HEAT INDUCIBLE LIPASE1 mitigates heat stress in Arabidopsis leaves and plays an important role in the turnover of monogalactosyldiacylglycerol in chloroplasts under heat stress.
Abstract
Under heat stress, polyunsaturated acyl groups, such as α-linolenate (18:3) and hexadecatrienoate (16:3), are removed from chloroplastic glycerolipids in various plant species. Here, we showed that a lipase designated HEAT INDUCIBLE LIPASE1 (HIL1) induces the catabolism of monogalactosyldiacylglycerol (MGDG) under heat stress in Arabidopsis thaliana leaves. Using thermotolerance tests, a T-DNA insertion mutant with disrupted HIL1 was shown to have a heat stress-sensitive phenotype. Lipidomic analysis indicated that the decrease of 34:6-MGDG under heat stress was partially impaired in the hil1 mutant. Concomitantly, the heat-induced increment of 54:9-triacylglycerol in the hil1 mutant was 18% lower than that in the wild-type plants. Recombinant HIL1 protein digested MGDG to produce 18:3-free fatty acid (18:3-FFA), but not 18:0- and 16:0-FFAs. A transient assay using fluorescent fusion proteins confirmed chloroplastic localization of HIL1. Transcriptome coexpression network analysis using public databases demonstrated that the HIL1 homolog expression levels in various terrestrial plants are tightly associated with chloroplastic heat stress responses. Thus, HIL1 encodes a chloroplastic MGDG lipase that releases 18:3-FFA in the first committed step of 34:6 (18:3/16:3)-containing galactolipid turnover, suggesting that HIL1 has an important role in the lipid remodeling process induced by heat stress in plants.
INTRODUCTION
Terrestrial plants increasingly suffer from high temperature stress due to climate change. Heat stress increases the fluidity of the membrane bilayer (Lee, 2000; Los and Murata, 2004). Plants have evolved a mechanism to change the membrane lipid composition and stabilize the membranes in response to heat stress. Heat stress induces a decrease in chloroplastic polyunsaturated acyl groups: α-linolenate (18:3) and hexadecatrienoate (roughanic acid) (16:3). These acyl groups are mostly covalently bonded to the glycerol backbones of monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG). These heat-induced metabolic changes are well conserved among various plant species, e.g., Atriplex lentiformis (Pearcy, 1978), Nerium oleander (Raison et al., 1982), Arabidopsis thaliana (Falcone et al., 2004; Higashi et al., 2015; Li et al., 2015), turf grasses (Poa pratensis, P. arachnifera, and Festuca arundinacea) (Su et al., 2009), and wheat (Triticum aestivum) (Narayanan et al., 2016).
MGDG is a major component of thylakoid membranes, and accounts for 50 mol% of the glycerolipid content in chloroplasts (Li-Beisson et al., 2013). In Arabidopsis leaves, MGDG acyl chains are composed approximately of 60 mol% of 18:3 and 30 mol% of 16:3 polyunsaturated fatty acids. Two MGDG biosynthetic pathways exist in plants. One of them, the prokaryotic pathway, is characterized by MGDG production with the almost exclusive presence of a 16:3 at the sn-2 position of the glycerol backbone, whereas the eukaryotic pathway synthesizes MGDG with 18:3 at the glyceryl sn-2 position. The expression of genes involved in the prokaryotic pathway is downregulated under heat stress (Higashi et al., 2015; Li et al., 2015). Fatty acid desaturases (FADs) introduce double bonds into MGDG saturated acyl chains (McConn and Browse, 1996; Routaboul et al., 2000). FAD8, a temperature-sensitive isoform of the plastidial ω-3 FADs in Arabidopsis, was reported to be unstable and degraded under heat stress (Matsuda et al., 2005). Thus, downregulation of the genes involved in the synthesis of MGDG and instability of the protein responsible for the introduction of double bonds into fatty acid chains cause a decrease in MGDG polyunsaturated acyl groups in chloroplasts under heat stress.
Although MGDG biosynthesis in leaves has been relatively well characterized, the catabolic process of MGDG turnover under stress conditions is unknown. The turnover rate of leaf membrane lipids is slow (Bao et al., 2000); therefore, lipid turnover is suggested to partly depend on the rate of de novo fatty acid synthesis (Falcone et al., 2004) and also be controlled by the activities of lipases, several of which are functionally redundant (Troncoso-Ponce et al., 2013). For instance, the production of jasmonate, a plant hormone, in leaves from polyunsaturated fatty acids is catalyzed by several redundant lipase genes (Ellinger et al., 2010). Two types of gene family have been reported to encode MGDG lipases in higher plants, which belong to DEFECTIVE IN ANTHER DEHISCENCE1-like (DAD1-like) proteins and patatin-like lipid acyl hydrolases. DAD1 has been found to be essential for pollen development by catalyzing the first committed step of the jasmonate biosynthetic pathway (Ishiguro et al., 2001). Some Arabidopsis patatin-like lipid acyl hydrolases are upregulated in leaves in response to water deficit and possibly contribute to membrane lipid degradation under drought stress (Matos et al., 2001, 2008). Although a large number of possible chloroplastic MGDG lipase genes have been identified in the Arabidopsis genome, the genes functioning in heat-stressed leaves remain unknown. To obtain further insight into chloroplastic membrane lipid remodeling under heat stress, it is important to identify the particular lipase genes responsible for the liberation of unsaturated fatty acids from MGDG.
We previously reported the changes in glycerolipid composition and gene expression of Arabidopsis leaves under heat stress (Higashi et al., 2015). The lipidomic data suggested that heat stress reduced the quantities of chloroplastic glycerolipids containing polyunsaturated acyl groups: MGDG, sulfoquinovosyldiacylglycerol (SQDG), and phosphatidylglycerol (PG). However, heat stress increased 18:3-containing phosphatidylcholine (PC), diacylglycerol (DAG), and triacylglycerol (TAG), which possibly serve as the acyl carrier of lipid turnover toward the β-oxidation of chloroplast-derived fatty acids. Transcriptome data suggested that heat stress induces the genes involved in lipid turnover, i.e., TAG synthesis, TAG degradation, and β-oxidation of fatty acids. Therefore, we hypothesized that some unidentified genes, which encode lipases localized in chloroplasts and are induced by heat stress, could participate in the catabolism of chloroplastic glycerolipids to liberate 18:3, leading to the synthesis of 18:3-containing TAG under heat stress.
In this study, we characterized a lipase designated HEAT INDUCIBLE LIPASE1 (HIL1) and proposed that it plays a considerable role in MGDG catabolism under heat stress. To select Arabidopsis candidate genes that affect glycerolipid composition under heat stress, we searched publicly available transcriptome data and omics databases. We focused on a single gene, HIL1, and examined the lipid composition of a T-DNA insertion mutant (hil1). We genetically complemented this mutant and analyzed it using the liquid chromatography-mass spectrometry (LC-MS)-based lipidomic platform. Using thermotolerance tests, we demonstrate that HIL1 plays a role in protecting leaves against heat stress. In addition to the lipidomic analysis, we elucidated the characteristic lipase activity of the HIL1 recombinant protein in vitro to liberate 18:3 from MGDG. Our results suggest that the lipase activity of HIL1 is responsible for the preferential turnover of chloroplastic 34:6-MGDG under heat stress. We present a general model of HIL1 function in chloroplastic galactolipid turnover in leaves as a conditionally vital component in heat stress responses across a variety of plant species.
RESULTS
Characteristic Expression Pattern of Lipase Genes under Heat Stress Delineates a Candidate Lipase Gene
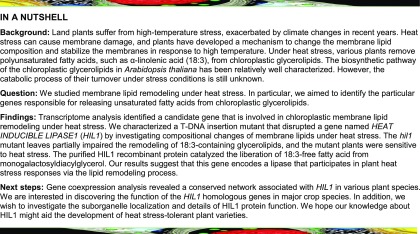
Approximately 300 putative lipase (acyl hydrolase) genes have been reported for Arabidopsis (Li-Beisson et al., 2013; Troncoso-Ponce et al., 2013). Among these, the expression levels of 181 genes were quantified in previous microarray data (Higashi et al., 2015). Hierarchical clustering analysis based on the expression patterns under different heat stresses resulted in the separation of 24 genes belonging to groups 9 and 10 (Figure 1A), which were induced by heat stress and tended to return to normal expression levels during temperature recovery (Table 1). Interestingly, groups 9 and 10 contain well-characterized lipases involved in phospholipid metabolism, phosphatidate phosphohydrolase 1 and 2 (PAH1 and PAH2) (Nakamura et al., 2009; Eastmond et al., 2010), and in TAG degradation, SUGAR-DEPENDENT1 (SDP1) and SDP1-LIKE (Eastmond, 2006; Kelly et al., 2013).
Figure 1.
Gene Expression of Putative Lipase Genes under Heat Stress.
(A) A tree diagram shows the similarity of expression patterns among the 181 Arabidopsis putative lipase genes under different heat stresses. Previous GeneChip data were used for the analysis (Higashi et al., 2015). Hierarchical clustering analysis classified the genes into 12 groups (red lines). Groups 9 and 10 (red font, enlargement in inset) contain genes (probe set IDs) induced by heat stress and returned to normal levels during temperature recovery (see Table 1 for detail).
(B) Microarray data of a heat-inducible lipase (At4g13550, HIL1), which belongs to group 10 (blue font, “254715_at”) in (A). Each data point was represented by a box and whisker plot (biological replicates of n = 3 to 9).
(C) The transcript levels of HIL1 were quantified by RT-qPCR. The y axis represents the relative expression level of HIL1 against “Control 08 h 17 d” calculated by the comparative CT (ΔΔCT) method. RNA content was normalized by EIF4A1 as an internal standard. Each data point expresses the mean of three experiments ± sd. The whole rosettes harvested from two to three different plants were pooled as one biological repeat.
Table 1. Putative Lipase Genes Responding to Heat Stress Clustered in Groups 9 and 10 in Figure 1A.
| AGI Code | Abbreviation | Probe Set ID | Description | Subcellular Localizationa |
|---|---|---|---|---|
| At1g19190 | 256039_at | α/β-Hydrolases superfamily protein | Cytosol | |
| At3g57140 | SDP1-likeb | 251654_at | Sugar dependent 1 like | Plasma membrane |
| At4g11840 | PLDγ3 | 254894_at | Phospholipase Dγ3 | Cytosol |
| At4g11830 | PLDγ2c | 254846_at | Phospholipase Dγ2 | Cytosol |
| 254893_at | ||||
| At5g62200 | 247459_at | Embryo specific protein 3 | Vacuole | |
| At3g14075 | 257006_at | Mono- diacylglycerol lipase like | Endoplasmic reticulum | |
| At3g50920 | LPPε1d | 252155_at | Phosphatidic acid phosphatase | Plastid |
| At1g52570 | PLDα2 | 262147_at | Phospholipase Dα2 | Cytosol |
| At1g02660 | PLIP2e | 260915_at | Plastid lipase 2 | Plasma membrane |
| At3g09560 | PAH1f | 258721_at | Phosphatidic acid phosphohydrolase 1 | Nucleus |
| At5g14310 | CXE16 | 250185_at | Carboxyesterase 16 | Plasma membrane |
| At2g16900 | 266536_at | Arabidopsis phospholipase-like protein family | Nucleus | |
| At5g42870 | PAH2f | 249168_at | Phosphatidic acid phosphohydrolase 2 | Nucleus |
| At4g00500 | 255677_at | α/β-Hydrolases superfamily protein | Plasma membrane | |
| At1g31480 | SGR2g | 256513_at | Shoot gravitropism 2 | Nucleus |
| At2g42450 | 265879_at | α/β-Hydrolases superfamily protein | Mitochondrion | |
| At1g05790 | 261312_at | Lipase class 3 family protein | Plasma membrane | |
| At5g04040 | SDP1b | 250877_at | Sugar dependent 1 | Nucleus |
| At5g37710 | 249577_at | α/β-Hydrolases superfamily protein | Plasma membrane | |
| At5g20060 | 246124_at | α/β-Hydrolases superfamily protein | Mitochondrion | |
| At3g55940 | 251768_at | Phosphoinositide-specific phospholipase C family protein | Mitochondrion | |
| At5g11650 | 250335_at | α/β-Hydrolases superfamily protein | Endoplasmic reticulum | |
| At1g75890 | 262681_at | GDSL-like lipase superfamily protein | Extracellular | |
| At4g13550 | HIL1h | 254715_at | Heat inducible lipase 1 | Plastid |
The 24 genes are listed in the order shown in Figure 1A, from left to right.
Subcellular localization was predicted using the SUBA3 website (http://suba.live) (as of May, 2018).
Wang et al. (2018).
This study.
We searched the web database SUBA (Hooper et al., 2014) to predict subcellular localization of the proteins encoded by the 24 genes in groups 9 and 10 (Table 1). At3g50920 (LPPε1) and At4g13550 (named HIL1) were predicted to encode chloroplast-localized proteins. LPPε1 has previously been characterized as a phosphatidic acid phosphatase in chloroplasts (Nakamura et al., 2007). HIL1 was annotated only as putative TAG lipase in the TAIR database (http://www.arabidopsis.org), but no detailed characterization has been made. The SUBA website mentioned three distinct proteomic studies, which reported peptide sequences of HIL1 from the purified chloroplastic fractions (Zybailov et al., 2008; Kong et al., 2011; Tomizioli et al., 2014).
