Abstract
Mechanical ventilation causes lung injury and systemic inflammatory responses in preterm sheep and is associated with bronchopulmonary dysplasia (BPD) in preterm infants. Budesonide added to surfactant decreased BPD by 20% in infants. We wanted to determine the effects of budesonide and surfactant on injury from high tidal volume (VT) ventilation in preterm lambs. Ewes at 125 ± 1 days gestational age had fetal surgery to expose fetal head and chest with placental circulation intact. Lambs were randomized to 1) mechanical ventilation with escalating VT to target 15 ml/kg by 15 min or 2) continuous positive airway pressure (CPAP) of 5 cmH2O. After the 15-min intervention, lambs were given surfactant 100 mg/kg with saline, budesonide 0.25 mg/kg, or budesonide 1 mg/kg. The fetuses were returned to the uterus for 24 h and then delivered and ventilated for 30 min to assess lung function. Budesonide levels were low in lung and plasma. CPAP groups had improved oxygenation, ventilation, and decreased injury markers compared with fetal VT lambs. Budesonide improved ventilation in CPAP lambs. Budesonide decreased lung weights and lung liquid and increased lung compliance and surfactant protein mRNA. Budesonide decreased proinflammatory and acute-phase responses in lung. Airway thickness increased in animals not receiving budesonide. Systemically, budesonide decreased monocyte chemoattractant protein-1 mRNA and preserved glycogen in liver. Results with 0.25 and 1 mg/kg budesonide were similar. We concluded that budesonide with surfactant matured the preterm lung and decreased the liver responses but did not improve lung function after high VT injury in fetal sheep.
Keywords: airway, bronchopulmonary dysplasia, inflammation, mechanical ventilation, preterm
INTRODUCTION
Lung inflammation and injury resulting from mechanical ventilation are central to the development of bronchopulmonary dysplasia (BPD) (26, 34, 35). Despite efforts to decrease the use of mechanical ventilation in very preterm infants, BPD rates have not declined (8, 10, 19). Systemic corticosteroids are the most effective agents to treat lung injury progressing to BPD, but therapy comes with the increased risk of adverse long-term outcomes (9). Inhaled budesonide, a commonly used medication to treat asthma, also decreased the risk of BPD when given daily during the first few weeks of life (3, 33). Recently, treatment of preterm infants with budesonide mixed with surfactant reduced the incidence of BPD by 20% without an increase in mortality or neurological deficits (38–40). Targeted administration of corticosteroids to the preterm lung may decrease systemic steroid effects.
The pharmacological properties of budesonide have been studied extensively in multiple lung diseases (37). Budesonide has very high affinity for glucocorticoid receptors (GR), nearly tenfold higher than dexamethasone. When budesonide is given into the airways, it is retained in lung tissue preferentially to other tissues because it is highly lipophilic and quickly solubilized into cellular lipids in the airways, where it is rapidly and reversibly esterified with oleic and palmitic acids (37). The budesonide esters, which have a low affinity for GR, then are slowly deesterified to free budesonide for prolonged exposure of the lung (5, 20). The half-life in the human fetal lung was ~4 h, but the physiological effects persisted for days (2). In a clinical trial, budesonide and surfactant decreased proinflammatory cytokine IL-8 levels in tracheal aspirates as long as 8 days after therapy (39). Systemically absorbed budesonide is rapidly metabolized on first pass through the liver by CYP3A enzymes to 16α-hydroxyprednisolone, minimizing systemic exposure (27, 29). Plasma levels of budesonide were low in infants given budesonide and surfactant by Yeh et al. (39), but other systemic effects were not explored. The combination of budesonide (0.25 mg/kg) and the surfactants beractant (100 mg/kg) or poractant (200 mg/kg), did not affect the surface tension properties or the clinical effectiveness in models of surfactant deficiency/inactivation (32, 40). The combination is easy to mix at the bedside, and the budesonide remains stable, in unconjugated forms, for over 24 h at room temperature (32, 39, 40). In addition, surfactant can effectively deliver budesonide to the airways and distal lungs of preterm infants (40).
We previously demonstrated in preterm fetal lambs that large tidal volume (VT) injury (up to 15 ml/kg) for 15 min caused airway epithelial changes, lung inflammation, lung maturation, and systemic responses in the liver (7, 15, 18). Mechanical ventilation with normal VT of 7–9 ml/kg for sheep caused less injury, and continuous positive airway pressure (CPAP) without VT did not initiate an inflammatory response (16, 30). For this study, we therefore evaluated the combination of budesonide (0.25 or 1 mg/kg) with surfactant on lung inflammation in the setting of mechanical ventilation or CPAP only. Using a fetal preterm sheep model (13) to isolate the initial injury from continued ventilation, we tested the hypothesis that the addition of budesonide to surfactant would improve lung function and decrease lung inflammation and systemic responses to mechanical ventilation-induced lung injury. Preterm sheep receiving budesonide and/or surfactant but no VT ventilation were used to test the hypothesis that budesonide would alter lung structure and maturation. Fetuses were returned to the uterus for 24 h and then delivered and ventilated for 30 min to evaluate physiological responses. The fetal model does not directly parallel clinical interventions in preterm infants; however, the model evaluates the progression of the 15-min ventilation injury and the effects of budesonide on that injury and systemic responses without the continued exposure of the lungs to oxygen or ventilation required with delivery and support of the animal. Understanding of the biological effects of budesonide and surfactant on the preterm lung is important for the identification of potential safety concerns.
METHODS
Maternal anesthesia and fetal exteriorization.
All animal experiments were performed with the approval of the Animal Ethics Committees of the University of Western Australia. Date-mated Merino-cross ewes at 125 ± 1 days gestational age (GA; term is ~150 days GA) were premedicated with a combination of acepromazine (0.03 mg/kg) and buprenorphine (0.01 mg/kg) intramuscularly 45 min before induction of anesthesia with a combination of ketamine (5 mg/kg) and midazolam (0.25 mg/kg iv). The tracheas of the ewes were intubated, and anesthesia was maintained with isoflurane (0.5–2% in 100% O2). Isoflurane will cross the placenta, anesthetize the fetus, and eliminate fetal breathing (14, 36). The fetal head and chest were exteriorized through a midline hysterotomy. Placental blood flow was maintained, and lung expansion could occur without effects from intrauterine pressure (15). A tracheostomy was performed on the fetal lamb, and a 4.5-mm cuffed endotracheal tube (ETT) was placed and secured. Fetal lung fluid (FLF) was then removed via the ETT using a syringe and suction catheter, and an aliquot of the FLF was snap-frozen.
Fetal interventions.
