Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrosing interstitial pneumonia of unknown cause with a median survival of only three years. Little is known about the mechanisms that precede the excessive collagen deposition seen in IPF, but cellular senescence has been strongly implicated in disease pathology. Senescence is a state of irreversible cell-cycle arrest accompanied by an abnormal secretory profile and is thought to play a critical role in both development and wound repair. Normally, once a senescent cell has contributed to wound repair, it is promptly removed from the environment via infiltrating immune cells. However, if immune clearance fails, the persistence of senescent cells is thought to drive disease pathology through their altered secretory profile. One of the major cell types involved in wound healing is fibroblasts, and senescent fibroblasts have been identified in the lungs of patients with IPF and in fibroblast cultures from IPF lungs. The question of what is driving abnormally high numbers of fibroblasts into senescence remains unanswered. The transcription factor signal transducer and activator of transcription 3 (STAT3) plays a role in a myriad of processes, including cell-cycle progression, gene transcription, as well as mitochondrial respiration, all of which are dysregulated during senescence. Activation of STAT3 has previously been shown to correlate with IPF progression and therefore is a potential molecular target to modify early-stage senescence and restore normal fibroblast function. This review summarizes what is presently known about fibroblast senescence in IPF and how STAT3 may contribute to this phenotype.
Keywords: fibroblast senescence, idiopathic pulmonary fibrosis, signal transducer and activator of transcription 3
INTRODUCTION
The interstitial lung diseases (ILDs) are a broad group of clinically defined lung disorders that are characterized by remodeling of the parenchyma and alveolar space through the chronic accumulation of extracellular matrix (ECM). This accumulation permanently inhibits oxygen transfer, leading to the characteristic symptoms of shortness of breath and exercise limitation and the clinical signs of fine basal inspiratory crackles on auscultation. Many ILDs have identifiable causes, such as inhaled proteins leading to hypersensitivity pneumonitis and drug therapies, including cancer chemotherapy; however, the causes of the majority of ILDs remain unknown. The most common disease in this group is idiopathic pulmonary fibrosis (IPF), affecting at least five million people globally and carrying with it a poor prognosis, with a median survival of three years, which is shorter than many cancers (87). Because of a greater understanding of the disease, advances in detection techniques, and an aging population, the incidence of IPF is increasing globally (34, 45).
Common risk factors for IPF include male sex (23, 37, 46), older age, with two-thirds of patients over 60 years of age at diagnosis (49, 86), and, interestingly, a history of cigarette smoking (6). Occupational exposures and gastroesophageal reflux (GERD) have been associated with the disease; the risk of developing IPF after metal and wood dust exposure increases proportionally with the number of years of exposure (6), whereas GERD is associated but not yet causally linked to the disease (reviewed in Ref. 31). All of the recognized risk factors for IPF share the ability to disrupt the lung epithelium once inhaled, which suggests that chronic respirable insults are sufficient to initiate the pathological changes of IPF in susceptible individuals.
Offsetting the dismal prognosis for people with IPF, two drugs (nintedanib and pirfenidone) have been shown to slow disease progression, reduce the decline in forced vital capacity, and increase longevity. As a result, in 2014, both were awarded FDA “breakthrough” designation and fast-track registration (50, 89). Nintedanib is a tyrosine kinase inhibitor, targeting receptors for vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), all of which are involved in transmembrane signaling and signal transduction (87). Tyrosine kinases play critical roles in the pathogenesis of IPF, with patients with IPF having an altered expression of specific tyrosine kinases that stimulate the migration and proliferation of lung fibroblasts (35, 61). The precise mode of action of pirfenidone remains unclear, predominantly because of its potential to exert an effect on numerous aspects of fibrosis. One decisive factor in the action of pirfenidone is thought to be its capacity to posttranscriptionally inhibit inflammatory cytokines and to enhance production of the anti-inflammatory cytokine interleukin-10 (IL-10) (73). Conclusions from the original patent application suggested that pirfenidone inhibits PDGF- and FGF-stimulated fibroblast proliferation and transforming growth factor-β (TGF-β)-stimulated procollagen synthesis from stromal cells (63).
Although these drugs only slow the relentless progression of the disease, they have demonstrated a survival benefit (90) and hence unequivocally demonstrated that IPF is treatable. However, neither drug reverses established fibrosis, and the majority of patients continue to decline, with lung transplant remaining the only definitive treatment.
IPF PATHOLOGY
The pathogenesis of IPF is proposed to occur as a result of repeated inhaled stimuli causing sequential injury to the lung epithelium (97). These injurious events are thought to result in disruption of the alveolar-capillary basement membrane, followed by excessive ECM deposition and fibrosis in the lung parenchyma (for a review of ECM in lung disease, see Ref. 11). In an IPF lung, fibrosis is typically heterogeneous with areas of relatively normal lung lying adjacent to areas of dense fibrosis (87). Despite this spatial heterogeneity, the fibrosis is thought to emanate from the parenchyma of the lower lobes (87).
Although little is known about the mechanisms that ultimately result in the excessive collagen deposition in IPF, cellular senescence has recently been strongly implicated in disease pathology. Cellular senescence is a state of cell-cycle arrest accompanied by an abnormal secretory profile, and, although in nonpathological states senescence is an important cellular pathway fundamental to embryonic development (71, 101) and preventing malignancies (17, 114), senescent cells are not a permanent feature in healthy tissue. In wound healing, for example, senescent fibroblasts appear early on in the stages of wound resolution, where they accelerate wound closure by inducing the differentiation of cells through their altered secretory profile (28).
Present understanding of IPF suggests that the disease is an interaction of genetic susceptibility, aging-associated processes, and repeated microinjury. The summation of these processes may affect certain cell types more substantially than others, with present evidence pointing toward fibroblasts and epithelial cells being primarily affected. This review will focus on the role of fibroblast senescence in the pathology of IPF.
The influence of the role of signal transducer and activator of transcription 3 (STAT3) in the acquisition of the senescent phenotype will also be explored in light of the numerous cellular processes that STAT3 regulates. STAT3 is a latent transcription factor, the activation of which is largely dependent on the IL-6 family of cytokines. Activation of STAT3 initiates the transcription of many of the factors that characterize the altered secretory profile of senescent cells, influences cell-cycle progression, and also plays a central role in mitochondrial respiration.
SENESCENCE
The senescent phenotype was first recognized in 1961 by Hayflick and Moorhead (40), who described the finite proliferative potential of fibroblasts in vitro. Fibroblasts underwent a limited number of population doublings before entering a state of permanent growth arrest, the gradual exhaustion of the replicative potential of cells now recognized as replicative senescence (RS). Since their discovery, senescent cells have been identified in embryological organs that are evolutionarily conserved through all the major animal lineages, suggesting that the phenotype is ancient (71, 93, 101).