Our previous microarray data (Higashi et al., 2015) indicated that HIL1 expression levels increased to 2.1-fold at 8 h heat stress and 2.0-fold at 24 h heat stress compared with that of the control at 8 and 24 h, respectively, and then decreased to control levels during recovery from heat stress (recovery at 8 and 24 h) (Figure 1B). RT-qPCR confirmed the microarray results (Figure 1C): The expression levels of HIL1 increased to 4.4-fold (Heat 08 h) and 3.3-fold (Heat 24 h) under heat stress compared with those under control conditions and decreased to control levels during temperature recovery. These results suggested that HIL1 could play a role in the adaptation of chloroplasts to heat stress.
Analysis of the Coding Sequence of HIL1 Suggests That HIL1 Encodes a Lipase Localized in Chloroplasts
Although the proteome databases suggested the chloroplastic localization of HIL1, the TAIR and RAFL databases (http://rarge.psc.riken.jp) predicted that coding sequences (CDSs) of HIL1 do not contain putative chloroplast transit peptide (cTP) sequences. To confirm the amino acid sequence of HIL1, we amplified the transcript of HIL1 by RT-PCR from Arabidopsis Col-0 leaf mRNA and conducted 5′ RACE. At least eight possible transcripts of splice variants were obtained from leaves under heat stress (Supplemental Figure 1). The isolated HIL1 CDS contained a 12-bp-long 5′ untranslated region (5′ UTR) (clone 1). 3′ RACE analysis suggested that the polyadenylation site of HIL1 was located 192 bp downstream of the stop codon.
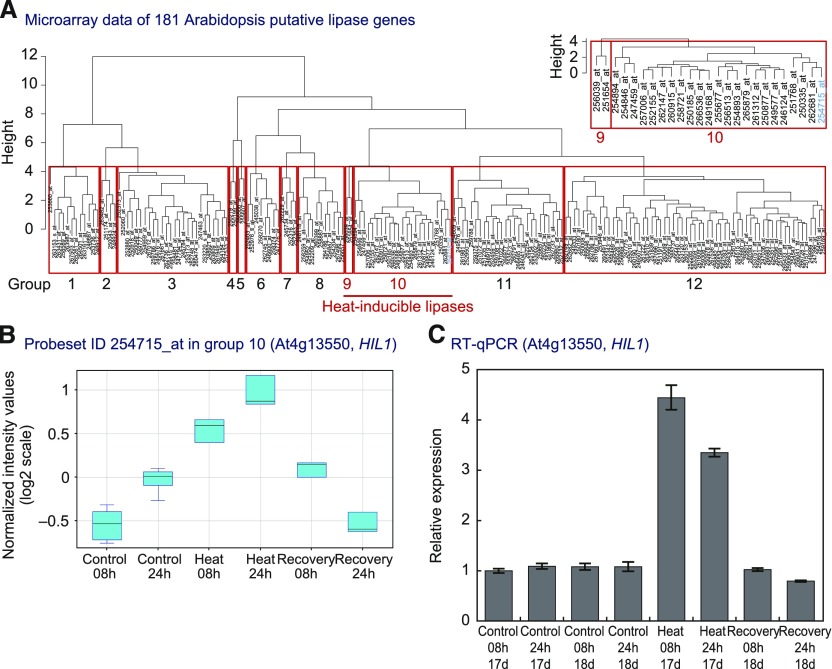
HIL1 encodes a polypeptide of 854 amino acid residues (Figure 2A). The polypeptide encoded by HIL1 contains a lipase-like domain and a C2 domain. The N-terminal 48 amino acid residues of HIL1 were predicted to be a cTP sequence by the ChloroP 1.1 server (https://www.cbs.dtu.dk/services/ChloroP). We confirmed that HIL1 mRNA encodes a putative cTP sequence region at exon 1, and there is no in-frame ATG upstream of the isolated full-length HIL1 CDS. Recently, a HIL1 CDS named At4g13550.2, the sequence of which is identical to that obtained in our study, but with a 5′ UTR that is slightly different, was reported in the Arabidopsis database (Araport 11, https://www.araport.org) (Cheng et al., 2017). These results suggest that HIL1 underwent alternative splicing at the region encoding the N-terminal amino acid sequences. We further demonstrated that HIL1 encodes a protein containing a putative cTP sequence.
Figure 2.
HIL1 Has Relatively Unique Characteristics among the Lipase Genes.
(A) Schematic representation of HIL1 structure. Regions of the putative cTP sequence, C2 domain, and lipase-like domain are shown in green, blue, and brown boxes, respectively.
(B) Sequence similarity between HIL1 and DAD1-like proteins. Arabidopsis genes that exhibit homology to the lipase-like domain of HIL1 were selected. Only the lipase-like domain sequences were used to generate the amino acid sequence alignment (Supplemental Data Set 1). Twelve DAD1-like proteins formed a group (blue). HIL1 is separated from this group (purple).
(C) Phylogenetic relationships among HIL1 and homologs in terrestrial plants. The phylogenetic tree was constructed from an alignment of deduced full-length amino acid sequences (Supplemental Data Set 1). The bar indicates amino acid substitutions per site. Bootstrap values (2000 replicates) > 70% are shown next to the branches.
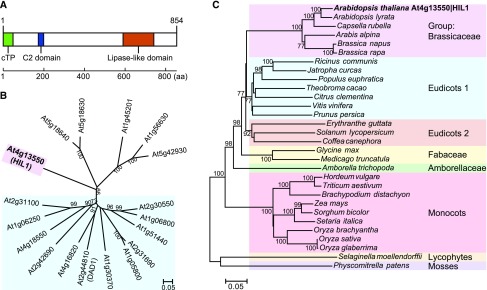
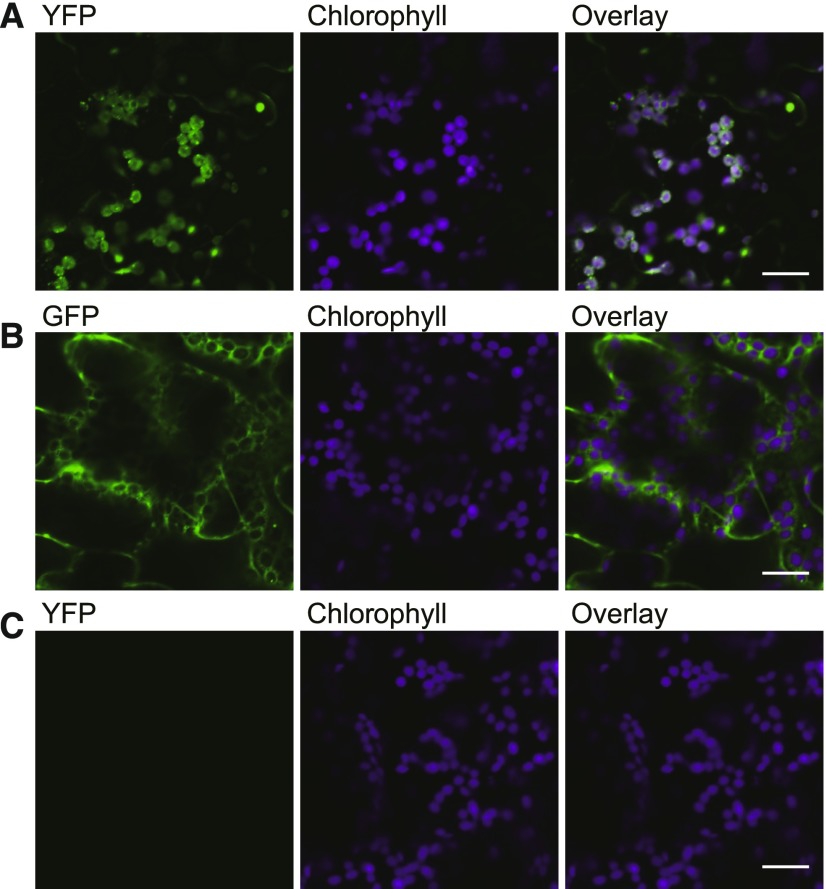
To confirm the chloroplastic targeting of HIL1, we fused the full-length amino acid sequence of HIL1 (M1-S854) to the N terminus of YFP and transiently expressed this fusion in Nicotiana benthamiana leaves under the control of the constitutive 35S promoter. Fluorescence derived from YFP of the HIL1-YFP fusion protein was associated with chlorophyll fluorescence (Figure 3A). Controls with a GFP and the p19 vector yielded no fluorescence in chloroplasts at the same signal level (Figures 3B and 3C). The chloroplastic localization of HIL1 was indicated from the result of the microscopy analysis in addition to the proteome database and the putative cTP sequence.
Figure 3.
Confocal Micrographs Showing Chloroplast Targeting of HIL1.
The HIL1-YFP fusion protein was transiently expressed in N. benthamiana leaves. HIL1-YFP (full-length HIL1 fused with YFP) (A), free GFP (B), and p19 negative control (C). Bars = 20 μm.
Homologous Genes of HIL1 Are Evolutionarily Conserved across Plant Species
Searching the TAIR database, we could not identify any homologous gene of HIL1 in Arabidopsis. Only the lipase-like domain of HIL1 (from V590 to R741) was similar to the lipase domain of the DAD1-like proteins (Hong et al., 2000; Ishiguro et al., 2001; Matsui et al., 2004; Seo et al., 2009). An unrooted phylogenetic tree of the 18 lipase-like domains from HIL1 and DAD1-like proteins (Figure 2B; Supplemental Data Set 1) indicated that the lipase-like domain sequence of HIL1 was located distal to that of the 12 DAD1-like proteins. Compared with the DAD1-like proteins, HIL1 has an additional N-terminal C2 domain and an unknown intermediate region (including I232 to Q577 encoded by exon 8). HIL1 also has a different exon-intron structure, with six introns in the lipase-like domain compared with no or one intron in DAD1-like genes. These sequence analyses suggest that HIL1 encodes a unique lipid acyl hydrolase different from the well-characterized DAD1-like proteins.
To investigate the evolutionary relationship, BLAST searches were conducted with the entire amino acid sequence of HIL1 on the UniProt database (http://www.uniprot.org). Phylogenetic analysis was performed on HIL1-like protein sequences from 30 plant species. The results suggest that homologous genes are widely distributed among terrestrial plants in Brassicaceae, Fabaceae, other eudicots, monocots, Amborellaceae, lycophytes, and mosses (Figure 2C; Supplemental Data Set 1). We mostly found only one HIL1 homolog from the same plant species. HIL1 homologs with lower sequence similarity were also found in microalgal species (Micromonas commoda). The HIL1 homolog in this microalga had 26% amino acid sequence identity with AtHIL1. These evolutionary studies indicated a possible widely indispensable function of HIL1-like proteins in terrestrial plants.
Characterization of a T-DNA Insertion Mutant and Its Genetically Complemented Plants Indicates a Physiological Role of HIL1 in Heat Tolerance
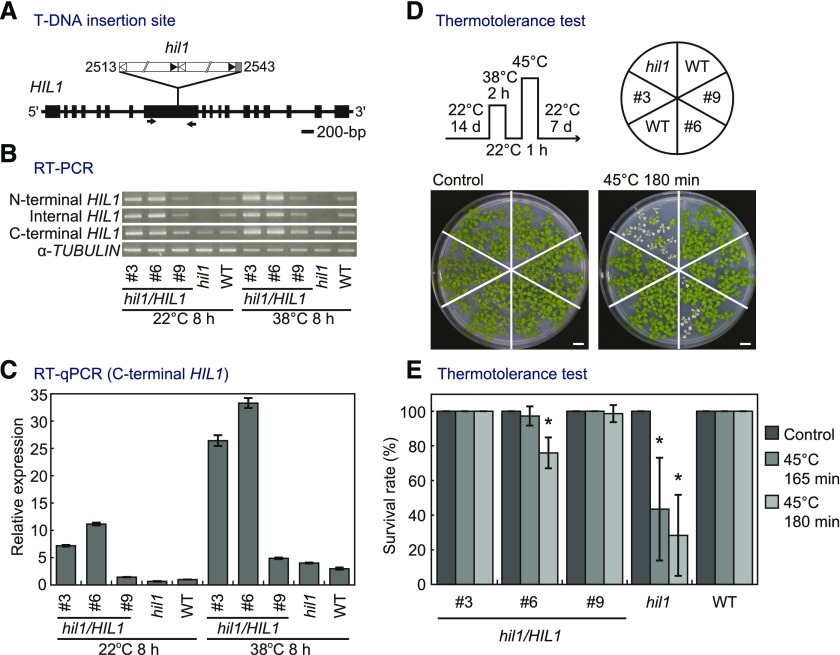
To define the in vivo function of HIL1, a T-DNA insertion mutant designated as hil1 was used for reverse genetic analysis. We isolated homozygous hil1 plants and determined the sole T-DNA insertion site at exon 8 of HIL1 (Figure 4A). The result of the DNA gel blot analysis indicated insertion of the T-DNA is at a single locus in the hil1 mutant (Supplemental Figure 2). For a complementation test of the T-DNA insertion mutant, we introduced the genomic sequence of HIL1 (7.4-kb length) into the homozygous hil1 mutant, resulting in hil1/HIL1 plants.
Figure 4.
Genomic Organization of the hil1 T-DNA Insertion Line, and the Impact of Insertional Mutation and Its Genetic Complementation on HIL1 Expression Levels and Thermotolerance.
(A) Schematic representation of HIL1 (At4g13550) with a T-DNA insertion mutant (hil1) used in this work. Exons are shown as boxes. White and black triangles show left and right borders, respectively. Numbers indicate the position of the T-DNA insertion from the start codon of HIL1. The arrows indicate primers used for RT-PCR (internal HIL1) in (B).
