A single-nucleotide polymorphism in a hairpin RNA promoter contributes to Alternaria alternata leaf spot resistance in apple and could serve as a marker to distinguish susceptible versus resistant apple cultivars.
Abstract
Apple leaf spot caused by the Alternaria alternata f. sp mali (ALT1) fungus is one of the most devastating diseases of apple (Malus × domestica). We identified a hairpin RNA (hpRNA) named MdhpRNA277 that produces small RNAs and is induced by ALT1 infection in ‘Golden Delicious’ apple. MdhpRNA277 produces mdm-siR277-1 and mdm-siR277-2, which target five resistance (R) genes that are expressed at high levels in resistant apple variety ‘Hanfu’ and at low levels in susceptible variety ‘Golden Delicious’ following ALT1 infection. MdhpRNA277 was strongly induced in ‘Golden Delicious’ but not ‘Hanfu’ following ALT1 inoculation. MdhpRNA277 promoter activity was much stronger in inoculated ‘Golden Delicious’ versus ‘Hanfu’. We identified a single-nucleotide polymorphism (SNP) in the MdhpRNA277 promoter region between ‘Golden Delicious’ (pMdhpRNA277-GD) and ‘Hanfu’ (pMdhpRNA277-HF). The transcription factor MdWHy binds to pMdhpRNA277-GD, but not to pMdhpRNA277-HF. Transgenic ‘GL-3’ apple expressing pMdhpRNA277-GD:MdhpRNA277 was more susceptible to ALT1 infection than plants expressing pMdhpRNA277-HF:MdhpRNA277 due to induced mdm-siR277 accumulation and reduced expression of the five target R genes. We confirmed that the SNP in pMdhpRNA277 is associated with A. alternata leaf spot resistance by crossing. This SNP could be used as a marker to distinguish between apple varieties that are resistant or susceptible to A. alternata leaf spot.
INTRODUCTION
Alternaria alternata leaf spot is a major fungal disease of apple (Malus × domestica). (Zhang et al., 2015). In general, when fungi attack apple leaves, they produce toxins and effectors, which disrupt plant metabolism (Hugot et al., 1999; Johnson et al., 2000; Abe et al., 2010), leading to H2O2 accumulation and cell death (Chung, 2012). In addition, fungal diseases result in black, necrotic leaf spots and moldy fruit cores (Otani et al., 1974; Johnson et al., 2000), an increased incidence of defoliation and fruit drop, and a decline in fruit quality and production (Sawamura and Yanase, 1963; Rotem, 1994; Rotondo et al., 2012; Shtienberg, 2012). Chemical treatments are commonly used to control fungal diseases, but such treatments have negative side effects, such as reduced food safety and environmental pollution. The most effective strategies for overcoming fungal diseases in apple are selecting pathogen-resistant apple resources and developing pathogen-resistant varieties through breeding. Therefore, it is crucial to explore the molecular mechanism underlying fungal disease resistance in apple.
When plants are infected with pathogens, plant transmembrane receptors recognize conserved fungal-oomycete cellulose binding elicitor proteins from the pathogen known as pathogen-associated molecular patterns (PAMPs) and activate a basal defense response, which leads to PAMP-triggered immunity. Whereas some pathogens deliver effector proteins that attenuate PAMP-triggered immunity, resistance (R) proteins in resistant hosts recognize cognate effectors and activate effector-triggered immunity (Dodds and Rathjen, 2010; Slootweg et al., 2010; Tameling et al., 2010; Heidrich et al., 2011a; Dangl et al., 2013; Niu et al., 2016). Most R proteins contain a nucleotide binding site (NB) and leucine-rich repeats (LRRs) and directly or indirectly recognize specific pathogen effectors (Sinapidou et al., 2004; Ashikawa et al., 2008; Lee et al., 2009; Loutre et al., 2009; Narusaka et al., 2009; Eitas and Dangl, 2010; Heidrich et al., 2011b; Brotman et al., 2013; Cesari et al., 2013; Steinbrenner et al., 2015). The NB domain, which is critical for ATP or GTP binding, provides energy that alters the protein’s conformation (Rairdan et al., 2008; Elmore et al., 2011), whereas the LRR domain perceives specific signals from the invading pathogen via a protein-protein interaction and activates a pathogen resistance response (Ellis et al., 1999; Belkhadir et al., 2004; DeYoung and Innes, 2006; McHale et al., 2006).
NB-LRR resistance proteins are grouped into two categories based on their N-terminal domains, Toll-like/Interleukin 1 receptor (TIR)-type NB-LRR and coiled-coil (CC)-type NB-LRR (CNL) proteins (Dangl and Jones, 2001; Meyers et al., 2002; Dong et al., 2016). Members of a subclass of CNLs with an N-terminal coiled-coil domain resembling that of RESISTANCE TO POWDERY MILDEW8 (RPW8) are referred to as CCR-NB-LRRs (Grant et al., 2003; Chini et al., 2004a; Chini and Loake, 2005; Peart et al., 2005; Bonardi et al., 2011; Collier et al., 2011; Jupe et al., 2012; Arya et al., 2014a; Dong et al., 2016). Studies on the roles of TIR-NB-LRR and CC-NB-LRR proteins in plant defense initially focused on Arabidopsis thaliana and tobacco (Nicotiana tabacum) (Bent et al., 1994; Whitham et al., 1994) and were followed by extensive studies in barley (Hordeum vulgare), tomato (Solanum lycopersicum), potato (Solanum tuberosum), rice (Oryza sativa), wheat (Triticum aestivum), maize (Zea mays), and other crops (Milligan et al., 1998; Halterman et al., 2001; Jordan et al., 2011; Bai et al., 2012; Saunders et al., 2012; Cesari et al., 2013; Harris et al., 2013; Inoue et al., 2013; Song et al., 2013; Stirnweis et al., 2014; Sueldo et al., 2015; Wang et al., 2015, 2016). To date, two CCR-NB-LRR proteins have been characterized: Nicotiana benthamiana N REQUIREMENT GENE1 (NRG1) (Peart et al., 2005; Collier et al., 2011) and Arabidopsis ACTIVATED DISEASE RESISTANCE1 (ADR1) (Chini et al., 2004a; Chini and Loake, 2005; Bonardi et al., 2011; Collier et al., 2011). CCR-NB-LRR proteins have been identified in the potato, soybean (Glycine max), and apple genomes by BLAST analysis, but their functions remain unknown (Meyers et al., 2003; Bonardi et al., 2011; Jupe et al., 2012; Arya et al., 2014b).
Disease resistance and susceptibility in plants are determined by the expression levels of NB-LRR genes. For instance, NB-LRR genes are expressed at higher levels in resistant than in susceptible varieties of wild strawberry (Fragaria vesca) and banana (Musa spp) during pathogen infection (Bai et al., 2013; Chen et al., 2016). Moreover, increasing NB-LRR gene expression improves plant disease resistance (Harris et al., 2013; Inoue et al., 2013). Disease-resistant and -susceptible cultivars might exhibit distinct patterns of NB-LRR expression via two mechanisms: (1) histone methylation in the promoters of these genes might affect the expression of NB-LRRs (Li et al., 2011; Xia et al., 2013) and (2) small RNAs might target TIR-NB-LRR or CC-NB-LRR genes, which affect plant immunity (He et al., 2008; Zhai et al., 2011; Li et al., 2012; Shivaprasad et al., 2012; Zhu et al., 2013; Arikit et al., 2014; Boccara et al., 2014; Liu et al., 2014; Ma et al., 2014; Ouyang et al., 2014; Wong et al., 2014; Deng et al., 2018). In support of the second mechanism, small RNAs are not induced in resistant plant varieties upon pathogen attack, causing their target TIR-NB-LRRs or CC-NB-LRRs to be highly expressed; by contrast, small RNAs are induced in susceptible varieties, leading to the suppression of their target TIR-NB-LRRs or CC-NB-LRRs (Ma et al., 2014; Ouyang et al., 2014). These studies highlight the importance of small RNAs that target critical genes involved in plant immune signaling. However, little is known about how small RNAs function in various plant varieties with different levels of disease resistance.
In this study, we identified MdhpRNA277, a hairpin RNA (hpRNA) that produces small RNAs and is induced in apple infected with A. alternata leaf spot fungus (ALT1). Both mdm-siR277-1 and mdm-siR277-2 target five R genes, MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5, which belong to the CCR-NB-LRR family. We detected a single-nucleotide polymorphism (SNP) in the MdhpRNA277 promoter, pMdhpRNA277, in resistant versus susceptible apple varieties and found that this SNP affects the binding of the transcription factor MdWHy to pMdhpRNA277, resulting in different levels of mdm-siR277-1 and mdm-siR277-2 production and differential expression of their target genes, as well as different levels of disease resistance.
RESULTS
The Production of MdhpRNA277-Derived mdm-siR277s Is Induced in ALT1-Infected Apple Leaves
We conducted next-generation sequencing of small RNAs from the leaves of ‘Golden Delicious’ (GD) apple (Malus × domestica), a representative A. alternata f. sp mali (ALT1)-susceptible apple cultivar that had been inoculated with ALT1 or mock-inoculated 24 h prior to sampling. BLAST analysis in miRBase (http://www.mirbase.org/) and the apple genome database (https://www.rosaceae.org/) revealed 58 microRNAs (miRNAs) with more than 2-fold differences in expression (log10 ≥ 2) between inoculated and mock-inoculated leaves (Daccord et al., 2017; Zhang et al., 2017).
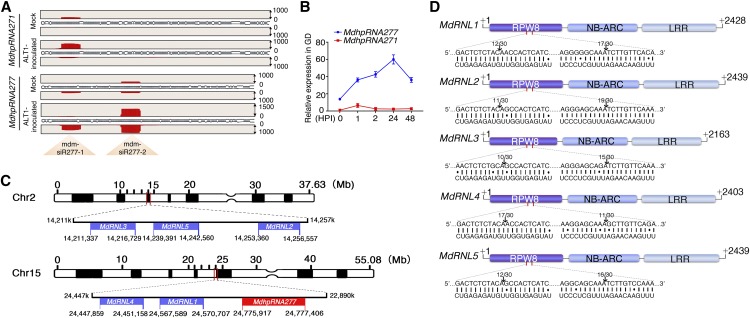
We identified a 21-nucleotide small RNA that was the most highly differentially expressed in inoculated leaves relative to the control. This small RNA initially caught our attention as a potential miRNA candidate. To identify the small RNA transcripts, we aligned the small RNA sequence to the apple genome and EST libraries by BLAST analysis and found that the small RNA is derived from two different genomic loci. RT-PCR and gene cloning confirmed that both loci were transcribed into mRNA transcripts that can form long hairpin structures and produce many small RNAs. We named these two long hairpin transcripts MdhpRNA277 and MdhpRNA271 (Figure 1A). Due to the long hairpin structures and diverse small RNAs generated, we reasoned that the ALT1-induced 21-nucleotide small RNA was not a miRNA but was instead a small-interfering RNA (siRNA). Based on bioinformatics analysis of next-generation sequencing data of small RNAs, we found that the long hpRNA gave rise to several small RNAs; however, two main regions of MdhpRNA277 were induced by infection with ALT1, whereas MdhpRNA271-derived small RNAs were not (Figure 1A). Also, RT-qPCR indicated that MdhpRNA277 production was highly induced by ALT1 inoculation in GD, whereas MdhpRNA271 was not (Figure 1B). Therefore, we focused our analysis on ALT1-induced MdhpRNA277, which is transcribed from chromosome 15 (Figures 1A to 1C). ALT1-induced small-RNA populations ranging from 21 to 24 nucleotides in size mainly accumulated at two regions of MdhpRNA277, i.e., mdm-siR277-1 and mdm-siR277-2 (Figure 1A).
Figure 1.
Identification of the ALT1-Induced Transcript, MdhpRNA277, Which Produces Two Small RNAs, mdm-siR277-1 and mdm-siR277-2, Which Target Five R Genes in GD Apple Leaves.
(A) Identification of the ALT1-induced long hairpin transcript, MdhpRNA277, and accumulation of mdm-siR277-1 and mdm-siR277-2 after ALT1 inoculation, as determined by small RNA sequencing. The numbers on the right refer to the number of reads, and the red areas refer to the read abundance.
(B) RT-qPCR to detect mRNA levels showing that MdhpRNA277 is induced, but MdhpRNA271 is not, in ALT1-inoculated GD seedlings. The numbers on the x axis indicate HPI. Spore inoculum concentration: 2 × 105 cfu/mL. Spore growth was measured at 6 d postinoculation (DPI). Error bars represent standard deviations calculated from three biological replicates (separate biological material) that were performed for each treatment.
(C) Chromosomal locations of the ALT1-induced MdhpRNA277 gene and the five target genes of mdm-siR277-1/2.