RS is considered in mitotic time as opposed to metabolic time; this is to say that it is not necessarily the chronological age of a cell that influences its path into senescence but rather the number of cell divisions. Senescent cells exhibit a blunted response to mitogenic stimuli (107), undergo growth arrest (16), and display increased activity of senescence associated-β-galactosidase (SA-β-Gal) (29).
In vitro, the telomeres of human somatic cells shorten by 30–200 bp with each mitotic event (39), and their specific role in conjunction with associated proteins is to prevent damage to the genome attributable to the “end replication problem.” The gradual degradation of telomeres, attributable to mitosis, ultimately results in a critical limit that is unable to effectively protect the DNA. Insufficient telomere length is thought to manifest in an increase in the incidence of double-strand breaks (DSBs) at the DNA ends. Activation of the p53 pathway is a common cellular response to DNA double-strand breaks, and, as a result, downstream targets of p53, including the cyclin-dependent kinase inhibitors p21waf1 and p16ink4D, serve as markers of senescence (84).
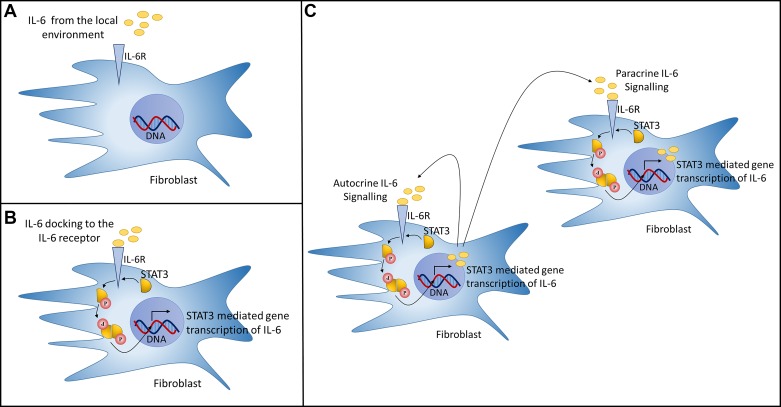
Although senescent cells do not undergo cell division, importantly, they remain viable and metabolically active (12, 32). It is this feature that is central to the proposed role of senescent cells in the pathogenesis of IPF, developing what has been described as the senescence-associated secretory phenotype (SASP) (21). The SASP plays important physiological roles and mediates several processes, such as cellular differentiation during wound repair. However, because of the characteristic secretory signature of the SASP, its persistence in the environment may contribute to the pathology of IPF through autocrine and paracrine interactions, such as cytokine-stimulated gene transcription (Fig. 1). The transcription factor NF-κB has increased activity with aging and aging-related chronic diseases (104) and has been shown to act as a regulator of the SASP, influencing the expression of more senescence-associated genes than p53 (18).
Fig. 1.
A scenario of cytokine-stimulated gene transcription mediated by the signal transducer and activator of transcription 3 (STAT3) activation pathway is shown. One of the core factors of the altered secretory profile of senescent fibroblasts is the cytokine IL-6, a major activator of the STAT3 pathway. A: IL-6 binding to the IL-6R on the fibroblast surface initiates an activation cascade. B: STAT3 recruitment to Janus kinase 1 (JAK) (not shown), a nonreceptor tyrosine kinase, results in STAT3 phosphorylation, dimerization, and translocation to the nucleus to mediate the transcription of target genes. C: persistent feedback loop potentially influencing autocrine and paracrine signaling has been identified where by STAT3-mediated transcription of sphingosine-1-phosphate receptor-1 (S1PR1) induces pSTAT3 through Jak2 activation (55). This positive feedback loop is thought to constituently activate STAT3, upregulating IL-6 transcription, ultimately resulting in its release to the local environment. A negative feedback mechanism for STAT3 does exist and is mediated by the suppressors of cytokine signaling (SOCS) proteins (118); however, loss of this negative feedback loop has been shown to be a feature of disease (4), and the SOCS/STAT3 silencing mechanism has also been reported to be diminished in idiopathic pulmonary fibrosis-derived fibroblasts (83).
CHARACTERISTICS OF SENESCENT FIBROBLASTS
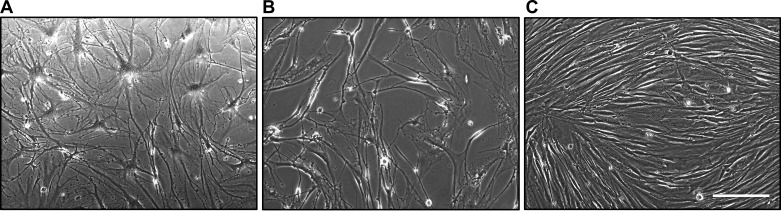
In vitro, late-passage fibroblasts adopt an enlarged flattened morphology thought to be indicative of RS (Fig. 2A). Fibroblasts derived from the lungs of patients with IPF display the same gross morphology even at early passage (Fig. 2B). In contrast, age-matched control fibroblasts of the same passage display a cigar-shaped morphology characteristic of the cell type (115) (Fig. 2C). As a result of the increased size of senescent fibroblasts, senescent cultures contain a lower cell density at confluence than younger cultures. The orientation of senescent fibroblasts in culture is characteristically random (Fig. 2, A and B) compared with the orderly parallel geometry to which early-passage nonpathological fibroblasts conform (Fig. 2C). Population-doubling times are also slower in IPF-derived fibroblasts (82, 88).
Fig. 2.
Morphology of late passage (P8) nonpathological fibroblasts (A), early passage (P4) idiopathic pulmonary fibrosis-derived fibroblasts (B), and early passage (P1) nonpathological fibroblasts (C) is shown. Scale bar = 200 µm.
Within the last few years, several publications have characterized IPF-derived fibroblasts and compared them to age-matched controls. All have concluded that IPF-derived fibroblasts exhibit multiple characteristics of senescence. Telomere length measurements have revealed that telomere attrition is a feature of fibroblasts derived from the lungs of patients with IPF (3). The expression of p21waf1 and p16ink4D has been shown to be increased in IPF, in both cultures of explanted fibroblasts (3, 96) and in fibroblasts of histological tissue sections (41, 96). The presence of the SASP has also been confirmed to be a core characteristic of IPF-derived fibroblasts (3, 88, 96). Gene transcripts of proinflammatory and profibrotic SASP factors, IL-6, IL-1β, basic FGF (FGFb), pro-α1-(I) collagen, and TGF-β have all been reported as being significantly increased in IPF lung fibroblasts (3, 88).