Fetal lambs were randomized to one of five interventions. They first received either 15 min of 1) CPAP (5 cmH2O) or 2) mechanical ventilation with escalating VT to a target of 15 ml/kg after 15 min of ventilation (fetal VT). Each animal was positioned prone on the abdomen of the ewe to maintain placental circulation. For Fetal VT lambs, Fabian ventilators (Acutronic, Switzerland) were used with initial settings of a rate of 40 breaths/min, inspiratory time of 0.7 s, positive end-expiratory pressure (PEEP) of 0 cmH2O, and an initial peak inspiratory pressure of 40 cmH2O. The weight of the animal was estimated, and peak inspiratory pressure was adjusted (max 55 cmH2O) to achieve a target of 6 ml/kg VT by 5 min of ventilation, 10–12 ml/kg by 10 min, and 15 ml/kg between 10 and 15 min (12, 15). Ventilator variables were chosen to induce airway and parenchymal lung injury (15). The CPAP and Fetal VT animals received 100% N2 to avoid oxygen exposure. After the 15-min intervention, the animals received either 1) surfactant (100 mg/kg; Curosurf, Chiesi) diluted to 10 ml with saline or surfactant with 2) budesonide (Pulmicort 0.5 mg/ml, Astra Zeneca) 0.25 mg/kg (Bud 0.25), or 3) 1 mg/kg (Bud 1); diluted to 10 ml with saline. For surfactant and budesonide dosing, 3 kg was used as the estimated fetal weight. After receiving the medication, the animals had the tracheostomy tube removed, the trachea was ligated to prevent loss of medication from the lungs, and the fetal lambs were returned to the uterus with recovery of the ewes.
Postnatal ventilation and monitoring.
Twenty-four hours after the fetuses were returned to the uterus, the ewes were anesthetized for caesarean delivery of the fetus. A tracheostomy was performed with a 4.5-mm cuffed ETT, after which fetal lung fluid was removed using an 8-Fr catheter and a 60-ml syringe. The lambs were then delivered, weighed, administered ketamine 10 mg/kg im, and ventilated for 30 min with Fabian ventilators (Acutronic, Switzerland) with 100% oxygen, heated, and humidified to 37°C (MR850 Humidifier; Fisher & Paykel Healthcare, Auckland, NZ). The ventilator settings for all lambs were initially set at a rate of 40 breaths/min, a peak inspiratory pressure (PIP) of 40 cmH2O, a PEEP of 5 cmH2O, and an inspiratory time of 0.5 s. The maximum PIP was 40 cmH2O and was adjusted to keep the VT between 8 and 9 ml/kg. An umbilical arterial catheter was placed. Ventilator settings and physiological data were recorded at 5-min intervals, and arterial blood gases were measured at 10, 20, and 30 min (Siemens Rapidlab 1265, Siemens, Australia). At 30 min, the animal was removed from the ventilator and the ET tube was clamped for 1 min to achieve complete lung collapse by oxygen absorption. Each ventilated lamb was then euthanized with pentobarbitone (100 mg/kg iv) and reweighed. Twin lambs that were not ventilated but exposed to the surgeries (unventilated controls) were delivered, euthanized, and weighed. After caesarean delivery of the lambs, the ewes were euthanized with pentobarbitone (160 mg/kg iv).
Tissue sampling.
Post-mortem inflation and deflation pressure volume curves were measured with stepwise changes in pressure to a maximum of 40 cmH2O (21). Bronchoalveolar lavage fluid (BALF) was collected by three repetitive saline lavages of the left lung and was used for cell counts and differential analysis. Cytospins were stained with Diff Quick (Fischer Scientific) for differential cell counts on 200 cells per slide (21). Tissues from the trachea, right lower peripheral lung, left main stem bronchi with surrounding lung parenchyma removed, posterior mediastinal lymph node (PMLN), and liver were snap-frozen for RNA isolation and formalin fixed for paraffin embedding (25). The right upper lobe was inflation fixed at 30 cmH2O with 10% formalin and then paraffin embedded (25). Lung tissue was dried in aa 65°C incubator for 72 h, and the ratio of wet weight to dry weight was determined once.
Quantitative RT-PCR.
Messenger RNA (mRNA) was extracted from the peripheral lung tissue of the right lower lobe and the liver with TRIzol (Invitrogen). cDNA was produced from 1 µg of mRNA using the Verso cDNA kit (Thermo Scientific). Custom Taqman gene primers (Life Technologies) for ovine sequences for ATP-binding cassette subfamily A member 3 (ABCA3), aquaporin-5 (AQ5), chitinase-3-like protein-1 (CHI3l1), connective tissue growth factor (CTGF), cysteine-rich angiogenic inducer 61 (Cyr61), early growth response protein-1 (Egr-1), epithelial sodium channel (ENaC), epiregulin (EREG), interleukin-1β (IL-1β), IL-6, monocyte chemoattractant protein-1 (MCP-1), serum amyloid A3 (SAA3), surfactant protein A (SFTPA), SFTPB, SFTPC, and SFTPD were used. Quantitative RT-PCR was performed with iTaq Universal mix (Bio-Rad) in a 15-µl reaction on a CFX Connect machine and software (Bio-Rad). 18S primers (Life Technologies) were used as the internal loading control.
Immunohistochemistry.
Immunostaining protocols used paraffin sections (4 µm) of formalin-fixed RUL, PMLN, or liver for H&E staining, periodic acid Schiff (PAS) staining, and immunohistochemistry for Ki67 (rabbit anti-human Ki67, no. MA5-15690, 1:250 dilution; Thermo Scientific) or FoxP3 (mouse anti-human (eBio7979) 1:100 dilution; Thermo Scientific) (22, 24). Slides for Ki67 were blinded, and 10 terminal airways (×40 on a Zeiss Axioskop 40) per animal were analyzed for Ki67+ epithelial cells and reported as percentage of cells. FoxP3 staining in lymphoid tissue of the PMLN were blinded and assigned a two-point scale (0 = no positive cells, 1 = occasional positive cell, 2 = large number of cells). PAS staining was blindly evaluated in the large airways (containing cartilaginous rings), bronchioles, and liver based on a two-point scale (0 = minimal staining, 1 = staining in ~50% of cells, 2 = majority of cells). Amylase treatment of some slides provided a negative control for PAS staining. Collagen was stained with Sirius Red-Fast Green FCF with picric acid. Images (×20) were evaluated with cellSens software (Olympus) using three regions of interest (106 × 60 pixels) per airway image (5 images per 15 regions per animal) and averages reported as percent collagen. For airway thickness, images were evaluated using a Leica LAS X to measure the distance from the apical epithelial cells to the bottom of the pink collagen region for three regions of each airway image (~15 measurements per airway size per animal).
Budesonide measurements.
