ABSTRACT
Surgical stress reduces concentrations of most proteins in serum and necessitates a rapid adjustment of hormones dependent on protein binding. Activation of vitamin D by renal 1α‐hydroxylation is dependent on protein binding because 1,25‐dihydroxyvitamin D (1,25(OH)2D3) is formed after megalin‐mediated reabsorption of 25‐hydroxyvitamin D (25OHD) bound to vitamin D binding protein (DBP). Postoperative alterations in serum concentrations of DBP and albumin may therefore impair 1,25(OH)2D3 production. Our objective was to determine sex‐specific changes in serum concentrations of vitamin D metabolites and sex steroids 2, 6, 24, and 48 hours and 3 weeks postoperatively. Fourteen women and eleven men aged 45 to 77 years without severe comorbidities undergoing unilateral total knee arthroplasty participated in this prospective study in a tertiary center for arthroplasty (trial ID: NCT02336932). The main outcome measures were total and free serum concentrations of 25OHD, 1,25(OH)2D3, 24,25‐dihydroxyvitamin‐D, DBP, albumin, sex hormone binding globulin (SHBG), calcium, and parathyroid hormone (PTH). Serum albumin and SHBG decreased postoperatively (Δalbumin48h −18% [−22%; −14%]). Unexpectedly, concentrations of DBP and 25OHD remained unaltered, but 1,25(OH)2D3 declined postoperatively. 1,25(OH)2D3 was 3 weeks after surgery −24% (−40%; −8%) lower than preoperative levels, whereas 24,25‐dihydroxyvitamin‐D remained unchanged in postmenopausal women. The calculated conversion rate of 25OHD to 1,25(OH)2D3 was strongly associated with serum 25‐OHD and PTH preoperatively, whereas serum calcium was most predictive postoperatively. In conclusion, surgery had no effect on serum concentrations of DBP, 25OHD, and PTH, whereas production of 1,25(OH)2D3 was markedly reduced. Further studies are needed to determine duration and putative outcome effects of this postoperative 1,25(OH)2D3 deficit in women, which in part may be due to discordance in CYP27B1 and CYP24A1 activity. © 2018 American Society for Bone and Mineral Research.
Keywords: VITAMIN D; FREE HORMONE; ELECTIVE SURGERY; 1,25(OH)2D3; SURGICAL STRESS
Introduction
Injury or trauma induces a series of metabolic, inflammatory, and endocrine changes known as the “surgical stress response.”1, 2, 3, 4, 5 A particular and abrupt event in response to surgery is a profound change in the distribution of proteins between the intra‐ and extravascular compartments.6, 7, 8 This redistribution gives rise to an instant reduction in the concentration of most circulating proteins, which necessitates immediate changes in factors dependent on protein binding in serum, for instance, calcium and steroid hormones.9
Elective orthopedic surgeries are common in elderly patients suffering from multiple comorbidities, including impaired bone remodeling, who may benefit from interventions facilitating bone formation, ingrowth of implants, or fracture repair.10 Vitamin D is known for its role in calcium homeostasis and bone health,11, 12 and studies have shown beneficial effects for fracture healing and callus formation in both animal models and humans.13, 14 Vitamin D is an atypical vitamin because it can be synthesized endogenously in the skin after UV‐B radiation that converts 7‐dehydrocholesterol into cholecalciferol (vitamin D). Cholecalciferol is not biologically active but has to be enzymatic modified to be fully activated. First, hepatic 25‐hydroxylation forms 25‐hydroxyvitamin D (25‐OHD), which in serum is associated with parathyroid hormone (PTH), calcium, and bone mineral density (BMD) and used clinically to determine vitamin D status.15 25‐OHD is not biologically active but undergoes renal 1α‐hydroxylation and 1,25‐dihydroxyvitamin D (1,25(OH)2D3) activates the vitamin D receptor.16 The critical and rate‐limiting step for activation of vitamin D is renal 1α‐hydroxylation, which is tightly regulated by PTH, fibroblast growth factor 23 (FGF23), sex steroids, interferons, and other factors.17, 18, 19 Noteworthy, all circulating vitamin D metabolites are transported by vitamin D binding globulin (DBP) or albumin in serum, and free available levels of vitamin D metabolites are much lower than other steroid hormones because of the strong binding to DBP.20, 21 One study showed that concentration of total 25‐OHD decreases within 24 hours after elective surgery,22 whereas others23 have shown that both total and free 25‐OHD remained low several months after elective surgery.24