(B) RT-PCR analysis of HIL1 transcripts in the wild-type plants (WT), hil1 mutant, and hil1 complemented with the genomic sequence of HIL1 (hil1/HIL1 #3, #6, and #9 T3 lines). Total RNA was extracted from the whole rosettes of 17-d-old plants under heat stress conditions at 38°C for 8 h (38°C 8 h). As a control, plants not subjected to the stress but grown under normal conditions at 22°C were examined at the same time point (22°C 8 h). HIL1 expression levels were examined at the N-terminal, internal (interrupted by the T-DNA insertion), and C-terminal regions. α-TUBULIN was used as the control.
(C) RT-qPCR of HIL1 transcripts in the wild type, hil1, hil1/HIL1 #3, #6, and #9. The y axis represents relative expression level of HIL1 against 22°C 8 h WT calculated by the comparative CT (ΔΔCT) method. RNA content was normalized by EIF4A1 as an internal standard. Each data point expresses the mean of three experiments ± sd. The whole rosettes harvested from two to three different plants were pooled as one biological repeat. Primers were designed at the C-terminal region of HIL1, which is located downstream of the T-DNA insertion site.
(D) and (E) The effects of heat shock on growth of the wild-type, hil1 mutant, and hil1/HIL1-complemented plants (#3, #6, and #9 T4 lines). Fourteen-day-old plants were treated at 38°C for 2 h, 22°C for 1 h, and 45°C for 165 or 180 min (45°C 165 min and 45°C 180 min), followed by recovery at 22°C for 7 d. Plants grown continuously at 22°C were examined as the normal growth condition (Control). Photographs of the whole rosettes were taken after the recovery periods (D). Survival rate was calculated based on the number of plants surviving after recovery (E). Each data point expresses the mean of more than four experiments ± sd. Significant differences from the wild-type plants were analyzed using Welch’s t test (*P < 0.001). Bars = 1 cm.
RT-PCR and RT-qPCR analyses were performed for HIL1 transcripts in leaves of 17-d-old wild-type, hil1 mutant, and complemented (hil1/HIL1 #3, #6, and #9) plants. HIL1 expression levels were examined by amplifying the N-terminal, internal, and C-terminal regions (Figures 4B and 4C). Transcripts for the N-terminal and internal regions were absent in the hil1 mutant, but the C-terminal region was still expressed, although it was expressed at lower levels than in the wild-type plants at 22°C (Figure 4C). HIL1 expression levels in the hil1/HIL1 #3 and #6 complemented plants were higher than those of the wild-type plants (6.9- and 10.4-fold, respectively, at 22°C). HIL1 expression level in the hil1/HIL1 #9 complemented plant was comparable to that of the wild-type plants. HIL1 expression increased under heat stress in the lines (Figure 4C). These results suggest that HIL1 expression levels changed with the inserted position of the complemented genomic DNA, and in all three cases, HIL1 expression was induced under heat stress.
The vegetative hil1 mutant exhibited no visible phenotypic changes under normal growth conditions. We conducted thermotolerance tests by recovery of plants after heat stress (Figure 4D). Plants were pretreated at 38°C for 2 h, then subjected to 45°C for 165 or 180 min. The wild-type and hil1/HIL1-complemented plants tolerated the heat shock, while the leaves of the hil1 mutant were severely bleached after recovery for 7 d. Survival rates of the hil1 mutant after heat stress were significantly reduced in comparison with those of the wild-type and hil1/HIL1-complemented plants (P < 0.001, Welch’s t test). We monitored the maximum photochemical efficiency of photosystem II (Fv/Fm) in leaves of the wild-type, hil1 mutant, and hil1/HIL1-complemented plants under short-term heat stress. Photosynthetic activity decreased with increased incubation time under heat stress, but that in the hil1 mutant was not significantly different from that in the wild-type and hil1/HIL1-complemented plants, at least during the first 5 h of heat stress (Supplemental Table 1). These results indicate that impairment of HIL1 in the hil1 mutant leads to significant growth defects on the recovery following exposure to heat shock, but does not affect measurable photosynthetic activity under a short-term heat stress.
The Accumulation Pattern of 18:3-Containing Glycerolipids Is Changed in the hil1 Mutant and hil1/HIL1-Complemented Plants
To determine the contribution of HIL1 to glycerolipid metabolism in vivo, we compared the glycerolipid composition in leaves of the wild-type, hil1 mutant, and complemented hil1/HIL1 plants (#3, #6 and #9) using the LC-MS-based lipidomic platform (Figure 5; Supplemental Figure 3). Samples were prepared from six separate cultures, where each has three or four biological replicates for the individual lines under normal temperature and heat stress conditions. A total of 96 lipid species were detected from Arabidopsis leaves (Supplemental Data Set 2), and from these, 62 lipid species were detected in all samples.
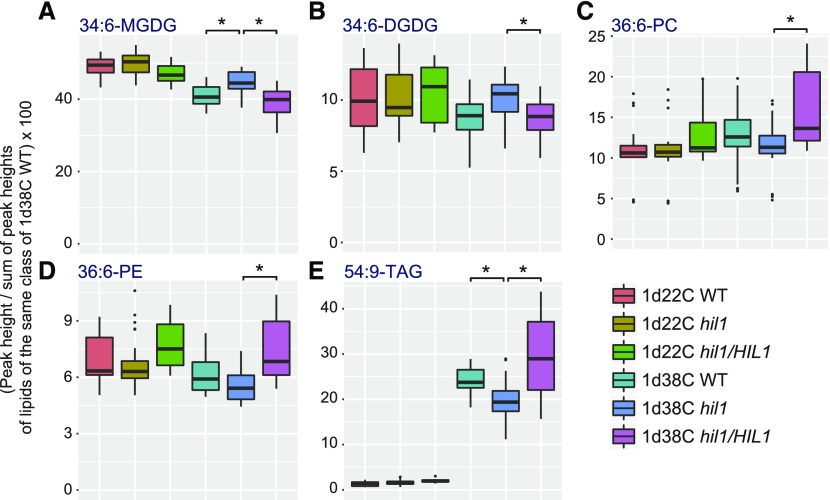
Figure 5.
Profiles of Characteristic Glycerolipid Species in Leaves of the Wild-Type, T-DNA Insertion Mutant, and Complemented Plants under Heat Stress.
Each data point of 34:6-MGDG (A), 34:6-DGDG (B), 36:6-PC (C), 36:6-PE (D), and 54:9-TAG (E) is represented by a box and whisker plot. Seventeen-day-old wild-type (WT), hil1 mutant, and hil1/HIL1-complemented plants were treated with 38°C for 1 d (1d38C WT, 1d38C hil1, and 1d38C hil1/HIL1). Crude lipid extracts from leaves were analyzed by LC-MS in the positive ion mode. Plants grown continuously at 22°C were examined as the normal growth condition (1d22C WT, 1d22C hil1, and 1d22C hil1/HIL1). Each data point expresses biological replicates of n ≥ 20. The #3, #6 and #9 hil1/HIL1-complemented plants were examined (Supplemental Data Set 2). The whole rosettes harvested from two to three different plants were pooled as one biological repeat. P values of Welch’s t test with < 0.001 (asterisk) are shown on each genetic variable under heat stress. The profiles of the other lipid species are shown in Supplemental Figure 3. Statistical results are in Supplemental Data Set 3.
Heat stress induced a decrease in 34:6-MGDG in leaves both of the wild-type and hil1 mutant plants (Figure 5A). But, this heat-induced reduction of 34:6-MGDG in the hil1 mutant was not as considerable as that in the wild-type plants (P < 0.001, Welch’s t test; Supplemental Data Set 3). The heat-induced decrease was recovered in the hil1/HIL1-complemented plants (P < 0.001, Welch’s t test). The quantity of 34:6-MGDG was not significantly changed between the wild-type and hil1/HIL1-complemented plants under heat stress (P = 0.041, Welch’s t test). In the wild-type plants, the quantity of 34:6-MGDG was further decreased or remained constant during temperature recovery from heat stress (Supplemental Figure 4) (Higashi et al., 2015). We continuously observed a higher quantity of 34:6-MGDG in the hil1 mutant during temperature recovery than that in the wild-type plants (Supplemental Figure 4). However, under normal growth conditions, 34:6-MGDG in the hil1 mutant was not significantly different from that in the wild-type plants (Figure 5A). These results indicate that the hil1 mutant was partially impaired by a decrease in 34:6-MGDG under heat stress and during recovery. This metabolic disorder was restored by the genomic insertion of HIL1 in the mutant. These data suggest that HIL1 is involved in 34:6-MGDG decomposition under heat stress.
In contrast with the 34:6-MGDG accumulation pattern, the quantity of 54:9-TAG drastically increased with heat stress (Figure 5E). The quantity of 54:9-TAG in the hil1 mutant under heat stress was lower than that in the wild-type (P < 0.001, Welch’s t test) and hil1/HIL1-complemented (P < 0.001, Welch’s t test) plants. The quantity of 54:9-TAG in the hil1/HIL1-complemented plants tended to be higher than that in the wild-type plants (P = 0.007, Welch’s t test). These results suggest that an induced increase of 54:9-TAG under heat stress was partially impaired in the hil1 mutant. This metabolic disorder was restored by the genomic insertion of HIL1 in the mutant, as was the case for 34:6-MGDG.
As shown in Supplemental Figure 3, the hil1 mutant accumulated 54:9-TAG at lower levels than the wild-type plants under heat stress (calculated as 18% decrease). This decrease of 54:9-TAG was higher than that of 54:8-TAG (7% decrease), 54:7-TAG (1% decrease), 54:6-TAG (1% increase), 52:7-TAG (15% decrease), and 52:5-TAG (8% decrease). Under heat stress, the hil1 mutant accumulated 19% less 52:6-TAG, which likely contains two 18:3 acyl chains, than the wild type. These results suggest that the increases of 18:3-containing TAG species under heat stress were preferentially impaired in the hil1 mutant.
We calculated the correlation coefficients of the detected lipid species using the data obtained under heat stress (Supplemental Figure 5). 34:6-MGDG exhibited a strong positive correlation to 34:6-DGDG (ρ = 0.87), whereas 34:6-MGDG showed a negative correlation to 36:6-phosphatidylethanolamine (36:6-PE) (ρ = –0.72), 34:3-MGDG (ρ = –0.72), 36:6-PC (ρ = –0.69), 54:9-TAG (ρ = –0.63), and 34:5-PC (ρ = –0.62). These correlation results suggest that heat stress induced a synchronized change among the seven lipid species, which is partially affected by HIL1. The accumulation pattern of 34:6-DGDG was similar to that of 34:6-MGDG, and the difference between the hil1 mutant and hil1/HIL1-complemented plants was significant under heat stress (P < 0.001, Welch’s t test) (Figure 5B). Likewise, the accumulation patterns of 36:6-PC and 36:6-PE under heat stress were similar to those of 54:9-TAG, and the differences between the hil1 mutant and hil1/HIL1-complemented plants were significant (P < 0.001, Welch’s t test) (Figures 5C and 5D). These lipidomic analyses suggest that the remodeling of 18:3 occurs from 34:6-MGDG and 34:6-DGDG toward 36:6-PC, 36:6-PE, and 54:9-TAG in Arabidopsis leaves under heat stress, and this process is partially mediated by the function of HIL1.
Lipidomic analysis of the hil1/HIL1-complemented plants (#3 and #6) under heat stress showed that the quantities of 34:6-MGDG and 54:9-TAG varied among different parental lines. HIL1 expression levels in the #3-2 and #6-2 hil1/HIL1-complemented T4 plants were higher than those in the #3-1 and #6-1 plants (Supplemental Figure 6A). The #3-2 and #6-2 plants under heat stress accumulated less 34:6-MGDG and more 54:9-TAG than the hil1 mutant (Supplemental Figure 6B). The heat stress-sensitive phenotype of the hil1 mutant was complemented in the #3-2 and #6-2 plants, but not in the #3-1 and #6-1 plants (Supplemental Figures 6C and 6D). These close associations of the observed phenotypic changes with the HIL1 expression levels and metabolic changes additionally support a protective function of HIL1 against heat stress.
Purified Recombinant HIL1 Exhibits Characteristic Lipase Activity with a Preference to Release 18:3 Free Fatty Acid
We examined the lipase activity of recombinant HIL1 produced by an Escherichia coli expression system. We fused the HIL1 mature sequence that lacks the region encoding the predicted cTP sequence, to the C terminus of the maltose binding protein (MBP) (named MBP-mHIL1). The recombinant protein was purified using affinity and size-exclusion chromatography to apparent homogeneity (Figure 6A). The MBP-tag was removed during the purification to yield mHIL1 protein. First, we incubated the purified MBP-mHIL1 protein with crude lipid extract of Arabidopsis leaves as a substrate and examined the degrees of change in the relative quantities of lipid species using the LC-MS-based lipidomic platform. The quantities of several glycerolipid species decreased significantly after incubation with the purified protein for 1 and 3 min (Supplemental Figure 7). The decreased lipid species belonged to lipid classes of MGDG, DGDG, SQDG, PG, PC, PE, and DAG. MGDG was the most susceptible lipid class to be cleaved by HIL1 in vitro. Because 34:6-MGDG and 36:6-MGDG are the predominant MGDG species in Arabidopsis leaves, 34:6-MGDG and 36:6-MGDG could be the actual substrates of HIL1 to change membrane lipid composition induced by heat stress. However, TAG and phosphatidylinositol (PI) species were not cleaved. This result is consistent with the results of the glycerolipid compositional changes under heat stress in vivo because the quantities of most TAG and PI species were elevated under heat stress in Arabidopsis leaves (Supplemental Figures 3 and 4) (Higashi et al., 2015). The incubation of crude lipid extract with HIL1 resulted in increases in the lipid species that were annotated as monogalactosylmonoacylglycerol (lyso-MGDG), lysophosphatidylcholine (lyso-PC), and lysophosphatidylethanolamine (lyso-PE) containing an acyl chain of 16:3, oleate (18:1), linoleate (18:2), or 18:3 (Supplemental Figure 7). This result suggests that the HIL1 recombinant protein digested MGDG, PC, and PE to produce lyso-lipid products. The degree of decreases in diacyl-glycerolipids was dependent on the length of the incubation period and the amount of proteins and substrates added to the assay. These results indicate that HIL1 encodes a lipase with relatively broad substrate specificity to leaf glycolipids and phospholipids with a preference for MGDG.