Plasma and lung tissue budesonide were extracted with ethyl acetate, dried under vacuum, and resuspended in 70 µl of a 1:1 methanol-water solution (11). Budesonide-d8 (50 ng/ml) was added to naïve lung tissue and extracted in a similar protocol for the standard curve. Budesonide analysis was performed with Agilent Technologies 1290 Infinity pumps and a 6400 Series Triple Quadrupole liquid chromatography-mass spectrometry (LC-MS) system, with an electrospray ionization source in positive ion mode. Twenty microliters of sample was injected in the MS and an Agilent Poroshell 2.1 × 50 mm C18, 2.6 μM column and a phenomenex biphenyl 3 × 150 mm, 2.6 μM column, were used to chromatographically separate analyses. The mobile phase for two-solvent gradient elution consisted of (A1) water + 0.1% formic acid, and then (B1) methanol + 0.1% formic acid, delivered at a flow-rate of 0.5 ml/min, with a gradient of 50 to 98% in 10 min, followed by reequilibration for 2 min, before subsequent injection. The MS was set to detect precursor and product budesonide ions (acquisition time 3.9 min), with masses of 431.5 and 323.0, respectively, and budesonide-d8 ions (acquisition time 3.83 min) with masses of 439.5 and 421.5, respectively. Using a signal-to-noise ratio of 10:1 as a cut-off, budesonide concentrations can be reliably measured to concentrations as low as 0.01 ng/ml.
Data analysis and statistics.
Results are shown as means ± SE and reported as fold increase over controls, with control values set at 1. The ventilation efficiency index (VEI) [3800/(PIP × rate × )] and oxygenation index (OI) [ × mean airway pressure)/] were calculated (28, 31). Statistics were analyzed with Prism 6 (GraphPad) using Student’s t-test, Mann-Whitney nonparametric test, or ANOVA tests as appropriate. Significance was accepted as P < 0.05.
RESULTS
There were no differences in gestational ages, sexes, or birth weights between groups (Table 1). Animals receiving fetal mechanical ventilation (Fetal VT) reached 5.7 ± 0.2 ml/kg at 5 min, 10.4 ± 0.4 ml/kg at 10 min, and 12.1 ± 0.6 ml/kg VT at the maximal PIP of 55 cmH2O for final 5 min of ventilation (Table 1). All animals survived the interventions, return to the uterus for 24 h, and delivery for newborn ventilation. At newborn delivery, lambs receiving only CPAP in utero had ample fetal lung fluid, whereas animals receiving mechanical ventilation had very little and thick fetal lung fluid. Free budesonide levels were low in the lung tissue and plasma, with values for the 1 mg/kg budesonide group proportionately higher than the 0.25 mg/kg groups (Table 1). The ventilation and CPAP groups had similar plasma and lung budesonide levels.
Table 1.
Budesonide values and physiological variables
| Budesonide |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | n | BW, kg | VT 15 min, ml/kg | Hgb at Birth, mg/dl | Lung, ng/g | Plasma, ng/ml | Lung Weight, g/kg | V40/Lung Wt, ml/g | Dry Wt/Wet Wt, %Original |
| CPAP + Saline | 5 | 2.9 | N/A | 12.7 ± 0.4 | N/A | N/A | 40 ± 2+ | 1.1 ± 0.1+ | 9.5 ± 0.4* |
| CPAP + Bud 0.25 mg/kg | 6 | 2.6 | N/A | 12.5 ± 0.6 | 19 ± 2 | 0.2 ± 0.04 | 32 ± 2* | 1.7 ± 0.1+* | 10.2 ± 0.2* |
| Fetal VT + Saline | 7 | 3.1 | 11.2 ± 1.3 | 11.6 ± 0.5*+ | N/A | N/A | 36 ± 1+ | 0.5 ± 0.1* | 10.4 ± 0.3* |
| Fetal VT + Bud 0.25 mg/kg | 8 | 3.0 | 11.9 ± 0.8 | 13.5 ± 0.4 | 24 ± 5 | 0.2 ± 0.02 | 25 ± 2t* | 0.7 ± 0.1+* | 14.5 ± 0.7+t |
| Fetal VT + Bud 1.0 mg/kg | 7 | 2.7 | 13.2 ± 0.9 | 13.6 ± 0.4 | 84 ± 12t | 0.8 ± 0.1t | 21 ± 1t+* | 0.9 ± 0.1+*t | 15.7 ± 0.7+t |
| Unventilated controls | 5 | 2.8 | N/A | 14.2 ± 0.5 | N/A | N/A | 28 ± 2 | 0.3 ± 0.1 | 11.8 ± 0.2t |
Values are means ± SE. BW, body weight; VT, tidal volume; V40, volume at 40 cmH2O; CPAP, continuous positive airway pressure; Bud, budesonide; N/A, not analyzed.
P < 0.05 vs. Controls;
P < 0.05 vs. CPAP + Saline;
P < 0.05 vs. Fetal VT +Saline.
Lung physiology.
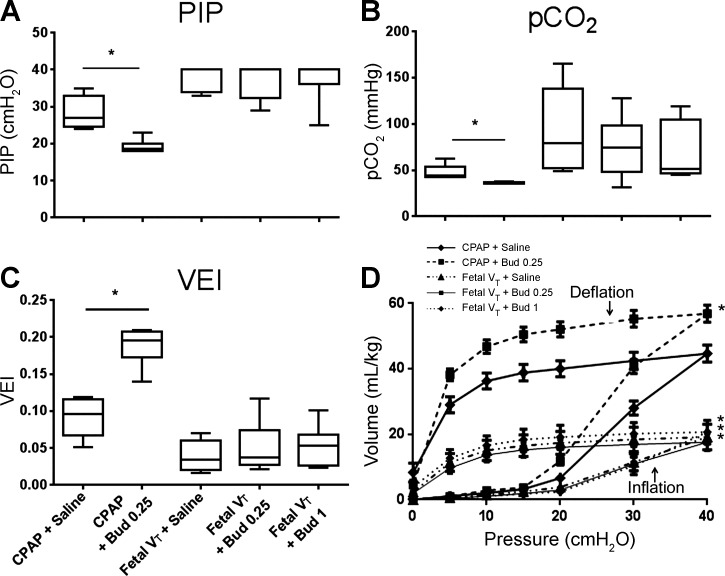
Oxygenation during postnatal ventilation with 100% O2 was significantly higher ( = 400 ± 32 mmHg, P < 0.05) for the animals receiving in utero CPAP than for the animals given in utero mechanical ventilation ( = 80 ± 16). Budesonide did not change the oxygenation for the CPAP or Fetal VT groups. OI in Fetal VT animals were very high (40 ± 11), signifying severe impairment of oxygenation. PIP targeted to 8–9 ml/kg could be reduced for the CPAP animals, and CPAP + Bud 0.25 mg/kg had significantly lower PIP than CPAP + Saline (Fig. 1A). The lambs receiving fetal VT injury required pressures close to 40 cmH2O for the 30-min ventilation period. Animals receiving CPAP only were able to be maintained with an average of 41 ± 2 mmHg compared with an average of 78 ± 8 for Fetal VT animals (P < 0.05; Fig. 1B). CPAP + Bud 0.25 animals had the lowest levels of any group. To quantify the degree of ventilator support required, we used the VEI, which incorporates the ventilation rate, peak pressure, and . The CPAP groups had higher VEI values than the Fetal VT animals (Fig. 1C). CPAP animals receiving budesonide had higher VEI measurements than with surfactant alone, signifying a maturational effect on the lungs. Of note, for all the ventilation variables, the Fetal VT injury groups were similar, with no budesonide effects at the 0.25 or 1 mg/kg doses. Hemoglobin level on blood gas at birth (Table 1) were lower in Fetal VT animals receiving saline, but not in Fetal VT animals receiving budesonide. Lactic acid levels were similar between groups at delivery and throughout the ventilation period.