Our hypothesis is that patients after elective surgery may develop a transient deficiency of calcitriol because of impaired 1α‐hydroxylation resulting in low levels of 1,25(OH)2D3. Activation of vitamin D is a tightly regulated process, but the decrease in circulating proteins in response to surgical stress may particularly influence 1α‐hydroxylation in the kidney because this conversion takes place intracellularly and uptake of the substrate has been shown to be dependent on protein binding.25 Normally, the complex of 25OHD‐DBP is filtrated out in the glomerulus and subsequently by a megalin‐ or cubulin‐dependent mechanism reabsorbed in the proximal tubulus cells, which enables intracellular 1α‐hydroxylation and subsequent release of 1,25(OH)2D3 into the systemic circulation.25 To our knowledge, no one has systematically measured DBP after surgical stress, and it remains to be shown whether serum DBP decreases similarly to albumin and sex hormone binding globulin (SHBG) or whether the liver compensates the acute decline in serum level of DBP. The altered protein level in serum may in theory facilitate a decline in serum levels of 1,25(OH)2D3 unless known regulators of 1α‐hydroxylase activity such as PTH, sex steroids, and interferons are able to compensate this impairment by upregulating 1α‐hydroxylase activity or downregulating CYP24A1.2, 3, 26 A putative transient decrease of 1,25(OH)2D3 may not be important clinically unless it is more persistent. A persistent impairment of 1α‐hydroxylase activity is important because it cannot be bypassed by treating patients with simple vitamin D supplementation. This may occur at a critical time for these orthopedic patients because optimal bone function is essential for primary bony fixation by ingrowth of uncemented prostheses to avoid aseptical loosening.27, 28 To demonstrate whether DBP is altered in response to surgical stress and if activation of vitamin D also is compromised, we conducted a detailed investigation of the changes in serum DBP, total and free vitamin D metabolites, calcium, and PTH and compared them with changes in sex steroids and gonadotropins before and after elective total knee arthroplasty (TKA).
Material and Methods
Study population and surgical procedure
A total of 28 patients (16 women and 12 men) were screened, but 2 women and 1 man were excluded because of serious comorbidities (2 rheumatoid arthritis and 1 renal failure), leaving 25 patients to be included (Supplemental Fig. S2). Participants were included from men and women scheduled for and operated from February 17, 2015, to January 27, 2016, with total knee arthroplasty (TKA) by an experienced surgeon (HH) at Copenhagen University Hospital, Hvidovre, Denmark. All participants were healthy, aged 45 to 77 years, and had a daily intake of cholecalciferol less than 15 μg (600 IE). Exclusion criteria were serious comorbidities such as liver or kidney failure, endocrine disease, or disorders related to vitamin D and calcium homeostasis. Moreover, no patients were treated with allopurinol, diuretics except thiazids, hormones, or immunosuppressive drugs. Patients were operated using the midline skin‐medial parapatellar incision to insert a tricompartmental cemented prosthesis. Patients followed a fast‐track protocol using optimized care principles (spinal anesthesia, tranexamic acid, local infiltration analgesia, no steroids, early mobilization within a few hours after surgery, multimodal opioid‐sparing analgesia, fixed functional discharge criteria, and discharge directly to home.29 The protocol was registered at http://ClinicalTrials.gov (NCT02336932) and the study was approved by the local ethics committee (H‐2‐2014‐104) and the Danish National Data Protection Agency.