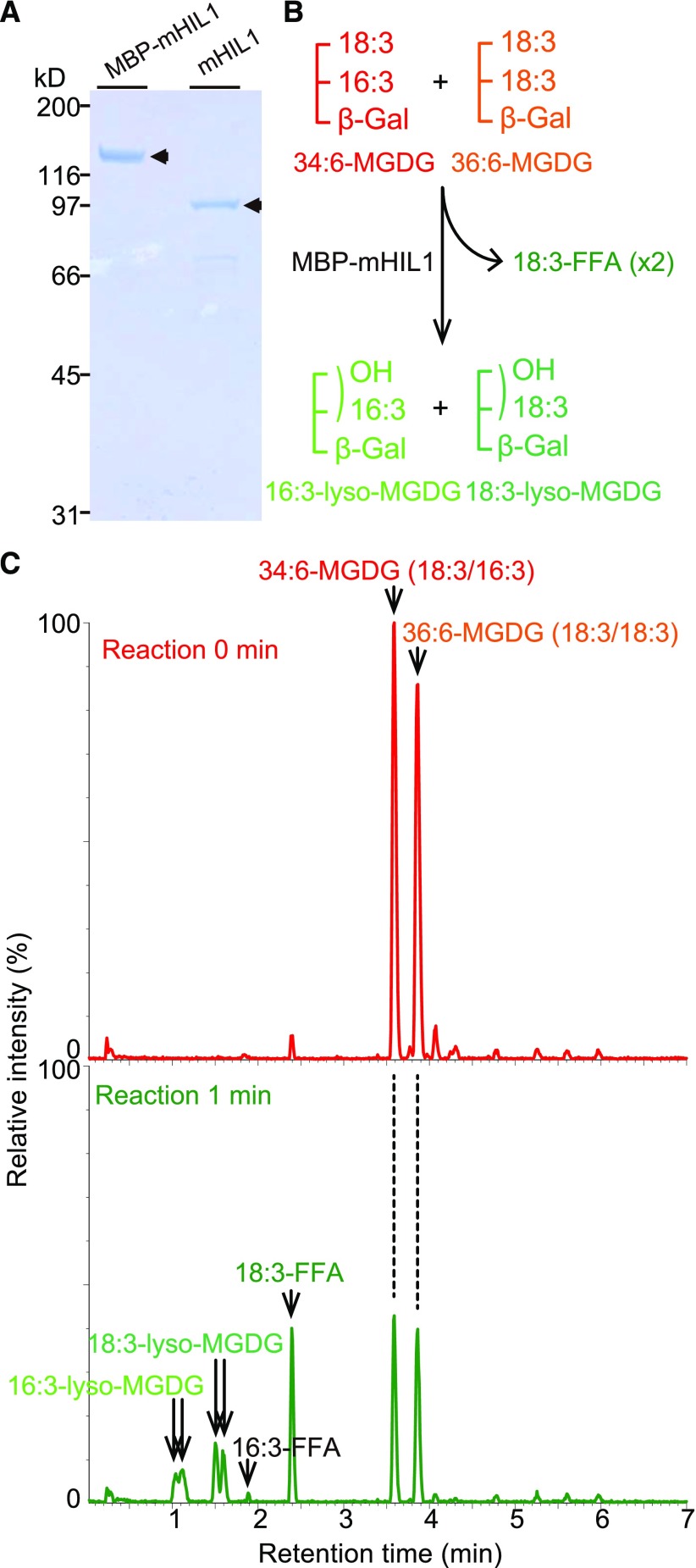
Figure 6.
The Recombinant HIL1 Protein Hydrolyzes MGDG to Liberate 18:3.
(A) The purified HIL1 recombinant proteins were visualized on a Coomassie blue-stained SDS-PAGE gel. Lane 1, the HIL1 mature sequence lacking the region encoding the putative cTP sequence was fused to the C terminus of MBP (MBP-mHIL1). Lane 2, MBP-mHIL1 was treated with Factor Xa prior to size-exclusion chromatography (mHIL1 removed MBP-tag).
(B) Schematic representation of the HIL1 lipase reaction.
(C) The purified protein (0.2 μg MBP-mHIL1) was incubated with the mixture of 34:6-MGDG and 36:6-MGDG as substrates. Lipids were extracted and subjected to LC-MS analysis. Specific activity was calculated as shown in Table 2. 18:3-FFA increased after 1 min incubation time. The peaks of putative 16:3-lyso-MGDG and 18:3-lyso-MGDG appeared after the 1 min reaction time. The base peak intensity chromatograms detected by the negative ion mode are shown.
When we used commercially available substrates for the lipase assay, incubation of the purified HIL1 recombinant protein with a mixture of 34:6-MGDG and 36:6-MGDG resulted in an increase in 18:3-free fatty acid (18:3-FFA) (Figures 6B and 6C). However, a decrease in 34:6-MGDG did not result in an associated increase in 16:3-free fatty acid (16:3-FFA) after 1 min of incubation, suggesting the preference to cleave 18:3-FFA from the glyceryl sn-1 position. The peaks of 16:3-lyso-MGDG and 18:3-lyso-MGDG appeared after 1 min of incubation (Figures 6B and 6C; Supplemental Figure 8) and decreased after 30 min of incubation (Supplemental Figure 8). Both 18:3-FFA and 16:3-FFA increased after 30 min of incubation. The doublet peaks of each lyso-MGDG product could be attributed to the sn-1 and sn-2 isomers. It was reported that 2-acyl-lyso-phospholipids undergo nonenzymatic isomerization to 1-acyl-lyso-phospholipids (Plückthun and Dennis, 1982; Okudaira et al., 2014; Sugasini and Subbaiah, 2017). Specific activity to the mixture of 34:6-MGDG and 36:6-MGDG was determined as 43.1 ± 1.7 and 29.1 ± 0.8 μmol min−1 mg−1 protein of MBP-mHIL1 and mHIL1 (removed MBP-tag), respectively (Table 2). 36:6-PE was also cleaved. Because PE is accumulated in extraplastidial membranes in Arabidopsis leaves (Li-Beisson et al., 2013), 36:6-PE could not be the substrate of chloroplastic HIL1 in vivo. 36:6-PG and SQDG were also cleaved. The other examined substrates, including hydrogenated 34:0- and 36:0-MGDG, were not cleaved under the same in vitro condition. These results indicate that the best substrate for HIL1 is MGDG with polyunsaturated acyl groups and releasing 18:3-FFA.
Table 2. Determination of Lipase Substrate of the HIL1 Recombinant Protein.
| Substrate | MBP-mHIL1 | mHIL1 Removed MBP-Tag |
|---|---|---|
| MGDG | 43.1 ± 1.7 | 29.1 ± 0.8 |
| Hydrogenated MGDG | N.D. | N.D. |
| DGDG | N.D. | N.D. |
| SQDG | 0.1 ± 0.0 | 0.3 ± 0.1 |
| 36:6-PG (18:3/18:3) | 1.8 ± 0.6 | 2.3 ± 0.6 |
| 36:0-PG (18:0/18:0) | N.D. | N.D. |
| 36:6-PC (18:3/18:3) | N.D. | N.D. |
| 34:2-PC (16:0/18:2) | N.D. | N.D. |
| 16:0-lyso-PC (16:0/OH) | N.D. | N.D. |
| 36:6-PE (18:3/18:3) | 26.4 ± 0.4 | 23.3 ± 0.6 |
| 34:1-PE (16:0/18:1) | N.D. | N.D. |
| 36:6-DAG (18:3/18:3) | N.D. | N.D. |
| 54:9-TAG (18:3/18:3/18:3) | N.D. | N.D. |
Data are presented as μmol min−1 mg−1 protein. Each substrate was incubated with the purified MBP-mHIL1 recombinant protein (0.2 μg) or mHIL1 protein removed MBP-tag (0.2 μg). Lipids were analyzed by LC-MS. The decreases in substrate were quantified in the positive ion mode. Each data point represents the mean value ± sd (n = 3). N.D., not detected.
The purified HIL1 protein had lipase activity with an optimized pH of 7.5 and temperature at 40°C (Supplemental Figures 9A and 9B). These results suggest that HIL1 prefers to digest lipid substrates under weak basic and high-temperature conditions. MBP-mHIL1 in the presence of 0.2% (v/v) Triton X-100 did not release free fatty acids from either the crude leaf lipids, mixture of 34:6-MGDG and 36:6-MGDG, or 36:6-PE (Supplemental Figure 9C). MBP-mHIL1 might be destabilized in the presence of the detergent.
When we expressed the full-length HIL1 including cTP sequence fused to MBP (MBP-HIL1) in E. coli, we observed much weaker lipase activity compared with MBP-mHIL1 (Supplemental Figure 10). Furthermore, MBP-HIL1 eluted as aggregates from the size-exclusion column. These results suggest that removal of the putative cTP sequence from HIL1 is necessary for proper HIL1 protein folding in the E. coli recombinant protein expression system.
Plants Conserve the Gene Coexpression Network of the HIL1 Homologs in Various Plant Species
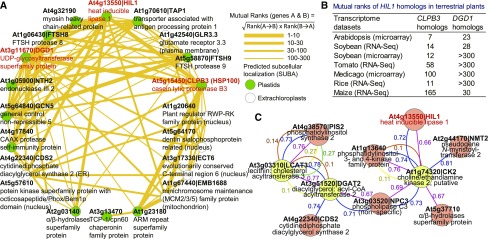
To obtain further insight into candidate genes that are possibly functionally related to HIL1 in a wide range of plants, gene coexpression analyses were conducted on the ATTED-II website (http://atted.jp, version Ath-m.6-0) (Obayashi et al., 2018). HIL1 was correlated with two known genes encoding casein lytic proteinase B3 (CLPB3) and digalactosyldiacylglycerol synthase 1 (DGD1) (Figure 7A). CLPB3 is a member of the heat shock protein 100-chaperone family and has been suggested to be involved in thylakoid membrane formation and protein reactivation in chloroplasts (Myouga et al., 2006; Pulido et al., 2016). Under heat stress, the CLPB3 chaperon protein possibly maintains folding of chloroplast-localized proteins including HIL1. DGD1 catalyzes the biosynthesis of DGDG from MGDG (Dörmann et al., 1999). The dgd1 mutants exhibit heat stress-sensitive phenotypes (Chen et al., 2006). An increase in DGDG synthesis under heat stress possibly results in the consumption of the substrate and reduction in MGDG content in the chloroplastic membrane (Figure 8). This compositional change may improve membrane stability because MGDG forms unstable lipid bilayers, whereas DGDG forms stable lipid bilayers (Lee, 2000). Therefore, both the well-characterized coexpressed genes possibly participate in chloroplastic heat stress responses together with HIL1. The network furthermore contains several uncharacterized genes, including some predicted chloroplast-localized proteins, as annotated in the SUBA database (colored green in Figure 7A). HIL1 and the coexpressed genes are suggested to coordinately play a role to protect vital functions in chloroplasts during heat stress.
Figure 7.
The Gene Coexpression Network of HIL1 and the Genes Involved in Chloroplastic Abiotic Stress Responses.
(A) The gene coexpression network of HIL1 in Arabidopsis was calculated on the ATTED-II website. Genes predicted to be localized to chloroplasts are marked with green dots. The mutual ranks represent nonparametric values reflecting the order of correlated genes. The values ≤300 are shown.
(B) The coexpression of the HIL1, CLPB3, and DGD1 homologs was conserved among plant species. The values were obtained from the ATTED-II website.
(C) Coexpression analysis of the putative heat-inducible genes involved in glycerolipid metabolism. One hundred and eighty Arabidopsis microarray CEL files under heat stress were selected as a data set for the calculation (Supplemental Table 4). LCAT3, DGAT2, and CK2 (yellow circles) were searched as queries. Seven genes (red circles) were coexpressed with the queries, which were possibly induced by heat stress. The links represent a positive association and values, which were calculated from five different methods (Pearson, blue; Spearman, red; CLR, brown; MRNET, yellow; ARACNE, green). The result was visualized using Cytoscape software.
