Abstract
Gastric cancer with metastases outside of the regional lymph nodes is deemed oncologically unresectable. Nevertheless, some metastatic lesions are technically resectable by applying established surgical techniques such as para‐aortic lymphadenectomy and hepatectomy. At the time of compilation of the Japanese gastric cancer treatment guidelines version 4, systematic reviews were conducted to see whether it is feasible to make any recommendation to dissect both the primary and metastatic lesions with intent to cure, possibly as part of multimodality treatment. Long‐term survivors were found among carefully selected groups of patients both in prospective and retrospective studies. In addition, there is a growing list of publications reporting encouraging outcomes of gastrectomy conducted after exceptionally good response to chemotherapy, usually among patients who underwent R0 resection. This type of surgery is often referred to as conversion surgery. It is sometimes difficult to define a clear borderline between curative surgery scheduled after neoadjuvant chemotherapy and the conversion surgery. This review summarizes what we knew after the literature reviews conducted at the time of compiling the Japanese guidelines and in addition reflects some new findings obtained thereafter through clinical trials and retrospective studies. Metastases were divided into three categories based on the major metastatic pathways: lymphatic, hematogenous, and peritoneal. In each of these categories, there were findings that could provide hope for patients with metastatic disease. These findings implied that the surgical technique that we already use could become more useful upon further developments in antineoplastic agents and drug delivery.
Keywords: conversion surgery, gastric cancer, metastasis, multimodality treatment
1. INTRODUCTION
Gastric adenocarcinoma remains a major health problem worldwide with 738 000 deaths annually.1 Although surgery is a mainstay of treatment for gastric cancer, indication for gastrectomy is limited to oncologically curable cancer.2 This is because gastrectomy is associated with relatively high morbidity and mortality, along with considerable decline in quality of life. Cancers, in general, can metastasize through three major pathways: lymphatic, hematogenous, and through direct dissemination of cancer cells from the tumor surface. Gastric adenocarcinoma has been feared as a particularly aggressive disease that has potential to spread through all of these pathways.3 Lymph node metastasis is the only pattern of disease spread that occurs during the earlier stages so that surgery with adequate lymphadenectomy has been believed to confer not only precise staging but also prognostic benefit.4 On the contrary, patients who harbor metastases to distant organs are considered to have little hope of cure even if the metastatic sites seem technically resectable, owing to rapid and almost inevitable growth of micrometastases that are likely to exist in such patients. This is in stark contrast with colorectal cancer in which hepatic and pulmonary metastases are often considered for surgery with intent to cure.
The present review attempts to identify situations where an aggressive surgical approach could be indicated for metastatic gastric cancer. There are two types of surgery for metastatic cancer. In one, the metastatic lesions are technically resectable, and a surgeon attempts complete resection of all lesions. The term “technically resectable” is ambiguous, may depend on the philosophy of each surgeon, and is extremely difficult to define. It may be more realistic to give the following as a typical example for each of the metastatic pathways: (i) cancer with a moderate number of swollen (≥1 cm) lymph nodes in the No. 16 a2/b1 regions which can be resected by the conventional technique of para‐aortic lymph node dissection such as the one explored in a Japan Clinical Oncology Group (JCOG) randomized trial to be described later in this article; (ii) cancer with a small number of liver metastases (typically ≥3) of a size and location that can be dissected by hepatectomy without exceptional considerations; and (iii) cancer with a small number of peritoneal deposits which can be coresected easily at the time of gastrectomy. Patients usually undergo systemic chemotherapy, often in the form of neoadjuvant chemotherapy to eradicate micrometastases5 and to avoid surgery in a cohort that suffers from rapid progression while chemotherapy is being given. In the other type, patients suffer from multiple metastases that are technically unresectable. Chemotherapy delivered to these patients sometimes results in complete or near‐complete response of the metastatic lesion, which could render the primary lesion ± the remainders of the metastases resectable. This type of surgery has recently been referred to as conversion surgery. Yoshida et al6 have recently created a comprehensive classification of gastrectomy for stage IV cancer which takes into consideration the indications for both types of surgery.