(D) mdm-siR277-1 and mdm-siR277-2 cleavage sites in the RPW8 domains of five target genes identified by 5′ RACE. The arrows indicate the positions of inferred cleavage sites, and the numbers above the sequences indicate the detected cleavage site of independent clones. MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 contain an N-terminal RPW8 domain and a C-terminal NB-LRR domain.
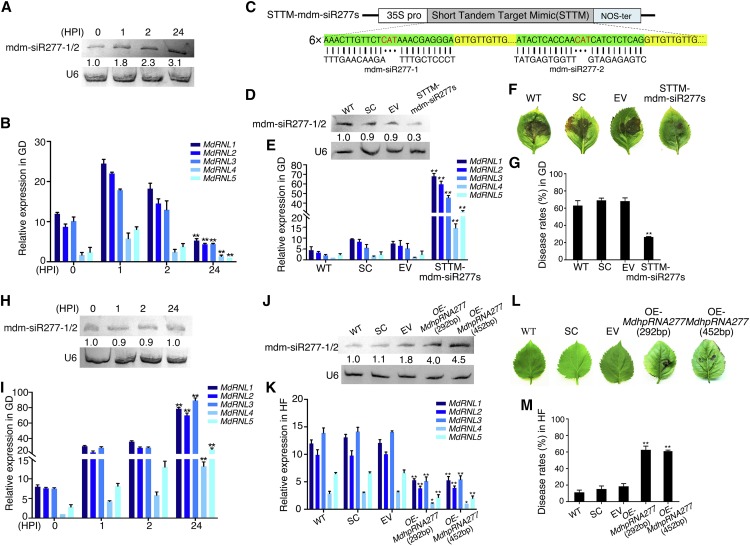
These mdm-siR277s were predicted to target several NB-LRR genes. Both mdm-siR277-1 and mdm-siR277-2, whose production is induced by ALT1, were predicted to target two different sites in the coding sequences of five RPW8-NB-LRR resistance genes (MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5), which are found on chromosomes 2 and 15 (Figures 1C and 1D). To verify that mdm-siR277-1 and mdm-siR277-2 target MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5, we performed a 5′ RACE assay in ALT-infected GD leaves. The RPW8 structural domains of all five mdm-siR277-targeted genes were cleaved (Figure 1D). Furthermore, in ALT1-inoculated GD leaves, endogenous mdm-siR277-1 and mdm-siR277-2 continued to be induced at 2 h postinoculation (HPI), and the mRNA levels of the target genes were correspondingly reduced (Figures 2A and 2B). These results strongly suggest that MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 are the direct targets of mdm-siR277-1 and mdm-siR277-2.
Figure 2.
mdm-siR277-1/2 Affects ALT1 Susceptibility in Apple by Regulating the Expression of Five R Genes.
(A) mdm-siR277-1/2 content in ALT1-inoculated GD plantlets, as revealed by RNA gel blot analysis. U6 was probed as a loading control. The signals were quantified, and relative abundances are shown below the blots.
(B) RT-qPCR showing the expression levels of five mdm-siR277-1/2 target genes in ALT1-inoculated GD plantlets. The numbers on the x axis indicate HPI.
(C) Schematic of constructs used to silence mdm-siR277-1/2 (STTM-mdm-siR277s) via Agrobacterium-mediated transient expression.
(D) mdm-siR277-1/2 content 4 d after agroinfiltration, as revealed by RNA gel blot analysis.
(E) RT-qPCR showing the mRNA levels of five target genes in mdm-siR277-1/2-silenced GD plantlets 48 h after ALT1 inoculation.
(F) Disease symptoms in GD leaves in which mdm-siR277-1/2 is silenced at 48 h after ALT1 inoculation.
(G) Disease rates in GD leaves in which mdm-siR277-1/2 is silenced at 48 h after ALT1 inoculation. In (D) to (G): WT, noninfiltrated GD plantlets; SC, scratch controls; EV, pFGC5941 empty vector; STTM-mdm-siR277s, GD plantlets with silenced mdm-siR277-1/2.
(H) mdm-siR277-1/2 content in ALT1-inoculated HF plantlets, as revealed by RNA gel blot analysis.
(I) RT-qPCR showing the expression levels of five mdm-siR277-1/2 target genes in ALT1-inoculated HF plantlets.
(J) mdm-siR277-1/2 content 4 d after agroinfiltration, as revealed by RNA gel blot analysis.
(K) The mRNA levels of five target genes, as revealed by RT-qPCR in mdm-siR277-1/2-overexpressing HF leaves 48 h after ALT1 inoculation.
(L) Phenotypes of HF leaves overexpressing mdm-siR277-1/2 at 48 h after ALT1 inoculation.
(M) Disease rates in HF leaves overexpressing mdm-siR277-1/2 at 48 h after ALT1 inoculation. In (J) to (M): WT, noninfiltrated HF plantlets; SC, scratch controls; EV, pFGC5941 empty vector; OE-MdhpRNA277 (452 bp), HF plantlets overexpressing whole transcript MdhpRNA277 (452 bp); OE-MdhpRNA277 (292 bp), HF plantlets overexpressing truncated transcript MdhpRNA277 (292 bp). Spore inoculum concentration: 2 × 105 cfu/mL. Spore growth was measured at 6 DPI. Error bars represent standard deviations calculated from three biological replicates (separate biological material). Student’s t test: **P < 0.01 and *P < 0.05.
mdm-siR277-1/2 Directly Participates in ALT1 Resistance
To investigate whether mdm-siR277-1 and mdm-siR277-2 are responsive to ALT1 infection in apple, we cloned the short tandem target mimic (STTM) of mdm-siR277-1 and mdm-siR277-2 (Tang et al., 2012; Yan et al., 2012; Reichel et al., 2015) into the binary vector pFGC5941 and introduced the resulting construct into GD leaves (via agroinfiltration) to suppress mdm-siR277-1 and mdm-siR277-2 expression (Figure 2C). RNA gel blot analysis 4 d after infiltration showed that mdm-siR277-1 and mdm-siR277-2 were silenced by STTM-mdm-siR277-1/2 in GD leaves (Figure 2D; the probe sequence of mdm-siR277-1 and mdm-siR277-2 is listed in Supplemental Table 1). Next, we inoculated GD leaves harboring STTM-mdm-siR277-1/2 with ALT1 4 d after infiltration. At 48 HPI, RT-qPCR analysis showed that the mRNA levels of the five target genes increased (Figure 2E).
In addition, exogenous STTM-mdm-siR277-1/2 treatment via agroinfiltration significantly suppressed ALT1 infection symptoms (Figure 2F) and reduced the disease rate (percentage; infected leaves/total number of leaves) (Figure 2G), suggesting that silencing of mdm-siR277-1 and mdm-siR277-2 increases resistance to ALT1 in the susceptible apple cultivar GD. By contrast, overexpression of the full-length transcript of MdhpRNA277 (452 bp) and a truncated transcript of MdhpRNA277 (including the two ALT1-induced mdm-siR277-1/2 sites, which together span 292 bp) in GD via Agrobacterium tumefaciens infiltration increased susceptibility to ALT1 in GD (Supplemental Figure 1). These results indicate that the expression levels of mdm-siR277-1 and mdm-siR277-2 affect the degree of resistance to ALT1 in a susceptible apple cultivar.
To investigate whether a series of mdm-siR277s derived from MdhpRNA277 contribute to differing degrees of ALT1 resistance in apple, we subjected 34 apple cultivars with different levels of ALT1 resistance to infection with ALT1 and compared their disease rates and RNA expression profiles (Supplemental Figure 2). The nucleotide sequences of mdm-siR277-1 and mdm-siR277-2 are identical between GD and ‘Hanfu’ (HF), as are the sequences of MdhpRNA277 and the target genes MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5. In contrast to our findings for the susceptible varieties, endogenous mdm-siR277-1 and mdm-siR277-2 were not induced after ALT1 inoculation, and the mRNA levels of the target genes increased significantly in the ALT1-inoculated resistant varieties (Figures 2H and 2I).
To investigate whether mdm-siR277-1 and mdm-siR277-2 are responsive to ALT1 infection in resistant apple varieties, we overexpressed the full-length transcript of MdhpRNA277 and a truncated transcript of MdhpRNA277 in HF (Dongguang × Fuji, HF), a representative resistant apple variety, via Agrobacterium infiltration. Four days after infiltration, RNA gel blot analysis showed that mdm-siR277-1 and mdm-siR277-2 were abundant in both the OE-MdhpRNA277 (452 bp) and OE-MdhpRNA277 (292 bp) lines (Figure 2J). Next, we inoculated HF leaves harboring OE-MdhpRNA277 (452 bp) and OE-MdhpRNA277 (292 bp) with ALT1 4 d after infiltration. At 48 h after ALT1 inoculation, RT-qPCR analysis showed that the mRNA levels of the five target genes decreased (Figure 2K) and the plants showed increased disease rates (Figures 2L and 2M). These results suggest that overexpression of mdm-siR277-1 and mdm-siR277-2 decreases ALT1 resistance in the resistant apple cultivar HF, and indicate that mdm-siR277-1 and mdm-siR277-2 levels are related to ALT1 resistance in apple.
MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 Are Targets of mdm-siR277-1 and mdm-siR277-2 That Enhance ALT1 Resistance
To investigate whether the five target genes participate in ALT1 resistance in apple, we overexpressed each gene in ALT1-susceptible GD leaves via agroinfiltration, inoculated the leaves with ALT1 4 d after agroinfiltration, and evaluated the disease rates in leaves at 48 HPI (Supplemental Figures 3A to 3D). Plants overexpressing MdRNL1, MdRNL2, MdRNL3, MdRNL4, or MdRNL5 showed significantly reduced disease rates compared with the control (Supplemental Figures 3A to 3D). Additionally, when the five target genes were silenced in the ALT1-resistant cultivar HF via agroinfiltration (Supplemental Figure 3E), MdRNL1-, MdRNL2-, MdRNL3-, MdRNL4-, and MdRNL5-suppressed leaves showed signs of infection at 48 HPI (Supplemental Figures 3F to 3H). These results indicate that MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 underlie the resistance to ALT1 infection in resistant apple cultivars.
SNP Diversity Contributes to the Activity of the MdhpRNA277 Promoter
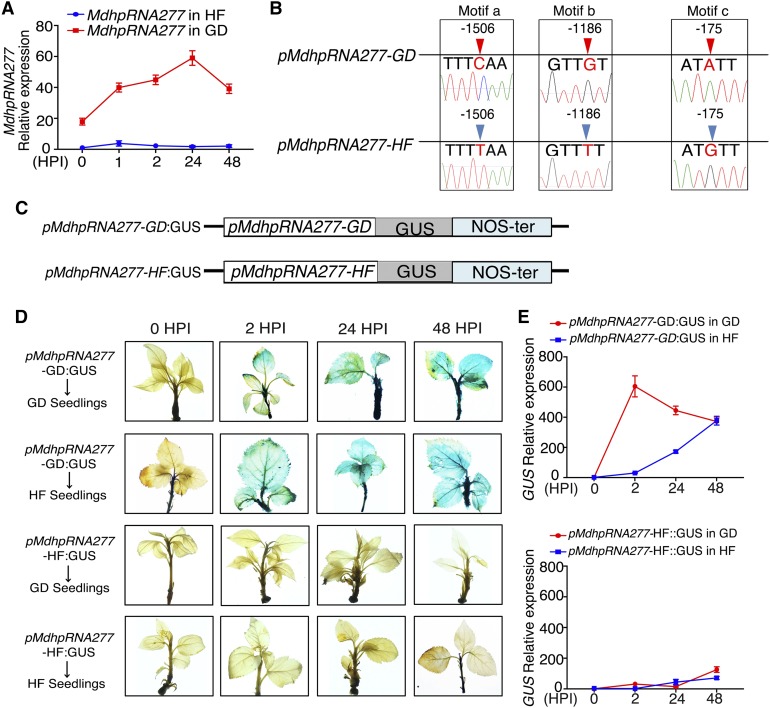
To explain why mdm-siR277-1 and mdm-siR277-2 are expressed differentially in the ALT1-susceptible (GD) versus ALT1-resistant (HF) apple cultivars, we compared the sequences and expression levels of MdhpRNA277 in the two cultivars. We cloned the MdhpRNA277 sequences from GD and HF and found that their sequences were identical; however, MdhpRNA277 was highly induced by ALT1 inoculation in GD but not in HF (Figure 3A). We confirmed these results by analyzing 34 apple cultivars with different levels of ALT1 resistance; MdhpRNA277 was expressed at high levels in the susceptible cultivars at 48 HPI, as observed in GD, but at low levels in the resistant cultivars, as in HF (Supplemental Figure 2A). We cloned the promoter sequence of MdhpRNA277 (1711 bp) and identified three SNPs (at −1506, −1186, and −175 bp) in the promoters of MdhpRNA277 isolated from GD (pMdhpRNA277-GD) and HF (pMdhpRNA277-HF) (Figure 3B).