Fig. 1.
Lung physiology after delivery and pressure-volume curves. CPAP, continuous positive airway pressure; VT, tidal volume; Bud 1, budesonide 1 mg/kg; Bud 0.25, budesonide 0.25 mg/kg. A: peak inspiratory pressures (PIP) for animals at 30 min of mechanical ventilation. B: values at 30 min. C: ventilation efficiency index (VEI) as calculated {VEI = [3800/(PIP·rate·)]} at 30 min. D: open chest pressure-volume inflation and deflation curves of post mortem lungs; n = 5–8 animals/group. *P < 0.05 vs. CPAP + Saline.
Lung volumes and weights.
The static lung gas volume at 40 cmH2O (V40) was higher in animals receiving CPAP alone (Fig. 1D) vs. Fetal VT. The V40 for CPAP animals exposed to budesonide was higher than for those exposed to surfactant alone, consistent with a maturational effect. All volumes were low, and there were no differences in pressure-volume curves between the Fetal VT animals. Lung weights per kilogram of bodyweight were similar in both the ventilated and CPAP animals that received only surfactant (Table 1). Budesonide decreased the lung weights of all lambs, with the largest decrease in animals receiving 1 mg/kg budesonide. The ratios of the lung volume (V40) to the lung weights (Table 1) demonstrate that CPAP animals exposed to budesonide had lighter lungs that held more gas volume. In animals receiving the Fetal VT, budesonide 1 mg/kg also had lighter lungs with higher volumes, but this effect was not significant for the 0.25 mg/kg budesonide dose. The decrease in weight in the lungs of Fetal VT animals may have been due primarily to differences in water content of the lungs, as the ratio of dry to wet weight (dry wt/wet wt) for peripheral lung tissue was lower in budesonide-exposed lungs (Table 1). Interestingly, these effects of budesonide on wet-to-dry weights did not occur in CPAP-only animals, and these animals had higher water content than controls.
Lung inflammation and acute-phase responses.
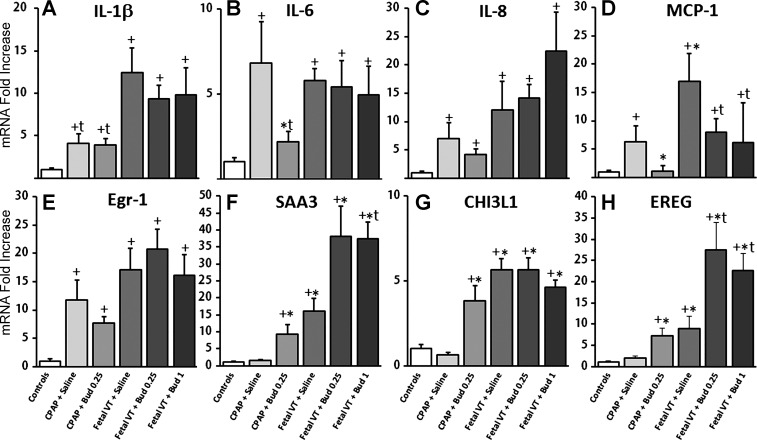
The mechanical ventilation after delivery increased mRNA for the proinflammatory cytokines (IL-1β, IL-6, IL-8, MCP-1; Fig. 2, A–D). IL-1β increased more with Fetal VT than with CPAP, but there was no effect with budesonide. IL-6 increased in all ventilated animals except animals receiving CPAP and budesonide 0.25 mg/kg. IL-8 increased in all groups compared with controls. MCP-1 decreased in both CPAP animals receiving budesonide 0.25 mg/kg and in Fetal VT animals receiving both budesonide 0.25 and 1 mg/kg compared with surfactant-treated animals (Fig. 2D). Fetal VT increased inflammatory cells in the BALF (66 ± 8 × 106/kg) compared with controls (0.5 ± 0.4 × 106/kg, P < 0.05), and the lung inflammatory cells were higher in the budesonide 0.25 mg/kg (104 ± 12 × 106/kg) and 1 mg/kg groups (160 ± 33 × 106/kg) compared with Fetal VT + Saline animals (P < 0.05). Neutrophils and monocytes were both increased with mechanical ventilation, with the highest levels in the animals exposed to budesonide 1 mg/kg. Acute-phase response mRNA for Egr-1 (Fig. 2E), CTGF, and Cyr61 mRNA increased in all animals without a difference with budesonide (CTGF and Cyr61 data not shown). Fetal VT increased mRNA for SAA3, CHI3L1, and EREG (Fig. 2, F–H). Budesonide increased SAA3, CHI3L1, and EREG mRNA in animals receiving CPAP only in utero and further increased SAA3 and EREG in animals receiving Fetal VT. There were no differences between the 0.25 and 1 mg/kg doses of budesonide for anti-inflammatory or acute phase responses.
Fig. 2.
Proinflammatory cytokine and acute-phase mRNA in peripheral lung tissue. CPAP, continuous positive airway pressure; VT, tidal volume; VT15, VT for 15 min; Bud 1, budesonide 1 mg/kg; Bud 0.25, budesonide 0.25 mg/kg. Interleukin (IL)-1β (A), IL-6 (B), IL-8 (C), and monocyte chemotactic protein-1 (MCP-1; D) mRNA in peripheral lung tissue. Acute-phase response genes early growth response protein-1 (Egr-1; E), serum amyloid A3 (SAA; F), chitinase-3-like protein-1 (CHI3L1; G), and epiregulin (EREG; H) mRNA responses in the lung. Values are means ± SE with unventilated control (UVC) set at 1; n = 5–8 animals/group. +P < 0.05 vs. UVC; *P < 0.05 vs. CPAP + Saline; tP < 0.05 vs. VT15 +Saline.
Airway thickness and proliferation.