2. LYMPH NODE METASTASIS
Before discussing metastases to various distant organs, it is necessary to discuss the value of lymph node dissection. Relevance of prophylactic lymph node dissection has been discussed thoroughly elsewhere,7, 8 and various guidelines currently support standard application of D2 dissection to treat resectable advanced gastric cancer.2, 9 However, cancer can spread beyond the boundary of standard D2 dissection. Thus, more extended lymphadenectomy had been proposed, but a campaign to enlarge the extent of prophylactic lymphadenectomy to include the para‐aortic lymph nodes turned out to be a failure in the JCOG9501 trial,10 a phase III trial in which the survival curve for the para‐aortic lymph node dissection group overlapped completely with that for the D2 dissection group.
Patients with metastasis to the para‐aortic lymph nodes are classified as stage IV.11 This denotes that metastases to the para‐aortic lymph nodes are considered as distant metastases. Para‐aortic lymph nodes in the peritoneal cavity are classified anatomically as a1, a2, b1, and b2 (Figure 1).11 During the 1980s, the technique for systematic dissection of a2/b1 lymph nodes was established and conducted experimentally in high‐volume hospitals throughout Japan. The JCOG9501 trial was conducted based on these techniques, and para‐aortic lymph node dissection was carried out safely with mortality and complication rates of 0.8% and 28.1%, respectively. One of the limitations of the trial was that the incidence of pathological para‐aortic lymph node metastasis among patients allocated to extended lymphadenectomy was unexpectedly low at 8.5%. In fact, the trial did not include patients who had enlarged lymph nodes in the para‐aortic region. Thus, survival benefit of para‐aortic nodal dissection cannot be denied among patients who had apparently swollen lymph nodes in the para‐aortic region. Sasako12 proposed a neoadjuvant strategy to this patient cohort as they likely suffer from micrometastases and may benefit from tumor shrinkage. After a series of phase II trials,12, 13, 14 neoadjuvant chemotherapy with S‐1 and cisplatin followed by gastrectomy and D2 plus para‐aortic lymph node dissection13 is considered the current standard of care in high‐volume centers in Japan.2 Para‐aortic lymph node dissection in this context denotes systematic dissection of the No. 16 a2/b1 region rather than sampling of apparently swollen nodes, as that was the strategy explored in the JCOG phase II trials. Absence of peritoneal deposits and negative peritoneal washing cytology through staging laparoscopy, absence of metastasis to other organs, and absence of cancer spread to the a1 or b2 regions and mediastinal/cervical lymph nodes have been prerequisites for participation in these phase II trials. Although not clarified in the eligibility criteria, it is likely that only patients with a moderate number of swollen nodes in the No. 16 a2/b1 region were entered into this trial.
Figure 1.