Figure 3.
Three SNPs in pMdhpRNA277-GD and pMdhpRNA277-HF Affect Respective Hairpin Gene Expression Levels.
(A) RT-qPCR to detect the mRNA levels of MdhpRNA277 in ALT1-inoculated GD and HF seedlings. Error bars represent standard deviations calculated from three biological replicates (separate biological material) that were performed for ALT1 treatment.
(B) SNPs in the promoter of MdhpRNA277 isolated from GD (pMdhpRNA277-GD) and HF (pMdhpRNA277-HF). Motif a, C-T base substitution at −1506 bp; motif b, G-T base substitution at −1186 bp; motif c, A-G base substitution at −175 bp.
(C) Schematic of constructs used to measure pMdhpRNA277-GD and pMdhpRNA277-HF activity.
(D) GUS staining of GD and HF seedlings infected with pMdhpRNA277-GD:GUS or pMdhpRNA277-HF:GUS after ALT1 inoculation. pMdhpRNA277-GD:GUS, GD and HF seedlings overexpressing pMdhpRNA277-GD:GUS; pMdhpRNA277-HF:GUS, GD and HF seedlings overexpressing pMdhpRNA277-HF:GUS.
(E) GUS mRNA levels in the seedling shown in (D), as revealed by RT-qPCR. Spore inoculum concentration: 2 × 105 cfu/mL. Spore growth was measured at 6 DPI.
To investigate whether the promoter activity of MdhpRNA277 is affected by the SNPs between GD and HF, we isolated pMdhpRNA277-GD and pMdhpRNA277-HF and introduced these sequences into the pCAMIBIA1305 vector upstream of the GUS reporter gene to generate pMdhpRNA277-GD:GUS and pMdhpRNA277-HF:GUS, respectively (Figure 3C). We introduced the constructs into 4-week-old GD and HF seedlings via agroinfiltration and inoculated the infiltrated seedlings with ALT1 4 d later. GUS histochemical staining and gene expression analysis showed that pMdhpRNA277-GD activity was induced in both GD and HF seedlings inoculated with ALT1, whereas pMdhpRNA277-HF was not induced in GD or HF seedlings (Figures 3D and 3E).
To identify which SNP is critical for pMdhpRNA277 activity, the three SNPs (motifs a, b, and c) from pMdhpRNA277-HF were mutated to the GD sequence and the mutated promoters were fused to GUS and used for agroinfiltration. When motif a or c in pMdhpRNA277-HF was mutated, GUS staining was not detected in infiltrated GD or HF seedlings after ALT1 inoculation (Supplemental Figure 4). When motif b in pMdhpRNA277-HF was mutated to the same sequence as in pMdhpRNA277-GD (T to G), strong GUS staining was detected in both infiltrated GD and HF seedlings after ALT1 inoculation (Figures 4A and 4B). By contrast, when motif b in pMdhpRNA277-GD was mutated to the same sequence as in pMdhpRNA277-HF (G to T), GUS staining was not detected in infiltrated GD or HF seedlings after ALT1 inoculation (Figures 4A and 4B). These results indicate that motif b (−1186 bp) is critical for the activity of the MdhpRNA277 promoter and that a G-to-T mutation in this motif eliminates the activity of this promoter (Supplemental Figure 5).
Figure 4.
Motif b in pMdhpRNA277 Is Critical for Its Activity.
(A) Schematic of the mutation in motif b (red arrowhead) in the promoters of MdhpRNA277-HF and MdhpRNA277-GD cloned in the pCAMIBIA1305 vector upstream of the GUS reporter gene to generate mpMdhpRNA277-HF-b:GUS and mpMdhpRNA277-GD-b:GUS, respectively.
(B) GUS staining of GD and HF leaves infected with mpMdhpRNA277-GD:GUS or mpMdhpRNA277-HF:GUS after ALT1 inoculation. Spore inoculum concentration: 2 × 105 cfu/mL. Spore growth was measured at 6 DPI.
The Transcription Factor MdWHy Fails to Recognize pMdhpRNA277-HF
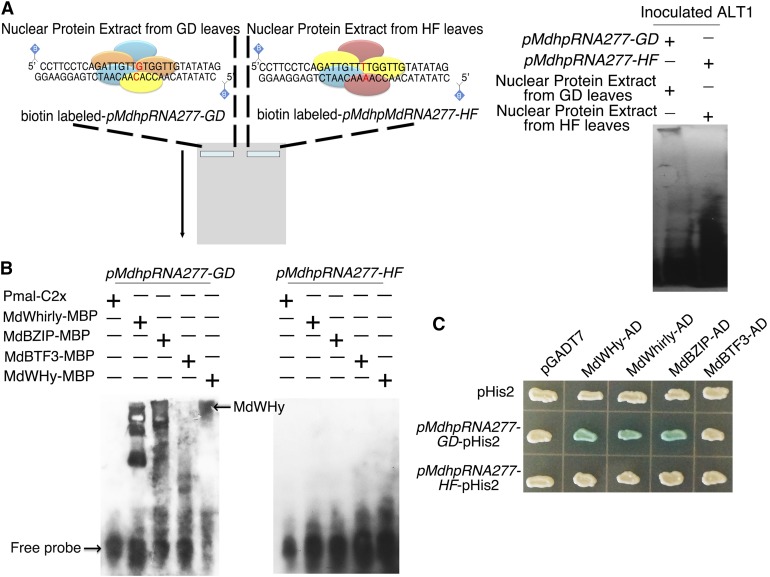
To investigate why a single nucleotide change eliminated the activity of pMdhpRNA277-HF, we incubated biotin-labeled forms of the promoter sequences (−1173 to −1203 bp) from pMdhpRNA277-GD and pMdhpRNA277-HF with nuclear protein extracts (NPEs) from ALT1-inoculated GD or HF leaves and subjected them to an electrophoretic mobility shift assay (EMSA) (Figure 5A). Whereas the labeled pMdhpRNA277-GD sequence bound to GD NPE, pMdhpRNA277-HF did not bind to HF NPE (Figure 5A).
Figure 5.
Three Transcription Factors Bind to pMdhpRNA277-GD, but Not to pMdhpRNA277-HF.
(A) Schematic diagram (left panel) of the method used to identify transcription factors via EMSA. The regions from −1173 to −1203 bp of pMdhpRNA277-GD and pMdhpRNA277-HF containing motif b were labeled with biotin and incubated with nuclear protein extracts from GD or HF leaves inoculated with ALT1. EMSA (left panel) to identify transcription factors that bind to pMdhpRNA277-GD (right side of the figure).
(B) EMSA showing MdWHy, MdWhirly, and MdbZIP binding to pMdhpRNA277-GD.
(C) Yeast one-hybrid assay showing the binding of MdWHy, MdWhirly, and MdbZIP to pMdhpRNA277-GD.
To identify potential proteins that bind to pMdhpRNA277-GD, we obtained four peptide fragments, MdWHy (Ciccarelli and Bork, 2005), MdWhirly (Desveaux et al., 2005), MdbZIP (Landschulz et al., 1988), and MdBTF3 (Wiedmann et al., 1994), by liquid chromatography-mass spectrometry. To test whether these four potential transcription factors bind to the pMdhpRNA277-GD promoter, the coding sequences of MdWHy, MdWhirly, MdbZIP, and MdBTF3 were heterologously expressed in bacteria as MBP fusion proteins, purified, and subjected to an EMSA using labeled pMdhpRNA277-GD and pMdhpRNA277-HF sequences as probes. MdWHy, MdWhirly, and MdbZIP bound to pMdhpRNA277-GD but not pMdhpRNA277-HF (Figure 5B), which was confirmed by yeast one-hybrid assays (Figure 5C).
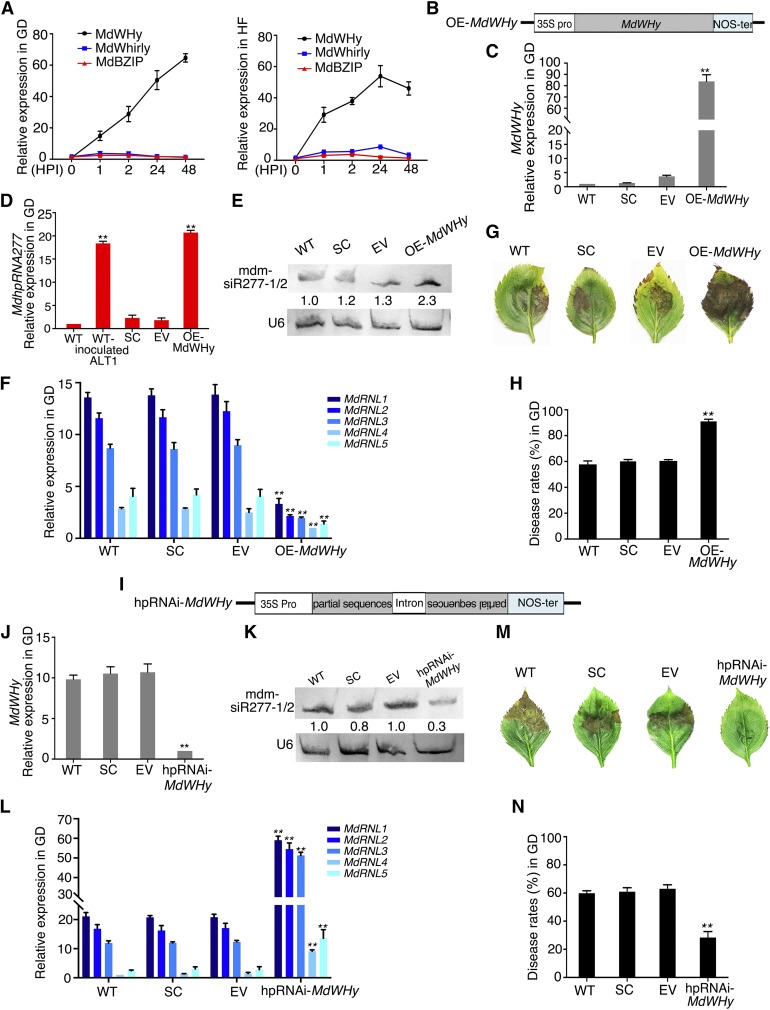
The Transcription Factor MdWHy Induces MdhpRNA277-Derived mdm-siR277-1/-2 Production and Contributes to ALT1 Susceptibility in Apple
To clarify which transcription factors directly respond to ALT1 infection, we investigated the expression of MdWHy, MdWhirly, and MdbZIP in ALT1-infected GD and HF leaves. RT-qPCR showed that MdWHy mRNA levels increased at 1 HPI with ALT1 in GD and HF leaves (Figure 6A). To investigate whether the activity of the transcription factor MdWHy directly corresponds to ALT1 susceptibility and resistance via regulating pMdhpRNA277-GD expression and abundance, we overexpressed MdWHy in GD leaves by agroinfiltration and measured the expression levels of mdm-siR277-1, mdm-siR277-2, and MdhpRNA277 in GD leaves at 4 d after infiltration (Figures 6B and 6C). Overexpression of MdWHy resulted in increased levels of mdm-siR277-1, mdm-siR277-2, and MdhpRNA277 (Figures 6D and 6E). Following inoculation with ALT1, MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 mRNA levels decreased, and plant sensitivity to ALT1 increased (Figures 6F to 6H). Furthermore, the expression levels of mdm-siR277-1 and mdm-siR277-2 decreased 4 d after MdWHy was silenced in GD via agroinfiltration with hpRNAi-MdWHy (Figures 6I to 6K). Following ALT1 inoculation, the symptoms were relieved and the RNA levels of the target genes MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 increased (Figures 6L to 6N). Moreover, when MdWHy was overexpressed in HF leaves via agroinfiltration, followed by ALT1 inoculation, ALT1 resistance was not altered in HF leaves (Supplemental Figure 6). These results indicate that the binding of MdWHy to pMdhpRNA277-GD induces the expression of mdm-siR277-1 and mdm-siR277-2 when susceptible varieties of apple are infected with ALT1. As MdWHy is unable to bind to pMdhpRNA277-HF, MdhpRNA277 is not induced when resistant varieties are infected with ALT1.
Figure 6.
Overexpression or Silencing of ALT1-Induced MdWHy Regulates ALT1 Resistance in GD, but Not in HF.
(A) The mRNA levels of MdWHy, MdWhirly, and MdbZIP in ALT1-inoculated GD or HF plantlets, as revealed by RT-qPCR.
(B) Schematic of constructs used to transiently overexpress MdWHy via Agrobacterium-mediated transformation.
(C) The mRNA level of MdWHy in GD plantlets, as revealed by RT-qPCR 4 d after infiltration of OE-MdWHy.