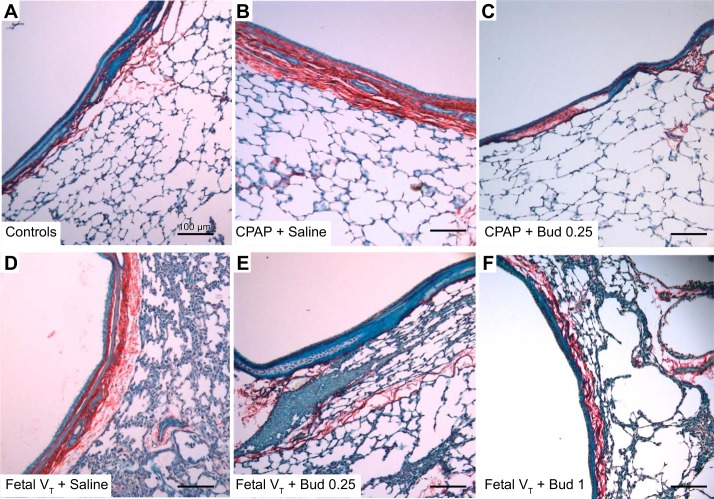
Large and small airway thickness was increased by both CPAP and Fetal VT in animals receiving surfactant plus saline (Fig. 3 and Table 2). The large cartilaginous airways of CPAP + Saline and Fetal VT + Saline had nearly twice the distance between the epithelial layer and the collagen staining adjacent to alveoli compared with the controls (Fig. 3D). The bronchioles were thicker in the animals receiving Fetal VT + Saline. The animals exposed to budesonide at 0.25 or 1 mg/kg did not have increased airway thickness relative to the unventilated controls. Quantification of collagen within the airways demonstrated increased collagen staining in animals receiving Fetal VT or CPAP + Saline, whereas the animals receiving budesonide 0.25 mg/kg had airway collagen similar to that of controls. Animals receiving 1 mg/kg budesonide had a small increase in collagen staining in the smaller airways, but not as large as without budesonide (Table 2). PAS staining demonstrated increased glycogen staining in the animals receiving mechanical ventilation without budesonide, with no increases over control animals for animals receiving budesonide. Ki67, a marker of proliferation, was increased in the smaller bronchioles of animals receiving CPAP + Saline and Fetal VT + Saline, but was less evident in the animals receiving budesonide (Table 2).
Fig. 3.
Large-airway images. Representative images of airways and peripheral lung stained with Sirius Red-Fast Green FCF with picric acid (×20). CPAP, continuous positive airway pressure; VT, tidal volume; Bud 1, budesonide 1 mg/kg; Bud 0.25, budesonide 0.25 mg/kg. A: unventilated controls have epithelial layer of ciliated cells and thin collagen layer (staining red) below epithelium and thin septum of the peripheral lung tissue. B: animals receiving CPAP + Saline have mild thickening of airways and mild congestion in peripheral lungs, finding not seen in animals receiving CPAP and budesonide (C). D: mechanical ventilation with VT 15 ml/kg caused congestion of the lungs and thickening of the airways. These changes were not seen with Bud 0.25 (E) or Bud 1 (F). Quantification of blinded segments provided in Table 2; n = 5 animals per group. Scale bar, 100 μm.
Table 2.
Airway thickness analysis, PAS staining, and Ki67
| Groups | Large Airway Thickness, μm | Large Airway Collagen, %Staining | Bronchiole Thickness, μm | Bronchiole Collagen, %Staining | PAS Staining Bronchioles, Out of 2 | Ki67+ Epithelium Bronchioles, %Staining |
|---|---|---|---|---|---|---|
| CPAP + Saline | 65.4 ± 8.9+ | 21.4 ± 5.7+ | 16.3 ± 4.1 | 51.3 ± 13.5+ | 1.6 ± 0.08+ | 60 ± 6+ |
| CPAP + Bud 0.25 | 35.1 ± 7.1 | 7.4 ± 1.6 | 14.1 ± 1.9 | 22.9 ± 10.6 | 0.5 ± 0.07 | 20 ± 2+* |
| Fetal VT + Saline | 70.1 ± 7.9+ | 29.4 ± 5.6+ | 21.3 ± 5.5+ | 84.8 ± 19.1+ | 1.9 ± 0.02+ | 62 ± 2+ |
| Fetal VT + Bud 0.25 | 30.1 ± 7.6 | 8.3 ± 1.7 | 13.2 ± 2.7 | 14.6 ± 1.6 | 0.6 ± 0.07t | 24 ± 9+t |
| Fetal VT + Bud 1.0 | 35.5 ± 4.1 | 18.2 ± 1.5* | 15.0 ± 0.4+ | 40.0 ± 8.1+ | 0.4 ± 0.03t | NA |
| Unventilated controls | 28.7 ± 11.4 | 3.6 ± 1.1 | 11.3 ± 1.2 | 13.0 ± 3.3 | 0.5 ± 0.05 | 0 ± 0 |
Values are means ± SE. VT, tidal volume; CPAP, continuous positive airway pressure; Bud, budesonide; PAS, periodic acid Schiff staining; NA, not analyzed.
P < 0.05 vs. Controls;
P < 0.05 vs. CPAP + Saline;
P < 0.05 vs. Fetal VT +Saline.
Lung maturation and surfactant mRNA.
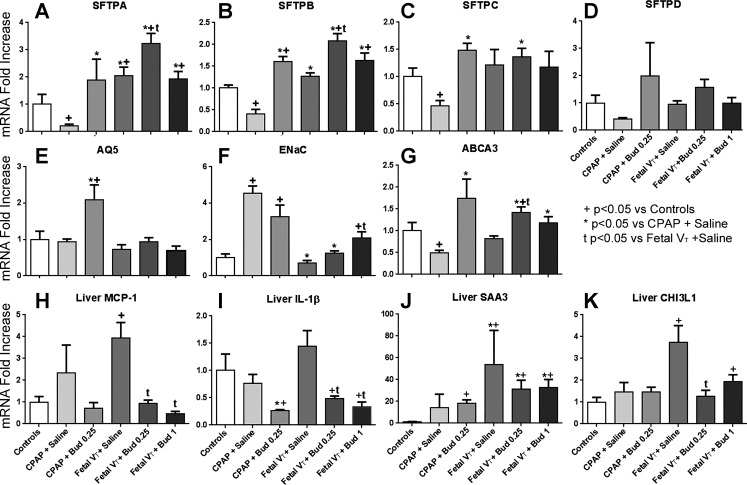
Surfactant protein A and B mRNAs were increased in animals exposed to budesonide (Fig. 4, A and B). There were consistent decreases in mRNA for SFTPA, SFTPB, and SFTPC in the CPAP + Saline groups compared with controls (Fig. 4). CPAP + Bud 0.25 did not have these decreases and had increased mRNA compared with controls. Animals receiving Fetal VT had small increases in some surfactant protein mRNA, which was further increased by budesonide for SFTPA and SFTPB. SFTPD did not change consistently for any of the groups. Fetal VT decreased this effect except in the animals receiving 1 mg/kg budesonide. AQ5 (Fig. 4E) was increased by budesonide in the CPAP animals, consistent with the decreased water content in the lungs of these animals (Table 1). The sodium channel ENaC mRNA (Fig. 4F) increased in both groups exposed to CPAP. ABCA3 mRNA had a similar mRNA pattern to that of surfactant proteins (Fig. 4G).