FIBROBLAST SENESCENCE: THE GOOD
The influence of senescent fibroblasts in wound repair is an important consideration in regard to IPF pathology because the disease process is thought to result from sequential injuries to the lung (epithelium). Successful wound resolution is crucial for maintaining tissue function and consists of several overlapping phases, the immediate response; the inflammatory response; the proliferation, migration, and contraction phase; and the resolution phase (comprehensively reviewed in Ref. 98). The whole process is orchestrated by resident cells that work in concert releasing cytokines and chemokines that set in motion a series of events that includes cellular migration and the synthesis and deposition of the ECM.
During normal wound healing, the proliferation, migration, and contraction phase marks the point when new granulation tissue is synthesized, facilitating the proliferation of fibroblasts. Within the granulation tissue, local fibroblasts differentiate into α-smooth muscle actin (α-SMA)-expressing fibroblasts, termed myofibroblasts (25, 91). The actin filaments play important roles in many cellular processes, including the generation of contractile force, enabling wound closure.
Studies have shown that senescent myofibroblasts accumulate as part of the normal process of wound healing, at least in animal models. Krizhanovsky et al. (51) examined the accumulation of senescent cells in a murine liver injury model. The senescent cells were identified as myofibroblasts that had initially proliferated in response to the injuring stimulus. Using knockout and transgenic mice, the authors determined that the senescent myofibroblasts present in the injured liver limited the accumulation of fibrotic tissue and facilitated the resolution of fibrosis.
The importance of senescent cells in wound healing has been notably elucidated by Jun and Lau (47), who described how CCN1, a secreted ECM-associated signaling protein, highly expressed at sites of wound repair can induce fibroblast senescence through its cell surface receptors, DNA damage response, and p53 activation, resulting in reactive oxygen species (ROS)-dependent activation of the p16INK4a/pRb pathway. The senescent fibroblasts were ultimately identified as myofibroblasts because of an abundance of α-SMA and were shown to accumulate in the granulation tissue of healing wounds, where they expressed antifibrotic genes. Thus, in this model, a worsening of fibrosis occurred in the absence of senescent cells.
Demaria et al. (28) confirmed that senescent fibroblasts were essential for optimal kinetics of granulation tissue formation. In this study, senescent fibroblasts appeared very early in the response to a cutaneous wound, where secreted factors such as PDGF-AA accelerated wound closure by inducing fibroblast-to-myofibroblast differentiation. Whether or not both senescent fibroblasts and senescent myofibroblasts are present at the site of injury is controversial; the distinction may simply be due to some authors identifying α-SMA expression, indicating a myofibroblast phenotype, whereas other groups do not extend their analysis to include identification of myofibroblasts.
Mellone et al. (64) showed that senescent fibroblasts obtained from head and neck and esophageal cancers are predominantly α-SMA positive. Interestingly, the study went on to demonstrate that fibroblasts induced into senescence developed molecular, ultrastructural, and contractile features typical of myofibroblasts. However, RNA sequencing of the two phenotypes revealed significant transcriptomic differences, particularly in genes associated with ECM deposition and organization, essentially suggesting that a senescent fibroblast is a nonfibrogenic, α-SMA-positive myofibroblast.
FIBROBLAST SENESCENCE: THE BAD
The senescent phenotype results in differential gene expression of bioactive mediators, such as inflammatory cytokines, growth factors (13), and ROS (80). The release of such factors by senescent fibroblasts has been shown capable of promoting the proliferation of preneoplastic and neoplastic epithelial cells (52, 78).
In another aging-associated pathology, venous leg ulcers (VLUs), the presence of senescent fibroblasts is thought to be detrimental to wound resolution. VLUs are chronic wounds that within a certain proportion of patients do not resolve successfully and instead persist for months and occasionally years (38). On the basis of clinical findings from VLU wound biopsies (65, 85, 100), it has been suggested that senescent fibroblasts inhibit wound resolution through the secretion of the proteolytic enzymes collagenase, elastase, and stromelysin (38). These ECM-degrading enzymes are particularly problematic for cutaneous wounds, which require sufficient ECM for the migration of cells, such as keratinocytes, to achieve wound resolution.
How the disruptive influence of senescent fibroblasts to cutaneous wound healing translates to IPF pathology is not immediately clear. The thickening of the ECM observed in IPF is likely a result of disturbances between ECM synthesis after injury and then ECM degradation after wound resolution. However, in VLUs, senescent fibroblasts prevent sufficient ECM deposition for wound healing to occur, whereas, in IPF, excessive ECM deposition occurs in the presence of senescent fibroblasts. It is tempting to suggest that senescent fibroblasts resident to areas of fibrosis are not the source of ECM deposition, either directly or through paracrine influence, and are instead attempting to mediate its resolution. Their lack of efficacy in clearing the ECM observed in an IPF lung may be due to the extent that it is deposited after each injuring event.
EPITHELIAL CELL SENESCENCE
Although epithelial cell senescence is outside the remit of this review, its potential to drive the pathogenesis of IPF should also be acknowledged. From histological tissue sections, epithelial senescence is also a well-documented feature of the disease (30, 41, 57, 66, 96), and, in a murine model, their targeted removal through the use of senolytics leads to an attenuation of profibrotic marker expression and increased epithelial cell function (57).
Similar to fibroblasts, senescent epithelial cells are also capable of signaling to other cells within their environment. Thus their profibrotic secretory profile (57, 66) could potentially drive the differentiation of resident lung fibroblasts to myofibroblasts (FMT), ultimately resulting in the excessive collagen deposition that characterizes the disease. Very recently, targeted removal of senescent epithelial cells with senolytic compounds was shown to attenuate SASP mediators and ECM markers, while increasing expression of alveolar epithelial markers ex vivo (57). This study provides the first tantalizing evidence to suggest that senescent epithelial cells contribute to fibrosis. The consequences of epithelial cell senescence might be twofold: 1) their presence may hinder reepithelialization attributable to their inability to divide, and 2) their profibrotic secretory profile (57, 66) may drive the differentiation of resident fibroblasts to a synthetic myofibroblast, thereby promoting excessive collagen deposition. However, like fibroblasts, although senescent epithelial cells are a prominent feature in IPF lungs, their influence and role in IPF pathology remain unclear.
Evidence for how cells become senescent in vivo is scant; however, several possibilities exist broadly centered on exposure to stress, termed stress-induced premature senescence (SIPS).
SIPS
Aside from telomere attrition resulting from mitosis, senescence can also be initiated through cellular insult. In vitro SIPS can be induced by a variety of treatment conditions that culminate in DNA damage, including chemical exposure, such as to hydrogen peroxide, irradiation, and ultraviolet light (107). Cells undergoing SIPS adopt a senescent phenotype earlier in mitotic time than RS but still share hallmark features of RS, including cell-cycle arrest and an altered secretory profile.
