Cell Fluorescence of each cell, the following equation was used: Corrected Total Cell Fluorescence = integrated density − (area of selected cell × mean fluorescence of background) (8, 40).
Statistical analysis.
All data are presented as means ± SE. Statistical differences between two groups were determined by Student's t-test and between more than two groups by one-way ANOVA, followed by Newman-Keuls Multiple Comparison post hoc analysis. A P value of ≤0.05 was considered statistically significant unless otherwise indicated. For in vivo studies, age-matched Kcnmb1+/+ mice were compared with Kcnmb1−/− mice. For in vitro studies, all experiments were repeated a minimum of five times.
RESULTS
BKCa channel β1 subunit expression is greatest in peripheral PASMCs.
To confirm deletion of Kcnmb1, genotyping was performed on DNA derived from tails and PASMCs from the peripheral vasculature of Kcnmb1+/+ and Kcnmb1−/− mice (6). Moroever, as shown in Fig. 1A, Kcnmb1 mRNA was detectable in PASMCs from Kcnmb1+/+, but not Kcnmb1−/− mice using primers specific for the boundary between exons 2 and 3. Western blot analysis of peripheral PASMCs demonstrated dramatically more expression of the β1 subunit in cells isolated from Kcnmb1+/+ compared with Kcnmb1−/− mice (Fig. 1B). In addition, isolated cells were examined for α-SMA expression, a marker of vascular SMCs, with 84.1 ± 3.9% Kcnmb1+/+ and 87.5 ± 2.9% Kcnmb1−/− cells staining positive for α-SMA expression (Fig. 1C). To determine whether BKCa channel β1 subunit distribution varied within the pulmonary vasculature, we measured β1 expression in PASMCs derived from the proximal (≥150 µm) and distal (≤150 µm) pulmonary vasculature. Under normoxic conditions, in Kcnmb1+/+ mice, β1 subunit expression was present in 84.6 ± 8.1% of α-SMA expressing cells derived from PAs ≤ 150 μm in diameter (Fig. 1D) and only 40.0 ± 6.0% of α-SMA expressing cells derived from the main PA.
Fig. 1.
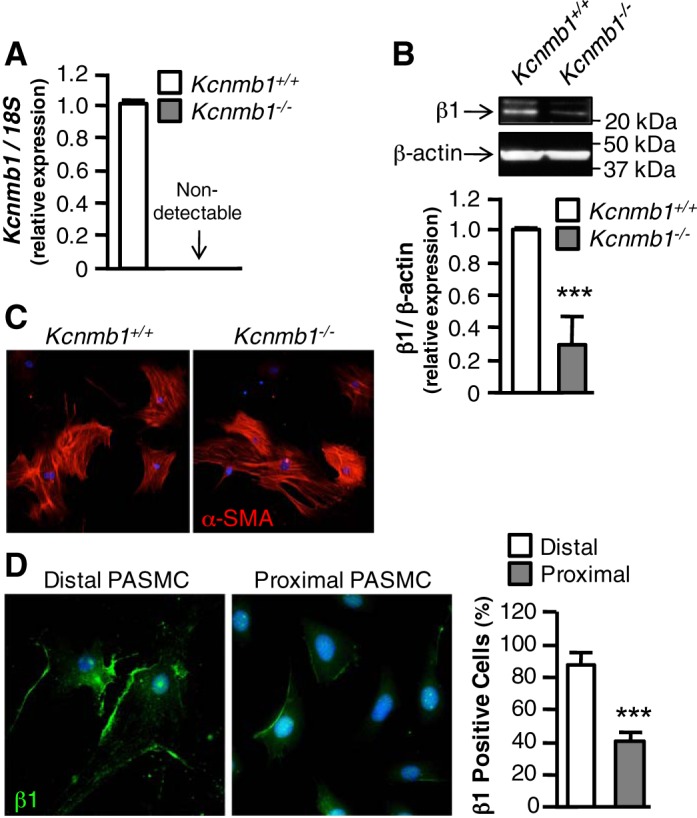
BK channel β1 subunit expression in peripheral pulmonary artery smooth muscle cells (PASMCs). A: expression of Kcnmb1 mRNA in peripheral PASMCs isolated from Kcnmb1+/+ and Kcnmb1−/− mice. Bars represent means ± SE (n = 3). B: expression of β1 in isolated PASMCs by Western immunoblot. β-actin serves as a loading control. Bars represent means ± SE (n = 3). ***P ≤ 0.001, Kcnmb1−/− vs. Kcnmb1+/+. C: expression of α-smooth muscle actin (α-SMA) protein in peripheral PASMC isolated from Kcnmb1+/+ and Kcnmb1−/− mice. α-SMA, red; nuclei, blue; magnification ×100. D: expression of β1 protein in isolated peripheral (distal) PASMC and proximal PASMC. β1, green; nuclei, blue; magnification ×200. Bars represent means ± SE (n = 3). ***P ≤ 0.001, proximal vs. distal PASMCs.
Deletion of the BKCa channel β1 subunit increases the pulmonary vascular response to acute and chronic hypoxia.
At baseline, under normoxic conditions, RVSP did not differ between Kcnmb1+/+ and Kcnmb1−/− mice. Acute hypoxia (5 min, 10% O2) increased RVSP in Kcnmb1−/− mice significantly (Fig. 2A), whereas the increase was not statistically significant in Kcnmb1+/+ mice. After 3 wk of chronic hypoxia, RVSP increased in both groups but was markedly more elevated in Kcnmb1−/− compared with Kcnmb1+/+ mice, with similar increases in RVSP in both male and female mice (Fig. 2B). In both Kcnmb1+/+ and Kcnmb1−/− mice, chronic hypoxia was associated with weight loss (Fig. 2C), an increase in hematocrit and heart rate (Table 1), and had no effect on left ventricular function. Male Kcnmb1−/− mice were significantly heavier than female Kcnmb1−/− mice.
Fig. 2.