Fig. 4.
Lung and liver mRNA values. CPAP, continuous positive airway pressure; VT, tidal volume; VT15, VT for 15 min; Bud 1, budesonide 1 mg/kg; Bud 0.25, budesonide 0.25 mg/kg. Lung (A–E): mRNA for surfactant proteins A (SFTPA; A), SFTPB (B), SFTPC (C), and SFTPD (D). Aquaporin 5 (AQ5; E) and epithelial sodium channel (ENaC; F) mRNA in the lung tissue. G: ATP-binding cassette sub-family A member 3 (ABCA3) transporter mRNA in lung. Liver (H–K): proinflammatory cytokine monocyte chemoattractant protein-1 (MCP-1; H) and IL-1β mRNA (I) in liver tissue. Acute-phase response genes serum amyloid A3 (SAA3; J) and chitinase-3-like protein-1 (CHI3L1; K) mRNA responses in the liver. Values are means ± SE with unventilatedcontrol (UVC) set at 1; n = 5–8 animals/group. +P < 0.05 vs. UVC; *P < 0.05 vs. CPAP + Saline; tP < 0.05 vs. VT15 +Saline.
Liver and PMLN responses.
The Fetal VT + Saline animals showed decreased glycogen staining (0.4 ± 0.3 out of 2, P < 0.05) within the liver compared with controls (1.9 ± 0.2) and animals receiving Fetal VT + Bud 0.25 (1.9 ± 0.2) or Fetal VT + Bud 1 (1.4 ± 0.3). The livers of animals that received budesonide were similar to those of unventilated controls, as assessed by blinded sections. Mechanical ventilation increased liver mRNA expression for the acute-phase genes SAA3 and CHI3L1, and proinflammatory cytokine MCP-1 (Fig. 3, H–K). Budesonide decreased MCP-1 and IL-1β mRNA in the liver and prevented the increase in CHI3L1. PMLN weights were lighter in Fetal VT animals receiving surfactant plus saline compared with controls or animals receiving budesonide (Table 3). FoxP3 immunostaining was found in the majority of Fetal VT in animals receiving surfactant plus saline (Table 3) but not in the PMLN of control, CPAP, or Fetal VT animals with budesonide.
Table 3.
PMLNs
| Group | PMLN Weight, g | FoxP3 (of 2) |
|---|---|---|
| CPAP + Saline | 0.43 ± 0.03 | 0.2 ± 0.2 |
| CPAP + Bud 0.25 | 0.50 ± 0.04 | 0 ± 0 |
| Fetal VT + Saline | 0.28 ± 0.03+* | 1.3 ± 0.4+* |
| Fetal VT + Bud 0.25 | 0.80 ± 0.07* | 0.2 ± 0.2t |
| Fetal VT + Bud 1.0 | 0.65 ± 0.09 | 0 ± 0t |
| Unventilated controls | 0.57 ± 0.05t | 0 ± 0t |
Values are means ± SE. PMLNs, posterior mediastinal lymph nodes; VT, tidal volume; CPAP, continuous positive airway pressure; Bud, budesonide.
P < 0.05 vs. Controls;
P < 0.05 vs. CPAP + Saline;
P < 0.05 vs. Fetal VT +Saline.
DISCUSSION
These experiments were designed to probe the responses of the injured preterm lung treated with surfactant and a potent corticosteroid within the clinical context of a renewed interest in corticosteroid treatment soon after birth to decrease BPD. Recently, randomized clinical trials have demonstrated that three administration approaches to early corticosteroid treatment can decrease BPD in extremely low-birth-weight infants. Ten-day infusions of low-dose hydrocortisone, prolonged aerosolized budesonide, or budesonide mixed with surfactant significantly decreased BPD (3, 4, 39). Although older trials had demonstrated that high-dose corticosteroids given shortly after birth could decrease BPD, there were metabolic and neurodevelopmental risks without decreased death (9). The newer trials exposed the infants to lower-dose and/or more lung-targeted corticosteroid treatments and are, in the aggregate, proof of principle that early treatment with postnatal corticosteroid can benefit very preterm infants with lung injury (1). The strategy of mixing budesonide with surfactant is particularly attractive, because it targets the lung only during routine surfactant therapy, and the concentrated corticosteroid within the lung can have prolonged effects on the lung with minimal systemic toxicity (39). However, there is minimal information about budesonide effects on the preterm injured lung.
For this initial evaluation, we wanted a defined injury to the preterm lung that could be evaluated for corticosteroid effects without the progressive injury that results from continued mechanical ventilation of the preterm lung. We (7, 15, 18) had previously demonstrated that 15 min of high VT ventilation of the fetal lung caused an acute and reproducible injury that induced inflammatory mediators within 30 min, had peak expression of inflammation at 3–6 h, and decreased inflammation by 24 h in utero after the injury. We chose a 24-h injury-to-evaluation interval to assess the effects of budesonide on lung physiology and the residual molecular signals of the initial injury. This model captures the clinical scenario of resuscitation-mediated lung injury followed by surfactant treatment but avoids the progressive injury from subsequent ventilation and oxygen exposure (15). The 30-min ventilation after 24 h in utero was simply to test lung function.
CPAP alone for 15 min does not injure the premature lungs in this model (30). Thus, by using CPAP on an externalized fetus, we could test for budesonide effects on the uninjured lung and control for the externalization of the fetus. Budesonide induced early maturation in the fetus, as indicated by large improvements in lung function with improved gas exchange, increased lung gas volumes measured by the pressure-volume curves, decreased lung weight per kilogram body weight, and increases in mRNA for surfactant proteins and AQ5 relative to the unventilated controls and/or CPAP without budesonide. We have no explanation as to why CPAP alone suppressed expression of the mRNA for the surfactant proteins. Although not previously reported, the large dose of surfactant given after the CPAP may have suppressed de novo surfactant synthesis. The cytokine and inflammatory mediator mRNA changes are complicated to interpret, because the CPAP or Fetal VT injury responses are superimposed on the responses to 30 min of ventilation 24 h later. We interpret the modest elevations of proinflammatory and acute-phase reaction genes in the CPAP groups to reflect the effects of the 30-min ventilation. Budesonide decreased MCP-1 and increased Egr-1 and EREG mRNA relative to CPAP alone. Most strikingly, budesonide protected the airways from the 30 min of ventilation to appear comparable to the unventilated controls. Therefore, budesonide had maturational benefits for the preterm lambs and some indications of protection to subsequent ventilation.