In vivo the definitive contribution of SIPS to disease pathogenesis is yet to be confirmed. However, several potential hypotheses to its origin have been proposed, including circulating immune cells and mitochondrial abnormalities within the affected cell. Immune-cell-induced senescence was first demonstrated by Braumuller et al. (9), who identified that T-helper-1 cells directly induced permanent growth arrest in cancerous pancreatic β-cells through the release of interferon-γ and tumor necrosis factor-α.
Several major pathways leading to SIPS likely emanate from the mitochondria. Mitochondrial dysfunction has been characterized by increases in mitochondrial mass (56), mitochondrial DNA mutations (112), and increases in ROS (27). Molecular and chemical downregulation of the mitochondrial Rieske iron sulfur protein (RISP), an enzyme located on the inner membrane, has been shown to induce senescence (68), as has the independent inhibition of complexes I, II, and III of the electron transport chain (ETC) (68, 112, 117). Misfiring ETC complexes are thought to result in increased ROS production, ultimately resulting in DSBs to the of the cell through chronic oxidative stress. An imbalance in cellular bioenergetics has also shown to influence the onset of senescence. Decreased ATP and increased AMP-activated protein kinase (AMPK) induce senescence by activating multiple signaling pathways (109).
SENESCENT CELL CLEARANCE
One of the defining features of senescent cells is their abnormal secretory profile, the SASP. The release of a specific array of chemokines and cytokines provides a unique signature, allowing senescent cells to signal to other cells within the local environment. This signaling, among other roles, facilitates their elimination by immune cells, which is arguably the most important function of the SASP, as the persistence of senescent cells is thought to drive age-related pathologies (103, 108) and disease (48, 114).
Senescent cells are cleared by both innate and adaptive immune responses; indeed both monocytes and macrophages are capable of clearing senescent cells (48), as are neutrophils and natural killer (NK) cells (114). Sagiv et al. (95) identified consistently upregulated NK cell ligands on the surface of senescent fibroblasts that promoted NK-mediated cytotoxicity. In addition to soluble factors and cell surface receptors, senescent fibroblasts have also been shown to engage in cell-cell contact, transferring proteins to NK and T cells via cytoplasmic bridges (7). Exocytosis of secretory granules is another method used by senescent cells to signal their NK cell-mediated destruction (94).
The frequency of senescent fibroblasts in the lungs of patients with IPF suggests that the senescent phenotype is inferring a resistance to normal senescent cell clearance. The mechanisms of evasion are presently unknown although possibilities include 1) immunosenescence, defined as changes to the immune system associated with age, and 2) a particular subset of senescent fibroblasts avoiding immune surveillance.
STAT3 SIGNALING PATHWAY IN IPF
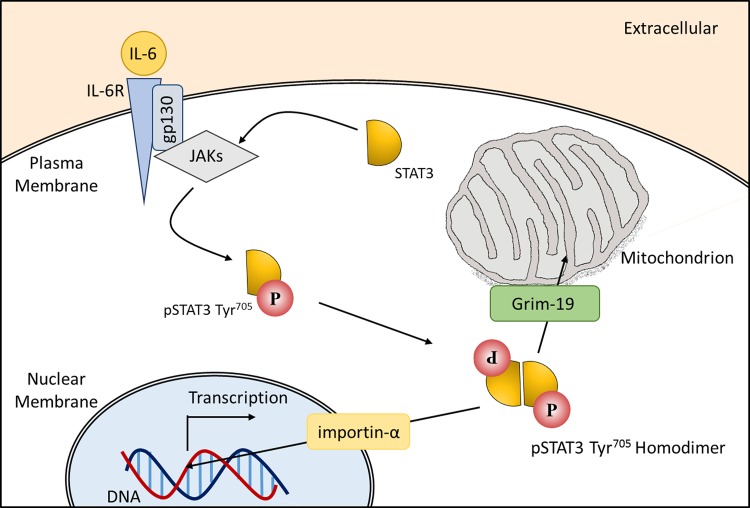
STAT3 is a member of a family of cytokine-responsive transcription factors and has been identified as being hyperphosphorylated in IPF-derived lung fibroblasts compared with age-matched controls (70, 82). Constitutively activated STAT3 has also been recognized as contributing to the pathology of several cancers (26, 120, 121) and inflammatory diseases (8, 36). As a latent transcription factor, STAT3 is found in the cytoplasm of nonstimulated cells. Following activation, STAT3 translocates to either the nucleus via an importin-α/importin-β1/Ran-mediated mechanism (19) to perform a gene regulatory role or to the mitochondria via GRIM-19 (102), where it associates with complexes of the ETC to facilitate optimal respiration (33, 110).
STAT3 activation is mediated by a number of extracellular stimuli that includes cytokines and growth factors (1). Major activators of STAT3 are the IL-6 family of cytokines that bind to the IL-6 receptor-α subunit (IL-6Rα) and glycoprotein 130 (gp130) (Fig. 3). IL-6/IL-6Rα binding results in phosphorylation and homodimerization of the signal-transducing receptor gp130. Janus kinase 1 (JAK1) or JAK2 activation follows from gp130 activation. Two JAK molecules of the same class are brought into close proximity, allowing the phosphate groups from gp130 to be transferred across to the newly localized JAKs. Cytoplasmic, nonphosphorylated, STAT3 contains an SH2 domain that facilitates its docking to the phosphorylated tyrosine residues on the JAKs. Once STAT3 is bound to a JAK, it becomes activated via phosphorylation at the tyrosine705 residue (pSTAT3705) near the COOH terminus. Serine phosphorylation has also been described at residue-727 (pSTAT3727); however, the serine kinase/kinases involved in activation are not well understood. Once phosphorylated, STAT3 homodimers form and translocate to the nucleus or to the mitochondria. The major negative regulators of STAT3 signaling are the SOCS proteins, in particular SOCS3, which prevent the phosphorylation of STAT3 through direct and indirect interactions with tyrosine kinase SH2 domains (2). A significant reduction in basal SOCS1 mRNA in IPF fibroblasts has previously been reported, as well as a trend toward reduced SOCS3 mRNA (83).
Fig. 3.
The Janus kinase 1/signal transducer and activator of transcription 3 (JAK/STAT3) signaling cascade initiated by IL-6 stimulation is shown. Upon IL-6 binding, the signal transduction pathway is initiated by glycoprotein 130 (gp130) followed by JAK activation (not shown); phosphorylated JAKs in turn mediate the recruitment and phosphorylation of STAT3. Phosphorylated STAT3 (pSTAT3) is then released from the receptor, and pSTAT3 homodimers form and translocate to the nucleus via an importin-α/importin-β1/Ran-mediated mechanism or to the mitochondria, where GRIM-19, a component of complex I in the electron transport chain, facilitates STAT3 localization to the inner membrane.