(D) The mRNA level of MdhpRNA277 in OE-MdWHy GD plantlets, as revealed by RT-qPCR.
(E) mdm-siR277-1/2 content in OE-MdWHy GD plantlets, as revealed by RNA gel blot analysis.
(F) RT-qPCR showing the mRNA levels of five target genes in MdWHy-overexpressing GD plantlets 48 h after ALT1 inoculation.
(G) Disease symptoms in GD leaves in which MdWHy is overexpressed at 48 h after ALT1 inoculation.
(H) Disease rates in GD leaves in which MdWHy is overexpressed at 48 h after ALT1 inoculation.
(I) Schematic of constructs used for silencing of MdWHy by Agrobacterium-mediated transient expression.
(J) The mRNA level of MdWHy 4 d after infiltration of hpRNAi-MdWHy in GD, as revealed by RT-qPCR.
(K) mdm-siR277-1/2 content in hpRNAi-MdWHy GD plantlets, as revealed by RNA gel blot analysis.
(L) Expression of MdRNL1/2/3/4/5, as revealed by RT-qPCR in GD plantlets in which MdWHy is silenced.
(M) Appearance of GD leaves in which MdWHy is silenced at 48 h after ALT1 inoculation.
(N) Disease rates in GD leaves in which MdWHy is silenced at 48 h after ALT1 inoculation. In (B) to (N): WT, noninfiltrated GD plantlets; SC, scratch controls; EV, pFGC5941 empty vector; OE-MdWHy, GD plantlets overexpressing MdWHy; hpRNAi-MdWhy, GD plantlets in which MdWHy is silenced. Spore inoculum concentration: 2 × 105 cfu/mL. Spore growth was measured at 6 DPI. Error bars represent standard deviations calculated from three biological replicates (separate biological material) performed for each treatment. Student’s t test: **P < 0.01.
Overexpressing pMdhpRNA277-GD:MdhpRNA277 Enhances ALT1 Susceptibility in Transgenic Apple
To confirm the notion that differential pMdhpRNA277 activity (pMdhpRNA277-GD and pMdhpRNA277-HF) alters resistance to ALT1 in ‘GL-3’, we genetically transformed plantlets from a high-regeneration capacity line isolated from ‘Royal Gala’ (Malus × domestica) named ‘GL-3’ (Dai et al., 2013) with the MdhpRNA277 sequence driven by the MdhpRNA277 promoter (pMdhpRNA277-GD or pMdhpRNA277-HF) (Supplemental Figures 7A to 7C). In ‘GL-3’ leaves, we suppressed mdm-siR277-1 and mdm-siR277-2 expression via transient transformation with STTM-mdm-siR277-1/2 via agroinfiltration. This suppression increased the mRNA levels of the five target genes and reduced the disease rate at 48 HPI with ALT1 (Supplemental Figures 7D to 7G). We then overexpressed truncated transcripts of MdhpRNA277 in ‘GL-3’ via Agrobacterium infiltration. At 48 h after ALT1 inoculation, the mRNA levels of the five target genes decreased and the plants showed symptoms of infection, including increased disease rates (Supplemental Figures 7H to 7K). These results suggest that mdm-siR277-1 and mdm-siR277-2 regulate the expression of MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5, which is related to ALT1 resistance in ‘GL-3’.
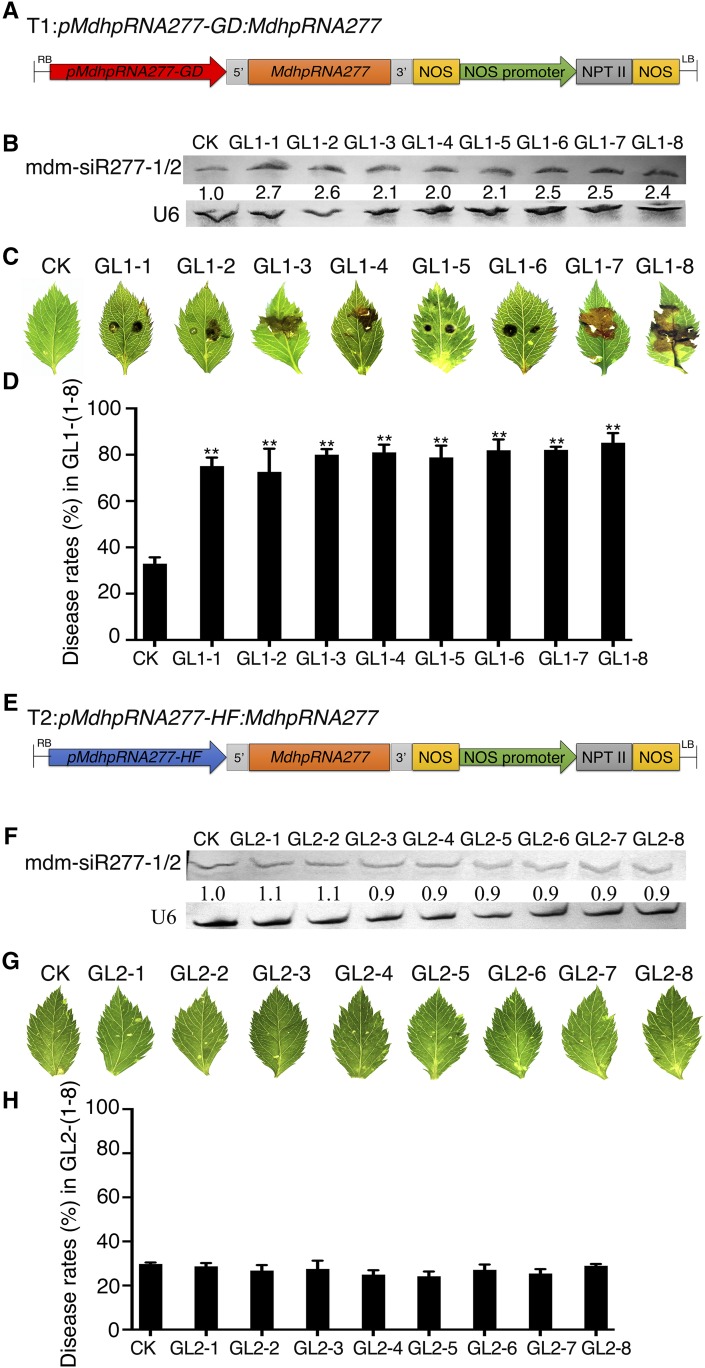
To investigate whether MdhpRNA277 harboring the GD promoter, but not the HF promoter, would induce mdm-siR277-1 and mdm-siR277-2 production, thereby increasing susceptibility to ALT1, we constructed the pMdhpRNA277-GD:MdhpRNA277 and pMdhpRNA277-HF:MdhpRNA277 binary vectors and transferred them to apple plants via transformation with Agrobacterium strain EHA105 (Figures 7A and 7E). Eight ‘GL-3’ apple lines, GL1-1–GL1-8, transgenically expressed pMdhpRNA277-GD:MdhpRNA277, and we confirmed the transgenic status of the lines by DNA gel blot analysis (Supplemental Figure 8). Forty-eight hours after inoculation with ALT1, RNA gel blot analysis showed that mdm-siR277-1 and mdm-siR277-2 were abundant and the mRNA levels of MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 were reduced in all GL1-1(-8) lines compared with untransformed plantlets (CK) (Figure 7B; Supplemental Figure 9). GL1-1(-8) showed symptoms of ALT1 infection, and the disease rates ranged from 72.65 to 85.21%, which were higher than those observed for GL2-1(-8), which harbored pMdhpRNA277-HF:MdhpRNA277 (24.19 to 28.96%) and had similar disease rates to those of CK (Figures 7C, 7D, 7G, and 7H).
Figure 7.
pMdhpRNA277-GD Is Induced by ALT1 Infection in Transgenic Apple, but pMdhpRNA277-HF Is Not.
(A) Schematic of the T1 construct used to stably overexpress MdhpRNA277 under the control of the pMdhpRNA277-GD promoter in GL-3 lines.
(B) RNA gel blot analysis of mdm-siR277-1/2 content at 48 HPI in GL-3 transgenic lines GL1-1(-8) compared with CK.
(C) Disease symptoms in 4-week-old GL1-1(-8) lines at 48 HPI.
(D) Disease rates in 4-week-old GL1-1(-8) lines at 48 HPI.
(E) Schematic of construct used to stably overexpress MdhpRNA277 under the control of the pMdhpRNA277-HF promoter in the GL-3 lines.
(F) RNA gel blot analysis of mdm-siR277-1/2 content at 48 HPI in GL-3 transgenic lines GL2-1(-8) compared with CK.
(G) Disease symptoms in 4-week-old GL2-1(-8) lines at 48 HPI.
(H) Disease rates in 4-week-old GL2-1(-8) lines at 48 HPI. Spore inoculum concentration: 2 × 105 cfu/mL. Spore growth was measured at 6 DPI. Error bars represent standard deviations calculated from three biological replicates (separate biological material) were performed for each treatment. Student’s t test: **P < 0.01.
Thus, MdhpRNA277 driven by the promoter from GD induced mdm-siR277-1 and mdm-siR277-2 production, thereby reducing MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 expression, resulting in increased ALT1 susceptibility in ‘GL-3’ transgenic apple lines. However, MdhpRNA277 driven by the promoter from HF did not induce mdm-siR277-1 or mdm-siR277-2 production, and the disease rates in transgenic plants harboring this construct did not differ from those of the control in the ‘GL-3’ background (Figure 7; Supplemental Figure 9).
The SNPs in pMdhpRNA277 Are Associated with A. alternata Leaf Spot Resistance in Various Apple Cultivars
We identified a potential SNP at −1186 bp (motif b) in pMdhpRNA277. To determine whether this SNP is associated with A. alternata leaf spot resistance in various apple cultivars, we sequenced motif b in 34 apple cultivars with different levels of ALT1 resistance (Supplemental Figure 2). A T residue in motif b was found in nine resistant cultivars, including HF, whereas a G residue was found in 11 susceptible cultivars, including GD. The 14 remaining varieties were heterozygous for T versus G (Supplemental Figure 2D).
To further validate that the SNP in pMdhpRNA277 is associated with A. alternata leaf spot resistance, we generated three additional populations via crosses between Han Fu × Yue Shuai, Golden Delicious × Nagafu 2, and Yue Guan × Nagafu 2. First, we determined the disease rates and genotypes (SNP site polymorphism at −1186 bp of pMdhpRNA277) of each parental line. The disease rate was 0% in Han Fu, 72.22% in Yue Shuai, 100% in Golden Delicious, 46.67% in Nagafu 2, and 36.66% in Yue Guan. We then analyzed the 55 progeny resulting from the cross Han Fu (TT) × Yue Shuai (GG). The plants fell into a single class (GT), with disease rates ranging from 15 to 50% (Supplemental Tables 2 and 3). The 60 progeny of Golden Delicious (GG) × Nagafu 2 (GT) fell into two classes (GG and GT); the 29 progeny carrying GG were susceptible to A. alternata leaf spot, and the observed ratios for genotype segregation fit the expected ratios (χ2 values of 0.8, P > 0.05) (Supplemental Tables 2 and 4). The segregation ratio of the three classes (TT, GT, and GG) of the 100 progeny from Yue Guan (GT) × Nagafu 2 (GT) was 21:55:24, which fit the expected ratio of 1:2:1, with χ2 values of 0.55. Of these, 21 progeny carrying TT showed resistance to A. alternata leaf spot, and 24 progeny carrying GG were susceptible to the pathogen (Supplemental Tables 2 and 5). These results suggest that the SNP identified in this study can be used as a marker of A. alternata leaf spot resistance/susceptibility in apple.
DISCUSSION
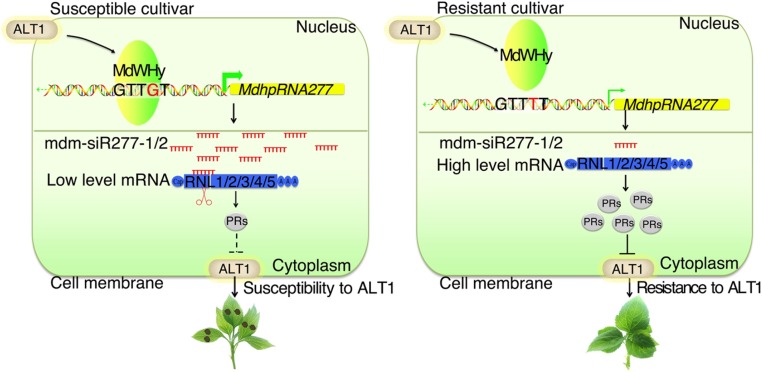
Apple is considered to be a model fruit tree due to its high productivity and worldwide economic value. However, apple production is limited by many types of fungal diseases. Apple A. alternata leaf spot, caused by A. alternaria f. sp mali, is one of the most devastating diseases of apple. Here, through a series of experiments, we developed a model explaining the molecular basis for different levels of disease resistance in various apple varieties. In resistant apple varieties, MdWHy is unable to bind to pMdhpRNA277-HF, and MdhpRNA277 transcription is suppressed, resulting in low levels of mdm-siR277-1 and mdm-siR277-2 production, high levels of MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 expression, and strong resistance to A. alternata leaf spot (Figure 8). By contrast, in susceptible varieties, MdWHy binds to pMdhpRNA277-GD, and MdhpRNA277 transcription is activated, resulting in high levels of mdm-siR277-1 and mdm-siR277-2 production and low levels of MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 expression, as well as weak resistance to A. alternata leaf spot (Figure 8).