Biochemical analysis
After verbal and written informed consent, blood samples were collected preoperatively and postoperatively 2, 6, 24, and 48 hours and finally at 3 weeks follow‐up. Blood samples were handled according to protocol, put on ice, and centrifuged before serum was frozen. Vitamin D metabolites (25‐OHD and 1,25(OH)2D3) were measured by isotope dilution liquid chromatography tandem mass spectrometry (LC‐MS/MS) as described previously.30, 31, 32 The used methodology separates 7α‐hydroxy‐4‐cholesten‐3‐one from 25‐OHD but not the epimer of 25‐OHD. The variations (2SD) for 25‐OHD2 and 1,25‐OH2D3 were 3 nmol/L and 16 pmol/L determined at 33 nmol/L and 87 pmol/L, respectively. 24,25‐dihydroxyvitamin D was measured using an ELISA (24R,25‐DVD3, MyBiosource, San Diego, CA, USA) with a CV% of 15. Sex steroids were determined by RIA (testosterone [Coat‐a‐Count, Siemens, Munich, Germany] and estradiol [Pantex, Santa Monica, CA, USA]). The detection limit and coefficients of variation (CV) were 0.23 nmol/L and 8% for testosterone and 18 pmol/L and 13% for estradiol. A time‐resolved immunofluorometric assay (Delfia, Wallac, Turku, Finland) was used for follicle‐stimulating hormone (FSH), luteinizing hormone (LH), and SHBG measurements, whereas DBP was determined using an immunoassay from Immundiagnostik (Bensheim, Germany). Inhibin B was determined using an enzyme‐linked immunoassay (InhibinB genII, Beckman Coulter, Brea, CA, USA). The CV of FSH, LH, SHBG, DBP, and Inhibin B assay used were <4%, <4%, <6%, <8%, and <11%, respectively. Free testosterone was calculated using the method of Vermeulen,33 free estrogen by Mazer method,34 and free and bioavailable vitamin D metabolites were calculated ad modum Bikle and Thadani, although only the latter method is presented here. Serum concentrations of albumin (CV 1.8%), calcium (CV 1.2%), parathyroid hormone (CV 3.4%), and alkaline phosphatase (CV 1.3%) were measured on Cobas from Roche Diagnostics A/S (Hvidovre, Denmark) as recommended by the manufacturer, and S‐Parathyroid hormone (CV% 3.4%) on Cobas e601. Albumin‐corrected calcium was calculated (total calcium + 0.020 × [41.3 – albumin]).
Statistics
Using a test level of 5%, power of 80%, and at least 10 participants of each sex enabled us to detect a 15% change in 25OHD and 1,25OH2D3 compared with the preoperative level. Means and standard deviations were calculated for all continuous variables. The mean for each variable was normalized to preoperative levels at each time point and stratified according to sex. All data presented in the figures are indexed to baseline levels and presented in Table 1. Two‐sided ANOVA with Dunnett's test were used to test for differences between time points and adjust for multiple comparisons. The conversion rate35, 36 (1000 × 1,25(OH)2D3/25‐OHD) was calculated preoperatively and 3 weeks postoperatively and presented as a function of substrate levels or serum concentrations of putative regulators, and the correlation coefficient was determined.
Table 1.
Baseline Characteristics of All Included Patients
| N (%)/Mean (SD) | Women | Men |
|---|---|---|
| Included (n) | 14 | 11 |
| Age (years) | 68 (7) | 67 (7) |
| Previous serious comorbidity | 0 (0) | 2 (4.3) |
| Current use of thiazide diuretics | 5 (36%) | 1 (9%) |
| DBP (μg/mL) | 383 (118) | 369 (63) |
| Total 25‐OH (nmol/L) | 57 (30) | 60 (31) |
| Free 25‐OH (pmol/L) | 14 (15) | 13 (7) |
| Total 1,25(OH)2D3 (pmol/L) | 70 (30) | 82 (34) |
| Free 1,25(OH)2D3 (pmol/L) | 0.29 (.23) | 0.31 (.16) |
| 24,25(OH)2D3 (nmol/L) | 13 (11) | 23 (27) |
| Albumin (g/L) | 44 (2) | 43 (3) |
| Albumin corrected calcium (mmol/L) | 2.30 (.07) | 2.29 (.12) |
| Phosphate (mmol/L) | 1.04 (.12) | 0.98 (.17) |
| Alkaline phosphatase (U/L) | 75 (22) | 63 (17) |
| PTH (pmol/L) | 5.3 (2.3) | 4.0 (1.6) |
| Testosterone (nmol/L) | 1.1 (0.5) | 10.1 (1.8) |
| Estradiol (pmol/L) | 69 (25) | 97 (24) |
| SHBG (nmol/L) | 46 (24) | 35 (14) |
| FSH (U/L) | 56.5 (16) | 5.9 (2) |
| LH (U/L) | 25.3 (10) | 3.9 (1) |
DBP = vitamin D binding protein; PTH = parathyroid hormone; SHBG = sex hormone binding globulin; FSH = follicle‐stimulating hormone; LH = luteinizing hormone.
Results
Baseline characteristics and standard care
Mean age was 67 years (Table 1). All patients had normal serum creatinine and estimated glomerular filtration rate (GFR) above the age‐matched lower reference, although 6 patients received thiazide diuretics for hypertension. The patients were on average vitamin D sufficient with a mean serum concentration of 25OHD and 1,25OH2D3 of 58 nmol/L and 75 pmol/L, respectively, although 4 patients had serum 25OHD <25 nmol/L (deficiency). PTH, albumin corrected, and total calcium were within the normal reference intervals, whereas patients with 25OHD <25 nmol/L had an expected compensated increase in serum PTH before the surgery. Preoperative concentrations of gonadotropins and sex steroids showed that all women were postmenopausal, illustrated by elevated FSH and LH levels and low serum concentrations of estradiol. All patients received similar treatment with 5.0 mL/kg/h isotonic 0.9% NaCl and 7.5 mL/kg/h Ringer lactat during surgery and immediately postoperatively (up to 12 hours after surgery) to secure cardiovascular homeostasis. None of the patients received blood or experienced any serious complications postoperatively.