STAT3 TRANSLOCATION TO THE NUCLEUS
In the nucleus STAT3 dimers mediate a transcriptional response of target genes, including IL-6 and EGFR, the antiapoptotic proteins B cell lymphoma-extra large (Bcl-xL), and Survivin (aka BIRC5), cyclin D1 (CCND1), and other transcription factors such as c-Myc and Twist-related protein 1 (TWIST1) (62, 111). It would appear that phosphorylation is not essential for nuclear translocation, as STAT3 is able to shuttle back and forth from the cytoplasm to the nucleus constitutively via a region located within the coiled-coil domain that is recognized by specific import carrier importin-α3 (60). Phosphorylation is also not absolutely essential for STAT3 to perform a gene-regulatory role; Yang et al. (116) demonstrated that nonphosphorylated STAT3 increased the expression of several genes not upregulated by pSTAT3. Nonphosphorylated STAT3 has been associated with an increased resistance to apoptosis through binding to regulatory regions of proapoptotic genes preventing their expression (105).
STAT3 TRANSLOCATION TO THE MITOCHONDRIA
Although most attention has been centered on its nuclear role, STAT3 also translocates to the mitochondria, where it influences cellular metabolism through facilitating the optimal functioning of the ETC (33, 110). Nonphosphorylated STAT3 and both pSTAT3-Tyr705 and pSTAT3-Ser727 have been identified in mitochondria (42, 110). Interestingly, only pSTAT3-Tyr705 was increased in the mitochondria during recovery from cardiac infarction, suggesting that tyrosine phosphorylation is key to influencing cellular processes in the mitochondria (42).
The mechanism by which STAT3 is transported to the mitochondria is yet to be confirmed, but an association with a component of the ETC, GRIM-19, has been proposed (102). Grim-19 is essential for the successful electron transfer activity of complex I, indicating that STAT3 is also likely to play an integral part in complex I function (44). Supporting this hypothesis, complex I and complex II efficiency is significantly decreased in STAT3-null cells (110). Phosphorylation at either TYR705 or Ser727 is sufficient for the optimal activities of complex I and II (110). Further support for the association of STAT3 with the inner mitochondria membrane comes from a study by Gough et al. (33), who demonstrated a 50% reduction in cellular ATP levels in STAT3-deficient cells.
Mitochondrial dysfunction is being increasingly recognized as a major contributing factor to aging-associated diseases, including senescence (54). Cells from patients with IPF have been shown to accumulate dysfunctional mitochondria (3, 10, 81), and evidence from animal models suggests that mitochondrial dysfunction plays a central role in the pathogenesis of experimental fibrosis (67, 99, 119). Álvarez et al. (3) reported that lung fibroblasts from patients with IPF displayed shorter telomere lengths and simultaneously dysfunctional mitochondria compared with age-matched controls. Reduced mitochondrial function results in a reduction of the critical coenzyme ATP (75). Reduced ATP production has also been identified as a feature of IPF fibroblasts (3), thought to be the result of lower basal respiration.
Mitochondrial dysfunction is unlikely to be the sole mechanism leading to the extent of senescent cells observed in IPF, but it likely plays a part because Correia‐Melo et al. (22) showed that senescent fibroblasts depleted of their mitochondria do not develop the characteristic SASP but do undergo cell-cycle arrest.
STAT3 ACTIVITY IN THE SENESCENT PHENOTYPE AND IPF PATHOLOGY
The activation of STAT3 is required for survival and growth of tumor cells (36); therefore, STAT3 is considered an oncogene. As a result, most of what is known about the role of STAT3 in senescence is derived from cancer studies, where the inhibition of the IL-6/STAT3 signaling pathway induces senescence (106, 122).
More recently, the STAT3/senescence axis has been identified as playing a role in the pathologies of wider-ranging diseases. For example, STAT3 activation has been correlated with the development of senescence in a murine model of end-stage renal disease, where inhibition of STAT3 activation resulted in downregulation of genes associated with senescence and the SASP (59). Pharmacological inhibition of the JAK-STAT pathway suppresses the SASP of preadipocytes and endothelial cells as well as SASP-induced adipose tissue inflammation (113). The IL-10/STAT3 signaling cascade has been shown to be a key regulator of the senescent phenotype in macrophages of the eye, and, through its inhibition, normal macrophage function is restored (72). Indirect evidence linking STAT3 to senescence comes from studies by Rodier et al. (92) and Kuilman et al. (53), who revealed that IL-6 is integral to the development of the senescent phenotype through regulating cell-cycle arrest and certain factors of the SASP.
The accumulation of ECM within the lung parenchyma is a pathological feature of IPF, and it appears that STAT3 has a part to play in the transcription of one of the major proteins, collagen I. STAT3 binds to the COL1A2 enhancer and is essential for RNA polymerase recruitment (79).
Oxidative stress is regarded as a contributing factor to the pathogenesis of IPF (reviewed in Ref. 5), and STAT3 has been shown to be a major component of the oxidative stress signaling pathway (14, 74). However, whether STAT3 activation is driving oxidative stress or is merely a result of oxidative stress is yet to be determined.
A STAT3-dependent mechanism has been shown to be capable of inferring fibroblasts with a resistance to apoptosis. Moodley et al. (69) showed that the resistance to induced apoptosis of IPF-derived lung fibroblasts was STAT3 mediated and that IL-6 enhanced Fas ligand (FasL)-induced apoptosis in control fibroblasts but conferred resistance to FasL-induced apoptosis in IPF fibroblasts. The successful resolution of fibrosis necessitates that fibroblast apoptosis occurs at the site of an injury, and these authors (69) implicate STAT3 in the extensive fibrosis observed in IPF through the persistence of apoptosis-resistant fibroblasts, also suggesting that IPF fibroblasts are inherently different from non-IPF fibroblasts.
More recent indirect evidence for a role of STAT3 in promoting fibrosis comes from Yu et al. (119), who identified that aerosolized thyroid hormone therapy significantly resolved lung fibrosis. The thyroid hormone 3,5,3′-triiodothyronine (T3) has previously been shown to significantly reduce STAT3 recruitment to its target promoters in response to IL-6 signaling (20).
It may be that the path to senescence is mediated by more than one pathway or potentially a combination of pathways, converging on several targets, maybe necessary for senescence induction. Cui et al. (24) found that microRNA-34a (miR34a) was capable of inducing senescence, and its expression was increased in the lungs of patients with IPF, particularly in fibroblasts. A causal relationship between mammalian target of rapamycin (mTOR) activation and lung cell senescence has also been identified, as mTOR inhibition has been shown to prevent senescence and inhibit the SASP (43).