Figure 8.
Model for the Role of SNPs in pMdhpRNA277 in Regulating A. alternata Leaf Spot Resistance in Apple.
In ALT1-susceptible apple cultivars, the transcriptional activity of transcription factor MdWHy is induced by ALT1 infection. MdWHy recognizes motif b (G) in the promoter of MdhpRNA277-GD, resulting in an increase in mdm-siR277-1/2 levels. mdm-siR277-1/2 then silence their target resistance genes MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5, resulting in disease susceptibility. By contrast, in ALT1-resistant apple cultivars, although MdWHy is also induced by ALT1 infection, MdWHy cannot bind to the promoter of MdhpRNA277-HF due to the replacement of a G residue with a T in motif b. Very low amounts of mdm-siR277-1/2 are produced, leading to the expression of target resistance genes MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5, thereby resulting in ALT1 resistance. The thick green arrow indicates high transcriptional activity of MdhpRNA277, while the thin green arrow indicates low transcriptional activity of MdhpRNA277.
Recently, increasing numbers of small RNAs with important roles in plant immunity have been discovered, such as miRNAs (He et al., 2008; Li et al., 2012; Shivaprasad et al., 2012; Zhao et al., 2013; Zhu et al., 2013; Boccara et al., 2014; Liu et al., 2014; Zhang et al., 2016) and phased siRNAs (phasiRNAs) (Zhai et al., 2011; Li et al., 2012; Arikit et al., 2014; Wong et al., 2014; Deng et al., 2018). Production of the miRNA csi-miR399 is induced in Las-positive citrus trees (infected with the bacterium causing Citrus Huanglongbing Disease), thereby suppressing PHOSPHATE2 expression and improving fruit appearance and productivity (Zhao et al., 2013). We previously found that Md-miR156ab and Md-miR395 suppress the expression of transcription factor genes MdWRKYN1 and MdWRKY26 and reduce the expression of several pathogenesis-related (PR) genes, resulting in susceptibility to ALT1 in the ALT1-susceptible apple cultivar, GD (Zhang et al., 2017). Several members of the WRKY transcription factor family are targeted by R proteins, causing these proteins to recognize W-box elements in the promoters of PR genes (Rushton et al., 1996; Eulgem et al., 1999, 2000; Sohn et al., 2014; Sarris et al., 2015). Thus, it may be worthwhile to investigate the relationship between ALT1-related WRKY genes (MdWRKYN1 and MdWRKY26) (Zhang et al., 2017) and the five ALT1-related R genes in apple (MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5). Moreover, the tobacco mosaic virus resistance gene N is regulated by the miRNA cluster miR6019/6020 in tobacco plants; 22-nucleotide miR6019 cleaves the N transcript at its TIR coding region and triggers phasiRNA production in an RDR6/DICER-LIKE4-dependent manner (Li et al., 2012; Deng et al., 2018). In soybean, miR393 and its phasiRNA regulate the expression of defense-associated R genes during Phytophthora sojae infection (Wong et al., 2014). Finally, miR482-mediated cleavage of NBS-LRR genes triggers the production of phased secondary small RNAs in tomato and cotton (Gossypium hirsutum) upon fungal pathogen attack (Zhu et al., 2013; Ouyang et al., 2014).
The small RNAs identified in this study, mdm-siR277-1 and mdm-siR277-2, which are derived from the conversion of single-stranded RNA into double-stranded hairpin RNA (Henderson et al., 2006; Axtell, 2013; Harmoko et al., 2013), are similar to miRNAs in terms of their hairpin structure, but they do not meet the criteria for annotation as miRNAs for several reasons (Taylor et al., 2017; Axtell and Meyers, 2018). First, the fold-back region of MdhpRNA277 is longer than 300 nucleotides, and it should therefore not be annotated as an miRNA-producing locus (Axtell and Meyers, 2018). Second, structural analysis of MdhpRNA277 revealed that it does not contain asymmetric bulges at the positions of mdm-siR277-1 and mdm-siR277-2, although such bulges should be present in miRNA precursors (Axtell and Meyers, 2018). Little is known about hpRNA loci, although DCL1, a typical member of a large class of endogenous dicer-like proteins, prefers substrates with hairpin-like structures and cleaves them at defined sites, perhaps targeting recently evolved or evolving miRNA genes (Henderson et al., 2006; Harmoko et al., 2013). Efforts directed at comprehensive genome-wide annotation of small RNA hpRNA loci are urgently needed (Axtell, 2013).
Furthermore, there are similarities between the 3′ region of MdhpRNA277 and R genes (the five target genes of mdm-siR277-1/2), suggesting that the long hairpin may have arisen from the duplication of R genes. Our results suggest that the regulation of R genes from this long hairpin locus involves the production of several small RNAs that target R genes, as two sites of mdm-siR277-1 and mdm-siR277-2 share high sequence similarity with their five target genes. MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 regulate ALT1 resistance in apple. MIR482/MIR2118 precursors share sequence similarity with NB-LRR genes, as well as MIR161 and MIR163 in Arabidopsis (Allen et al., 2004; Xia et al., 2015). Inverted duplication events based on complete or partial sequences of a gene occur as follows: The sequence is duplicated to form a long, paired stem-loop structure; the sequence gradually accumulates mutations and becomes shorter, reaching the canonical length of an MIRNA precursor (<300 bp); the miRNA processing enzyme DCL1 is then recruited to the sequence (Allen et al., 2004; Voinnet, 2009; Xia et al., 2015). This evolutionary history shows that miRNAs can indeed evolve from their target genes. Indeed, the hairpin RNA MdhpRNA277 may gradually develop sequence mutations and become shorter, eventually evolving into an MIRNA precursor and giving rise to a completely novel, “young” miRNA that might be obscured by mutation and selection over millions of years (Allen et al., 2004; Xia et al., 2015).
MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 promote resistance to ALT1 in HF following infection. MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 are members of CCR-NB-LRR, a small subclass of the NB-LRR protein family. Few CCR-NB-LRR proteins have been found in plants, and little is known about this subclass (Collier and Moffett, 2009). CCR-NB-LRR proteins containing N-terminal RPW8 domains have been identified via BLAST analyses of conserved NB-ARC or LRR peptide sequences against the Arabidopsis, N. benthamiana, potato, soybean, and apple genomes, but their functions remain unknown (Meyers et al., 2003; Bonardi et al., 2011; Jupe et al., 2012; Arya et al., 2014b). Studies on the roles of CCR-NB-LRR proteins have solely focused on NRG1 in N. benthamiana and ADR1 in Arabidopsis (Chini et al., 2004b; Peart et al., 2005; Bonardi et al., 2011; Collier et al., 2011), both of which are helper NB-LRRs that must form a complex with other proteins to ensure R protein function (Peart et al., 2005; Collier et al., 2011; Dong et al., 2016). However, there are no reports of CCR-NB-LRR proteins that physically interact with NB-LRR proteins to mediate defense responses (Eitas and Dangl, 2010; Collier et al., 2011). Thus, it may be worth searching for proteins that interact with MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5.
In this study, we identified an SNP at −1186 bp (motif b) in pMdhpRNA277 by analyzing 34 apple cultivars with different levels of ALT1 resistance and three additional populations derived from crosses and showed that this SNP could be used to distinguish resistant from susceptible cultivars. Resistant cultivars exhibited a T residue in motif b (leaf disease rate ≤ 15%); susceptible cultivars had a G residue at this position (leaf disease rate ≥ 50%); and neutral cultivars were heterozygous (T/G) at this position (15% < leaf disease rate > 50%) (Supplemental Tables 2 to 5). Therefore, this SNP could be used as a marker to distinguish among apple varieties that are resistant or susceptible to A. alternata leaf spot. The ability to identify apple lines that are resistant to this devastating disease makes this SNP a valuable tool for the apple breeding industry.
METHODS
Plant Materials and Growth Conditions
Apple (Malus domestica cv ‘Golden Delicious’, Malus domestica cv ‘Hanfu’) plants were grown by tissue culture on Murashige and Skoog (MS) medium containing 0.6 mg L−1 6-benzylaminopurine and 0.15 mg L−1 1-naphthylacetic acid in a climate-controlled culture room at 25 ± 1°C under fluorescent lights at ∼100 μmol photons m−2 s−1 (Philips Lighting TL5 28W/865) with a 16/8-h light/dark photoperiod .The plants were transferred to fresh medium every 4 weeks. Approximately four-week-old apple seedlings were infiltrated with Agrobacterium tumefaciens and used in the fungal infection experiments when the fifth leaf of the seedling had expanded (leaf length: 2 cm), as described previously (Bai et al., 2011).
Leaves were sampled from ∼7- to 8-year-old trees of 34 apple cultivars grown at the National Germplasm Repository of Apple (Institute of Pomology of Chinese Academy of Agricultural Sciences, CAAS, Xingcheng, Liaoning Province, China) (Supplemental Figure 2). The crossing parents Han Fu, Yue Shuai, Golden Delicious, Nagafu 2, and Yue Guan were 15-year-old trees, and the hybrid populations derived from Han Fu × Yue Shuai and Golden Delicious × Nagafu 2 were 6x-year-old trees at the time of the experiment. The hybrid population of Yue Guan × Nagafu 2 comprised 3-year-old trees (Supplemental Tables 2 to 5); all of these trees were grown at the Liaoning Institute of Pomology. Leaves (5 cm in length) were collected from the field in May of 2016 and 2017 and placed on moist filter paper in a culture dish within 30 min. A moist cotton ball was wrapped around the base of each petiole, and the leaves were incubated at a relative humidity of 75% and a temperature of 25°C. The leaves remained fresh for at least 5 d.
A line with a high regeneration capacity isolated from ‘Royal Gala’ (Malus × domestica) named ‘GL-3’ (Dai et al., 2013) was used for genetic transformation. In vitro-grown ‘GL-3’ plants were subcultured every 4 weeks on MS medium supplemented with 0.6 mg L−1 6-benzylaminopurine and 0.15 mg L−1 1-naphthylacetic acid at 25 ± 1°C under fluorescent lights at ∼100 μmol photons m−2 s−1 (Philips Lighting TL5 28W/865) with a 16/8-h light/dark photoperiod .
Fungal Growth and Infection Assay
Plant pathogenic ALT1 (Alternaria alternata f. sp mali) strains were provided by LiYun Guo (China Agricultural University). The fungus was grown on potato dextrose agar medium at 25°C for 6 d. Inoculum spores were suspended in deionized water at 2×105 colony-forming units (cfu)/mL, and yields were assessed by microscopy (Olympus CX31RTSF).
The infection rates of plants grown on MS medium were determined as described below (“Agrobacterium-Mediated Transient Expression Assays with pFGC5941 Constructs” and “Isolation and Identification of the MdhpRNA277 Promoter”).
Leaves were collected (length: 5 cm) from plants grown in the field and injected with a 2 × 105 cfu/mL pathogenic spore suspension of ALT1 at two spots per leaf. After inoculation, the leaves were returned to the culture dishes and placed in an incubator (75% humidity; 25°C) under conditions that maintained freshness for at least 5 d. At 48 HPI, the leaf disease rates (%: infected leaves/total number of leaves) were calculated and the expression levels of the genes determined. The experiments were repeated three times, and t test analysis was performed. Three replicates of ∼30 inoculated leaves derived from different plants were conducted to reduce the experimental error. The plants were graded into three types according to the degree of infection in leaves: resistant (leaf disease rate ≤ 15%), neutral (15% < leaf disease rate > 50%), and susceptible (leaf disease rate ≥ 50%).
Total RNA Extraction and RNA Gel Blot Analysis of Small RNAs
Total RNA was isolated from apple leaves using a modified cetyltrimethylammonium bromide method (Li et al., 2009) and treated with DNase I (Invitrogen) to remove DNA contamination. RNA integrity was verified by electrophoresis on a 1.2% agar gel, and the RNA concentration was measured using an ND-1000 NanoDrop spectrophotometer (Thermo Fisher Scientific). RNA gel blot analysis was performed as previously reported (Rio, 2014) with a Digoxin Hybridization Detection Kit following the manufacturer’s instructions (Mylab; DIGD-120). Approximately 60 μg of RNA was separated in a 15% polyacrylamide gel containing 7 M urea and electrically transferred to Hybond-N+ membranes (GE Healthcare). The 5′ end-modified digoxin-labeled mdm-siR277-1 and mdm-siR277-2 probes were synthesized by the Taihe Biotech Sequence Company. Primers are listed in Supplemental Table 1.