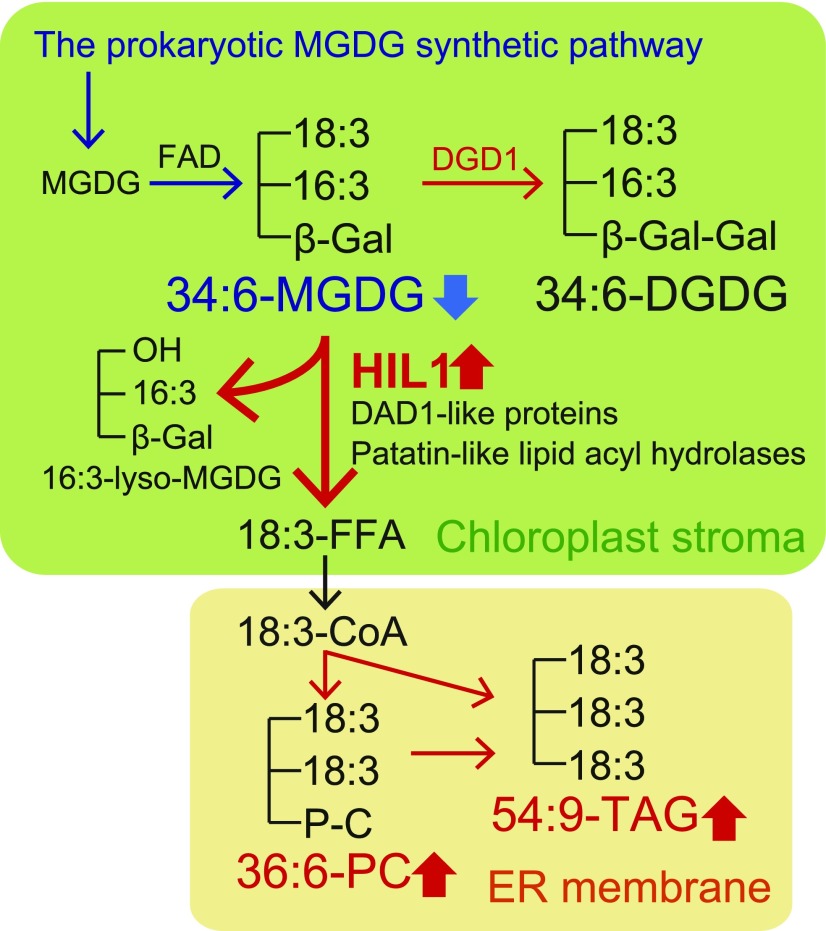
Figure 8.
Proposed Role of HIL1 in Leaf MGDG Turnover under Heat Stress.
34:6-MGDG is synthesized in the prokaryotic pathway and by fatty acid desaturases (FAD). HIL1 is responsible for the release of 18:3-FFA from 34:6-MGDG in chloroplasts. Other lipases of DAD1-like proteins and patatin-like lipid acyl hydrolases could also work in this process. The released 18:3-FFA is converted to 36:6-PC and 54:9-TAG in the endoplasmic reticulum (ER). Red arrows indicate the increased pathways under heat stress suggested from the lipidomic analysis in this study and the transcriptome analysis (Higashi et al., 2015), whereas blue arrows indicate the decreased pathways under heat stress. To improve thylakoid membrane stability, HIL1 may coordinately work with the coexpressed gene encoding DGD1. DGD1 synthesizes DGDG from MGDG (Dörmann et al., 1999).
We also found that HIL1 homologs from soybean (Glycine max), tomato (Solanum lycopersicum), Medicago truncatula, rice (Oryza sativa), and maize (Zea mays) were coexpressed with the CLPB3 homologs, and the soybean and maize HIL1 homolog was also coexpressed with the DGD1 homologs (Figure 7B). These results suggest that the gene coexpression relationship among the HIL1, CLPB3, and DGD1 homologs is well conserved in plant species.
We examined more specific gene coexpression networks for selected Arabidopsis genes possibly related to glycerolipid biosynthesis (Li-Beisson et al., 2013; Troncoso-Ponce et al., 2013). A database has been developed to find more heat stress-responsive genes involved in glycerolipid metabolism. The correlation coefficients were calculated from 180 published microarray CEL files (Supplemental Table 4), which were related to various high-temperature conditions in Arabidopsis. To draw a gene coexpression network under heat stress, we used three genes whose expression levels increased more than 3-fold under heat stress (Kilian et al., 2007; Weston et al., 2011; Hanada et al., 2013) as queries: lecithin:cholesterol acyltransferase 3 (LCAT3), diacylglycerol:acyl-CoA acyltransferase 2 (DGAT2), and a putative choline kinase (CK2, At1g74320). The gene coexpression network contained seven genes associated with the query genes (Figure 7C). HIL1 was included in the network and possibly was induced by various heat stress experiments. The network contained three genes encoding proteins involved in PI biosynthesis: cytidine diphosphate diacylglycerol synthase 2 (CDS2), phosphatidylinositol synthase 2 (PIS2), and a putative phosphatidylinositol-3- and 4-kinase family protein (At1g13640). These three genes might be responsible for a relative increase in the quantity of PI among phospholipid classes under heat stress at 38°C in leaves (Supplemental Figure 4) (Higashi et al., 2015). The putative lipase gene (At5g37710) in Figure 7C was also classified in the heat-inducible lipase group in Table 1. These results again indicate that HIL1 is a major component of the gene network responding to heat stress.
DISCUSSION
HIL1 Plays a Role in the Turnover of Chloroplastic MGDG Polyunsaturated Acyl Groups under Heat Stress
MGDG is the main constituent of thylakoid membranes. MGDG is considered important under heat stress, as the quantity of polyunsaturated acyl groups (16:3 and 18:3) of mainly MGDG in chloroplasts significantly decreases under these conditions. The isolation of Arabidopsis mutants provided some clues about the function of MGDG (Falcone et al., 2004; Routaboul et al., 2012). In this study, an Arabidopsis lipase gene, HIL1, was identified using an extensive lipidomic analysis integrated with transcriptome and public proteome databases.
We selected HIL1, which was induced by heat stress and localized in chloroplasts, and showed that impairment of HIL1 in the hil1 mutant resulted in a heat stress-sensitive phenotype (Figure 4D; Supplemental Figure 6C). HIL1 is involved in the degradation of MGDG derived from the prokaryotic pathway as a turnover of 18:3 under heat stress, where the liberated 18:3-FFA is suggested to be partly converted to TAG. This conclusion is based on the observation that leaves of the hil1 mutant exhibited relatively higher accumulation of 34:6-MGDG and lower accumulation of 54:9-TAG under heat stress than those of the wild-type plants (Figure 5). The difference in 34:6-MGDG accumulation between the wild-type and mutant plants was relatively small but statistically significant under heat stress, but not significant under normal temperature conditions. We confirmed that genetic complementation of HIL1 restores the defect of MGDG turnover and the heat-sensitive phenotype in the hil1 mutant. Therefore, HIL1 possibly plays functional roles in the turnover of MGDG derived from the prokaryotic pathway in the chloroplastic membrane under heat stress, as summarized in Figure 8.
The hil1 mutant described in this work demonstrates the usefulness of lipidomic analysis in identifying metabolic mutants that tend to have subtle differences in target metabolites from the wild-type plants. Based on the correlation coefficients of lipid species, we successfully indicated a relationship between MGDG turnover and TAG synthesis, where lipid species exhibited a negative correlation under heat stress (Supplemental Figure 5). In addition, the correlation analysis revealed the possible involvement of 34:6-DGDG, 36:6-PC, and 36:6-PE in MGDG turnover (Supplemental Figure 5). Though other types of experiments, such as pulse-chase labeling, are required to underpin the lipidomic observation and to further understand the precursor-product relationship of lipid metabolites under heat stress in detail, the remodeling of 18:3 from chloroplastic 34:6-MGDG to 54:9-TAG possibly occurred through or together with the formation of 18:3-containing phospholipids in the endoplasmic reticulum (Figure 8). Our findings demonstrate that HIL1 could trigger and partly regulate this turnover process across organelles.
In Vitro Lipase Properties of HIL1 Are Consistent with HIL1 Functions Suggested from in Vivo Lipidomic Studies
The E. coli recombinant protein of HIL1 cleaved most diacyl-glycolipid and -phospholipid species with a preference for MGDG in vitro (Supplemental Figure 7). MGDG with polyunsaturated acyl groups was cleaved with the highest specific activity of all examined substrates (Table 2). These results were consistent with the impairment of a decrease in 34:6-MGDG content observed in leaves of the hil1 mutant under heat stress (Figure 5). However, the HIL1 protein did not cleave TAG and PI species in vitro (Table 2; Supplemental Figure 7). This lipase substrate preference is in agreement with the inducible TAG and PI accumulation in leaves under heat stress (Supplemental Figures 3 and 4) (Higashi et al., 2015). The specific activity of HIL1 toward MGDG was high (Table 2), which is comparable to that of the published Arabidopsis TAG lipases of AtLip1 (At2g15230) and SDP1 (At5g04040) toward TAG (El-Kouhen et al., 2005; Eastmond, 2006).
Plants synthesize MGDG that contains 16:3 at the glyceryl sn-2 position (Kunst et al., 1989; Li et al., 2015). Therefore, the in vitro lipase activity suggests that HIL1 cleaves 34:6-MGDG at the glyceryl sn-1 position to liberate 18:3-FFA (Figure 6). In addition, incubation of HIL1 with crude leaf lipid extract resulted in formation of lyso-PC and lyso-PE species with 18:2 and 18:3 and decreases in PC and PE species (Supplemental Figure 7). However, no increase in lyso-PC and lyso-PE species with 16:0 was observed. In Arabidopsis leaves, 16:0 is mostly located at the glyceryl sn-1 position of PC and PE (Li et al., 2015), suggesting the recombinant HIL1 cleaved PC and PE at the glyceryl sn-1 position to produce lyso-lipids with a 18:2 or 18:3 acyl chain. This preference of HIL1 to cleave acyl groups at the glyceryl sn-1 position of glycerolipids is the same as for DAD1 acyl hydrolase (Ishiguro et al., 2001), which has a similar lipase-like domain as HIL1. In addition, HIL1 does not cleave saturated FFAs of MGDG, PE, and PG, even at the glyceryl sn-1 position (Table 2), suggesting a preference for the glyceryl sn-1 position and for the cleaving of MGDG, PE, and PG with unsaturated acyl groups.
Because the HIL1 recombinant protein exhibited lipase activity with broad substrate specificity in vitro (Table 2; Supplemental Figure 7), its substrate preference in vivo should be further investigated considering the local lipid environment to which HIL1 can access. Though further experiments, such as chloroplast import and fractionation studies, could confirm the detailed subcellular and suborganellar localization of native HIL1, confocal laser scanning microscopy analysis indicates that recombinant HIL1-YFP fusion protein is localized in chloroplasts (Figure 3). Chloroplastic localization of HIL1 is also supported by three proteomic studies (Zybailov et al., 2008; Kong et al., 2011; Tomizioli et al., 2014). The recombinant protein of HIL1 without the cTP sequence exhibited higher lipase activity than the full-length protein (Supplemental Figure 10). The optimum pH and temperature of HIL1 are compatible with those in chloroplast stroma under heat stress (Supplemental Figure 9). These biochemical data collectively suggest that HIL1 exhibits optimized lipase activity toward MGDG polyunsaturated acyl groups in heat-stressed chloroplasts as the mature form after cleavage of the putative cTP sequence.
The recombinant HIL1 protein cleaved the mixture of 34:6-MGDG and 36:6-MGDG, and 36:6-PE (Table 2, Figure 6). These substrates share no common structural moieties except for 18:3 acyl chains. MGDG and PE with polyunsaturated acyl groups can form an inverted hexagonal phase in vitro (Seddon, 1990). The inverted hexagonal phase is the most common nonlamellar phases. The fatty acyl chains point outwards so that the surfaces are hydrophobic (Lee, 2000). Lipids in the inverted hexagonal phase cannot build biological membrane bilayer. These results raise the possibility that HIL1 might be activated when hydrophobic interactions form between HIL1 and glycerolipids under a phenomenon that gives rise to an abnormal membrane structure.
HIL1 Redundantly Functions with Other Lipases and Is Important in the Heat Stress Response
HIL1 apparently functions under normal and high-temperature conditions because HIL1 expression was observed under both conditions (Figures 1 and 3). However, the hil1 mutant showed no significant difference in the glycerolipid content under normal growth conditions compared with the wild-type plants (Figure 5). The redundant Arabidopsis MGDG lipase genes possibly compensated for the lack of HIL1 lipase activity in hil1 leaves under normal temperature conditions. Several Arabidopsis enzymes were reported to digest MGDG in vitro using recombinant proteins (Ishiguro et al., 2001; La Camera et al., 2005; Yang et al., 2007; Hyun et al., 2008; Matos et al., 2008; Seo et al., 2009; Wang et al., 2017). Recently, a plastid lipase PLASTID LIPASE1 (PLIP1, At3g61680) was reported to be responsible for PG turnover in chloroplasts (Wang et al., 2017). PLIP1 overexpression plants exhibited a decrease in PG and a concomitant decrease in 16:3-containing MGDG under normal growth conditions. These MGDG lipases possibly catabolize MGDG in vivo (Figure 8).
HIL1 was upregulated by heat stress (Figures 1 and 3). The other MGDG lipases mentioned above and examined in our microarray analysis (Table 1) were not induced by heat stress. HIL1 exhibits a characteristic amino acid sequence and exon-intron structure that sets it apart from the remaining members of the lipase family (Figure 2). Therefore, we propose that HIL1 could have evolved in terrestrial plants to be functionally distinct from the other MGDG lipases in heat-stressed leaves. Because the necessity of lipid remodeling under heat stress is higher than that under normal temperature conditions, HIL1 probably plays a major role in the rapid lipid turnover under heat stress by removing 18:3 from MGDG.