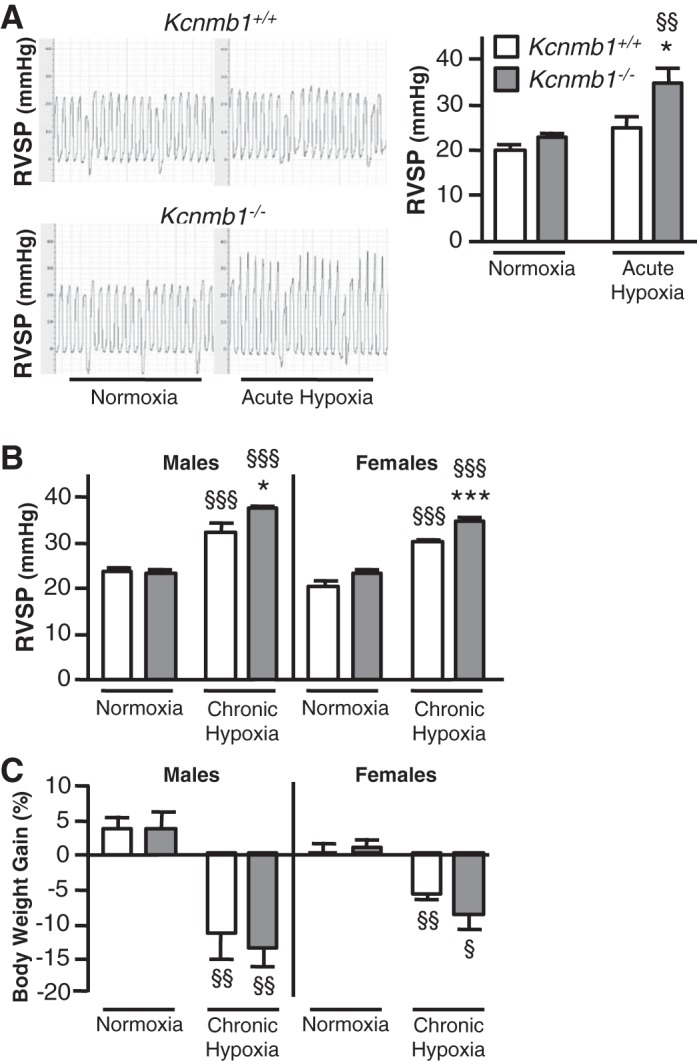
Depletion of β1 subunit increases pulmonary vascular tone. A: right ventricular systolic pressure (RVSP) of Kcnmb1+/+ and Kcnmb1−/− mice in response to acute hypoxia (10% O2, 5 min). Representative RVSP tracings from individual mice exposed to acute hypoxia. Bars represent means ± SE (n = 5–6 per group). *P ≤ 0.05, Kcnmb1−/− vs. Kcnmb1+/+; §§P ≤ 0.01, hypoxia vs. normoxia. B: RVSP of Kcnmb1+/+ and Kcnmb1−/− mice in normoxia (21% O2, 3 wk) and following chronic hypoxia (10% O2, 3 wk). Bars represent means ± SE (males, n = 4–5 per group; females, n = 6 per group). *P ≤ 0.05, ***P ≤ 0.001, Kcnmb1−/− vs. Kcnmb1+/+; §§§P ≤ 0.001, hypoxia vs. normoxia. C: analysis of weight gain under normoxic and hypoxic conditions. Percent body weight gain was determined for Kcnmb1+/+ and Kcnmb1−/− mice exposed to 21% O2 for 3 wk (males, n = 8 per group; females, n = 7 per group) or 10% O2 for 3 wk (males, n = 7 per group; females, n = 8 per group). §P ≤ 0.05, §§P ≤ 0.01, hypoxia vs. normoxia.
Table 1.
Hemodynamic assessments of Kcnmb1+/+ and Kcnmb1−/− mice
| Normoxia |
Chronic Hypoxia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Kcnmb1+/+ | n | Kcnmb1−/− | n | Kcnmb1+/+ | N | Kcnmb1−/− | N | |
| Hematocrit, % | 46.5 ± 1.0 | 12 | 48.3 ± 0.3 | 11 | 64.0 ± 1.6§§§ | 13 | 65.8 ± 1.4§§§ | 15 |
| Heart rate, beats/min | 579 ± 18.0 | 15 | 570 ± 14.7 | 15 | 674 ± 12.1§§§ | 15 | 650 ± 11.6§§§ | 15 |
| Blood pressure, mmHg | 109 ± 3.1 | 15 | 106 ± 4.1 | 15 | 98.5 ± 3.2§ | 15 | 101 ± 3.7 | 15 |
Values are means ± SE; n = mice/group.
P < 0.05;
P < 0.001, chronic hypoxia vs. normoxia.
β1 subunit expression is limited to arterioles less than 150 μm in diameter.