Identification and Cloning of mdm-siR277-1/2-Targeted Genes
A search for novel siRNA-targeted genes was conducted by sequence alignment with the apple genome from http://plantgrn.noble.org/psRNATarget. Detailed annotation information about the targeted genes, including nucleotide sequences, chromosome locations, and predicted protein domains, was obtained from the apple genome sequence https://iris.angers.inra.fr/gddh13/the-apple-genome-downloads.html and https://www.rosaceae.org databases (Daccord et al., 2017). The cleaved miRNA target genes used for 5′ RACE were obtained as described (Liu et al., 2010) using a 5′ RACE kit following the manufacturer’s instructions (GIBCO BRL Life Technologies). Oligonucleotides used for cloning are listed in Supplemental Table 1.
RT-qPCR Assay
Total RNA was extracted from apple leaves using an EASY Spin Kit (Biomed; RA106-02), amplified using oligo(dT) primers and reverse transcribed into cDNA (Takara) to detect mRNA expression levels (Supplemental Table 6). RT-qPCR was performed using SuperReal PreMix Plus (SYBR Green) (Tiangen; FP205) under the following cycling conditions: 40 cycles of 95°C for 10 s and 60°C for 30 s (Applied Biosystems 7500). Relative RNA levels were calculated using the 2−ΔΔCt method and normalized using Actin as a reference (Livak and Schmittgen, 2001). Specific primers recognizing MdhpRNA277, MdhpRNA271, and the mdm-siR277-1/2 targets (MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5) are listed in Supplemental Tables 6 and 7.
A total of nine leaves from three different plants subjected to the same treatment were collected as one sample for RNA isolation, reverse transcription, and RT-qPCR. The same number of noninfiltrated GD/HF/‘GL-3’ seedlings and EV (pFGC5941)-infiltrated GD/HF/‘GL-3’ plants served as controls. Three technical replicates were performed for each cDNA sample, and three biological repeats (separate biological materials) were performed for each treatment. Student’s t test was used to evaluate statistical significance.
Agrobacterium-Mediated Transient Expression Assays with pFGC5941 Constructs
Agrobacterium-mediated transient expression was performed using the pFGC5941 construct driven by the 35S promoter. The loss-of-function mdm-siR277-1/2 sequence was cloned into the NcoI/BamHI restriction site of the pFGC5941 binary vector containing the STTM of the mdm-siR277-1/2 sequence (Yan et al., 2012). Constructs used to overexpress MdhpRNA277(452 bp), MdhpRNA277(292 bp), MdRNL1/2/3/4/5, and the synonymous mutated mdm-siR277-1/2 target site versions of MdRNL1/2/3/4/5, MdWHy, MdWhirly, and MdbZIP (OE-MdhpRNA277[452 bp], OE-MdhpRNA277[292 bp], OE-MdRNL1/2/3/4/5, OE-mMdRNL1/2/3/4/5, OE-MdWHy, OE-MdWhirly, and OE-MdbZIP, respectively) were also produced by cloning the full-length coding sequences of the genes into the NcoI/BamHI site of the pFGC5941 binary vector (Figures 2C and 6B; Supplemental Figures 1A and 3A). Loss-of-function MdRNL1, MdRNL2, MdRNL3, MdRNL4, MdRNL5, and MdWHy constructs were produced by cloning the reverse partially specific sequences of these genes into pFGC5941, carrying NcoI/SawI and XbaI/BamHI digestion sites, to produce siRNAs (hpRNAi-MdRNL1/2/3/4/5 and hpRNAi-MdWHy, respectively) (Supplemental Figure 3E; Figure 6I). Primers are listed in Supplemental Table 7.
The binary constructs were transformed into Agrobacterium strain GV3101 by heat shock transformation (Bai et al., 2011). Following the addition of 4 μL of 100 μM acetosyringone, the vector-transformed Agrobacterium in 10 mL YEP was cultured at 28°C with shaking at 130 rpm for 12 to 16 h until OD600 = 1. The Agrobacterium was then suspended in liquid medium (10 mM MES-KOH [pH 5.2], 10 mM MgCl2, and 100 μM acetosyringone) and incubated for 2 h before use.
The pFGC5941 constructs were infiltrated into the leaves of 4-week-old in vitro-grown plantlets by injection at two spots per leaf (left side and right side); each construct was infiltrated into more than 10 apple plantlets (∼50 leaves). The infiltrated plantlets were returned to MS medium and cultured in a growth room at 25 ± 1°C under fluorescent lights at ∼100 μmol photons m−2 s−1 (Philips Lighting TL5 28W/865) with a 16/8-h light/dark photoperiod. Four days after Agrobacterium infiltration, nine leaves (length: 2 cm) from three different plantlets were sampled, and RNA was extracted from the infiltrated regions of the leaves and used for gene expression analysis. The expression efficiency of exogenous vectors delivered via agroinfiltration was high, and exogenous gene expression lasted up to 7 d. In addition to the leaves used for gene expression analysis, more than 30 leaves from 10 plantlets were inoculated with ALT1 pathogenic spore suspension (2 × 105 cfu/mL) at the same two spots 4 d after Agrobacterium infiltration, and the plantlets were returned to MS medium and cultured for 48 h. To calculate the disease rate, ∼30 leaves from 10 4-week-old GD/HF/‘GL-3’ apple seedlings were infiltrated with Agrobacterium harboring various constructs. The same number of noninfiltrated GD/HF/‘GL-3’ seedlings and EV (pFGC5941)-infiltrated GD/HF/‘GL-3’ plants served as controls. Leaf phenotypes and leaf disease rates in ALT1-infected plants were examined at 48 HPI and performed using three independent biological replicates, and Student’s t test was used to evaluate the statistical significance of differences in leaf disease rates. The leaves were not detached from the plantlets during infiltration and inoculation, and photographs of leaves on whole plantlets were taken at 48 HPI. This Agrobacterium infiltration and ALT1 inoculation method was used for the experiments shown in Figures 2 and 6 and Supplemental Figures 1, 4, and 8.
Isolation and Identification of the MdhpRNA277 Promoter
The promoter sequence of MdhpRNA277 was PCR-amplified based on the apple genome sequence (https://iris.angers.inra.fr/gddh13/the-apple-genome-downloads.html) (Daccord et al., 2017) and analyzed using the online software tool PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Primers are listed in Supplemental Table 7.
A transient expression assay was performed to test the activity of the promoters, as previously described (Bai et al., 2011). Briefly, pMdhpRNA277-GD, pMdhpRNA277-HF, and pMdhpRNA277-HF harboring a mutation in motif a (T-C), b (T-C), and c (G-A), respectively, and pMdhpRNA277-GD with a mutation in motif b (C-T), carrying BamHI/NcoI restriction sites, were ligated into the pCAMBIA1305 vector harboring the GUS gene, generating constructs pMdhpRNA277-GD:GUS, pMdhpRNA277-HF:GUS, mpMdhpRNA277-HF:GUS-a, mpMdhpRNA277-HF:GUS-b, mpMdhpRNA277-HF:GUS-c, and mpMdhpRNA277-GD:GUS-b, respectively (Figures 3C and 4A; Supplemental Figure 4A). The GUS constructs were introduced into Agrobacterium strain GV3101, which was vacuum-infiltrated into tissue-cultured ‘Golden Delicious’ and ‘Hanfu’ plants.
The binary constructs were transformed into Agrobacterium strain GV3101 by heat-shock transformation as described above. The Agrobacterium was suspended in liquid medium (10 mM MES-KOH [pH 5.2], 10 mM MgCl2, and 100 μM acetosyringone) and incubated for 2 h before use. The pCAMBIA1305 constructs were infiltrated into the leaves of 4-week-old tissue-cultured plantlets under a vacuum of 65 kPa for 20 min, and the infiltrated plantlets were returned to the MS medium. Four days after agroinfiltration, the plantlets were inoculated with a pathogenic spore suspension of ALT1 under a vacuum of 65 kPa for 10 min and returned once again to the MS medium. Two days later, whole plantlets were incubated in GUS staining solution. This Agrobacterium infiltration and ALT1 inoculation method was used for the experiments shown in Figures 3 and 4 and Supplemental Figure 4.
GUS Histochemical Staining
Tissue-cultured apple plants were incubated in GUS staining solution (0.2 M NaH2PO4·2H2O, 0.2 M NaH2PO4·12H2O, 100 mM X-Gluc, and 10% methyl alcohol) at 37°C for 2 d, followed by destaining in 70% ethyl alcohol prior to examination.
Nuclear Protein Extraction
Nuclear protein was extracted using a plant nuclei extraction kit (Sigma-Aldrich; CELYTPN1). Leaf tissue (20 g) in 60 mL lysis buffer containing 1 M DTT was passed through a filter mesh by centrifugation (18,000g; 10 min; 4°C). The material was resuspended in lysis buffer containing 1:100 (v/v) protease inhibitor cocktail solution plus 1% Triton X-100, followed by centrifugation (12,000g; 5 min; 4°C). The pellet (containing protein) was combined with 5 mM DTT and 1:100 (v/v) protease inhibitor cocktail and stored at −70°C.
Protein Purification
The coding sequences of MdWHy, MdWhirly, MdbZIP, and MdBTF3 were individually cloned into pMal-c2x vectors and transformed into Escherichia coli Transetta (DE3) cells. The E. coli cells were incubated at 16°C for 16 h and resuspended in cell buffer (10 mM Tris-HCl [pH 7.4] and 30 mM NaCl), followed by ultrasonication at 35% power for 20 min (Xinzhi sonicator; JY92-2N) and centrifugation (12,000g; 1 h; 4°C). The supernatant was incubated in rinsed MBP Amylose Beads (NEB; E8035) at 4°C for 1 h. After discarding the supernatant, the beads were rinsed three times with a solution of 200 mM NaCl, 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, and 1 mM DTT, and the purified protein was used for further experiments.
EMSAs
The 5′ biotin-labeled pMd-MIRLn12-277-GD or pMd-MIRLn12-277-HF DNA sequences were annealed in 10× annealing buffer (100 mM Tris-HCl [pH 7.5], 10 mM EDTA, and 1 M NaCl) at 75°C for 30 min and stored at −20°C. The EMSAs were performed using a LightShift chemiluminescent EMSA kit (Thermo Fisher Scientific; 20148) according to the manufacturer’s protocol.
Production and Analysis of Stable Transformants
The vector for MdhpRNA277 overexpression driven by pMdhpRNA2777-GD was named pMdhpRNA277-GD:MdhpRNA277. The vector for MdhpRNA277 overexpression driven by pMdhpRNA277-HF was named pMdhpRNA277-HF:MdhpRNA277. ‘GL-3’ was stably transformed with these constructs as described previously (Holefors et al., 1998). Transgenic plants were confirmed by DNA gel blot analysis. The mdm-siR277-1/2 levels in all transgenic and control lines were determined by RNA gel blot analysis. The mRNA levels of MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 in all transgenic and control lines were determined by RT-qPCR.
DNA Gel Blot Analysis
Fifteen micrograms of genomic DNA was digested with HindIII at 37°C overnight, separated on a 1% (w/v) agarose gel in 1× Tris-acetate-EDTA buffer, and blotted onto a positively charged nylon membrane (GE Healthcare) by capillary transfer with 20× SSC (0.15 M NaCl and 0.015 M trisodium citrate dihydrate, pH 7.0) as the transfer buffer (Southern, 1975). The blotted DNA was hybridized with digoxigenin-labeled probes. The probes were synthesized by PCR using a PCR DIG probe synthesis kit (Mylab) and the primers NptII-F and NptII-R for nptII (listed in Supplemental Table 1) using a Digoxin Hybridization Detection Kit (Mylab; DIGD-120) following the manufacturer’s instructions.
Accession Numbers
Sequence data from this article can be found in the Genome Database for Rosaceae website (https://www.rosaceae.org/) under the following accession numbers: MdRNL1 (MD15G1278100), MdRNL-2 (MD02G166300), MdRNL3 (MD02G1165600), MdRNL4 (MD15G1277700), MdRNL5 (MD02G1166100), MdBTF3 (MD06G1239900), MdbZIP (MD05G1275200), MdWHy (MD15G1142200), and MdWhirly (MD16G1077800). Next-generation sequencing data from this article can be found in the Gene Expression Omnibus website (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE115664.
Supplemental Data
Supplemental Figure 1. Overexpression of MdhpRNA277 in GD leaves increases ALT1 susceptibility.