Postoperative changes in serum albumin, SHBG, vitamin D binding protein, and vitamin D metabolites
As expected, serum concentrations of albumin and SHBG decreased significantly (p < 0.05) postoperatively (Fig. 1). The decline in serum albumin was already detectable 2 hours postoperatively, reaching nadir after 48 hours (Δalbumin48h) −18% (−22%; −14%) compared with baseline levels. The largest decrease in SHBG levels of −17% (−33.4%; −0.2%) was found 2 hours postoperatively, and a decrease between 10% and 20% also persisted after 48 hours in both sexes. Interestingly, the decline in DBP was not statistically significant at any time point. A tendency toward lower DBP concentration was found in men only after 24 hours ΔDBP −17% (−36%; 2%). Total 25‐OHD concentrations had a downward trend from 2 to 24 hours postoperatively, although not statistically significantly after adjustment for multiple testing. After 48 hours, serum 25‐OHD remained low in men, where 25OHD48h was −19% (−33%; −8%), whereas serum concentrations were higher than baseline levels in women (Fig. 2). Total 25‐OHD reached baseline levels after 3 weeks in both sexes. Free 25‐OHD concentrations were remarkably identical in both sexes, and the percentage of free 25OHD did not change significantly during the study period. Moreover, concentrations of total, free, bioavailable, and the percentage of free 25OHD were not different when compared at baseline and 3 weeks after surgery. Noteworthy, total 1,25(OH)2D3 declined over time but was only significantly different from baseline level 3 weeks after the surgery, Δ1,25D3W −20% (−33%; −7%). The drop in 1,25(OH)2D3 concentration was only robustly found in women with a −24% (−40%; −8%) drop compared with baseline, whereas men had a −15% (−36%; 6%) and not statistically significant decline 3 weeks postoperatively. Total calcium decreased modestly in both sexes from 2 to 48 hours postoperatively (p < 0.05), but albumin corrected calcium remained constant throughout the study period. Surprisingly, both total and albumin corrected calcium were significantly higher 3 weeks after surgery. This was partly mirrored by alkaline phosphatase (AP), which decreased from 2 to 48 hours postoperatively followed by an increase in both sexes (ΔAPwomen3w 18% [−5%; 42%], ΔAPmen3w 55% [15%; 95%]). No significant changes were found in serum phosphate or PTH levels, although PTH levels tended to be 25% lower 3 weeks after the surgery (p = 0.09). Interestingly, 24,25‐dihydroxyvitamin D levels decreased significantly in men (Fig. 2) 3 weeks after surgery (p = 0.032), whereas women experienced no significant changes postoperatively. 24,25‐dihydroxyvitamin D was the only vitamin D metabolite measured with an ELISA, and serum 24,25‐dihydroxyvitamin D was positively associated with 25OHD, 1,25(OH)2D3, calcium, FSH, and estradiol and negatively associated with PTH and phosphate (p < 0.05).
Figure 1.
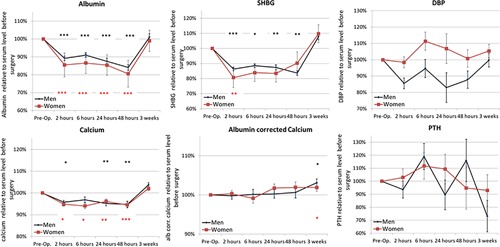
Serum concentrations of circulating proteins, calcium, and PTH in response to surgery. Data are presented as mean ± SEM normalized to the average serum concentration preoperatively and stratified according to sex. *p < 0.05, **p < 0.01, ***p < 0.001 (black asterisks = men; red asterisks = women).
Figure 2.

Changes in serum concentrations of total, free, and bioavailable 25‐OHD and 1,25(OH)2D3 and 24,25(OH)2D3 after the surgical stress response. Data are presented as mean ± SEM normalized to the average concentration preoperatively and stratified according to sex. *p < 0.05, **p < 0.01, ***p < 0.001 (black asterisks = men; red asterisks = women).