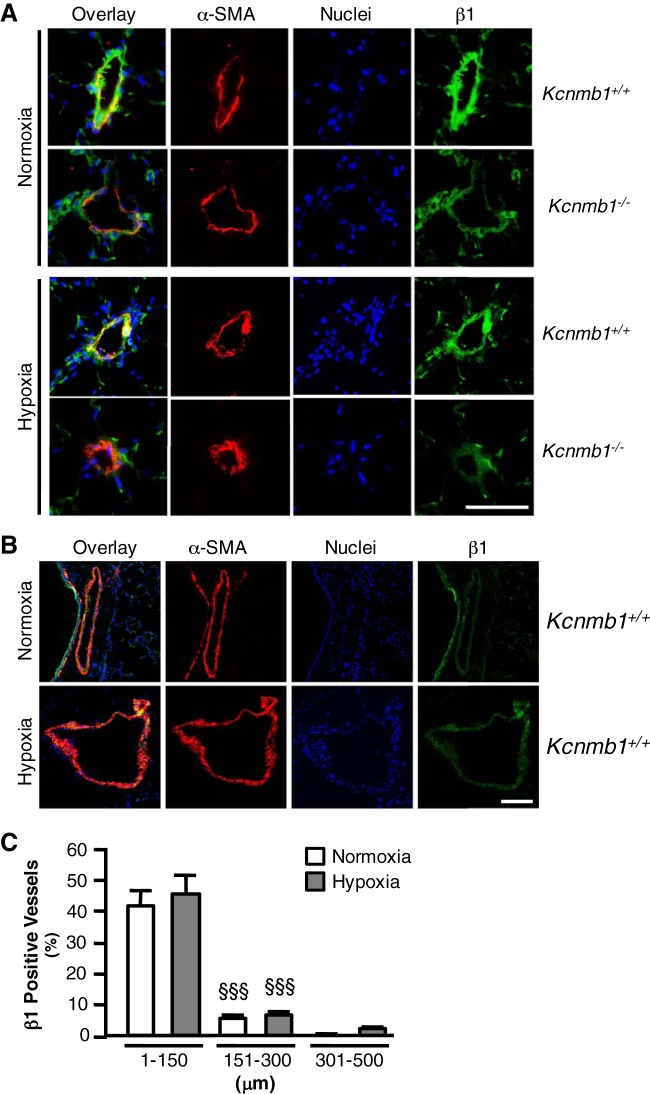
As shown in Fig. 3A, immunostaining of the pulmonary vasculature demonstrated that in Kcnmb1+/+ mice β1 subunit expression was present under normoxic and hypoxic conditions for vessels less than 150 μm, whereas there was no expression under normoxic conditions and no hypoxic induction in Kcnmb1−/− mice. The images shown in Fig. 3B demonstrate that in Kcnmb1+/+ mice larger arteries do not express β1 under normoxic or hypoxic conditions. Quantification of β1-positive vessels by diameter demonstrates that β1 is mainly expressed in vessels under 150 μm (Fig. 3C). There was no significant difference for the percentage of vessels positive for β1 expression in relation to vessel size between normoxic and hypoxic Kcnmb1+/+ mice.
Fig. 3.
BK channel β1 subunit expression in peripheral arterioles. A: β1 expression in pulmonary tissues from adult Kcnmb1+/+ and Kcnmb1−/− mice exposed to hypoxia (10% O2) or normoxia (21% O2) for 3 wk. α-smooth muscle actin (α-SMA), red; β1, green; nuclei, blue; yellow, colocalization; magnification ×200; scale bar 100 μm. B: proximal pulmonary arteries (PAs) do not significantly express the β1 subunit in Kcnmb1+/+ mice under normoxic (21% O2) or hypoxic (10% O2) conditions. α-SMA, red; β1, green; nuclei, blue; yellow, colocalization; magnification ×200; scale bar 100 μm. C: quantification of the percentage of β1 positive vessels by vessel diameter (μm) for Kcnmb1+/+ mice. A miniumum of 10 fields at ×200 magnification were counted per condition (normoxia and chronic hypoxia). Bars represent means ± SE (n = 6 per group). No significant differences exist between Kcnmb1+/+ mice exposed to either normoxia (21% O2) or chronic hypoxia (10% O2). Significant differences exist between β1 positive vessels in regards to vessel diameter. §§§P ≤ 0.001, 151–300 μm vs. 1–150 μm normoxia; §§§P ≤ 0.001, 151–300 μm vs. 1–150 μm hypoxia.
Deletion of the BKCa channel β1 subunit does not increase hypoxic pulmonary vascular remodeling.
Chronic hypoxia increased medial thickness in both Kcnmb1+/+ and Kcnmb1−/− mice. Despite higher RVSP in Kcnmb1−/− compared with Kcnmb1+/+ mice, the degree of muscularization was similar in both groups (Fig. 4A). To explore more fully the potential of differences in muscularization, PAs ≤ 150 µm in diameter were assessed as either NM, PM, or FM. As shown in Fig. 4B, chronic hypoxia increased peripheral muscularization to a similar degree in both Kcnmb1−/− and Kcnmb1+/+ mice. In addition, Fig. 4C demonstrates that chronic hypoxia resulted in a similar reduction in the number of distal pulmonary arteries for both Kcnmb1−/− and Kcnmb1+/+ mice. Altogether, the loss of the β1 subunit in the Kcnmb1−/− mice promotes increased vascular tone without increasing vascular remodeling.
Fig. 4.
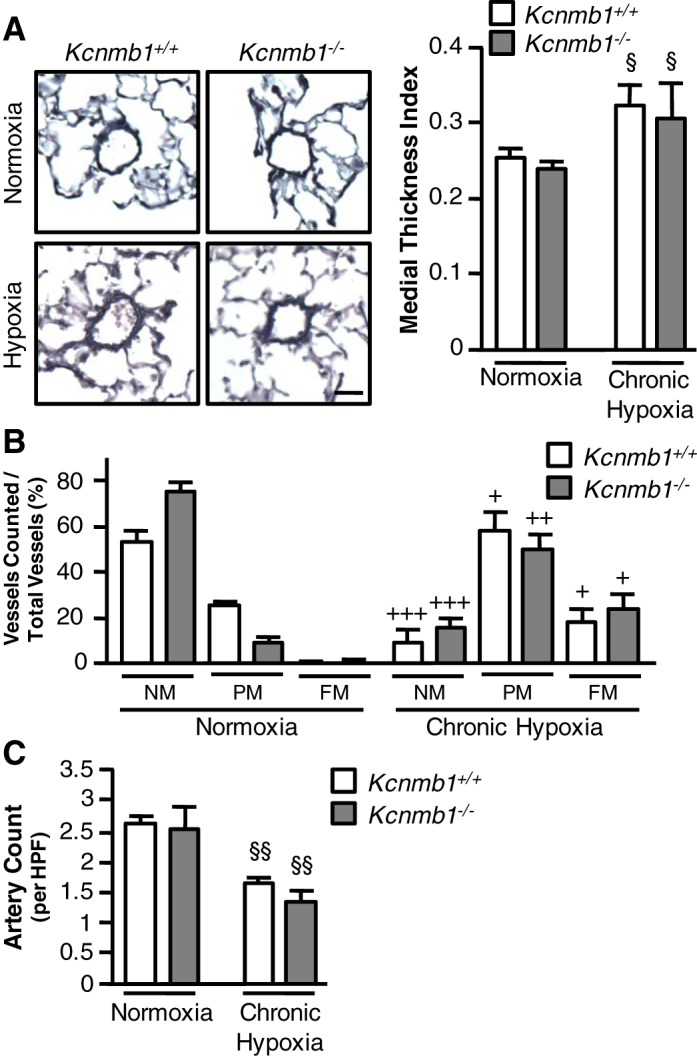
Morphometric analysis of pulmonary vasculature in Kcnmb1−/− mice. A: Movat pentachrome-stained pulmonary tissues from Kcnmb1+/+ and Kcnmb1−/− mice exposed to normoxia or chronic hypoxia to examine muscularization of peripheral pulmonary arteries (PAs). Magnification ×200; scale bar 25 μm. Quantification of peripheral PA muscularization expressed as the medial thickness index of PA ≤ 100 μm in external diameter (n = 5 per group with 10 fields assessed per mouse). Bars represent means ± SE, §P ≤ 0.05, normoxia vs. hypoxia. B: α-SMA and Von Willebrand Factor (VWF) stained peripheral vessels were counted and categorized into nonmuscularized (NM), partially muscularized (PM), or