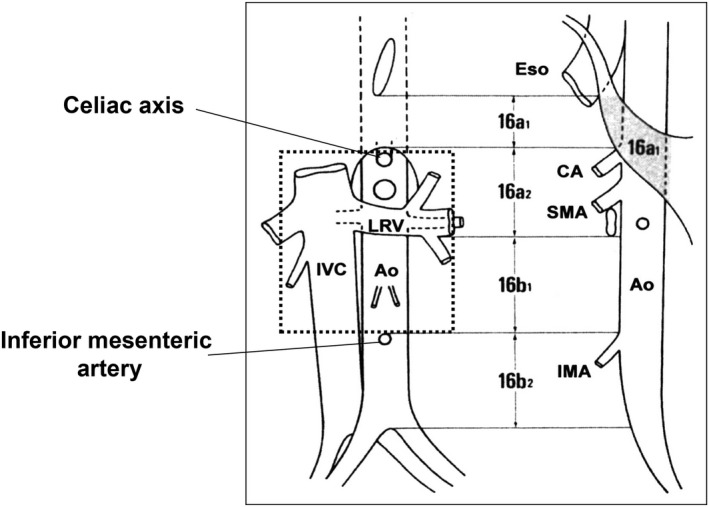
Anatomical extent of para‐aortic lymph node dissection that was carried out as a part of prophylactic dissection. 16a2 denotes para‐aortic lymph nodes distributed between the celiac axis and the lower border of the left renal vein, and b1 denotes those between the lower border of the left renal vein and inferior mesenteric artery
Dissection of mediastinal lymph nodes could be indicated and may, in some cases, even be recommended for patients with junctional cancer, given the presence of long‐term survivors among patients with metastases to such lymph nodes.15 Furthermore, patients with para‐aortic lymph node metastases beyond the a2 and b1 regions could still be indicated for surgery pending marked shrinkage of these nodes during chemotherapy which, originally, would have been cause for palliation. In such cases, surgeons often carry out limited or standard lymph node station dissection in order to evaluate the histological responses, but do not necessarily dissect all lymph nodes that have been swollen. This type of surgery has recently been referred to as conversion surgery.6 Although this term has not been defined explicitly, it could be characterized as follows: (i) surgery is not preplanned as in the case of neoadjuvant strategy, but is proposed after exceptional response of the metastatic lesions to chemotherapy; (ii) resection of all metastatic lesions that had originally been detected is not necessarily required, especially when complete response of such lesions was achieved; and (iii) surgery is indicated when information through imaging studies, laboratory data, and other modalities suggests possibility of R0 resection. There are sporadic reports of long‐term survivors among the responders who underwent conversion surgery mainly for, but not restricted to, distant lymphatic metastases in retrospective analyses (Table 1).16, 17, 18, 19
Table 1.
Four retrospective case series looking at conversion surgery after chemotherapy for advanced/metastatic gastric cancer
| First author | Time of accrual | Eligibility | Chemotherapeutic regimen | Indication for surgery | No. of patients who received chemotherapy | No. of patients who received gastrectomy | % treated by gastrectomy | No. of patients who received R0 resection | Rate of R0 resection among all surgeries | MST of patients who received surgery | MST of the R0 resected patients |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamaguchi16 | 2001‐2013 | cStage IV | Various doublets/triplets | R0 resection possible | 259 | 84 | 32.4 | 43 | 51.2 | 30.5 | 41.3 |
| Fukuchi17 | 2003‐2013 | Any one of the following: cT4b, P1, H1, CY1, M1 | S‐1/CDDP or S‐1/paclitaxel | R0 resection possible | 151 | 40 | 26 | 32 | 80 | 53 | 62 |
| Sato18 | 2002‐2014 | cStage IV | S‐1/CDDP/docetaxel | R0 resection possible | 100 | 33 | 33 | 28 | 85 | 47.8 | 47.9 |
| Ishigami19 | 2005‐2011 | P1 or CY1 | S‐1/paclitaxel IV/paclitaxel IP | CY0, disappearance of shrinkage of peritoneal mets | 100 | 64 | 64 | 44 | 68.8 | 30.5 | NA |
The fourth series was restricted to patients who underwent intraperitoneal chemotherapy for peritoneal metastases.
IP, intraperitoneal chemotherapy; mets, metastases; MST, median survival time (months); NA, not available.
Approximately 30% of patients who received chemotherapy underwent surgery. Series with higher incidence of R0 resection were associated with superior survival time.17, 18
3. HEPATIC METASTASIS
Gastric cancer cells that enter the bloodstream first enter the portal vein to reach the liver. These cells are considered to sometimes pass through the liver and lung to eventually metastasize to more remote sites such as bone, adrenal gland, and the brain, showing their diversity and that of the microenvironments where they eventually establish themselves as metastases. Anatomically speaking, however, the liver is the most logical and frequent site of hematogenous metastasis from gastric cancer. Liver metastases often emerge as multiple nodules occupying both lobes, sometimes accompanied by other distant metastases, and are usually considered incurable. Thus, hepatic metastases that undergo hepatectomy are carefully selected based on the number and size of metastatic nodules and biological characteristics of the primary cancer.