Sex steroids and gonadotropins after surgery
The decline in SHBG levels postoperatively was followed 24 hours after surgery exclusively in men of a decline in total and free testosterone comprising −52% (−73%; −32%) and −50% (−73; −32%), respectively. Interestingly, concentrations of total and free testosterone were not restored to baseline levels but remained 20% lower for testosterone (p = 0.051) and 23% lower for free testosterone (p = 0.083) when assessed 3 weeks postoperatively. Serum estradiol also decreased in men 24 hours after surgery, although not reaching statistical significance. In contrast, the percentage of free sex steroids increased for both testosterone and estradiol in both sexes during the first 2 days but was normalized 3 weeks after surgery. Total and free concentrations of testosterone and estradiol remained at a low but stable level in the postmenopausal women at all time points, but a marked decline in serum concentrations of FSH was found in both sexes. The decrease was huge and started already after 2 hours in the postmenopausal women and persisted up to 48 hours after surgery in both men and women (Fig. 3). The decline in LH was most pronounced at 24 hours −57% (−87; −26%) in women, whereas the men had no significant decrease in LH. Consequently, testosterone/LH ratio was significantly lower in men 24 and 48 hours after surgery (ΔT/LH24h −42% [−80%; −4%], ΔT/LH24h −41% [−79%; −4%]), whereas the 24% lower T/LH level 3 weeks after surgery was insignificant after adjustment of multiple comparison.
Figure 3.

Changes in serum concentrations of sex steroids and gonadotropins after the surgery. (A) Total, free, and percentage free estradiol. (B) Total, free, and percentage free testosterone. (C) Gonadotropins and testosterone/LH ratio. Data are presented as mean ± SEM normalized to the average concentration preoperatively and stratified according to sex. *p < 0.05, **p < 0.01, ***p < 0.001.
Formation of 1,25(OH)2D3 before and after surgery
Conversion of 25OHD to 1,25(OH)2D3 was evaluated as a crude estimation of the actual conversion rate (1000 × 1,25(OH)2D3/25‐OHD), and the calculated conversion rate was lower after surgery. As expected, 1α‐hydroxylase activity was dependent upon vitamin D status, so men and women with vitamin D deficiency (<25 nmol/L) had a conversion rate three‐ to fivefold higher than patients with high vitamin D status (>75 nmol/L). In women, the conversion rate was particularly high when vitamin D status was low and the dependence on substrate availability was much more pronounced in women compared with men (Fig. 4). The conversion rate clearly differed between sexes, and other hormones were also clearly different. Women had much higher FSH levels, and FSH was weakly inversely associated with the conversion rate postoperatively. Men, unlike women, had high levels of sex steroids, but free testosterone levels were not associated with the conversion rate before the surgery. After surgery, a positive association (p < 0.05) between free testosterone and the conversion rate was found in men, whereas there was no association between sex steroids and the conversion rate in women (data not shown).
Figure 4.
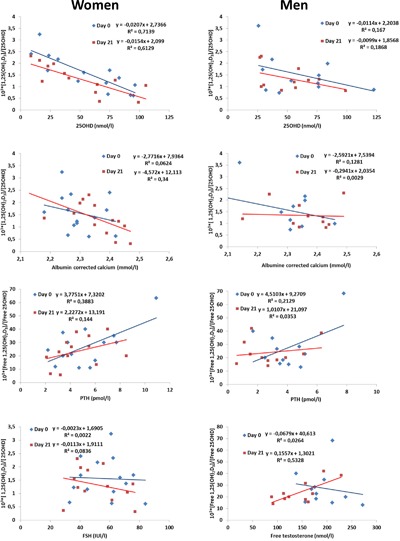
Changes in formation of 1,25(OH)2D3 before and after surgery. The conversion rate (1000 × 1,25(OH)2D3/25‐OHD) preoperatively and 3 weeks postoperatively as a function of 25‐OHD, albumin corrected calcium, PTH, FSH, and testosterone. Data are presented with correlation coefficient and formula.