fully muscularized (FM). Bars represent means ± SE of the number of α-SMA-positive PA ≤ 150 μm in external diameter (n = 3–6 per group with 12 fields assessed per mouse). Within each group, significance was determined as follows: +++P ≤ 0.001, normoxia vs. hypoxia; Kcnmb1−/− PM, ++P ≤ 0.01 normoxia vs. hypoxia; Kcnmb1−/− FM, +P ≤ 0.05, normoxia vs. hypoxia. C: quantification of α-SMA-positive distal PA ≤ 150 μm in external diameter per high-powered field (HPF) in Kcnmb1+/+ and Kcnmb1−/− mice exposed to normoxia or chronic hypoxia. Bars represent means ± SE of the number of α-SMA positive distal PA (n = 5–6 per group with 12 fields assessed per mouse). §§P ≤ 0.01, normoxia vs. hypoxia.
Deletion of the BKCa channel β1 subunit increases PASMC focal adhesion complexes and Rho kinase expression.
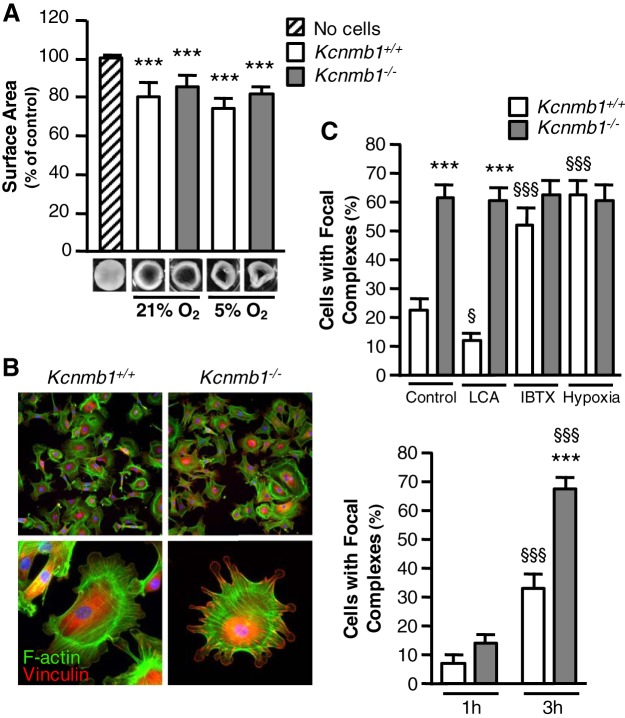
To confirm that isolated peripheral PASMCs from both Kcnmb1−/− and Kcnmb1+/+ mice can sense and directly respond to acute hypoxia, collagen gel contraction assays were performed. PASMCs from both genotypes decreased collagen gel surface area significantly under both normoxic and hypoxic conditions (Fig. 5A). Given that focal complexes (large dynamic protein complexes) can modulate the contractile state of vascular SMCs, we measured focal adhesion complex formation by staining for the common focal adhesion linker protein vinculin to further probe the pulmonary vascular response to hypoxia in Kcnmb1−/− compared with Kcnmb1+/+ mice. Focal complexes, as demonstrated by costaining of both F-actin and vinculin (44), were much more prevalent in PASMCs from Kcnmb1−/− compared with Kcnmb1+/+ mice within 3 h postseeding (Fig. 5B). We next quantified the number of focal complexes in isolated PASMCs after exposing the cells to BKCa agonists, antagonists, and hypoxia. Treatment with LCA, an inducer of β1-mediated BKCa channel activation (7), decreased the number of focal adhesion complexes in Kcnmb1+/+ PASMCs and, as expected in the absence of the β1 subunit, had no effect on Kcnmb1−/− PASMCs (Fig. 5C). Conversely, treatment with iberiotoxin, a specific BKCa channel antagonist (18), significantly increased focal complexes in Kcnmb1+/+ mice and caused no further increase in Kcnmb1−/− cells (Fig. 5C), arguing for inactivation of the BKCa channel as a determinant of focal adhesion formation. Hypoxia increased focal complexes in Kcnmb1+/+ cells to a similar degree as in Kcnmb1−/− cells.
Fig. 5.
Focal complex expression is greater in pulmonary artery smooth muscle cells (PASMCs) from Kcnmb1−/− compared with Kcnmb1+/+. A: isolated PASMC from both Kcnmb1+/+ and Kcnmb1−/− contract in collagen gel contraction assays. Bars represent means ± SE (n = 3). ***P ≤ 0.001, all samples vs. control (no cells). B: representative images of the expression of focal complexes in isolated peripheral PASMCs 3 h postseeding. Vinculin, red; F-actin, green; nuclei, blue; magnification ×100. Enlarged images, magnification ×200. Quantification of the percentage of cells expressing focal complexes. Bars represent means ± SE (n = 3). ***P ≤ 0.001, 3 h Kcnmb1−/− vs. 3 h Kcnmb1+/+; §§§P ≤ 0.001, 3 h vs. 1 h. C: quantification of the percentage of cells expressing focal complexes in isolated PASMCs left untreated (control), or treated with lithocholic acid (LCA; 45 μM for 30 min), iberiotoxin (IBTX; 55 nM for 1 h), or 5% O2 (hypoxia for 1 h) 3 h postseeding. Bars represent means ± SE (n = 3). ***P ≤ 0.001, Kcnmb1−/− vs. Kcnmb1+/+; §P ≤ 0.05, §§§P ≤ 0.001, treated vs. control.