Adam et al20 analyzed a retrospective series of a large number of resected non‐colorectal and non‐neuroendocrine liver metastases into three categories, stratified by the 5‐year survival rate of the patients who underwent hepatectomy. Gastric cancer was classified into the intermediate group where the 5‐year survival rate was approximately 25%. Most reports of hepatectomy for gastric cancer metastasis are single‐institution case series consisting of 20‐30 cases accumulated over more than two decades.21 By systematically reviewing these reports, the Japanese Gastric Cancer Treatment Guidelines committee came to a conclusion that a solitary nodule, lack of other non‐curative factors, and favorable stage in terms of cT and cN categories of the primary tumor were among the factors that indicate better prognosis.21
More recently, larger case series have been published through multi‐institutional studies and combined analysis of data from high‐volume hospitals (Table 2) that reported a 5‐year survival rate of approximately 30%.22, 23, 24 Treatment selected in these studies was usually hepatectomy, but the amount of the liver resected varied widely depending not only on the number or size of the lesions but also on the preference of the surgeons. Two of these studies included radiofrequency ablation (RFA) as alternative treatment modalities.22, 24 As the indication for RFA was limited based on the diameter and location of the metastatic tumors, it is currently impossible to see whether RFA could replace surgery. However, RFA did seem to show efficacy for a well‐selected cohort of patients in these studies. Regardless of the details of treatment modalities selected, patients who were indicated for surgery in these studies usually had ≤3 nodules and, in one of the studies, a subset with a solitary nodule apparently had an outstanding outcome.22 When discussing the number of metastatic nodules, the influence of the evolution of novel imaging modalities cannot be ignored as, again, most of the studies accrued patients over more than a decade. In this respect, one recent single‐institution study was convincing because the authors looked only at patients with ≤3 nodules which were invariably diagnosed using enhanced magnetic resonance imaging (MRI). The 5‐year survival rate in that study also exceeded 30%.25 Based on these studies, the Japanese guidelines decided not to deny the possibility of carrying out hepatectomy for those with a “small number” of metastatic nodules.2
Table 2.
Four recent Asian retrospective series of surgically treated hepatic metastasis from gastric cancer
| First author/year | No. of cases | Type of study | Accrual | Characteristics | No. of metastatic nodules | 5‐year survival rate (%) | Prognostic factors |
|---|---|---|---|---|---|---|---|
| Oki 201622 | 94 | Retrospective Multi‐institutional | 2000‐2010 | Includes 25 cases treated by RFA | Solitary: 56 (60%), 2 nodules: 19, 3 nodules: 9, ≥4 nodules: 10 | 42 | Solitary metastasis, primary tumor >pN2 |
| Kinoshita 201523 | 256 | Retrospective Five institutions | 1990‐2010 | All cases underwent hepatectomy | Solitary: 168 (66%), 2 nodules: 44, 3 nodules: 18, ≥4 nodules: 26 | 31 | >2 metastatic nodules, primary tumor ≥pT3 |
| Guner 201624 | 98 | Retrospective Single institution | 1998‐2013 | Includes 30 cases treated by RFA | Solitary: 67 (68%), 2 nodules: 20, ≥3 nodules: 11 | 30 | Tumor diameter >3 cm |
| Tatsubayashi 201725 | 28 | Retrospective Single institution | 2004‐2014 | All cases underwent contrast‐enhanced MRI | Solitary: 20 (71%), 2 nodules: 7, 3 nodules: 1 | 32 | Not identified |
Five‐year survival rate was ≥30% in all series.
MRI, magnetic resonance imaging; RFA, radiofrequency ablation.