The Removal of MGDG Polyunsaturated Acyl Groups by HIL1 Possibly Results in Enhanced Thylakoid Membrane Stability under Heat Stress
The hil1 mutant exhibited a lower thermotolerance than the wild-type and genetically complemented hil1/HIL1 plants (Figure 4D; Supplemental Figure 6), suggesting that HIL1 is involved in protecting against severe heat shock in plants. The photosynthetic capabilities of the hil1 mutant under heat stress were not significantly different from those of the wild-type and hil1/HIL1-complemented plants (Supplemental Table 1). Thus, the heat stress-sensitive phenotype of the hil1 mutant is not likely attributed to the inhibition of photosynthesis under the short-term heat stress. These phenotypes still need to be further corroborated by various thermotolerance tests on the HIL1 genetic materials, for example, under recurrent cycles of heat shock, and detailed analyses of photosynthetic function. In addition, it should be noted that global warming is not only elevating the extremes of high temperature but also increasing basal temperatures.
Our lipidomic analysis indicates that the growth defect of the heat-stressed hil1 mutant is attributable to the lower turnover of 34:6-MGDG, which contains 16:3 and 18:3, in hil1 chloroplast membranes under heat stress. High temperature causes membrane disorder. In vitro experiments have shown that MGDG containing polyunsaturated acyl groups becomes more susceptible in the nonlamellar phase with increased temperature (Seddon, 1990) because the inverted hexagonal structure is a typical topology of glycerolipid molecules displayed by polyunsaturated MGDG. In this study, MGDG containing polyunsaturated acyl groups (16:3 and 18:3) were digested by the recombinant HIL1 protein (Table 2). As demonstrated by the lipidomic analysis, impairment of HIL1 in the hil1 mutant negatively affected catabolism of 34:6-MGDG under heat stress. Therefore, removing MGDG polyunsaturated acyl groups by HIL1 may improve thylakoid membrane bilayer stability under heat stress.
Heat and Some Abiotic Stresses Induce HIL1 Expression
Because HIL1 catalyzes the first step in MGDG turnover, it can be the prime target for the regulation of this important pathway under heat stress. Gene coexpression analysis suggested the existence of a conserved gene association network of HIL1, CLPB3, and DGD1 in various plant species (Figure 7). Published microarray results suggested that the expression level of the HIL1 homolog in soybean was elevated by heat stress at 41 and 44°C in leaves (Supplemental Figure 11A) (Weston et al., 2011). HIL1 function has possibly been maintained during the evolution of terrestrial plants, and works cooperatively with CLPB3 and DGD1 as indispensable genes for heat abiotic stress responses in chloroplasts (Figure 8).
The prediction that HIL1 participates in some abiotic stress responses was reinforced by examining the expression patterns of HIL1 under various environmental perturbations using public microarray databases, eFP browser (http://bar.utoronto.ca) (Winter et al., 2007), and Genevestigator (Genevisible) (https://genevisible.com) (Hruz et al., 2008). In shoots, HIL1 expression was consistently increased during heat stress (Supplemental Figure 11B). HIL1 expression was also increased under osmotic stress, high light, and treatment with phytoprostane A1, which is an oxylipin possibly derived from 18:3 (Supplemental Figure 11C), indicating a role for lipid remodeling also in the response to these other stresses. Other abiotic stresses, i.e., cold, salt, drought, genotoxic, oxidative, UV-B, and wound stresses, did not affect HIL1 expression. Expression levels of HIL1 in the quadruple mutant of the transcription factors involved in heat stress HSFA1a, b, d, and e (the QK mutant; At1g32330, At3g02990, At4g17750, and At5g16820) (Supplemental Figure 11C) (Liu et al., 2011) was not increased during heat stress. This suggests that HSFA1 regulates HIL1 transcription under heat stress.
Lipidomic Analysis and Integration with Public Omics Databases Facilitate Gene Annotation in Arabidopsis
Recent metabolomic studies identified metabolic changes of more than 100 Arabidopsis mutants (Hur et al., 2013; Fukushima et al., 2014; Kim et al., 2015). In this study, we focused on glycerolipid accumulation and assessed genetic function under short-term heat stress. The extensive lipidomic analysis of hil1 leaves and complementation test suggested a relationship between HIL1 function and the metabolic changes of chloroplastic 34:6-MGDG under heat stress. The lipidomic analysis platform was also used to examine in vitro lipase activity of the HIL1 recombinant protein. Our study demonstrates the usefulness of LC-MS-based lipidomic analysis for functional characterization of lipase genes specialized in plant species.
In this study, we used public omics databases to isolate candidate genes involved in heat stress responses. Gene coexpression analysis revealed a conserved network of HIL1 in various plant species (Figure 7). The specific gene coexpression network of the published microarray data was quite useful to delineate the heat-inducible candidate genes, including HIL1 (Figure 7C). The gene annotation was accelerated by using proteomics databases to predict the subcellular localization of candidate genes. The selection of candidate genes was based on current knowledge about glycerolipid metabolism in plants (Li-Beisson et al., 2013; Troncoso-Ponce et al., 2013). Integration of this published information contributed to generating the hypothesis, and we performed a lipidomic analysis of the hil1 mutant and genetically complemented plants under heat stress. Finding solutions to deal with the consequences of global warming is a major social challenge of our time. Omics approaches could assist in developing rational strategies to generate heat stress-tolerant plants.
METHODS
Plant Materials
Arabidopsis thaliana ecotype Col-0 plants were used in this study. An Arabidopsis T-DNA-tagged line of SALK_016719, namely hil1, was obtained from the ABRC. The homozygous mutant was identified using a PCR-based method (Alonso et al., 2003; Watanabe et al., 2008). The primers used for screening for the homozygous hil1 mutant are listed in Supplemental Table 2. PCR products were sequenced to determine the exact insertion points.
The plant growth conditions were the same as that described previously (Higashi et al., 2015). In brief, plants were grown on Murashige and Skoog medium agar plates with 0.5% sucrose in a growth chamber at 22°C under 16/8 h light (40 to 75 μmol m−2 s−1 of fluorescent lamp)/dark cycle. Seventeen-day-old plants were subjected to heat stress at 38°C. The plants were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until use. For each treatment and time point, rosettes (whole upper-ground parts) from two to three different plants (∼50 mg wet weight) were harvested and pooled, and then examined as biological replicates.
Chemicals
The following compounds were purchased to perform in vitro lipase assay: MGDG and SQDG from Lipid Products; the hydrogenated MGDG from Matreya; DGDG, 36:6-PG, 36:0-PG, 36:6-PC, 34:2-PC, 16:0-lyso-PC, 36:6-PE, and 34:1-PE from Avanti Polar Lipids; and 36:6-DAG and 54:9-TAG from Nu-Chek-Prep.
Complementation Test
A 7.4-kb consecutive nucleotide sequence of HIL1, which contains the promoter and 5′ UTR (1130 bp), CDS with introns (5627 bp), and the 3′ untranslated and terminator regions (628 bp), was amplified by PCR using Arabidopsis genomic DNA as template (Supplemental Table 2), cloned into the pENTR/D-TOPO vector (Thermo Fisher Scientific) as an entry vector, and sequenced to confirm the absence of PCR errors. This genomic fragment was introduced into the pGWB1 binary vector (GenBank: AB289764.1) (Nakagawa et al., 2007) by the LR clonase reaction (Thermo Fisher Scientific). Transformation into Agrobacterium tumefaciens and Arabidopsis (hil1 mutant) and selection of transformants were performed as described previously (Yonekura-Sakakibara et al., 2007). The obtained T3 and T4 lines were subjected to lipidomic analysis.
Backcrossing of the hil1 Mutant
To prepare the hil1 mutant used in the fourth to sixth experiments of the lipidomic analysis (see the “Lipidomic Analysis” section and Supplemental Data Set 2 for detail), the hil1 mutant was backcrossed to the wild-type plants, where pollen from the hil1 mutant was attached to the wild-type Col-0 flowers. The backcrossed plants were identified by genomic DNA PCR with gene-specific and T-DNA border primers (Supplemental Table 2). Homozygous offspring were obtained after the backcrossing.
Thermotolerance Test
Plants were grown on Murashige and Skoog medium agar plates containing 0.5% sucrose in a growth chamber at 22°C under 16/8-h light/dark cycle. Fourteen-day-old plants were subjected to heat stress in the growth chamber at 38°C for 2 h, 22°C for 1 h, and 45°C for the indicated times. After the heat stress treatment, the plants continued to be grown at 22°C for 7 d. The hil1/HIL1-complemented T4 lines were examined.
RT-PCR Analysis
Total RNA in leaves of the wild-type, hil1 mutant, and hil1/HIL1-complemented plants was extracted using the RNeasy Plant Mini Kit (Qiagen). cDNA was generated using Superscript III (Thermo Fisher Scientific). The primers used for RT-PCR are listed in Supplemental Table 2. The primers for α-TUBULIN were designed as described by Watanabe et al. (2008). The PCR program for amplification was as follows: 95°C for 2 min; 24 (for α-TUBULIN) or 26 cycles (for HIL1) of 95°C for 30 s, 60°C for 30 s, and 72°C for 1.5 min; and final extension at 72°C for 5 min.
Quantitative Real-Time PCR
Accumulation of HIL1 transcripts was measured by RT-qPCR with a StepOnePlus Real-Time PCR system with SYBR Green Master Mix (Applied Biosystems). cDNAs prepared for the RT-PCR analysis were used as templates. Primer pairs to amplify HIL1 were designed using Primer Express software (Applied Biosystems). The primers for eukaryotic translation initiation factor 4A1 (EIF4A1) were designed as described by Okazaki et al. (2009). Real-time PCR was performed in triplicate (technical replicates).
DNA Gel Blot Analysis
Genomic DNA of the wild-type, hil1 mutant, and backcrossed homozygous hil1 mutant plants was extracted using the method described on the TAIR website. The extraction buffer contains 220 mM Tris-HCl, pH 8.0, 140 mM sorbitol, 22 mM EDTA, 800 mM NaCl, 1% (w/v) sodium lauroyl sarcosinate, and 0.8% (w/v) cetyltrimethylammonium bromide. Genomic DNA (15 μg) was digested with restriction enzymes overnight, separated by agarose gel electrophoresis (0.7% [w/v] agarose), and transferred to nylon membranes. DNA gel blot analysis was performed using the DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Diagnostics) following the manufacturer’s protocol. For probe preparation, part of the Salk T-DNA sequence was cloned into the pCR4Blunt-TOPO vector (Thermo Fisher Scientific) and sequenced (Supplemental Table 2). The plasmid was digested by BamHI and HindIII. The fragment was extracted from a 1.5% (w/v) agarose gel and used to synthesize a DIG-labeled probe.
5′ and 3′ RACE
The 5′ UTR and 3′ UTR of HIL1 were amplified and cloned using a GeneRacer Kit (Thermo Fisher Scientific). Total RNA was obtained from the whole rosettes of 14-d-old plants under heat stress at 38°C for 3 h. The primers are listed in Supplemental Table 2. The reaction mixtures of the first PCR were used as the templates for the nested PCR. The amplified fragments were cloned into the pCR4Blunt-TOPO vector (Thermo Fisher Scientific) and sequenced.
Lipidomic Analysis
To confirm the reproducibility of the lipidomic analysis results, the plant cultures were repeated six times in total (see Supplemental Data Set 2 for more sample information). The method for lipid extraction and the LC-MS conditions were described previously (Okazaki et al., 2013; Okazaki and Saito, 2018). For lipidomic analysis in this study, the data set recorded in the positive ion mode was used. The total lipid was extracted from the aerial parts of plants. Levels of lipid species were normalized. Peak height in each lipid species was divided by the mean of sum of peak heights of lipid species of the same lipid classes detected from the wild-type plants under heat stress (1d38C WT), where the lipid species detected from the all measurements were summed. This normalization was conducted individually on the six plant growth periods (experiments 1 to 6). The value of zero was eliminated when constructing the box and whisker plot. Significance levels were calculated between the wild-type and hil1 mutant plants, between the hil1 mutant and hil1/HIL1-complemented plants, and between the wild-type and hil1/HIL1-complemented plants by Welch’s t test (two-sided).
Spearman’s rank correlation coefficients of lipid species were calculated among 81 measurements only under heat stress using the R program (http://www.r-project.org), where peak height in each lipid species was normalized as peak height divided by mean of peak heights of the same lipid species of the wild-type plants (1d38C WT) in the same experiment. Hierarchical clustering of the lipid species was conducted by the complete linkage method based on Euclidean distance.
Expression and Purification of Recombinant HIL1 in Escherichia coli
The CDS of HIL1 was amplified from the cDNAs obtained from the whole rosettes of 14-d-old Arabidopsis plants under heat stress at 38°C and normal growth conditions. The primers are listed in Supplemental Table 2. The obtained PCR products were cloned into either the pENTR/D-TOPO or pCR4Blunt-TOPO vector (Thermo Fisher Scientific) and sequenced. The mature sequence lacking the region encoding the putative cTP sequence at the N terminus was amplified by PCR (Supplemental Table 2) and subcloned into the protein expression vector of pMAL-c5x (New England Biolabs), yielding the MBP-mHIL1 construct (MBP-C49-S854 of HIL1).
The MBP-mHIL1 expression construct was transformed into E. coli BL21 star (DE3) cells (Thermo Fisher Scientific). Transformed E. coli was grown at 37°C while being shaking at 180 rpm in 1 liter of rich broth, containing 10 g tryptone, 5 g yeast extract, 5 g NaCl, 2 g glucose, and 100 μg mL−1 ampicillin, until the absorbance at 600 nm reached ∼0.5. After protein induction with 0.3 mM isopropyl β-d-1-thiogalactopyranoside, the culture was grown at 29°C for 2 h with shaking. E. coli cells were harvested, frozen in liquid nitrogen, and stored at −80°C until purification.