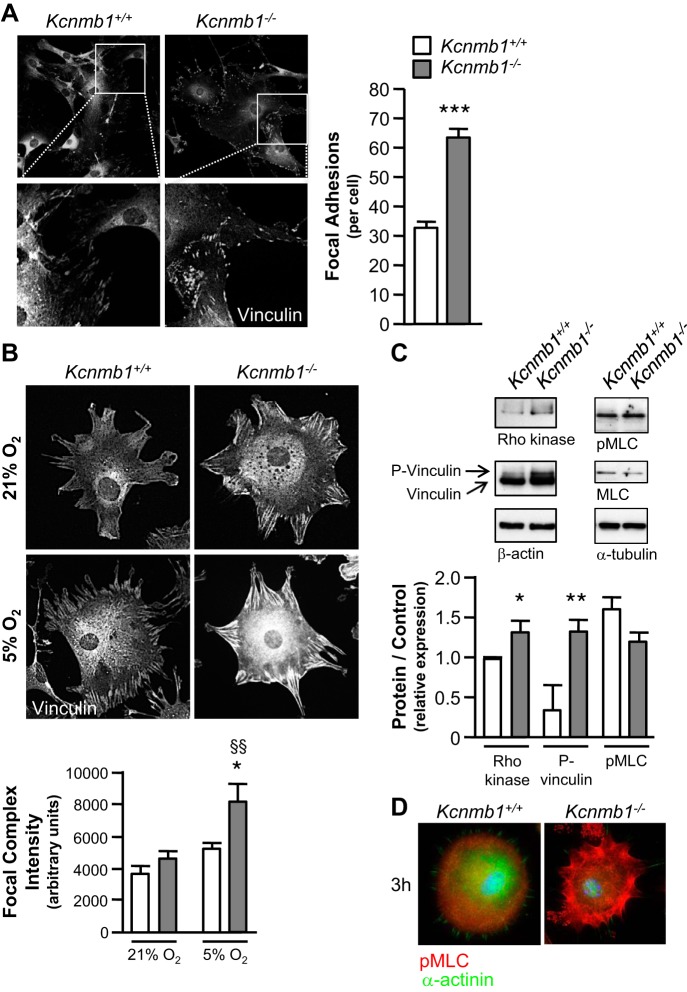
As demonstrated in Fig. 6A, focal adhesions were present in PASMCs from both Kcnmb1+/+ and Kcnmb1−/− mice 24 h postseeding. However, for each cell demonstrating focal adhesions, there were substantially more in Kcnmb1−/− compared with Kcnmb1+/+ PASMCs. With exposure to hypoxia, focal complexes increased to a greater degree in Kcnmb1−/− compared with Kcnmb1+/+ PASMCs 3 h postseeding (Fig. 6B). Given that vinculin controls focal adhesion formation (21) and Rho kinase activity can potentiate focal adhesion formation (3), we performed immunoblotting (Fig. 6C), which revealed more phospho-vinculin and Rho kinase expression in Kcnmb1−/− compared with Kcnmb1+/+ PASMCs.
Fig. 6.
Hypoxia induces greater focal complex expression in Kcnmb1−/− compared with Kcnmb1+/+ pulmonary artery smooth muscle cells (PASMCs). A: expression of focal adhesions in isolated peripheral PASMCs from Kcnmb1+/+ and Kcnmb1−/− mice. Quantification of the number of focal adhesions per cell 24 h postseeding as visualized by vinculin expression, magnification ×200. Bars represent means ± SE (n = 3). ***P ≤ 0.001, Kcnmb1−/− vs. Kcnmb1+/+. B: focal complex expression in peripheral PASMCs. Hypoxia (5% O2) increases focal complexes to a greater degree in Kcnmb1−/− compared with Kcnmb1+/+ PASMCs. Quantification of focal complex intensity per cell (arbitrary units) 3 h postseeding as visualized by vinculin expression, magnification ×200. Bars represent means ± SE (n = 3). *P ≤ 0.05, 5% O2 Kcnmb1−/− vs. 5% O2 Kcnmb1+/+; §§P ≤ 0.01, 5% O2 Kcnmb1−/− vs. 21% O2 Kcnmb1−/−. C: Western immunoblots of Rho kinase, vinculin, phospho-vinculin (P-vinculin), β-actin, phospho- myosin light chain (pMLC), MLC, and α-tubulin expression in isolated PASMC. Rho kinase and phospho-vinculin expression are increased in PASMC from Kcnmb1−/− compared with Kcnmb1+/+ 3 h postseeding. Bars represent means ± SE (n = 3). *P ≤ 0.06, **P ≤ 0.01, Kcnmb1−/− vs. Kcnmb1+/+. D: representative images of the expression of pMLC in isolated peripheral PASMC 3 h postseeding. pMLC, red; α-actinin, green; nuclei, blue; magnification ×400.
To further investigate the mechanism underlying the increased tone in Kcnmb1−/− compared with Kcnmb1+/+ mice, we measured MLC and pMLC expression (Fig. 6C). The ratio of pMLC to MLC did not differ between Kcnmb1+/+ and Kcnmb1−/− PASMCs. Upon further examination, pMLC localized in the cytoplasm for Kcnmb1+/+ cells while pMLC in the Kcnmb1−/− cells localized at the cell periphery within 3 h postseeding, where actin bundles are formed (Fig. 6D). At 24 h postseeding, both cell types maintained similar distribution patterns of pMLC with pMLC associating with actin fibers throughout the cell (data not shown).