The 15 min of escalating VT achieved the goal of causing a substantial and uniform lung injury (15, 18). The VT injury initially targeted the small airways and subsequently propagated throughout the fetal lungs (7, 12). Fetal lambs have the ability to repair ventilator-induced injury by 15 days after injury, and we have seen early activation of repair mechanisms by 24 h after injury (6, 7). We were surprised at the severity of physiological abnormalities at 24 h. Despite surfactant treatment after the injury, oxygenation and gas exchange were very poor even with a peak inspiratory pressure of 40 cmH2O. Lung gas volumes were ~45% of the CPAP plus surfactant group. The injury completely removed the surfactant effects, presumably because of severe inhibition of function, as the trachea had been ligated to prevent loss of the surfactant and budesonide. Furthermore, the budesonide did not suppress IL-1β, IL-6, or IL-8 expression, although MCP-1 was deceased for Fetal VT injury alone. The acute-phase response genes SAA3 and EREG were increased in the budesonide-treated groups. Budesonide prevented the airway changes that resulted from the 30-min ventilation and decreased PAS staining and Ki67-positive cells in the bronchioles, all effects that could benefit the preterm lung. However, contrary to our hypothesis, budesonide did not uniformly decrease indicators of inflammation in these immature lungs.
As indicators of a lung maturation response to lung injury, SFTPA and SFTPB expression increased relative to the unventilated controls or CPAP. We (18) previously reported that ventilation injury could increase indicators of lung maturation within 24 h in the fetal lung. Budesonide further increased SFTPA and SFTPB expression above that achieved with injury alone, another potential benefit to budesonide treatment after lung injury.
We also evaluated liver responses to lung injury and budesonide. We had previously demonstrated systemic effects of VT injury in this model (15). There are substantial concerns about systemic effects of postnatal corticosteroid treatments (9). CPAP alone did not change acute-response gene expression in the liver, although there was great variability for SAA3 mRNA expression. Budesonide decreased IL-1β expression. As previously reported, the Fetal VT injury increased indicators of acute-phase responses in the liver, and budesonide suppressed the IL-1β, MCP-1, and CHI3L1 mRNA responses. Changes in liver glycogen (PAS staining) with mechanical ventilation could have been due to changes in gluconeogenesis, but these findings would need to be further explored. In future studies, systemic effects of budesonide in the preterm lamb model should be evaluated for other organ systems.
We evaluated the 0.25 mg/kg dose of budesonide because that dose was used in the clinical trials (39, 40). The higher dose was selected to detect more potential benefit. There was no advantage of a higher dose, as all indicators of budesonide effects were similar. The lung and blood levels of budesonide provide minimal guidance to dosing as the single measurements were 24 h after treatment with surfactant and after 30 min of ventilation. Nevertheless, the blood level of 0.2 ng/ml for the 0.25 mg/kg dose is equivalent to ~2 ng/ml dexamethasone or 40 ng/ml cortisol. This level is a substantial exposure, which is in the range of corticosteroid exposures that cause lung maturation in sheep (23) and represent stress cortisol levels for the premature infant. The lung tissue budesonide level is substantially higher at roughly 20 ng/g, which would convert to ~400 ng/ml of cortisol.
Budesonide had large physiological and maturational effects on the uninjured fetal lung treated with surfactant. With lung injury just before treatment, budesonide did not improve lung function or have much of an effect on inflammation when assessed 24 h later. We (17) previously reported that antenatal corticosteroids greatly decreased post-delivery lung injury, but large doses of dexamethasone or cortisol given just before the injury had little effect. A similar result applies for budesonide in surfactant for lung function. However, other indicators such as protection of airways, induced lung maturation, and decreased systemic effects suggest benefits that could contribute to less BPD.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01-HD-072842 (to A. H. Jobe) and Chiesi Farmaceutici S.p.A. (to A. H. Jobe and N. H. Hillman).
DISCLOSURES
F. Salomone is employed by Chiesi Farmaceutical, who provided the surfactant for the experiments. He was not involved in the interpretation of the results, but he and Chiesi approved the manuscript. All other authors do not have conflicts of interest.
AUTHOR CONTRIBUTIONS
T.B.K., M.W.K., F.S., G.C.M., A.H.J., and N.H.H. conceived and designed research; T.B.K., E.R., M.W.K., M.S., H.U., S.W., G.C.M., A.H.J., and N.H.H. performed experiments; T.B.K., E.R., A.F.S., A.H.J., and N.H.H. analyzed data; T.B.K., A.F.S., A.H.J., and N.H.H. interpreted results of experiments; T.B.K., A.H.J., and N.H.H. prepared figures; T.B.K., M.W.K., A.H.J., and N.H.H. drafted manuscript; T.B.K., E.R., M.W.K., A.F.S., F.S., G.C.M., A.H.J., and N.H.H. edited and revised manuscript; T.B.K., E.R., M.W.K., A.F.S., F.S., M.S., H.U., S.W., G.C.M., A.H.J., and N.H.H. approved final version of manuscript.
REFERENCES
- 1.Bancalari E, Jain D, Jobe AH. Prevention of bronchopulmonary dysplasia: are intratracheal steroids with surfactant a magic bullet? Am J Respir Crit Care Med 193: 12–13, 2016. doi: 10.1164/rccm.201509-1830ED. [DOI] [PubMed] [Google Scholar]
- 2.Barrette AM, Roberts JK, Chapin C, Egan EA, Segal MR, Oses-Prieto JA, Chand S, Burlingame AL, Ballard PL. Antiinflammatory effects of budesonide in human fetal lung. Am J Respir Cell Mol Biol 55: 623–632, 2016. doi: 10.1165/rcmb.2016-0068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V, Van den Anker JN, Meisner C, Engel C, Schwab M, Halliday HL, Poets CF; NEUROSIS Trial Group . Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med 373: 1497–1506, 2015. doi: 10.1056/NEJMoa1501917. [DOI] [PubMed] [Google Scholar]
- 4.Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, Zupan-Simunek V, Coursol A, Beuchée A, Bolot P, Andrini P, Mohamed D, Alberti C; PREMILOC Trial Study Group . Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet 387: 1827–1836, 2016. doi: 10.1016/S0140-6736(16)00202-6. [DOI] [PubMed] [Google Scholar]
- 5.Brattsand R, Miller-Larsson A. The role of intracellular esterification in budesonide once-daily dosing and airway selectivity. Clin Therapeut 25, Suppl C: C28–C41, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 301: L917–L926, 2011. doi: 10.1152/ajplung.00207.2011. [DOI] [PubMed] [Google Scholar]
- 7.Deptula N, Royse E, Kemp MW, Miura Y, Kallapur SG, Jobe AH, Hillman NH. Brief mechanical ventilation causes differential epithelial repair along the airways of fetal, preterm lambs. Am J Physiol Lung Cell Mol Physiol 311: L412–L420, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JL; Victorian Infant Collaborative Study Group . Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med 377: 329–337, 2017. doi: 10.1056/NEJMoa1700827. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev (5): CD001146, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Fischer HS, Bührer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 132: e1351–e1360, 2013. doi: 10.1542/peds.2013-1880. [DOI] [PubMed] [Google Scholar]