For purification, the E. coli pellet was resuspended in a column buffer, containing 20 mM Tris-HCl, pH 7.5, and 200 mM NaCl. After sonication and centrifugation at 15,000g at 4°C for 20 min, the resulting supernatant was gently mixed with a 1.5 mL bed volume of amylose resin (New England Biolabs). The resin was washed three times and transferred to a disposable open column. Resin-bound protein was eluted with 2 mL of the column buffer with 10 mM maltose and frozen in liquid nitrogen. The amylose resin-purified protein was thawed and loaded onto a gel filtration column (Superdex 200 increase 10/300GL; GE Healthcare) and eluted in the column buffer at 0.5 mL min−1 at room temperature. MBP-tag was removed from MBP-mHIL1 by Factor Xa (New England Biolabs) prior to size-exclusion chromatography. The purified protein was immediately subjected to a lipase assay. The protein was not frozen after size-exclusion chromatography. The purified proteins were visualized on a Coomassie blue-stained SDS-PAGE gel.
For E. coli recombinant protein expression of full-length HIL1, the corresponding sequence was amplified by PCR (Supplemental Table 2) and subcloned into the pMAL-c5x protein expression vector (New England Biolabs), yielding the MBP-HIL1 construct (MBP-M1-S854 of HIL1). The recombinant protein was expressed and purified by amylose resin under the same procedure as that for MBP-mHIL1.
Assay of Lipase Activity
Crude lipid extract of Arabidopsis leaves (from 100 to 990 μg freeze-dried leaves per sample) and the purchased pure chemicals (19 nmol per sample) were used as a substrate for the in vitro lipase assay. The crude lipid extract was prepared by the method described by Okazaki and Saito (2018). Dried substrate was resuspended with a reaction buffer containing 100 mM Tris-HCl, pH 7.5, and 5% (w/v) gum arabic, by a tip-type sonicator. Lipase activity measurements without any detergents were reported in some papers (Eastmond, 2004; Matsui et al., 2004).
The substrate solution (50 μL) was mixed with the purified protein (50 μL) and incubated at 30°C with shaking (500 rpm). The reaction was stopped by freezing in liquid nitrogen. Protein quantities of 1, 0.2, and 0.05 μg gel filtration-purified MBP-mHIL1 and mHIL1 (removed MBP-tag) were examined. After an incubation period of up to 60 min, lipid was reextracted and subjected to LC-MS. The decreases in substrates after the incubation period were quantified based on the standard curve of each substrate compound to calculate specific activities. It was considered that equal molar amounts of both lipid species, 34:6-MGDG and 36:6-MGDG, were present in the mixture. For the SQDG substrate, the decreases in 34:3-SQDG and 36:6-SQDG were quantified.
The enzyme reaction products were analyzed by LC-MS as described above. The data set recorded in the positive ion mode was used for quantification. For MGDG reaction products, chromatograms in the negative ion mode were shown in Figure 6 and Supplemental Figure 8. The value in each lipid species was divided by the mean of sum of values of the lipid species of the same lipid classes detected at 0 min reactions, where the lipid species detected from all the measurements were used. Lipid species of putative lyso-lipids were selected based on the mass-to-charge ratios and retention times. The peak heights of the lyso-MGDG, lyso-PC, and lyso-PE species were normalized by those of 34:6-MGDG, 36:6-PC, and 36:6-PE at 0 min reactions, respectively.
The MBP-HIL1 construct, different pH and temperatures, and detergents were examined by the spectrophotometric method. For quantification of free fatty acids produced by the protein, a diagnostic kit (NEFA C-Test; Wako Pure Chemical) was used with α-linolenic acid (Sigma-Aldrich) as a standard. Free fatty acids in the reaction mixture were extracted by the method of Bligh and Dyer (1959), dried by SpeedVac, and incubated with the kit solutions of 600 μL in total, and measured colorimetrically at 550 nm. The following buffer was used for the pH experiment: 100 mM MES, pH 6, BisTris, pH 6.5 to 7, MOPS, pH 7.5, Tris-HCl, pH 8 to 9, and glycine-NaOH, pH 9.5 to 10. For the pH experiment, protein was eluted by 5 mM Tris-HCl, pH 7.5 and 200 mM NaCl from the gel filtration column. For the temperature experiment, the crude leaf lipid extract was resuspended with 100 mM MOPS, pH 7.5, and 5% (w/v) gum arabic.
Phylogenetic Analysis
The BLASTP program from TAIR (http://www.arabidopsis.org) and UniProt (http://www.uniprot.org) was used for sequence homology searches of HIL1. Only the “lipase class 3” domain sequence of each Arabidopsis gene was used to generate the tree diagram. The HIL1 homologs in other plant species contained the lipase domain, C2 domain, and a similar N-terminal sequence as that of HIL1. Amino acid sequences with a sequence identity of >95% in the same plant species were omitted from the phylogenetic analysis. The NCBI database (http://www.ncbi.nlm.nih.gov) was used to confirm sequences of the HIL1 homologs and find gene models (Supplemental Table 3). The deduced amino acid sequences were aligned by the MUSCLE method (Edgar, 2004). A phylogenetic tree was constructed with MEGA 6.06 software (Tamura et al., 2011) using the neighbor-joining method with bootstrap procedures (2000 replicates). The alignment data are available in Supplemental Data Set 1.
Subcellular Localization of HIL1
The CDS of HIL1 without the stop codon was amplified by PCR, and cloned into the pENTR/D-TOPO vector (Thermo Fisher Scientific) (Supplemental Table 2). The HIL1 sequence was introduced into a binary vector of pH35GY to generate a YFP fusion (Nakagawa et al., 2007; Okazaki et al., 2009) by the LR clonase reaction (Thermo Fisher Scientific), yielding the HIL1-YFP construct (35Spro: HIL1-YFP).
For transient expression in Nicotiana benthamiana, 4-week-old plants were transformed by the agroinfiltration method (Leuzinger et al., 2013). Agrobacterium strain GV3101 carrying 35Spro:HIL1-YFP and pGWB6 empty vector (GenBank: AB289769.1) (35Spro:GFP; OD600 = 0.2) was coinfiltrated with Agrobacterium carrying the p19 silencing suppressor (OD600 = 0.1), respectively. For the negative control, Agrobacterium carrying the p19 construct (OD600 = 0.3) was infiltrated alone. Three days after infiltration, the subcellular localization of the HIL1-YFP fusion protein was analyzed with a Zeiss LSM700 inverted confocal laser scanning microscope with a 20× dry objective. Excitation for YFP/GFP was 488 nm and emission was detected with a 490- to 635-nm band-pass filter. Chlorophyll was excited at 639 nm, and emission was detected with a 640-nm long-pass filter. Composite figures were made using ImageJ software using the LSM toolbox plug-in (Schneider et al., 2012).
Photosynthetic Activity
Arabidopsis plants were grown under the same conditions as those for lipidomic analysis. Seventeen-day-old wild-type, hil1 mutant, and hil1/HIL1-complemented plants of #6 and #9 were subjected to heat stress at 38°C for 0, 1, 3, and 5 h, and then incubated in the dark for 15 min. The method for the photosynthetic activity measurement was described by Myouga et al. (2008).
Transcriptome Data Analysis
Microarray data of the 181 candidate lipase genes was obtained from Higashi et al. (2015). Hierarchical clustering was calculated among six variables of conditions (Control 08 h, Control 24 h, Heat 08 h, Heat 24 h, Recovery 08 h, and Recovery 24 h) by the complete linkage method based on Euclidean distance (k = 12) using the R program (http://www.r-project.org).
Gene Coexpression Analysis across Heat Stress Microarray Data Sets and Network Inferences
Coexpression analysis was initially performed on the website ATTED-II (http://atted.jp). To search for more heat stress-responsive genes involved in glycerolipid metabolism, 10 independent data sets (180 microarray CEL files in total) sampled from Arabidopsis (Supplemental Table 4) were collected. All CEL files for publicly available data sets based on the GeneChip Arabidopsis Genome ATH1 Array were downloaded from the NCBI GEO (Barrett et al., 2013) and ArrayExpress (Kolesnikov et al., 2015). The raw CEL files within each data set were preprocessed and summarized using Robust Multichip Average (Irizarry et al., 2003) with the “affy” package (Gautier et al., 2004) in the R program (http://www.r-project.org). Any probe sets at risk of cross-hybridization (*x_at and *s_at) and probe sets with the prefix “AFFX” were eliminated. This process resulted in an expression data matrix consisting of 21,685 probe sets. Gene coexpression was assessed using both the Pearson and Spearman correlation coefficients with the R program. Based on integrated network inference (Fukushima and Kusano, 2014), three information-theoretic inference methods, ARACNE (Margolin et al., 2006), CLR (Faith et al., 2007), and MRNET (Meyer et al., 2007), were used to infer the regulatory relationship between genes. These inferences were based on the “minet” package (Meyer et al., 2008). A pair of genes that showed values of Pearson > 0.71, Spearman > 0.66, CLR > 0.05, MRNE > 0.09, and ARA > 0.27 were connected by a line. The result was visualized using Cytoscape software version 2.8 (Shannon et al., 2003).
Accession Numbers
Sequence data from this article can be found in TAIR (http://www.arabidopsis.org) under the following accession numbers: α-TUBULIN (At5g19770), CK2 (At1g74320), CLPB3 (At5g15450), DAD1 (At2g44810), DGD1 (At3g11670), EIF4A1 (At3g13920), HIL1 (At4g13550), LCAT3 (At3g03310), and PLIP1 (At3g61680). The other sequences reported in this article can be found in the GenBank/EMBL/DDBJ and UniProt databases under the accession numbers listed in Supplemental Table 3.
Supplemental Data
Supplemental Figure 1. N-terminal splicing variants of HIL1.
Supplemental Figure 2. DNA gel blot analysis of the wild-type, hil1 mutant, and backcrossed hil1 mutant plants.
Supplemental Figure 3. Lipid composition in leaves of the wild-type, T-DNA insertion mutant, and complemented plants under heat stress.
Supplemental Figure 4. Lipid composition in leaves of the wild-type and T-DNA insertion mutant plants during temperature recovery.
Supplemental Figure 5. Spearman’s rank correlation coefficients of glycerolipid species under heat stress.
Supplemental Figure 6. The hil1/HIL1-complemented plants with high HIL1 expression levels complemented the heat stress-sensitive phenotype of the hil1 mutant.
Supplemental Figure 7. In vitro lipase assay of the recombinant HIL1 using Arabidopsis leaf crude lipid extract as a substrate.
Supplemental Figure 8. The recombinant HIL1 protein hydrolyzes MGDG and lyso-MGDG.
Supplemental Figure 9. Optimum pH, temperature, and detergent test of the HIL1 recombinant protein.
Supplemental Figure 10. In vitro properties of the mature and full-length HIL1 recombinant proteins.
Supplemental Figure 11. Public microarray data of various perturbations for expression changes of HIL1 in Arabidopsis and the HIL1 homolog in soybean.
Supplemental Table 1. Analysis of photosynthetic activity in leaves of the wild-type, hil1 mutant, and hil1/HIL1-complemented plants under heat stress
Supplemental Table 2. Primers used in this study
Supplemental Table 3. Gene accession numbers used in this study
Supplemental Table 4. Data set used for constructing the heat stress-related coexpression network
Supplemental Data Set 1. Text file of the amino acid sequence alignment of HIL1 and its homologs used in Figure 2.
Supplemental Data Set 2. Lipidomic data from plants.
Supplemental Data Set 3. P values calculated from the lipidomic data.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
HSFA1 Gramene: At1g32330, At3g02990, At4g17750, At5g16820
HSFA1 Araport: At1g32330, At3g02990, At4g17750, At5g16820
Acknowledgments
We thank the ABRC for distributing seeds for a T-DNA-tagged line. We thank T. Nakagawa at Shimane University for providing the pGWB vector series. We thank RIKEN CSRS and RIKEN BSI for Sanger sequencing. This research was supported in part by Japan Science and Technology Agency, Strategic International Collaborative Research Program (SICORP); by Integrated Lipidology Program of RIKEN; and by the Japan Science and Technology Agency KAKENHI program (Grant 17K07663 to A.F.).
AUTHOR CONTRIBUTIONS
Y.H. and K.Sa. conceived the project. Y.H., Y.O., and K.Sa. designed the experiments. Y.H. performed molecular experiments and data analysis. Y.H., Y.O., and K.T. carried out lipidomic measurements. A.F. performed gene coexpression analysis across heat stress microarray data sets. E.K. and Y.H. performed subcellular localization analysis. F.M., K.Sh., and Y.H. performed photosynthetic activity measurements. Y.H. and K.Sa. wrote the manuscript. All authors commented on the manuscript.
Footnotes
Articles can be viewed without a subscription.