DISCUSSION
The present series of experiments provides evidence that the β1 subunit of the BKCa channel modulates the response of the pulmonary vasculature to both acute and chronic hypoxia. In keeping with an oxygen sensing role, the β1 subunit of the BKCa channel expression is relatively enriched in PASMCs derived from the peripheral vasculature (13, 25). In mice without the β1 subunit of the BKCa channel, pulmonary artery pressure is unaffected in ambient oxygen, whereas the pulmonary vascular response to both acute and chronic hypoxia is accentuated, even in the absence of an increase in vascular remodeling. Consistent with the whole animal response to hypoxia, isolated PASMCs from Kcnmb1−/− mice sense and respond directly to hypoxia. These results support the notion that the β1 subunit of the BKCa channel plays a previously undescribed role in limiting the pulmonary vascular response to both acute and chronic hypoxia in both female and male mice. Moreover, in the absence of the β1 subunit of the BKCa channel, PASMCs manifest more focal adhesions which may underlie the accentuated response to hypoxia. Taken together, the observations that β1 subunit expression is limited to the peripheral pulmonary vascular SMCs, and that hypoxia increases RVSP and focal adhesion expression more in Kcnmb1−/− compared with Kcnmb1+/+ mice in the absence of accentuated muscularization, support the overall proposition that the β1 subunit modulates the pulmonary vascular response to hypoxia.
These results underscore the importance of the β1 subunit of the BKCa channel in fine tuning the reponse of the pulmonary vasculature to hypoxia. The importance of the β1 subunit of the BKCa channel in coupling Ca2+ release from an intracellular store with activation of the channel, membrane hyperpolarization, and a decrease in vascular tone has been demonstrated in the systemic (6, 29), cerebral (24), and coronary (28) circulations. In the lungs, beyond the fetal (12) and perinatal stages (42), the physiological significance of the β1 subunit of the BKCa channel has been less well proven, though a gain-of-function polymorphism mitigates asthma severity (39) and it is expressed in PASMCs (47). These findings are consistent with a role for the β1 subunit of the BKCa channel in modulating the pulmonary vascular response to hypoxia by coupling Ca2+ sparks to channel activation and the regulation of PASMC tone (26).
Further evidence of fhe functional significance of the the β1 subunit of the BKCa channel in PASMCs includes the observations that in Kcnmb1−/− mice, focal complexes and Rho kinase expression were increased relative to PASMCs from Kcnmb1+/+ mice. Focal adhesion complexes represent macromolecular assemblies that permit transmission of mechanical forces to adjacent cells and the underlying extracellular matrix promoting both contraction and migration (11). In the absence of the β1 subunit of the BKCa channel, PASMCs were more contracted in appearance with dramatically more focal complexes. Consistent with this construct, compared with Kcnmb1+/+ PASMCs, in response to hypoxia Kcnmb1−/− cells manifested more focal adhesions per cell and relatively more phospho-vinculin and Rho kinase expression, molecules that play a role in focal adhesion formation. Moreover, the observations that LCA, a β1 subunit agonist (7), had no effect on Kcnmb1−/− and decreased focal-adhesion complex expression in Kcnmb1+/+ PASMCs support a specific role for the β1 subunit in focal complex formation. The observation that antagonism of BKCa channel increased focal complex expression in both cell populations but to a greater degree in Kcnmb1+/+ PASMCs offers still more support for a role for the BKCa channel in focal complex formation. In keeping with the overall physiological phenotype, the increase in focal complexes in response to hypoxia was significantly greater in Kcnmb1−/− PASMCs.
The overall notion that the the β1 subunit of the BKCa channel modulates the response of the pulmonary circulation to changes in O2 tension is consistent with prior observations. From a developmental perspective, in PASMC quantal release of Ca2+ from ryanodine-sensitive stores, or calcium sparks, to activate the BKCa channel is confined to the neonatal stage (30). In neonatal, but not adult, pulmonary circulation, SMC resting membrane potential is determined by the BKCa channel (32). Given that biologically imperative neonatal pulmonary vasodilation results from O2, nitric oxide (1), ventilation (9), and shear stress (15), physiological stimuli that act through BKCa channel activation (12, 41, 42), increasing BKCa channel sensitivity via augmented β1 subunit expression favors dilation and mitigates constriction. From the perspective of molecular regulation, hypoxia increases β1 subunit expression by direct interaction between hypoxia-inducible factor-1α (HIF-1α) and hypoxia response elements in the promoter region of the Kcnmb1 gene (2). In the normally low O2 tension intrauterine environment, PASMC β1 subunit expression is relatively enriched in the fetus and neonate likely as a result of high levels of HIF-1α expression, perhaps owing to differential prolyl hydroxylase activity (34).
From the standpoint of pulmonary vascular tone beyond the neonatal period, the present data demonstrate that the β1 subunit plays a physiologically significant role in mitigating vasoconstriction. Chronic and acute hypoxic pulmonary vasoconstriction are accentuated in Kcnmb1−/− mice, even while vascular remodeling is unaffected. The cellular underpinnings of the heightened vasoconstriction may include a more robust hypoxia-induced increase in PASMC cytosolic calcium ([Ca2+]i) in the absence of the β1 subunit (2). The increase in Rho kinase activity in PASMCs without the β1 subunit may further (33) increase the sensitivity of MLC kinase to Ca2+ and increase SMC tone for any given amount of pMLC (38). Moreover, the strikingly apparent increase in focal complexes in Kcnmb1−/− PASMCs will also promote constriction, and over time, even cell migration (11). The observation that the β1 subunit is well expressed in SMCs derived from ≤150-µm vessels is consistent with this notion as hypoxia causes contraction and an increase in cytosolic Ca2+ in SMCs derived from smaller vessels (≤150 µm) (36). The physiological importance of the β1 subunit of the BKCa channel might be greater still in the context of chronic hypoxia, either pathological, such as in pulmonary parenchymal disease, or at high altitude.