Supplemental Figure 2. SNP sites, disease rates, and expression of MdhpRNA277 and MdRNL-1/-2/-3/-4/-5 in 34 ALT1-inoculated apple cultivars with various levels of resistance to ALT1.
Supplemental Figure 3. MdRNL1, MdRNL2, MdRNL3, MdRNL4, and MdRNL5 contribute to ALT1 resistance in apple.
Supplemental Figure 4. Motifs a and c are not related to the activity of the MdhpRNA277 promoter.
Supplemental Figure 5. Schematic diagram of the location of the SNP (−1186 bp) in the MdhpRNA277 transcript.
Supplemental Figure 6. MdWHy overexpression does not affect the resistance of ‘Hanfu’ plantlets to ALT1.
Supplemental Figure 7. mdm-siR277-1/2 regulate the expression of five R genes affecting ALT1 susceptibility in ‘GL-3’.
Supplemental Figure 8. Phenotypes of transgenic ‘GL-3’ plants, which were confirmed by DNA gel blot analysis.
Supplemental Figure 9. Expression levels of five target genes in transgenic ‘GL-3’ plants at 48 HPI with ALT1, as revealed by RT-qPCR.
Supplemental Table 1. Primer sequences used for small RNA 5′ RACE and to produce the RNA gel blot, DNA gel blot, and EMSA probes.
Supplemental Table 2. Segregation of genotypes in the progeny of three crossing populations.
Supplemental Table 3. SNP diversity, genotypes, and phenotypes of 55 crossing progenies from Han Fu (TT) × Yue Shuai (GG).
Supplemental Table 4. SNP diversity, genotypes, and phenotypes of 60 crossing progenies from Golden Delicious (GG) × Nagafu 2 (GT).
Supplemental Table 5. SNP diversity, genotypes, and phenotypes of 100 crossing progenies from Yue Guan (GT) × Nagafu 2 (GT).
Supplemental Table 6. Primer sequences used for RT-qPCR.
Supplemental Table 7. Primer sequences used to clone small RNA primary transcripts and genes.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Liyun Guo from China Agricultural University for providing the apple A. alternata leaf spot (Alternaria alternata f. sp. mali) fungal strains. We thank Zhihong Zhang (Shenyang Agricultural University), the Fruit Tree Research Institute of the Chinese Academy of Agricultural Sciences, and Liaoning Institute of Pomology for providing the plant materials. This work was supported by the National Natural Science Foundation of China (31630066).
AUTHOR CONTRIBUTIONS
Q.Z., C.M., W.L., and T.L. conceived this project and designed all experiments. Q.Z., Y.Z., Z.G., and S.W. performed experiments. Q.Z., Y.W., L.H., and S.W. contributed to apple transformation. Q.Z. and X.D. analyzed experimental data. Q.Z. and T.L. contributed to writing and revising the manuscript.
References
- Abe K., Iwanami H., Kotoda N., Moriya S., Takahashi S. (2010). Evaluation of apple genotypes and Malus species for resistance to Alternaria blotch caused by Alternaria alternata apple pathotype using detached-leaf method. Plant Breed. 129: 208–218. [Google Scholar]
- Allen E., Xie Z., Gustafson A.M., Sung G.H., Spatafora J.W., Carrington J.C. (2004). Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat. Genet. 36: 1282–1290. [DOI] [PubMed] [Google Scholar]
- Arikit S., Xia R., Kakrana A., Huang K., Zhai J., Yan Z., Valdés-López O., Prince S., Musket T.A., Nguyen H.T., Stacey G., Meyers B.C. (2014). An atlas of soybean small RNAs identifies phased siRNAs from hundreds of coding genes. Plant Cell 26: 4584–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya P., Kumar G., Acharya V., Singh A.K. (2014a). Genome-wide identification and expression analysis of NBS-encoding genes in Malus x domestica and expansion of NBS genes family in Rosaceae. PLoS One 9: e107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya P., Kumar G., Acharya V., Singh A.K. (2014b). Genome-wide identification and expression analysis of NBS-encoding genes in Malus x domestica and expansion of NBS genes family in Rosaceae. PLoS One 9: e107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikawa I., Hayashi N., Yamane H., Kanamori H., Wu J., Matsumoto T., Ono K., Yano M. (2008). Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180: 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell M.J. (2013). Classification and comparison of small RNAs from plants. Annu. Rev. Plant Biol. 64: 137–159. [DOI] [PubMed] [Google Scholar]
- Axtell M.J., Meyers B.C. (2018). Revisiting criteria for plant microRNA annotation in the era of big data. Plant Cell 30: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Kasai A., Yamada K., Li T., Harada T. (2011). A mobile signal transported over a long distance induces systemic transcriptional gene silencing in a grafted partner. J. Exp. Bot. 62: 4561–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Liu J., Chang C., Zhang L., Maekawa T., Wang Q., Xiao W., Liu Y., Chai J., Takken F.L., Schulze-Lefert P., Shen Q.H. (2012). Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 8: e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T.T., Xie W.B., Zhou P.P., Wu Z.L., Xiao W.C., Zhou L., Sun J., Ruan X.L., Li H.P. (2013). Transcriptome and expression profile analysis of highly resistant and susceptible banana roots challenged with Fusarium oxysporum f. sp. cubense tropical race 4. PLoS One 8: e73945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y., Nimchuk Z., Hubert D.A., Mackey D., Dangl J.L. (2004). Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. Plant Cell 16: 2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent A.F., Kunkel B.N., Dahlbeck D., Brown K.L., Schmidt R., Giraudat J., Leung J., Staskawicz B.J. (1994). RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265: 1856–1860. [DOI] [PubMed] [Google Scholar]
- Boccara M., Sarazin A., Thiébeauld O., Jay F., Voinnet O., Navarro L., Colot V. (2014). The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog. 10: e1003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi V., Tang S., Stallmann A., Roberts M., Cherkis K., Dangl J.L. (2011). Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc. Natl. Acad. Sci. USA 108: 16463–16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman Y., et al. (2013). Dual resistance of melon to Fusarium oxysporum races 0 and 2 and to Papaya ring-spot virus is controlled by a pair of head-to-head-oriented NB-LRR genes of unusual architecture. Mol. Plant 6: 235–238. [DOI] [PubMed] [Google Scholar]
- Cesari S., et al. (2013). The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 25: 1463–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.R., Brurberg M.B., Elameen A., Klemsdal S.S., Martinussen I. (2016). Expression of resistance gene analogs in woodland strawberry (Fragaria vesca) during infection with Phytophthora cactorum. Mol. Genet. Genomics 291: 1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A., Loake G.J. (2005). Motifs specific for the ADR1 NBS-LRR protein family in Arabidopsis are conserved among NBS-LRR sequences from both dicotyledonous and monocotyledonous plants. Planta 221: 597–601. [DOI] [PubMed] [Google Scholar]
- Chini A., Grant J.J., Seki M., Shinozaki K., Loake G.J. (2004a). Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 38: 810–822. [DOI] [PubMed] [Google Scholar]
- Chini A., Grant J.J., Seki M., Shinozaki K., Loake G.J. (2004b). Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 38: 810–822. [DOI] [PubMed] [Google Scholar]
- Chung K.R. (2012). Stress response and pathogenicity of the necrotrophic fungal pathogen Alternaria alternata. Scientifica (Cairo) 2012: 635431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli F.D., Bork P. (2005). The WHy domain mediates the response to desiccation in plants and bacteria. Bioinformatics 21: 1304–1307. [DOI] [PubMed] [Google Scholar]
- Collier S.M., Moffett P. (2009). NB-LRRs work a “bait and switch” on pathogens. Trends Plant Sci. 14: 521–529. [DOI] [PubMed] [Google Scholar]
- Collier S.M., Hamel L.P., Moffett P. (2011). Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol. Plant Microbe Interact. 24: 918–931. [DOI] [PubMed] [Google Scholar]
- Daccord N., et al. (2017). High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 49: 1099–1106. [DOI] [PubMed] [Google Scholar]
- Dai H.Y., Li W.R., Han G.F., Yang Y., Ma Y., Li H., Zhang Z.H. (2013). Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Hortic. (Amsterdam) 164: 202–208. [Google Scholar]
- Dangl J.L., Jones J.D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411: 826–833. [DOI] [PubMed] [Google Scholar]
- Dangl J.L., Horvath D.M., Staskawicz B.J. (2013). Pivoting the plant immune system from dissection to deployment. Science 341: 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Wang J., Tung J., Liu D., Zhou Y., He S., Du Y., Baker B., Li F. (2018). A role for small RNA in regulating innate immunity during plant growth. PLoS Pathog. 14: e1006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desveaux D., Maréchal A., Brisson N. (2005). Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci. 10: 95–102. [DOI] [PubMed] [Google Scholar]
- DeYoung B.J., Innes R.W. (2006). Plant NBS-LRR proteins in pathogen sensing and host defense. Nat. Immunol. 7: 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds P.N., Rathjen J.P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Dong O.X., Tong M., Bonardi V., El Kasmi F., Woloshen V., Wünsch L.K., Dangl J.L., Li X. (2016). TNL-mediated immunity in Arabidopsis requires complex regulation of the redundant ADR1 gene family. New Phytol. 210: 960–973. [DOI] [PubMed] [Google Scholar]
- Eitas T.K., Dangl J.L. (2010). NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Curr. Opin. Plant Biol. 13: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.G., Lawrence G.J., Luck J.E., Dodds P.N. (1999). Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore J.M., Lin Z.J., Coaker G. (2011). Plant NB-LRR signaling: upstreams and downstreams. Curr. Opin. Plant Biol. 14: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P.J., Schmelzer E., Hahlbrock K., Somssich I.E. (1999). Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J. 18: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P.J., Robatzek S., Somssich I.E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5: 199–206. [DOI] [PubMed] [Google Scholar]
- Grant J.J., Chini A., Basu D., Loake G.J. (2003). Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol. Plant Microbe Interact. 16: 669–680. [DOI] [PubMed] [Google Scholar]
- Halterman D., Zhou F., Wei F., Wise R.P., Schulze-Lefert P. (2001). The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f. sp. hordei in barley and wheat. Plant J. 25: 335–348. [DOI] [PubMed] [Google Scholar]
- Harmoko R., Fanata W.I.D., Yoo J.Y., Ko K.S., Rim Y.G., Uddin M.N., Siswoyo T.A., Lee S.S., Kim D.Y., Lee S.Y., Lee K.O. (2013). RNA-dependent RNA polymerase 6 is required for efficient hpRNA-induced gene silencing in plants. Mol. Cells 35: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C.J., Slootweg E.J., Goverse A., Baulcombe D.C. (2013). Stepwise artificial evolution of a plant disease resistance gene. Proc. Natl. Acad. Sci. USA 110: 21189–21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.F., Fang Y.Y., Feng L., Guo H.S. (2008). Characterization of conserved and novel microRNAs and their targets, including a TuMV-induced TIR-NBS-LRR class R gene-derived novel miRNA in Brassica. FEBS Lett. 582: 2445–2452. [DOI] [PubMed] [Google Scholar]
- Heidrich K., Wirthmueller L., Tasset C., Pouzet C., Deslandes L., Parker J.E. (2011a). Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334: 1401–1404. [DOI] [PubMed] [Google Scholar]
- Heidrich K., Wirthmueller L., Tasset C., Pouzet C., Deslandes L., Parker J.E. (2011b). Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334: 1401–1404. [DOI] [PubMed] [Google Scholar]
- Henderson I.R., Zhang X., Lu C., Johnson L., Meyers B.C., Green P.J., Jacobsen S.E. (2006). Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat. Genet. 38: 721–725. [DOI] [PubMed] [Google Scholar]
- Holefors A., Xue Z.T., Welander M. (1998). Transformation of the apple rootstock M26 with the rolA gene and its influence on growth. Plant Sci. 136: 69–78. [DOI] [PubMed] [Google Scholar]
- Hugot K., Aime S., Conrod S., Poupet A., Galiana E. (1999). Developmental regulated mechanisms affect the ability of a fungal pathogen to infect and colonize tobacco leaves. Plant J. 20: 163–170. [DOI] [PubMed] [Google Scholar]
- Inoue H., Hayashi N., Matsushita A., Xinqiong L., Nakayama A., Sugano S., Jiang C.J., Takatsuji H. (2013). Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein-protein interaction. Proc. Natl. Acad. Sci. USA 110: 9577–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.D., Johnson L., Itoh Y., Kodama M., Otani H., Kohmoto K. (2000). Cloning and characterization of a cyclic peptide synthetase gene from Alternaria alternata apple pathotype whose product is involved in AM-toxin synthesis and pathogenicity. Mol. Plant Microbe Interact. 13: 742–753. [DOI] [PubMed] [Google Scholar]