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Bao X., Focke M., Pollard M., Ohlrogge J. (2000). Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J. 22: 39–50. [DOI] [PubMed] [Google Scholar]
- Barrett T., et al. (2013). NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 41: D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- Chen J., Burke J.J., Xin Z., Xu C., Velten J. (2006). Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant Cell Environ. 29: 1437–1448. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y., Krishnakumar V., Chan A.P., Thibaud-Nissen F., Schobel S., Town C.D. (2017). Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 89: 789–804. [DOI] [PubMed] [Google Scholar]
- Dörmann P., Balbo I., Benning C. (1999). Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 284: 2181–2184. [DOI] [PubMed] [Google Scholar]
- Eastmond P.J. (2004). Cloning and characterization of the acid lipase from castor beans. J. Biol. Chem. 279: 45540–45545. [DOI] [PubMed] [Google Scholar]
- Eastmond P.J. (2006). SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J., Quettier A.L., Kroon J.T., Craddock C., Adams N., Slabas A.R. (2010). Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell 22: 2796–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kouhen K., Blangy S., Ortiz E., Gardies A.M., Ferté N., Arondel V. (2005). Identification and characterization of a triacylglycerol lipase in Arabidopsis homologous to mammalian acid lipases. FEBS Lett. 579: 6067–6073. [DOI] [PubMed] [Google Scholar]
- Ellinger D., Stingl N., Kubigsteltig I.I., Bals T., Juenger M., Pollmann S., Berger S., Schuenemann D., Mueller M.J. (2010). DONGLE and DEFECTIVE IN ANTHER DEHISCENCE1 lipases are not essential for wound- and pathogen-induced jasmonate biosynthesis: redundant lipases contribute to jasmonate formation. Plant Physiol. 153: 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith J.J., Hayete B., Thaden J.T., Mogno I., Wierzbowski J., Cottarel G., Kasif S., Collins J.J., Gardner T.S. (2007). Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. PLoS Biol. 5: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D.L., Ogas J.P., Somerville C.R. (2004). Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima A., et al. (2014). Metabolomic characterization of knockout mutants in Arabidopsis: Development of a metabolite profiling database for knockout mutants in Arabidopsis. Plant Physiol. 165: 948–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima A., Kusano M. (2014). A network perspective on nitrogen metabolism from model to crop plants using integrated ‘omics’ approaches. J. Exp. Bot. 65: 5619–5630. [DOI] [PubMed] [Google Scholar]
- Gautier L., Cope L., Bolstad B.M., Irizarry R.A. (2004). affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315. [DOI] [PubMed] [Google Scholar]
- Hanada K., et al. (2013). Small open reading frames associated with morphogenesis are hidden in plant genomes. Proc. Natl. Acad. Sci. USA 110: 2395–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Okazaki Y., Myouga F., Shinozaki K., Saito K. (2015). Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 5: 10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Wang T.W., Hudak K.A., Schade F., Froese C.D., Thompson J.E. (2000). An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence. Proc. Natl. Acad. Sci. USA 97: 8717–8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C.M., Tanz S.K., Castleden I.R., Vacher M.A., Small I.D., Millar A.H. (2014). SUBAcon: a consensus algorithm for unifying the subcellular localization data of the Arabidopsis proteome. Bioinformatics 30: 3356–3364. [DOI] [PubMed] [Google Scholar]
- Hruz T., Laule O., Szabo G., Wessendorp F., Bleuler S., Oertle L., Widmayer P., Gruissem W., Zimmermann P. (2008). Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinforma. 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur M., Campbell A.A., Almeida-de-Macedo M., Li L., Ransom N., Jose A., Crispin M., Nikolau B.J., Wurtele E.S. (2013). A global approach to analysis and interpretation of metabolic data for plant natural product discovery. Nat. Prod. Rep. 30: 565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun Y., et al. (2008). Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev. Cell 14: 183–192. [DOI] [PubMed] [Google Scholar]
- Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264. [DOI] [PubMed] [Google Scholar]
- Ishiguro S., Kawai-Oda A., Ueda J., Nishida I., Okada K. (2001). The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Morita M.T., Fukaki H., Yamauchi Y., Uehara M., Niihama M., Tasaka M. (2002). SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.A., van Erp H., Quettier A.L., Shaw E., Menard G., Kurup S., Eastmond P.J. (2013). The sugar-dependent1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 162: 1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J., Whitehead D., Horak J., Wanke D., Weinl S., Batistic O., D’Angelo C., Bornberg-Bauer E., Kudla J., Harter K. (2007). The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 50: 347–363. [DOI] [PubMed] [Google Scholar]
- Kim T., Dreher K., Nilo-Poyanco R., Lee I., Fiehn O., Lange B.M., Nikolau B.J., Sumner L., Welti R., Wurtele E.S., Rhee S.Y. (2015). Patterns of metabolite changes identified from large-scale gene perturbations in Arabidopsis using a genome-scale metabolic network. Plant Physiol. 167: 1685–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov N., et al. (2015). ArrayExpress update--simplifying data submissions. Nucleic Acids Res. 43: D1113–D1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R.P., Siu S.O., Lee S.S., Lo C., Chu I.K. (2011). Development of online high-/low-pH reversed-phase-reversed-phase two-dimensional liquid chromatography for shotgun proteomics: a reversed-phase-strong cation exchange-reversed-phase approach. J. Chromatogr. A 1218: 3681–3688. [DOI] [PubMed] [Google Scholar]
- Kunst L., Browse J., Somerville C. (1989). A mutant of Arabidopsis deficient in desaturation of palmitic Acid in leaf lipids. Plant Physiol. 90: 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Camera S., Geoffroy P., Samaha H., Ndiaye A., Rahim G., Legrand M., Heitz T. (2005). A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J. 44: 810–825. [DOI] [PubMed] [Google Scholar]
- Lee A.G. (2000). Membrane lipids: it’s only a phase. Curr. Biol. 10: R377–R380. [DOI] [PubMed] [Google Scholar]
- Leuzinger K., Dent M., Hurtado J., Stahnke J., Lai H., Zhou X., Chen Q. (2013). Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. J. Vis. Exp. 77: e50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zheng Q., Shen W., Cram D., Fowler D.B., Wei Y., Zou J. (2015). Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell 27: 86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y., et al. (2013). Acyl-lipid metabolism. The Arabidopsis Book 11: e0161, doi/10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.C., Liao H.T., Charng Y.Y. (2011). The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 34: 738–751. [DOI] [PubMed] [Google Scholar]
- Los D.A., Murata N. (2004). Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666: 142–157. [DOI] [PubMed] [Google Scholar]
- Margolin A.A., Nemenman I., Basso K., Wiggins C., Stolovitzky G., Dalla Favera R., Califano A. (2006). ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics 7 (Suppl 1): S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos A.R., d’Arcy-Lameta A., França M., Pêtres S., Edelman L., Kader J., Zuily-Fodil Y., Pham-Thi A.T. (2001). A novel patatin-like gene stimulated by drought stress encodes a galactolipid acyl hydrolase. FEBS Lett. 491: 188–192. [DOI] [PubMed] [Google Scholar]
- Matos A.R., Gigon A., Laffray D., Pêtres S., Zuily-Fodil Y., Pham-Thi A.T. (2008). Effects of progressive drought stress on the expression of patatin-like lipid acyl hydrolase genes in Arabidopsis leaves. Physiol. Plant. 134: 110–120. [DOI] [PubMed] [Google Scholar]
- Matsuda O., Sakamoto H., Hashimoto T., Iba K. (2005). A temperature-sensitive mechanism that regulates post-translational stability of a plastidial omega-3 fatty acid desaturase (FAD8) in Arabidopsis leaf tissues. J. Biol. Chem. 280: 3597–3604. [DOI] [PubMed] [Google Scholar]
- Matsui K., Fukutomi S., Ishii M., Kajiwara T. (2004). A tomato lipase homologous to DAD1 (LeLID1) is induced in post-germinative growing stage and encodes a triacylglycerol lipase. FEBS Lett. 569: 195–200. [DOI] [PubMed] [Google Scholar]
- McConn M., Browse J. (1996). The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis Mutant. Plant Cell 8: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P.E., Kontos K., Lafitte F., Bontempi G. (2007). Information-theoretic inference of large transcriptional regulatory networks. EURASIP J. Bioinform. Syst. Biol. 2007: 79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P.E., Lafitte F., Bontempi G. (2008). minet: A R/Bioconductor package for inferring large transcriptional networks using mutual information. BMC Bioinformatics 9: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myouga F., Motohashi R., Kuromori T., Nagata N., Shinozaki K. (2006). An Arabidopsis chloroplast-targeted Hsp101 homologue, APG6, has an essential role in chloroplast development as well as heat-stress response. Plant J. 48: 249–260. [DOI] [PubMed] [Google Scholar]
- Myouga F., Hosoda C., Umezawa T., Iizumi H., Kuromori T., Motohashi R., Shono Y., Nagata N., Ikeuchi M., Shinozaki K. (2008). A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20: 3148–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tsuchiya M., Ohta H. (2007). Plastidic phosphatidic acid phosphatases identified in a distinct subfamily of lipid phosphate phosphatases with prokaryotic origin. J. Biol. Chem. 282: 29013–29021. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Koizumi R., Shui G., Shimojima M., Wenk M.R., Ito T., Ohta H. (2009). Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc. Natl. Acad. Sci. USA 106: 20978–20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S., Tamura P.J., Roth M.R., Prasad P.V., Welti R. (2016). Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 39: 787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T., Aoki Y., Tadaka S., Kagaya Y., Kinoshita K. (2018). ATTED-II in 2018: a plant coexpression database based on investigation of the statistical property of the mutual rank index. Plant Cell Physiol. 59: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y., Saito K. (2018). Plant lipidomics using UPLC-QTOF-MS. Methods Mol. Biol. 1778: 157–169. [DOI] [PubMed] [Google Scholar]
- Okazaki Y., Shimojima M., Sawada Y., Toyooka K., Narisawa T., Mochida K., Tanaka H., Matsuda F., Hirai A., Hirai M.Y., Ohta H., Saito K. (2009). A chloroplastic UDP-glucose pyrophosphorylase from Arabidopsis is the committed enzyme for the first step of sulfolipid biosynthesis. Plant Cell 21: 892–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y., Kamide Y., Hirai M.Y., Saito K. (2013). Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 9 (suppl. 1): 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira M., Inoue A., Shuto A., Nakanaga K., Kano K., Makide K., Saigusa D., Tomioka Y., Aoki J. (2014). Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 55: 2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy R.W. (1978). Effect of growth temperature on the fatty acid composition of the leaf lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiol. 61: 484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plückthun A., Dennis E.A. (1982). Acyl and phosphoryl migration in lysophospholipids: importance in phospholipid synthesis and phospholipase specificity. Biochemistry 21: 1743–1750. [DOI] [PubMed] [Google Scholar]
- Pulido P., Llamas E., Llorente B., Ventura S., Wright L.P., Rodríguez-Concepción M. (2016). Specific Hsp100 chaperones determine the fate of the first enzyme of the plastidial isoprenoid pathway for either refolding or degradation by the stromal Clp protease in Arabidopsis. PLoS Genet. 12: e1005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J.K., Roberts J.K., Berry J.A. (1982). Correlations between the thermal stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of the higher plant, Nerium oleander, to growth temperature. Biochim. Biophys. Acta 688: 218–228. [Google Scholar]
- Routaboul J.M., Fischer S.F., Browse J. (2000). Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol. 124: 1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul J.M., Skidmore C., Wallis J.G., Browse J. (2012). Arabidopsis mutants reveal that short- and long-term thermotolerance have different requirements for trienoic fatty acids. J. Exp. Bot. 63: 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J.M. (1990). Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta 1031: 1–69. [DOI] [PubMed] [Google Scholar]
- Seo Y.S., Kim E.Y., Kim J.H., Kim W.T. (2009). Enzymatic characterization of class I DAD1-like acylhydrolase members targeted to chloroplast in Arabidopsis. FEBS Lett. 583: 2301–2307. [DOI] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K., Bremer D.J., Jeannotte R., Welti R., Yang C. (2009). Membrane lipid composition and heat tolerance in cool-season turfgrasses, including a hybrid bluegrass. J. Am. Soc. Hortic. Sci. 134: 511–520. [Google Scholar]
- Sugasini D., Subbaiah P.V. (2017). Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group. Greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species. PLoS One 12: e0187826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizioli M., et al. (2014). Deciphering thylakoid sub-compartments using a mass spectrometry-based approach. Mol. Cell. Proteomics 13: 2147–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso-Ponce M.A., Cao X., Yang Z., Ohlrogge J.B. (2013). Lipid turnover during senescence. Plant Sci. 205-206: 13–19. [DOI] [PubMed] [Google Scholar]
- Wang K., Froehlich J.E., Zienkiewicz A., Hersh H.L., Benning C. (2017). A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell 29: 1678–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Guo Q., Froehlich J.E., Hersh H.L., Zienkiewicz A., Howe G.A., Benning C. (2018). Two abscisic acid-responsive plastid lipase genes involved in jasmonic acid biosynthesis in Arabidopsis thaliana. Plant Cell 30: 1006–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Mochida K., Kato T., Tabata S., Yoshimoto N., Noji M., Saito K. (2008). Comparative genomics and reverse genetics analysis reveal indispensable functions of the serine acetyltransferase gene family in Arabidopsis. Plant Cell 20: 2484–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston D.J., Karve A.A., Gunter L.E., Jawdy S.S., Yang X., Allen S.M., Wullschleger S.D. (2011). Comparative physiology and transcriptional networks underlying the heat shock response in Populus trichocarpa, Arabidopsis thaliana and Glycine max. Plant Cell Environ. 34: 1488–1506. [DOI] [PubMed] [Google Scholar]
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G.V., Provart N.J. (2007). An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Devaiah S.P., Pan X., Isaac G., Welti R., Wang X. (2007). AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea. J. Biol. Chem. 282: 18116–18128. [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K., Tohge T., Niida R., Saito K. (2007). Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. J. Biol. Chem. 282: 14932–14941. [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang C., Bedair M., Welti R., Sumner L.W., Baxter I., Wang X. (2011). Suppression of phospholipase Dγs confers increased aluminum resistance in Arabidopsis thaliana. PLoS One 6: e28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B., Rutschow H., Friso G., Rudella A., Emanuelsson O., Sun Q., van Wijk K.J. (2008). Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]