From a developmental standpoint, the present findings may possess meaningful implications. At birth, the resistance of the pulmonary circulation declines precipitously with components of the O2- (12), ventilation- (42), and shear stress-induced (41) pulmonary vasodilation mediated by activation of the BKCa channel. Activation of the channel via release of intracellular Ca2+ from ryanodine-sensitive stores is developmentally regulated (30, 31). From a teleological perspective, greater β1 subunit expression, promoted by the relatively low O2 tension fetal environment, increases the Ca2+ sensitivity of the BKCa channel and the likelihood of channel activation, membrane hyperpolarization, and vasodilation. Whether the PASMC β1 subunit expression changes in association with vessel size with maturation remains unknown.
Though the data included in this report are derived from a murine model of hypoxia-induced pulmonary hypertension, the implications may be more generalizable. Given that the β1 subunit of the BKCa channel is under the transcriptional control of HIF-1α, the response of PASMCs to hypoxia entails an increase in β1 subunit expression (2, 33), which ought to decrease tone. In PASMCs from humans with pulmonary hypertension, HIF-1α expression, the predominant HIF isoform in PASMCs, is decreased, and pMLC and contractility are increased (5). Thus, if the normal pulmonary vascular response to hypoxia is augmented, a decrease in HIF-1α expression in PASMCs may be playing an etiologic role in multiple separate, yet interactive ways. The findings in human PASMCs are consistent with results in a murine model wherein HIF-1α expression was selectively deleted in SM22α-expressing cells, which caused an increase in pulmonary vascular tone and an augmented response to hypoxia (5), but these results diverge from Ball et al. (4) wherein selective deletion of HIF-1α using an inducible myosin heavy chain promoter resulted in decreased pulmonary vascular tone in reponse to chronic hypoxia. Taken together, these findings point to a role for the β1 subunit of the BKCa channel in the pulmonary vascular response to hypoxia.
In conclusion, the findings in this report point to a role for the β1 subunit of the BKCa channel in modulating the response of PASMCs to hypoxia. Evidence in support of this notion includes the accentuated physiological response to acute and chronic hypoxia in the absence of augmented pulmonary vascular remodeling. Moreover, the observations that β1 subunit expression is relatively enriched in the peripheral pulmonary vasculature and that Rho kinase activity and focal adhesion complex expression are increased in PASMCs without the β1 subunit offer further support for a role in vasoregulation. Moreover, the present results outline a potential mechanistic link between HIF-1α activity, BKCa channel β1 subunit expression, and tone in the microcirculation. The expression pattern of the β1 subunit of the BKCa channel raises the potential that it can be exploited as a novel and previously unaddressed therapeutic target in the pulmonary vasculature.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-0706280 (to D. N. Cornfield) and HL-5R01-HL-122918-02 (to C. M. Alvira), and funding from the Burroughs Wellcome Fund (to D. N. Cornfield).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.B., C.M.A., and D.N.C. conceived and designed research; E.A.B., L.L., and S.L.B. performed experiments; E.A.B., L.L., and S.L.B. analyzed data; E.A.B., C.M.A., and D.N.C. interpreted results of experiments; E.A.B. and L.L. prepared figures; E.A.B. drafted manuscript; C.M.A. and D.N.C. edited and revised manuscript; E.A.B., R.B., C.M.A., and D.N.C. approved final version of manuscript.
REFERENCES
- 1.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 259: H1921–H1927, 1990. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 2.Ahn YT, Kim YM, Adams E, Lyu SC, Alvira CM, Cornfield DN. Hypoxia-inducible factor-1α regulates KCNMB1 expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 302: L352–L359, 2012. doi: 10.1152/ajplung.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275: 1308–1311, 1997. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 4.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med 189: 314–324, 2014. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes EA, Chen CH, Sedan O, Cornfield DN. Loss of smooth muscle cell hypoxia inducible factor-1α underlies increased vascular contractility in pulmonary hypertension. FASEB J 31: 650–662, 2017. doi: 10.1096/fj.201600557R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 7.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol 72: 359–369, 2007. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- 8.Burgess A, Vigneron S, Brioudes E, Labbé JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA 107: 12564–12569, 2010. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassin S, Dawes GS, Mott JC, Ross BB, Strang LB. The vascular resistance of the fetal and newly ventilated lung of the lamb. J Physiol 171: 61–79, 1964. doi: 10.1113/jphysiol.1964.sp007361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang T, Wu L, Wang R. Altered expression of BK channel beta1 subunit in vascular tissues from spontaneously hypertensive rats. Am J Hypertens 19: 678–685, 2006. doi: 10.1016/j.amjhyper.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun 307: 355–361, 2003. doi: 10.1016/S0006-291X(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 12.Cornfield DN, Reeve HL, Tolarova S, Weir EK, Archer S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc Natl Acad Sci USA 93: 8089–8094, 1996. doi: 10.1073/pnas.93.15.8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM. Acute hypoxia increases cytosolic calcium in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 265: L53–L56, 1993. doi: 10.1152/ajplung.1993.265.1.L53. [DOI] [PubMed] [Google Scholar]
- 14.Cox DH, Aldrich RW. Role of the β1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol 116: 411–432, 2000. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawes GS, Mott JC, Widdicombe JG, Wyatt DG. Changes in the lungs of the new-born lamb. J Physiol 121: 141–162, 1953. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essin K, Gollasch M. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotechnol 2009: 135249, 2009. doi: 10.1155/2009/135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Fernández JM, Tomás M, Vázquez E, Orio P, Latorre R, Sentí M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest 113: 1032–1039, 2004. doi: 10.1172/JCI200420347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia ML, Hanner M, Knaus HG, Koch R, Schmalhofer W, Slaughter RS, Kaczorowski GJ. Pharmacology of potassium channels. Adv Pharmacol 39: 425–471, 1997. doi: 10.1016/S1054-3589(08)60078-2. [DOI] [PubMed] [Google Scholar]