At this juncture, there is not enough available evidence. Despite the mounting evidence suggesting STAT3 signaling is important in senescence, whether it drives the onset of senescence or whether it becomes dysregulated as a consequence of senescence (123) remains unknown.
SUMMARY AND FUTURE DIRECTIONS
In nonpathological states, the transient nature of senescent cells allows them to play important roles in cellular processes, such as embryonic development, growth-arresting premalignant cells, and optimal wound resolution. However, the persistence of senescent cells appears to manifest in age-associated diseases such as IPF. Why the onset of the senescent phenotype should normally support tissue homeostasis but drive disease pathology when present in a subset of fibroblasts is unclear. It may be that immune clearance of senescent fibroblasts is compromised as a result of a preexisting pathology or because of a late-onset genetic manifestation in a similar fashion to Huntington’s disease (76).
The potential to target senescent cells for removal may be realized with a class of drugs called senolytics, which promote apoptosis in senescent cells through disabling crucial prosurvival pathways (15, 124). However, caution on their use must be raised in light of research highlighting epithelial cell senescence as a pathological feature in IPF (57). Given that end-stage IPF shows ablation of the alveolar epithelium, drugs that indiscriminately target senescent cells may speed up the pathogenesis of an already aggressive disease. In this case, targeting senescent fibroblasts specifically will likely prove a better strategy. A nanoparticle-based targeting approach involving receptor-ligand interaction may prove fruitful, and such therapeutics are presently being tailored toward the epithelium in respiratory diseases such as asthma. However, targeting fibroblasts will be more difficult given the lack of specific markers to unequivocally identify the cell type.
Both RS and SIPS are likely involved in IPF pathology. The incidence of RS cells is an unavoidable consequence of aging (108), and SIPS cells likely accumulate in the lung as a result of tissue-damaging agents. However, whether these cells are contributing to the excessive fibrosis observed in IPF is yet to be determined. Evidence from animal models suggests that more severe fibrosing occurs in the absence of senescent cells. It remains to be confirmed whether these findings translate into human studies.
In summary, the available literature suggests that senescent fibroblasts are a key aspect of IPF pathology. However, to date, reports linking STAT3 activation and the pathology of IPF are sparse, but studies by O'Donoghue et al. (77) and Pechkovsky et al. (82) indicate that STAT3 dysregulation is a feature of at least a subtype of patients with IPF. Even though evidence for an association is presently lacking, encouragement is drawn from studies linking STAT3 hyperphosphorylation in cancers and also from well-studied roles of STAT3 in nonpathological cellular processes, which ultimately become abnormal during senescence. Future studies should seek to elucidate the molecular mechanisms that lead to the onset of the senescent phenotype, which may help to define whether a factor such as STAT3 can be targeted to rescue the normal phenotype of senescent fibroblasts.
GRANTS
This research was supported by NHMRC grant 1099569 and by the Lung Foundation Australia David Wilson Scholarship (D. Waters) and by the NHMRC Centre for Research Excellence in Pulmonary Fibrosis (CRE-PF). D. W. Waters and K. E. C. Blokland are Fellows of the CRE-PF. J. Burgess was supported by a Rosalind Franklin Fellowship cofunded by the University of Groningen and the European Union.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.W., K.B., and P.P. prepared figures; D.W., K.B., P.P., J.K.B., M.S., C.G., and D.A.K. drafted manuscript; D.W., J.K.B., S.M., C.P., C.G., and D.A.K. edited and revised manuscript; D.A.K. approved final version of manuscript.
REFERENCES
- 1.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci 1171: 59–76, 2009. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 22: 503–529, 2004. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 3.Álvarez D, Cárdenes N, Sellarés J, Bueno M, Corey C, Hanumanthu VS, Peng Y, D’Cunha H, Sembrat J, Nouraie M, Shanker S, Caufield C, Shiva S, Armanios M, Mora AL, Rojas M. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 313: L1164–L1173, 2017. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltayiannis G, Baltayiannis N, Tsianos EV. Suppressors of cytokine signaling as tumor repressors. Silencing of SOCS3 facilitates tumor formation and growth in lung and liver. J BUON 13: 263–265, 2008. [PubMed] [Google Scholar]
- 5.Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 103: 1245–1256, 2009. doi: 10.1016/j.rmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, Waldron JA; Collaborating Centers . Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Am J Epidemiol 152: 307–315, 2000. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 7.Biran A, Perelmutter M, Gal H, Burton DGA, Ovadya Y, Vadai E, Geiger T, Krizhanovsky V. Senescent cells communicate via intercellular protein transfer. Genes Dev 29: 791–802, 2015. doi: 10.1101/gad.259341.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15: 91–102, 2009. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Häring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Röcken M. T-helper-1-cell cytokines drive cancer into senescence. Nature 494: 361–365, 2013. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 10.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol 240: 397–409, 2016. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calhoun C, Shivshankar P, Saker M, Sloane LB, Livi CB, Sharp ZD, Orihuela CJ, Adnot S, White ES, Richardson A, Le Saux CJ. Senescent cells contribute to the physiological remodeling of aged lungs. J Gerontol A Biol Sci Med Sci 71: 153–160, 2016. doi: 10.1093/gerona/glu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campisi J. Cancer, aging and cellular senescence. In Vivo 14: 183–188, 2000. [PubMed] [Google Scholar]
- 14.Carballo M, Conde M, El Bekay R, Martín-Nieto J, Camacho MJ, Monteseirín J, Conde J, Bedoya FJ, Sobrino F. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem 274: 17580–17586, 1999. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- 15.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83, 2016. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J 332: 43–50, 1998. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725–730, 2005. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 25: 2125–2136, 2011. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cimica V, Chen HC, Iyer JK, Reich NC. Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-β1. PLoS One 6: e20188, 2011. doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras-Jurado C, Alonso-Merino E, Saiz-Ladera C, Valiño AJ, Regadera J, Alemany S, Aranda A. The thyroid hormone receptors inhibit hepatic interleukin-6 signaling during endotoxemia. Sci Rep 6: 30990, 2016. doi: 10.1038/srep30990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A, Rushton MD, Charles M, Jurk D, Tait SW, Czapiewski R, Greaves L, Nelson G, Bohlooly-Y M, Rodriguez-Cuenca S, Vidal-Puig A, Mann D, Saretzki G, Quarato G, Green DR, Adams PD, von Zglinicki T, Korolchuk VI, Passos JF. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 35: 724–742, 2016. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 150: 967–972, 1994. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 24.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol 56: 168–178, 2017. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7: 301–311, 2014. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darnell JE. Validating Stat3 in cancer therapy. Nat Med 11: 595–596, 2005. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 27.Davalli P, Mitic T, Caporali A, Lauriola A, D’ Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev 18: 2016, 2016. doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge R-M, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733, 2014. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Disayabutr S, Kim EK, Cha SI, Green G, Naikawadi RP, Jones KD, Golden JA, Schroeder A, Matthay MA, Kukreja J, Erle DJ, Collard HR, Wolters PJ. miR-34 miRNAs regulate cellular senescence in type II alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One 11: e0158367, 2016. doi: 10.1371/journal.pone.0158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahim A, Crooks M, Hart SP. Gastroesophageal reflux and idiopathic pulmonary fibrosis: a review. Pulm Med 2011: 634613, 2011. doi: 10.1155/2011/634613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gey C, Seeger K. Metabolic changes during cellular senescence investigated by proton NMR-spectroscopy. Mech Ageing Dev 134: 130–138, 2013. doi: 10.1016/j.mad.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324: 1713–1716, 2009. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax 61: 980–985, 2006. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimminger F, Günther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J 45: 1426–1433, 2015. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15: 103–113, 2009. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajari Case A, Johnson P. Clinical use of nintedanib in patients with idiopathic pulmonary fibrosis. BMJ Open Respir Res 4: e000192, 2017. doi: 10.1136/bmjresp-2017-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence–implications for treatment. Int Wound J 2: 364–368, 2005. doi: 10.1111/j.1742-4801.2005.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460, 1990. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 40.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621, 1961. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 41.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6: 231ra47, 2014. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109: 1302–1308, 2011. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 43.Houssaini A, Breau M, Kebe K, Abid S, Marcos E, Lipskaia L, Rideau D, Parpaleix A, Huang J, Amsellem V, Vienney N, Validire P, Maitre B, Attwe A, Lukas C, Vindrieux D, Boczkowski J, Derumeaux G, Pende M, Bernard D, Meiners S, Adnot S. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 3: e93203, 2018. doi: 10.1172/jci.insight.93203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang G, Lu H, Hao A, Ng DC, Ponniah S, Guo K, Lufei C, Zeng Q, Cao X. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol Cell Biol 24: 8447–8456, 2004. doi: 10.1128/MCB.24.19.8447-8456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc 11: 1176–1185, 2014. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 46.Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med 150: 670–675, 1994. doi: 10.1164/ajrccm.150.3.8087336. [DOI] [PubMed] [Google Scholar]
- 47.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010. [Erratum in Nat Cell Biol 12: 1249, 2010.] doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, Iken M, Vucur M, Weiss S, Heikenwalder M, Khan S, Gil J, Bruder D, Manns M, Schirmacher P, Tacke F, Ott M, Luedde T, Longerich T, Kubicka S, Zender L. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479: 547–551, 2011. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 49.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 50.King TEJ Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 51.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667, 2008. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98: 12072–12077, 2001. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031, 2008. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 54.Lane RK, Hilsabeck T, Rea SL. The role of mitochondrial dysfunction in age-related diseases. Biochim Biophys Acta 1847: 1387–1400, 2015. doi: 10.1016/j.bbabio.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 16: 1421–1428, 2010. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee HC, Yin PH, Chi CW, Wei YH. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci 9: 517–526, 2002. doi: 10.1007/BF02254978. [DOI] [PubMed] [Google Scholar]
- 57.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, Ota C, Costa R, Schiller HB, Lindner M, Wagner DE, Günther A, Königshoff M. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J 50: 50, 2017. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung JY, Wilson HL, Voltzke KJ, Williams LA, Lee HJ, Wobker SE, Kim WY. Sav1 loss induces senescence and Stat3 activation coinciding with tubulointerstitial fibrosis. Mol Cell Biol 37: 37, 2017. doi: 10.1128/MCB.00565-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L, McBride KM, Reich NC. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc Natl Acad Sci USA 102: 8150–8155, 2005. doi: 10.1073/pnas.0501643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu YY, Zhao XK, Yu L, Qi F, Zhai B, Gao CQ, Ding Q. Interaction of Src and alpha-V integrin regulates fibroblast migration and modulates lung fibrosis in A preclinical model of lung fibrosis. Sci Rep 7: 46357, 2017. doi: 10.1038/srep46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macias E, Rao D, Digiovanni J. Role of stat3 in skin carcinogenesis: insights gained from relevant mouse models. J Skin Cancer 2013: 684050, 2013. doi: 10.1155/2013/684050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margolin SB. Treatment of cytokine growth factor caused disorders. US Patent US006090822A, July 18, 2000.
- 64.Mellone M, Hanley CJ, Thirdborough S, Mellows T, Garcia E, Woo J, Tod J, Frampton S, Jenei V, Moutasim KA, Kabir TD, Brennan PA, Venturi G, Ford K, Herranz N, Lim KP, Clarke J, Lambert DW, Prime SS, Underwood TJ, Vijayanand P, Eliceiri KW, Woelk C, King EV, Gil J, Ottensmeier CH, Thomas GJ. Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis. Aging (Albany NY) 9: 114–132, 2016. doi: 10.18632/aging.101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg 28: 876–883, 1998. doi: 10.1016/S0741-5214(98)70064-3. [DOI] [PubMed] [Google Scholar]
- 66.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–L401, 2011. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell C, Robin MA, Mayeuf A, Mahrouf-Yorgov M, Mansouri A, Hamard M, Couton D, Fromenty B, Gilgenkrantz H. Protection against hepatocyte mitochondrial dysfunction delays fibrosis progression in mice. Am J Pathol 175: 1929–1937, 2009. doi: 10.2353/ajpath.2009.090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moiseeva O, Bourdeau V, Roux A, Deschênes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol 29: 4495–4507, 2009. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol 29: 490–498, 2003. doi: 10.1165/rcmb.2002-0262OC. [DOI] [PubMed] [Google Scholar]
- 70.Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, Mutsaers SE, Thompson PJ, Knight DA. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol 163: 345–354, 2003. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118, 2013. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, Apte RS. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun 6: 7847, 2015. doi: 10.1038/ncomms8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakazato H, Oku H, Yamane S, Tsuruta Y, Suzuki R. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur J Pharmacol 446: 177–185, 2002. doi: 10.1016/S0014-2999(02)01758-2. [DOI] [PubMed] [Google Scholar]
- 74.Ng IH, Yeap YY, Ong LS, Jans DA, Bogoyevitch MA. Oxidative stress impairs multiple regulatory events to drive persistent cytokine-stimulated STAT3 phosphorylation. Biochim Biophys Acta 1843: 483–494, 2014. doi: 10.1016/j.bbamcr.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med (Encinitas) 13: 35–43, 2014. [PMC free article] [PubMed] [Google Scholar]