- 11.Gazzotti T, Barbarossa A, Zironi E, Roncada P, Pietra M, Pagliuca G. An LC-MS/MS method for the determination of budesonide and 16α-hydroxyprednisolone in dog plasma. MethodsX 3: 139–143, 2016. doi: 10.1016/j.mex.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillman NH, Kallapur SG, Pillow JJ, Moss TJ, Polglase GR, Nitsos I, Jobe AH. Airway injury from initiating ventilation in preterm sheep. Pediatr Res 67: 60–65, 2010. doi: 10.1203/PDR.0b013e3181c1b09e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillman NH, Kemp MW, Miura Y, Kallapur SG, Jobe AH. Sustained inflation at birth did not alter lung injury from mechanical ventilation in surfactant-treated fetal lambs. PLoS One 9: e113473, 2014. doi: 10.1371/journal.pone.0113473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman NH, Kemp MW, Noble PB, Kallapur SG, Jobe AH. Sustained inflation at birth did not protect preterm fetal sheep from lung injury. Am J Physiol Lung Cell Mol Physiol 305: L446–L453, 2013. doi: 10.1152/ajplung.00162.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 176: 575–581, 2007. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillman NH, Moss TJ, Nitsos I, Jobe AH. Moderate tidal volumes and oxygen exposure during initiation of ventilation in preterm fetal sheep. Pediatr Res 72: 593–599, 2012. doi: 10.1038/pr.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillman NH, Pillow JJ, Ball MK, Polglase GR, Kallapur SG, Jobe AH. Antenatal and postnatal corticosteroid and resuscitation induced lung injury in preterm sheep. Respir Res 10: 124, 2009. doi: 10.1186/1465-9921-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillman NH, Polglase GR, Pillow JJ, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol 300: L232–L241, 2011. doi: 10.1152/ajplung.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, Buzas JS. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr 171: e164396, 2017. doi: 10.1001/jamapediatrics.2016.4396. [DOI] [PubMed] [Google Scholar]
- 20.Jendbro M, Johansson CJ, Strandberg P, Falk-Nilsson H, Edsbäcker S. Pharmacokinetics of budesonide and its major ester metabolite after inhalation and intravenous administration of budesonide in the rat. Drug Metab Dispos 29: 769–776, 2001. [PubMed] [Google Scholar]
- 21.Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore Ervin M, Padbury JF, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med 162: 1656–1661, 2000. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 22.Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, Kramer BW, Newnham JP, Ikegami M, Jobe AH. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med 179: 955–961, 2009. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp MW, Saito M, Usuda H, Molloy TJ, Miura Y, Sato S, Watanabe S, Clarke M, Fossler M, Scmidt A, Kallapur SG, Kramer BW, Newnham JP, Jobe AH. Maternofetal pharmacokinetics and fetal lung responses in chronically catheterized sheep receiving constant, low-dose infusions of betamethasone phosphate. Am J Obstet Gynecol 215: e771–e775, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med 171: 73–77, 2005. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 25.Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 164: 982–988, 2001. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- 26.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, Cotten CM, Wilson-Costello DE, Shankaran S, Van Meurs KP, Davis AS, Gantz MG, Finer NN, Yoder BA, Faix RG, Carlo WA, Schibler KR, Newman NS, Rich W, Das A, Higgins RD, Walsh MC; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 183: 1715–1722, 2011. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore CD, Roberts JK, Orton CR, Murai T, Fidler TP, Reilly CA, Ward RM, Yost GS. Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos 41: 379–389, 2013. doi: 10.1124/dmd.112.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL. Lung surfactant replacement in premature lambs with extracted lipids from bovine lung lavage: effects of dose, dispersion technique, and gestational age. Pediatr Res 19: 569–577, 1985. doi: 10.1203/00006450-198506000-00014. [DOI] [PubMed] [Google Scholar]
- 29.O'Connell EJ. Review of the unique properties of budesonide. Clin Therapeut 25, Suppl C: C42–C60, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RY, Royse E, Kemp MW, Miura Y, Noe A, Jobe AH, Hillman NH. Distending pressure did not activate acute phase or inflammatory responses in the airways and lungs of fetal, preterm lambs. PLoS One 11: e0159754, 2016. doi: 10.1371/journal.pone.0159754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polglase GR, Hillman NH, Ball MK, Kramer BW, Kallapur SG, Jobe AH, Pillow JJ. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation. Pediatr Res 65: 67–71, 2009. doi: 10.1203/PDR.0b013e318189487e. [DOI] [PubMed] [Google Scholar]
- 32.Ricci F, Catozzi C, Ravanetti F, Murgia X, D’Aló F, Macchidani N, Sgarbi E, Di Lallo V, Saccani F, Pertile M, Cacchioli A, Catinella S, Villetti G, Civelli M, Amadei F, Stellari FF, Pioselli B, Salomone F. In vitro and in vivo characterization of poractant alfa supplemented with budesonide for safe and effective intratracheal administration. Pediatr Res 82: 1056–1063, 2017. doi: 10.1038/pr.2017.171. [DOI] [PubMed] [Google Scholar]
- 33.Shinwell ES, Portnov I, Meerpohl JJ, Karen T, Bassler D. Inhaled corticosteroids for bronchopulmonary dysplasia: a meta-analysis. Pediatrics 138: e20162511, 2016. doi: 10.1542/peds.2016-2511. [DOI] [PubMed] [Google Scholar]
- 34.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID III, Watterberg KL, Saha S, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 313: L1101–L1153, 2017. doi: 10.1152/ajplung.00343.2017. [DOI] [PubMed] [Google Scholar]
- 36.Ueki R, Tatara T, Kariya N, Shimode N, Hirose M, Tashiro C. Effect of decreased fetal perfusion on placental clearance of volatile anesthetics in a dual perfused human placental cotyledon model. J Anesth 28: 635–638, 2014. doi: 10.1007/s00540-013-1777-3. [DOI] [PubMed] [Google Scholar]
- 37.van den Brink KI, Boorsma M, Staal-van den Brekel AJ, Edsbäcker S, Wouters EF, Thorsson L. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol 66: 27–35, 2008. doi: 10.1111/j.1365-2125.2008.03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkataraman R, Kamaluddeen M, Hasan SU, Robertson HL, Lodha A. Intratracheal administration of budesonide-surfactant in prevention of bronchopulmonary dysplasia in very low birth weight infants: a systematic review and meta-analysis. Pediatr Pulmonol 52: 968–975, 2017. doi: 10.1002/ppul.23680. [DOI] [PubMed] [Google Scholar]
- 39.Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, Tsai CH, Lin HC. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med 193: 86–95, 2016. doi: 10.1164/rccm.201505-0861OC. [DOI] [PubMed] [Google Scholar]
- 40.Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, Pyati S, Tsai CH. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics 121: e1310–e1318, 2008. doi: 10.1542/peds.2007-1973. [DOI] [PubMed] [Google Scholar]