Discussion
This prospective study shows that activation of vitamin D is compromised after TKA and serum 1,25(OH)2D3 remains persistently low 3 weeks after surgery in women. The decline in 1,25(OH)2D3 occurs despite a stable concentration of DBP in the postoperative phase. We speculate that the unchanged DBP in women is caused by increased hepatic mobilization. It is unlikely that DBP exempt redistribution exclusively in women because the decline in serum concentrations of albumin and SHBG resembles the decrease in serum DBP, albumin, and SHBG observed in men in the postoperative phase. Moreover, the marked and persistent decrease in serum 1,25(OH)2D3 after elective surgery may be of clinical interest irrespectively of the precise mechanism of action because correction of this deficit theoretically requires supplementation with activated vitamin D (etalpha/calcitriol) rather than cholecalciferol. In the search for a causal explanation, diminished substrate availability is an obvious question; however, this is unlikely because both total and free serum 25OHD concentrations were similar in samples taken preoperatively and 3 weeks postoperatively. This implies that the observed 1,25(OH)2D3 deficit may not be resolved by vitamin D supplementation alone, which is the routine treatment recommended today. Moreover, our data show that the normal dependency of substrate availability for generation of 1,25(OH)2D3 is altered postoperatively. After surgery, serum calcium is the most predictive factor, whereas 25OHD and PTH are the best predictors preoperatively.
DBP concentration and handling clearly differs between sexes, and the observed changes in protein binding are therefore the best and simplest explanation for the compromised 1‐alpha hydroxylase activity in women postoperatively. We observed no compensatory increase in PTH to maintain conversion of 25OHD at a sufficient level to reach 1,25(OH)2D3 concentrations at preoperative levels. The lack of PTH response indicates that calcium in conjunction with other factors may blunt the normal PTH response. Calcium is a known regulator of 1‐alpha hydroxylase activity and PTH secretion.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Albumin corrected calcium increased significantly after surgery and could theoretically augment the observed 35% decrease in serum 1,25(OH)2D3.37, 38, 39 Regulation of 1‐alpha hydroxylase activity is complicated, but a prevailing hypothesis is that one regulator, for instance, circulating FGF‐23 concentration in various disease states, prevails over other signals, including PTH and calcium. FGF23 lowers 1‐alpha hydroxylase but concomitantly induces CYP24A1 transcription, which in turn would lead to accelerated catabolism of vitamin D metabolites and thereby lower serum concentration of all vitamin D metabolites,40 including 25OHD. In fact, serum 24,25‐dihydroxyvitamin D decreased only in men after surgery and the persistently high activity of CYP24A1 in women concomitantly with lower CYP27B1 implies that a larger fraction of the substrate pool is inactivated because of increased CYP24A1 activity, which alone or in combination with decreased CYP27B1 activity may be the cause for low 1,25(OH)2D3. FGF23 could theoretically induce these changes but is unlikely to be a key factor because no major changes in serum phosphate were found, which indicates that other factors also could be involved, such as inflammatory cytokines known to regulate vitamin D metabolism.
Impaired megalin‐mediated cellular uptake would in theory lead to diminished serum concentrations of 1,25(OH)2D3 but also low 25OHD and DBP. The latter was not observed and therefore not supportive for our initial hypothesis. Instead, the difference in sex steroids and/or gonadotropins between sexes could also be responsible because the women were postmenopausal, whereas the men had normal circulating sex steroids and gonadotropin levels. Sex steroids have previously been shown to induce 1α‐hydroxylase activity and formation of 1,25(OH)2D3,41 but a clinical importance of sex steroids as regulators of 1α‐hydroxylation has to our knowledge not been demonstrated in humans. However, previous work in humans has shown that estradiol‐progesterone treatment increases both total 1,25(OH)2D3 and DBP markedly in non‐pregnant women,42 which implies that estrogen is a potent regulator of activation of vitamin D, or maybe it is the gonadotropins that also are influenced by sex steroids that mediate non‐gonadal effects. Our data indicate that sex steroids and FSH were associated with CYP24A1 activity in both sexes, but further studies are warranted to demonstrate if this link is causal. All the included men experienced a decline in total and free testosterone postoperatively. This decline was compensated by an insignificant increase in LH levels, which resulted in a lower testosterone/LH ratio.
The clinical questions remaining are whether the decrease in 1,25(OH)2D3 in women and decline in serum testosterone in men are of clinical importance and if correction of these hormones would be beneficial for patients with a new bone implant. In this context, studies in rodents have shown that administration of vitamin D metabolites after experimental fracture significantly improved the mechanical strength of the fractured bone.19 Vitamin D deficiency is common in patients undergoing knee replacement and associated with outcome.43 Moreover, a small randomized clinical trial in patients with proximal humerus fractures showed increased callus formation in patients receiving vitamin D and calcium over the first 6 weeks compared with placebo.14 Approximately 5% to 10% do not obtain final union of their knee prosthesis. Etiology is mechanical, anatomical, surgical, or biological factors, and a recent study suggested that up to 85% of patients with nonunion had undiagnosed metabolic or endocrine abnormalities.44
In conclusion, generation of 1,25(OH)2D3 is impaired after elective knee replacement, resulting in low circulating levels of activated vitamin D, which may influence the postoperative phase in women having a new bone implant. The main clinical question is to determine whether the decrease in 1,25(OH)2D3 is clinically relevant and should be corrected postoperatively to avoid a functional vitamin D deficiency despite normal serum 25OHD levels.