- Jordan T., Seeholzer S., Schwizer S., Toller A., Somssich I.E., Keller B. (2011). The wheat Mla homologue TmMla1 exhibits an evolutionarily conserved function against powdery mildew in both wheat and barley. Plant J. 65: 610–621. [DOI] [PubMed] [Google Scholar]
- Jupe F., Pritchard L., Etherington G.J., Mackenzie K., Cock P.J., Wright F., Sharma S.K., Bolser D., Bryan G.J., Jones J.D., Hein I. (2012). Identification and localisation of the NB-LRR gene family within the potato genome. BMC Genomics 13: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W.H., Johnson P.F., McKnight S.L. (1988). The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240: 1759–1764. [DOI] [PubMed] [Google Scholar]
- Lee S.K., et al. (2009). Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 181: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Pignatta D., Bendix C., Brunkard J.O., Cohn M.M., Tung J., Sun H., Kumar P., Baker B. (2012). MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 109: 1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.F., Li X.F., Han ZhH., Shu H.R., Li T.Zh. (2009). Molecular analysis of two Chinese pear (Pyrus bretschneideri Rehd.) spontaneous self-compatible mutants, Yan Zhuang and Jin Zhui. Plant Biol. (Stuttg.) 11: 774–783. [DOI] [PubMed] [Google Scholar]
- Li Y., Dong O.X., Johnson K., Zhang Y. (2011). MOS1 epigenetically regulates the expression of plant Resistance gene SNC1. Plant Signal. Behav. 6: 434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhang L., Sun J., Luo Y., Wang M.B., Fan Y.L., Wang L. (2010). A simple artificial microRNA vector based on ath-miR169d precursor from Arabidopsis. Mol. Biol. Rep. 37: 903–909. [DOI] [PubMed] [Google Scholar]
- Liu J., Cheng X., Liu D., Xu W., Wise R., Shen Q.H. (2014). The miR9863 family regulates distinct Mla alleles in barley to attenuate NLR receptor-triggered disease resistance and cell-death signaling. PLoS Genet. 10: e1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Loutre C., Wicker T., Travella S., Galli P., Scofield S., Fahima T., Feuillet C., Keller B. (2009). Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J. 60: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Ma C., Lu Y., Bai S., Zhang W., Duan X., Meng D., Wang Z., Wang A., Zhou Z., Li T. (2014). Cloning and characterization of miRNAs and their targets, including a novel miRNA-targeted NBS-LRR protein class gene in apple (Golden Delicious). Mol. Plant 7: 218–230. [DOI] [PubMed] [Google Scholar]
- McHale L., Tan X., Koehl P., Michelmore R.W. (2006). Plant NBS-LRR proteins: adaptable guards. Genome Biol. 7: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers B.C., Morgante M., Michelmore R.W. (2002). TIR-X and TIR-NBS proteins: two new families related to disease resistance TIR-NBS-LRR proteins encoded in Arabidopsis and other plant genomes. Plant J. 32: 77–92. [DOI] [PubMed] [Google Scholar]
- Meyers B.C., Kozik A., Griego A., Kuang H., Michelmore R.W. (2003). Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15: 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S.B., Bodeau J., Yaghoobi J., Kaloshian I., Zabel P., Williamson V.M. (1998). The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10: 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka M., Shirasu K., Noutoshi Y., Kubo Y., Shiraishi T., Iwabuchi M., Narusaka Y. (2009). RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. 60: 218–226. [DOI] [PubMed] [Google Scholar]
- Niu D., Lii Y.E., Chellappan P., Lei L., Peralta K., Jiang C., Guo J., Coaker G., Jin H. (2016). miRNA863-3p sequentially targets negative immune regulator ARLPKs and positive regulator SERRATE upon bacterial infection. Nat. Commun. 7: 11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H., Nishimura S., Kohmoto K. (1974). Nature of Specific Susceptibility to Alternaria kikuchiana in Nijisseiki Cultivar among Japanese Pears (III): Chemical and thermal protection against effect of host-specific toxin. Jpn. J. Phytopathol. 40: 59–66. [Google Scholar]
- Ouyang S., Park G., Atamian H.S., Han C.S., Stajich J.E., Kaloshian I., Borkovich K.A. (2014). MicroRNAs suppress NB domain genes in tomato that confer resistance to Fusarium oxysporum. PLoS Pathog. 10: e1004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart J.R., Mestre P., Lu R., Malcuit I., Baulcombe D.C. (2005). NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr. Biol. 15: 968–973. [DOI] [PubMed] [Google Scholar]
- Rairdan G.J., Collier S.M., Sacco M.A., Baldwin T.T., Boettrich T., Moffett P. (2008). The coiled-coil and nucleotide binding domains of the Potato Rx disease resistance protein function in pathogen recognition and signaling. Plant Cell 20: 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel M., Li Y., Li J., Millar A.A. (2015). Inhibiting plant microRNA activity: molecular SPONGEs, target MIMICs and STTMs all display variable efficacies against target microRNAs. Plant Biotechnol. J. 13: 915–926. [DOI] [PubMed] [Google Scholar]
- Rio D.C. (2014). Northern blots for small RNAs and microRNAs. Cold Spring Harb. Protoc. 2014: 793–797. [DOI] [PubMed] [Google Scholar]
- Rotem J. (1994). The Genus Alternaria: Biology, Epidemiology, and Pathogenicity. (St. Paul, MN: American Phytopathological Society; ). [Google Scholar]
- Rotondo F., Collina M., Brunelli A., Pryor B.M. (2012). Comparison of Alternaria spp. collected in Italy from apple with A. mali and other AM-toxin producing strains. Phytopathology 102: 1130–1142. [DOI] [PubMed] [Google Scholar]
- Rushton P.J., Torres J.T., Parniske M., Wernert P., Hahlbrock K., Somssich I.E. (1996). Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 15: 5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Sarris P.F., et al. (2015). A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161: 1089–1100. [DOI] [PubMed] [Google Scholar]
- Saunders D.G.O., Breen S., Win J., Schornack S., Hein I., Bozkurt T.O., Champouret N., Vleeshouwers V.G.A.A., Birch P.R.J., Gilroy E.M., Kamoun S. (2012). Host protein BSL1 associates with Phytophthora infestans RXLR effector AVR2 and the Solanum demissum Immune receptor R2 to mediate disease resistance. Plant Cell 24: 3420–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura K., Yanase H. (1963). Studies on spotted disease of apples. 2. On Alternaria sp., causal organism of Alternaria blotch and Japanese name of the disease. Bull. Hort. Res. Stn. Japan 1: 77–94. [Google Scholar]
- Shivaprasad P.V., Chen H.M., Patel K., Bond D.M., Santos B.A., Baulcombe D.C. (2012). A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24: 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtienberg D. (2012). Effects of host physiology on the development of core rot, caused by alternaria alternata, in Red Delicious apples. Phytopathology 102: 769–778. [DOI] [PubMed] [Google Scholar]
- Sinapidou E., Williams K., Nott L., Bahkt S., Tör M., Crute I., Bittner-Eddy P., Beynon J. (2004). Two TIR:NB:LRR genes are required to specify resistance to Peronospora parasitica isolate Cala2 in Arabidopsis. Plant J. 38: 898–909. [DOI] [PubMed] [Google Scholar]
- Slootweg E., et al. (2010). Nucleocytoplasmic distribution is required for activation of resistance by the potato NB-LRR receptor Rx1 and is balanced by its functional domains. Plant Cell 22: 4195–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K.H., Segonzac C., Rallapalli G., Sarris P.F., Woo J.Y., Williams S.J., Newman T.E., Paek K.H., Kobe B., Jones J.D.G. (2014). The nuclear immune receptor RPS4 is required for RRS1SLH1-dependent constitutive defense activation in Arabidopsis thaliana. PLoS Genet. 10: e1004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.Y., Kim C.Y., Han M., Ryu H.S., Lee S.K., Sun L., He Z., Seo Y.S., Canal P., Ronald P.C., Jeon J.S. (2013). Differential requirement of Oryza sativa RAR1 in immune receptor-mediated resistance of rice to Magnaporthe oryzae. Mol. Cells 35: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E.M. (1975). Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98: 503–517. [DOI] [PubMed] [Google Scholar]
- Steinbrenner A.D., Goritschnig S., Staskawicz B.J. (2015). Recognition and activation domains contribute to allele-specific responses of an Arabidopsis NLR receptor to an oomycete effector protein. PLoS Pathog. 11: e1004665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnweis D., Milani S.D., Brunner S., Herren G., Buchmann G., Peditto D., Jordan T., Keller B. (2014). Suppression among alleles encoding nucleotide-binding-leucine-rich repeat resistance proteins interferes with resistance in F1 hybrid and allele-pyramided wheat plants. Plant J. 79: 893–903. [DOI] [PubMed] [Google Scholar]
- Sueldo D.J., Shimels M., Spiridon L.N., Caldararu O., Petrescu A.J., Joosten M.H., Tameling W.I. (2015). Random mutagenesis of the nucleotide-binding domain of NRC1 (NB-LRR Required for Hypersensitive Response-Associated Cell Death-1), a downstream signalling nucleotide-binding, leucine-rich repeat (NB-LRR) protein, identifies gain-of-function mutations in the nucleotide-binding pocket. New Phytol. 208: 210–223. [DOI] [PubMed] [Google Scholar]
- Tameling W.I.L., Nooijen C., Ludwig N., Boter M., Slootweg E., Goverse A., Shirasu K., Joosten M.H.A.J. (2010). RanGAP2 mediates nucleocytoplasmic partitioning of the NB-LRR immune receptor Rx in the Solanaceae, thereby dictating Rx function. Plant Cell 22: 4176–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Yan J., Gu Y., Qiao M., Fan R., Mao Y., Tang X. (2012). Construction of short tandem target mimic (STTM) to block the functions of plant and animal microRNAs. Methods 58: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.S., Tarver J.E., Foroozani A., Donoghue P.C. (2017). MicroRNA annotation of plant genomes - Do it right or not at all. BioEssays 39: 10.1002/bies.201600113. [DOI] [PubMed] [Google Scholar]
- Voinnet O. (2009). Origin, biogenesis, and activity of plant microRNAs. Cell 136: 669–687. [DOI] [PubMed] [Google Scholar]
- Wang G.F., Ji J., El-Kasmi F., Dangl J.L., Johal G., Balint-Kurti P.J. (2015). Molecular and functional analyses of a maize autoactive NB-LRR protein identify precise structural requirements for activity. PLoS Pathog. 11: e1004674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ye X., Liu H., Liu X., Wei C., Huang Y., Liu Y., Tu J. (2016). Both overexpression and suppression of an Oryza sativa NB-LRR-like gene OsLSR result in autoactivation of immune response and thiamine accumulation. Sci. Rep. 6: 24079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S., Dinesh-Kumar S.P., Choi D., Hehl R., Corr C., Baker B. (1994). The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78: 1101–1115. [DOI] [PubMed] [Google Scholar]
- Wiedmann B., Sakai H., Davis T.A., Wiedmann M. (1994). A protein complex required for signal-sequence-specific sorting and translocation. Nature 370: 434–440. [DOI] [PubMed] [Google Scholar]
- Wong J., et al. (2014). Roles of small RNAs in soybean defense against Phytophthora sojae infection. Plant J. 79: 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R., Xu J., Arikit S., Meyers B.C. (2015). Extensive families of miRNAs and PHAS loci in Norway spruce demonstrate the origins of complex phasiRNA networks in seed plants. Mol. Biol. Evol. 32: 2905–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Cheng Y.T., Huang S., Win J., Soards A., Jinn T.L., Jones J.D., Kamoun S., Chen S., Zhang Y., Li X. (2013). Regulation of transcription of nucleotide-binding leucine-rich repeat-encoding genes SNC1 and RPP4 via H3K4 trimethylation. Plant Physiol. 162: 1694–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Gu Y., Jia X., Kang W., Pan S., Tang X., Chen X., Tang G. (2012). Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 24: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J., et al. (2011). MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 25: 2540–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.X., Tian Y., Cong P.H. (2015). Proteome analysis of pathogen-responsive proteins from apple leaves induced by the Alternaria blotch Alternaria alternata. PLoS One 10: e0122233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Li Y., Zhang Y., Wu C., Wang S., Hao L., Wang S., Li T. (2017). Md-miR156ab and Md-miR395 target WRKY transcription factors to influence apple resistance to leaf spot disease. Front. Plant Sci. 8: 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xia R., Kuang H., Meyers B.C. (2016). The diversification of plant NBS-LRR defense genes directs the evolution of microRNAs that target them. Mol. Biol. Evol. 33: 2692–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., et al. (2013). Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for citrus huanglongbing disease. Mol. Plant 6: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.H., Fan L., Liu Y., Xu H., Llewellyn D., Wilson I. (2013). miR482 regulation of NBS-LRR defense genes during fungal pathogen infection in cotton. PLoS One 8: e84390. [DOI] [PMC free article] [PubMed] [Google Scholar]