- 76.Nopoulos PC. Huntington disease: a single-gene degenerative disorder of the striatum. Dialogues Clin Neurosci 18: 91–98, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Donoghue RJ, Knight DA, Richards CD, Prêle CM, Lau HL, Jarnicki AG, Jones J, Bozinovski S, Vlahos R, Thiem S, McKenzie BS, Wang B, Stumbles P, Laurent GJ, McAnulty RJ, Rose-John S, Zhu HJ, Anderson GP, Ernst MR, Mutsaers SE. Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from Smad3-mediated lung fibrosis. EMBO Mol Med 4: 939–951, 2012. doi: 10.1002/emmm.201100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papaioannou I, Xu S, Denton CP, Abraham DJ, Ponticos M. STAT3 controls COL1A2 enhancer activation cooperatively with JunB, regulates type I collagen synthesis post-transcriptionally and is essential for lung myofibroblast differentiation. Mol Biol Cell 29: 84–95, 2018. doi: 10.1091/mbc.E17-06-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6: 347, 2010. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, Choi AMK, Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10: e0121246, 2015. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pechkovsky DV, Prêle CM, Wong J, Hogaboam CM, McAnulty RJ, Laurent GJ, Zhang SS, Selman M, Mutsaers SE, Knight DA. STAT3-mediated signaling dysregulates lung fibroblast-myofibroblast activation and differentiation in UIP/IPF. Am J Pathol 180: 1398–1412, 2012. doi: 10.1016/j.ajpath.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 83.Prele C, Iosifidis T, McAnulty R, Badrian B, Jamieson S, Pearce D, Ernst M, Thompson P, Laurent G, Knight D, Mutsaers S. LSC Abstract – Investigating SOCS-mediated regulation of STAT signalling in idiopathic pulmonary fibrosis (IPF). Eur Respir J 48: PP2028, 2016. doi: 10.1183/13993003.congress-2016.PP202. [DOI] [Google Scholar]
- 84.Qian Y, Chen X. Senescence regulation by the p53 protein family. Methods Mol Biol 965: 37–61, 2013. doi: 10.1007/978-1-62703-239-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raffetto JD, Mendez MV, Phillips TJ, Park HY, Menzoian JO. The effect of passage number on fibroblast cellular senescence in patients with chronic venous insufficiency with and without ulcer. Am J Surg 178: 107–112, 1999. doi: 10.1016/S0002-9610(99)00134-8. [DOI] [PubMed] [Google Scholar]
- 86.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schünemann HJ; American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association . An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 192: e3–e19, 2015. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 88.Ramos C, Montaño M, García-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24: 591–598, 2001. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 89.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 90.Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, Brozek J, Beyene J, Schünemann H. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med 14: 18, 2016. doi: 10.1186/s12916-016-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rockey DC, Weymouth N, Shi Z. Smooth muscle α actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS One 8: e77166, 2013. doi: 10.1371/journal.pone.0077166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979, 2009. [Erratum in Nat Cell Biol 11: 1272, 2009. Dosage error in article text.] doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romer AS, Parsons TS. The Vertebrate Body. Philadelphia, PA: Saunders, 1977. [Google Scholar]
- 94.Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 32: 1971–1977, 2013. doi: 10.1038/onc.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sagiv A, Burton DGA, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 8: 328–344, 2016. doi: 10.18632/aging.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Selman M, King TE Jr, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians . Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 98.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci 122: 3209–3213, 2009. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell 14: 774–783, 2015. doi: 10.1111/acel.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stanley A, Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg 33: 1206–1211, 2001. doi: 10.1067/mva.2001.115379. [DOI] [PubMed] [Google Scholar]
- 101.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130, 2013. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 102.Tammineni P, Anugula C, Mohammed F, Anjaneyulu M, Larner AC, Sepuri NB. The import of the transcription factor STAT3 into mitochondria depends on GRIM-19, a component of the electron transport chain. J Biol Chem 288: 4723–4732, 2013. doi: 10.1074/jbc.M112.378984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123: 966–972, 2013. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ, Robbins PD. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 122: 2601–2612, 2012. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Timofeeva OA, Tarasova NI, Zhang X, Chasovskikh S, Cheema AK, Wang H, Brown ML, Dritschilo A. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci USA 110: 1267–1272, 2013. doi: 10.1073/pnas.1211805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tkach M, Coria L, Rosemblit C, Rivas MA, Proietti CJ, Díaz Flaqué MC, Beguelin W, Frahm I, Charreau EH, Cassataro J, Elizalde PV, Schillaci R. Targeting Stat3 induces senescence in tumor cells and elicits prophylactic and therapeutic immune responses against breast cancer growth mediated by NK cells and CD4+ T cells. J Immunol 189: 1162–1172, 2012. doi: 10.4049/jimmunol.1102538. [DOI] [PubMed] [Google Scholar]
- 107.Toussaint O, Dumont P, Remacle J, Dierick JF, Pascal T, Frippiat C, Magalhaes JP, Zdanov S, Chainiaux F. Stress-induced premature senescence or stress-induced senescence-like phenotype: one in vivo reality, two possible definitions? Sci World J 2: 230–247, 2002. doi: 10.1100/tsw.2002.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Deursen JM. The role of senescent cells in ageing. Nature 509: 439–446, 2014. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang W, Yang X, López de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem 278: 27016–27023, 2003. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 110.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Function of mitochondrial Stat3 in cellular respiration. Science 323: 793–797, 2009. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82: 241–250, 1995. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 112.Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23: 303–314, 2016. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 112: E6301–E6310, 2015. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445: 656–660, 2007. [Erratum in Nature 473: 544, 2011.] doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Zeische R, Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY) 7: 664–672, 2015. doi: 10.18632/aging.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res 65: 939–947, 2005. [PubMed] [Google Scholar]
- 117.Yoon YS, Byun HO, Cho H, Kim BK, Yoon G. Complex II defect via down-regulation of iron-sulfur subunit induces mitochondrial dysfunction and cell cycle delay in iron chelation-induced senescence-associated growth arrest. J Biol Chem 278: 51577–51586, 2003. doi: 10.1074/jbc.M308489200. [DOI] [PubMed] [Google Scholar]
- 118.Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res Ther 7: 100–110, 2005. doi: 10.1186/ar1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JPW, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo e Drigo R, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24: 39–49, 2017. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9: 798–809, 2009. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs 18: 45–56, 2009. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yun UJ, Park SE, Jo YS, Kim J, Shin DY. DNA damage induces the IL-6/STAT3 signaling pathway, which has anti-senescence and growth-promoting functions in human tumors. Cancer Lett 323: 155–160, 2012. doi: 10.1016/j.canlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 123.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA 104: 16158–16163, 2007. [Erratum in Proc Natl Acad Sci USA 104: 19655, 2007.] doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 9: 955–963, 2017. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]