Disclosures
All authors state that they have no conflicts of interest.
Supporting information
Supporting Figures S1.
Acknowledgments
We are thankful for the help of all the nurses and technicians at Hvidovre Hospital and Brian Vendelboe for valuable technical assistance.
Funding was provided by Rigshospitalet, Novo‐Nordisk Foundation, Åse and Ejnar Danielsens Foundation, Danish Agency for Science, Technology, and Innovation, and Lundbeck Foundation for Fast‐track Hip and Knee Arthroplasty.
Authors’ roles: Conception: MBJ and HK. Funding and study design: HK, MBJ, and HH. Patient handling: HH. Analysis: AJ, PJB, and MBJ. Statistics: MBJ. Drafting of manuscript: MBJ and HK with input from all authors.
References
- 1. Veenhof AA, Sietses C, van Hoogstraten IM, et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011; 26:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kehlet H. The surgical stress response: should it be prevented? Can J Surg. 1991; 34:565–7. [PubMed] [Google Scholar]
- 3. Manzanilla MA, Fonseca F, Manzanilla MA Jr, Crespo RS. Effect of surgical stress on blood levels of calcium and phosphorus. J Int Coll Surg. 1953; 20:573–8. [PubMed] [Google Scholar]
- 4. Wang C, Chan V, Yeung RT. Effect of surgical stress on pituitary‐testicular function. Clin Endocrinol. 1978; 9:255–66. [DOI] [PubMed] [Google Scholar]
- 5. Ilias I, Tzanela M, Mavrou I, et al. Thyroid function changes and cytokine alterations following major surgery. Neuroimmunomodulation. 2007; 14:243–7. [DOI] [PubMed] [Google Scholar]
- 6. Smale BF, Hobbs CL, Mullen JL, Rosato EF. Serum protein response to surgery and starvation. J Parenter Enteral Nutr. 1982; 6:395–8. [DOI] [PubMed] [Google Scholar]
- 7. Hubner M, Mantziari S, Demartines N, Pralong F, Coti‐Bertrand P, Schafer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016; 2016:8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labgaa I, Joliat GR, Kefleyesus A, et al. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open. 2017; 7:e013966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brandt MR, Kehlet H, Skovsted L, Hansen JM. Rapid decrease in plasma‐triiodothyronine during surgery and epidural analgesia independent of afferent neurogenic stimuli and of cortisol. Lancet. 1976; 2:1333–6. [DOI] [PubMed] [Google Scholar]
- 10. Kremers HM, Lewallen EA, van Wijnen AJ, Lewallen DG. Clinical factors, disease parameters, and molecular therapies affecting osseointegration of orthopedic implants. Curr Mol Biol Rep. 2016; 2:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosen CJ, Gallagher JC. The 2011 IOM report on vitamin D and calcium requirements for North America: clinical implications for providers treating patients with low bone mineral density. J Clin Densitom. 2011; 14:79–84. [DOI] [PubMed] [Google Scholar]
- 12. van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone. 2008; 42:260–6. [DOI] [PubMed] [Google Scholar]
- 13. Fu L, Tang T, Miao Y, Hao Y, Dai K. Effect of 1,25‐dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. 2009; 44:893–8. [DOI] [PubMed] [Google Scholar]
- 14. Doetsch AM, Faber J, Lynnerup N, Watjen I, Bliddal H, Danneskiold‐Samsoe B. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo‐controlled study. Calcif Tissue Int. 2004; 75:183–8. [DOI] [PubMed] [Google Scholar]
- 15. Lorenzen M, Boisen IM, Mortensen LJ, Lanske B, Juul A, Blomberg JM. Reproductive endocrinology of vitamin D. Mol Cell Endocrinol. 2017; 453:103–12. [DOI] [PubMed] [Google Scholar]
- 16. Gray RW, Omdahl JL, Ghazarian JG, DeLuca HF. 25‐Hydroxycholecalciferol‐1‐hydroxylase. Subcellular location and properties. J Biol Chem. 1972; 247:7528–32. [PubMed] [Google Scholar]
- 17. Blomberg Jensen M.Vitamin D and male reproduction. Nat Rev Endocrinol. 2014; 10:175–86. [DOI] [PubMed] [Google Scholar]
- 18. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010; 78:140–5. [DOI] [PubMed] [Google Scholar]