- 19.Ghatnekar A, Chrobak I, Reese C, Stawski L, Seta F, Wirrig E, Paez-Cortez J, Markiewicz M, Asano Y, Harley R, Silver R, Feghali-Bostwick C, Trojanowska M. Endothelial GATA-6 deficiency promotes pulmonary arterial hypertension. Am J Pathol 182: 2391–2406, 2013. doi: 10.1016/j.ajpath.2013.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gollasch M, Tank J, Luft FC, Jordan J, Maass P, Krasko C, Sharma AM, Busjahn A, Bähring S. The BK channel beta1 subunit gene is associated with human baroreflex and blood pressure regulation. J Hypertens 20: 927–933, 2002. doi: 10.1097/00004872-200205000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 179: 1043–1057, 2007. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim FY, Barnes EA, Ying L, Chen C, Lee L, Alvira CM, Cornfield DN. Pulmonary artery smooth muscle cell endothelin-1 expression modulates the pulmonary vascular response to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 308: L368–L377, 2015. doi: 10.1152/ajplung.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1α in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res 112: 1230–1233, 2013. doi: 10.1161/CIRCRESAHA.112.300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Löhn M, Lauterbach B, Haller H, Pongs O, Luft FC, Gollasch M. beta(1)-Subunit of BK channels regulates arterial wall [Ca2+] and diameter in mouse cerebral arteries. J Appl Physiol (1985) 91: 1350–1354, 2001. doi: 10.1152/jappl.2001.91.3.1350. [DOI] [PubMed] [Google Scholar]
- 25.Madden JA, Dawson CA, Harder DR. Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol (1985) 59: 113–118, 1985. doi: 10.1152/jappl.1985.59.1.113. [DOI] [PubMed] [Google Scholar]
- 26.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 27.Nisancioglu MH, Betsholtz C, Genové G. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res 70: 5109–5115, 2010. doi: 10.1158/0008-5472.CAN-09-4245. [DOI] [PubMed] [Google Scholar]
- 28.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel β1 subunit decrease with coronary artery ageing in the rat. J Physiol 559: 849–862, 2004. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000. doi: 10.1161/01.RES.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 30.Porter VA, Reeve HL, Cornfield DN. Fetal rabbit pulmonary artery smooth muscle cell response to ryanodine is developmentally regulated. Am J Physiol Lung Cell Mol Physiol 279: L751–L757, 2000. doi: 10.1152/ajplung.2000.279.4.L751. [DOI] [PubMed] [Google Scholar]
- 31.Porter VA, Rhodes MT, Reeve HL, Cornfield DN. Oxygen-induced perinatal pulmonary vasodilation is mediated by ryanodine-sensitive activation of a calcium-sensitive K+ channel. Am J Physiol Lung Cell Mol Physiol 281: L1379–L1385, 2001. doi: 10.1152/ajplung.2001.281.6.L1379. [DOI] [PubMed] [Google Scholar]
- 32.Reeve HL, Archer SL, Weir EK, Cornfield DN. Maturational changes in K+ channel activity and oxygen sensing in the ovine pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 275: L2019–L1025, 1998. [Google Scholar]
- 33.Resnik E, Herron J, Fu R, Ivy DD, Cornfield DN. Oxygen tension modulates the expression of pulmonary vascular BKCa channel α- and β-subunits. Am J Physiol Lung Cell Mol Physiol 290: L761–L768, 2006. doi: 10.1152/ajplung.00283.2005. [DOI] [PubMed] [Google Scholar]
- 34.Resnik ER, Herron JM, Lyu SC, Cornfield DN. Developmental regulation of hypoxia-inducible factor 1 and prolyl-hydroxylases in pulmonary vascular smooth muscle cells. Proc Natl Acad Sci USA 104: 18789–18794, 2007. doi: 10.1073/pnas.0706019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 57: 811–821, 1985. doi: 10.1161/01.RES.57.6.811. [DOI] [PubMed] [Google Scholar]
- 36.Salvaterra CG, Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L323–L328, 1993. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- 37.Saqueton CB, Miller RM, Porter VA, Millla CM, Cornfield DN. NO causes perinatal pulmonary vasodilation through K+ channel activation and requires intracellular calcium release. Am J Physiol Lung Cell Mol Physiol 276: L925–L932, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 275: 21722–21729, 2000. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 39.Seibold MA, Wang B, Eng C, Kumar G, Beckman KB, Sen S, Choudhry S, Meade K, Lenoir M, Watson HG, Thyne S, Williams LK, Kumar R, Weiss KB, Grammer LC, Avila PC, Schleimer RP, Burchard EG, Brenner R. An african-specific functional polymorphism in KCNMB1 shows sex-specific association with asthma severity. Hum Mol Genet 17: 2681–2690, 2008. doi: 10.1093/hmg/ddn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steffen A, Ladwein M, Dimchev GA, Hein A, Schwenkmezger L, Arens S, Ladwein KI, Margit Holleboom J, Schur F, Victor Small J, Schwarz J, Gerhard R, Faix J, Stradal TE, Brakebusch C, Rottner K. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci 126: 4572–4588, 2013. doi: 10.1242/jcs.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storme L, Rairigh RL, Parker TA, Cornfield DN, Kinsella JP, Abman SH. K+-channel blockade inhibits shear stress-induced pulmonary vasodilation in the ovine fetus. Am J Physiol Heart Circ Physiol 276: L220–L228, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Tristani-Firouzi M, Martin EB, Tolarova S, Weir EK, Archer SL, Cornfield DN. Ventilation-induced pulmonary vasodilation at birth is modulated by potassium channel activity. Am J Physiol Heart Circ Physiol 271: H2353–H2359, 1996. doi: 10.1152/ajpheart.1996.271.6.H2353. [DOI] [PubMed] [Google Scholar]
- 43.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 285: 1929–1931, 1999. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 44.Yamin R, Morgan KG. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J Physiol 590: 4145–4154, 2012. doi: 10.1113/jphysiol.2012.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ying L, Becard M, Lyell D, Han X, Shortliffe L, Husted CI, Alvira CM, Cornfield DN. The transient receptor potential vanilloid 4 channel modulates uterine tone during pregnancy. Sci Transl Med 7: 319ra204, 2015. doi: 10.1126/scitranslmed.aad0376. [DOI] [PubMed] [Google Scholar]
- 46.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103: 691–696, 1999. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng YM, Park SW, Stokes L, Tang Q, Xiao JH, Wang YX. Distinct activity of BK channel β1-subunit in cerebral and pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 304: C780–C789, 2013. doi: 10.1152/ajpcell.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]