- 19. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008; 29:726–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz JB, Lai J, Lizaola B, et al. Variability in free 25(OH) vitamin D levels in clinical populations. J Steroid Biochem Mol Biol. 2014; 144 Pt A:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25‐hydroxyvitamin D in serum and its regulation by albumin and the vitamin D‐binding protein. J Clin Endocrinol Metab. 1986; 63:954–9. [DOI] [PubMed] [Google Scholar]
- 22. Louw JA, Werbeck A, Louw ME, Kotze TJ, Cooper R, Labadarios D. Blood vitamin concentrations during the acute‐phase response. Crit Care Med. 1992; 20:934–41. [DOI] [PubMed] [Google Scholar]
- 23. Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25‐hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011; 93:1006–11. [DOI] [PubMed] [Google Scholar]
- 24. Zaloga GP, Butterworth JF. Hypovitaminosis D in hospitalized patients: a marker of frailty or a disease requiring treatment? Anesth Analg. 2014; 119:613–8. [DOI] [PubMed] [Google Scholar]
- 25. Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25‐(OH) vitamin D3. Cell. 1999; 96:507–15. [DOI] [PubMed] [Google Scholar]
- 26. Sarti A, De Gaudio AR, Messineo A, Cuttini M, Ventura A. Glomerular permeability after surgical trauma in children: relationship between microalbuminuria and surgical stress score. Crit Care Med. 2001; 29:1626–9. [DOI] [PubMed] [Google Scholar]
- 27. Roizen MF, Roizen JD. Vitamin D and your patients: don't accept wimpy. Anesth Analg. 2014; 119:503–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peersman G, Bellemans J, Richart T. Is oral vitamin D a risk factor for prosthetic loosening? Acta Orthop Belg. 2009; 75:2–7. [PubMed] [Google Scholar]
- 29. Husted H. Fast‐track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012; 83:1–39. [DOI] [PubMed] [Google Scholar]
- 30. Blomberg Jensen M, Gerner Lawaetz J, Andersson AM, et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum Reprod. 2016; 31(8):1875–85. [DOI] [PubMed] [Google Scholar]
- 31. Blomberg Jensen M, Bjerrum PJ, Jessen TE, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011; 26:1307–17. [DOI] [PubMed] [Google Scholar]
- 32. Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jorgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab. 2018; 103(3):870–81. [DOI] [PubMed] [Google Scholar]
- 33. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999; 84:3666–72. [DOI] [PubMed] [Google Scholar]
- 34. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009; 74:512–9. [DOI] [PubMed] [Google Scholar]
- 35. Bollehuus HL, Rehfeld A, de Neeergaard R, et al. Selection of high‐quality spermatozoa may be promoted by activated vitamin D in the woman. J Clin Endocrinol Metab. 2017; 102(3):950–61. [DOI] [PubMed] [Google Scholar]
- 36. Pasquali M, Tartaglione L, Rotondi S, et al. Calcitriol/calcifediol ratio: an indicator of vitamin D hydroxylation efficiency? BBA Clin. 2015; 3:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med. 2008; 40:82–91. [DOI] [PubMed] [Google Scholar]
- 38. Hoenderop JG, Chon H, Gkika D, et al. Regulation of gene expression by dietary Ca2+ in kidneys of 25‐hydroxyvitamin D3‐1 alpha‐hydroxylase knockout mice. Kidney Int. 2004; 65:531–9. [DOI] [PubMed] [Google Scholar]
- 39. Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25‐hydroxyvitamin D3‐1alpha‐hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci U S A. 1999; 96:8253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Semin Nephrol. 2013; 33:158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanaka Y, Castillo L, DeLuca HF. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc Natl Acad Sci U S A. 1976; 73:2701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D‐binding protein on the serum concentration of 1,25‐dihydroxyvitamin D3. Significance of the free 1,25‐dihydroxyvitamin D3 concentration. J Clin Invest. 1981; 67:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shin KY, Park KK, Moon SH, Yang IH, Choi HJ, Lee WS. Vitamin D deficiency adversely affects early post‐operative functional outcomes after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017; 25:3424–30. [DOI] [PubMed] [Google Scholar]
- 44. Brinker MR, O'Connor DP, Monla YT, Earthman TP. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007; 21:557–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures S1.