There are multi‐institutional retrospective analyses from European countries, also showing that not all hepatectomies are futile.26, 27 However, their conclusions tended to be reserved, indicating that a prospective study is needed to justify hepatectomy even in favorable situations. The only consensus between the east and the west is that some form of chemotherapy is needed prior to or after hepatectomy (or gastrectomy plus hepatectomy in cases of simultaneous metastasis) in order to eliminate the micrometastases that are likely to exist. When designing an international prospective trial to explore optimal multimodality treatment for “resectable” liver metastasis, however, the European investigators continue to show prudent attitudes and insist that a control arm to be treated by chemotherapy alone is necessary to confirm the prognostic impact of hepatectomy.
4. PERITONEAL METASTASIS
Peritoneal metastasis is considered to occur from cancer cells that are shed from the serosal surface or disseminated during surgical manipulation. As meticulous systematic lymphadenectomy has been the standard in cancer surgery for a long time in Japan, locoregional recurrences, especially recurrences to regional lymph nodes that should have been resected, have been considered regrettable and are actually infrequent. Thus, in Japan and Korea, the pattern of recurrence after curative surgery has long been peritoneal dissemination.28, 29 In addition, there are patients who suffer from peritoneal disease at their first clinical visit, especially among patients with linitis plastica type cancer.30, 31 Peritoneal deposits are whitish nodules typically seen in the omentum, fatty pads of the transverse colon, mesocolon and peritoneal surfaces adjacent to the stomach. They will eventually spread to all parts of the abdomen, constitute stone‐hard nodules, and result in obstruction of the bowel, ureter and biliary tract, causing feeding problems, ileus, hydronephrosis, and jaundice. Massive ascites is another problem observed in a certain subset of patients with peritoneal disease.
In gastric cancer, peritoneal metastases have been deemed incurable even if only a few nodules are found at the time of laparotomy. Furthermore, presence of free cancer cells in the peritoneal cavity detected by cytological examination of the peritoneal washes predicts recurrences as peritoneal carcinomatosis with a high positive predictive value.32, 33, 34, 35, 36 These characteristics prompted several surgeons to carry out staging laparoscopy for selected patients and in clinical trials to accurately stage patients and avoid futile laparotomy.12, 13, 14, 37 In addition, as a result of the peculiar pharmacokinetics of systemically delivered anticancer drugs in the peritoneal cavity38 and lack of lesions that are measurable by imaging studies, peritoneal disease has not been eligible in phase II studies for new drugs in which overall response rates were often used as a surrogate endpoint. Taken together, this resulted in paucity of treatment options with evidence for this particular type of metastatic gastric cancer.
Cytological examination of the peritoneal washes obtained at laparotomy or laparoscopy is a useful means of detecting peritoneal disease before a metastatic nodule grows to be macroscopically detectable. Presence of cancer cells, termed CY1, denotes poor prognosis, and CY1 is regarded as equivalent to M1 and P1 (presence of visible peritoneal metastasis) in the Japanese Classification of Gastric Carcinoma.11 Thus, patients with CY1 status fall into the stage IV category. As staging laparoscopy continues not to be standard practice for all cases of advanced gastric cancers in Japan,2 surgeons are informed of the CY status during surgery, or even after surgery, at institutions where services for immediate cytopathological evaluation are unavailable. Whether to stop gastrectomy at the confirmation of CY1 or to continue has been a matter of debate, particularly for linitis plastica type cancer.30 More recently, S‐1 was found to significantly decrease the incidence of recurrence of peritoneal disease in a pivotal randomized trial comparing postoperative S‐1 with surgery alone for stage II/III cancer.39 Furthermore, in a one‐arm study to explore efficacy of postoperative S‐1 for patients with CY1/P0 or CY1/P1 status (here, P1 denotes a few deposits that could easily be coresected), the 5‐year survival rate was 26% whereas there was no long‐term survivor among the historical controls.40 Thus, it was after the classification of S‐1 that surgeons began to continue with gastrectomy for the CY1 population with some confidence.41, 42 Accordingly, the current Japanese guidelines discuss the possibility of a multimodality treatment that includes gastrectomy for patients with CY1 cancer in the absence of other non‐curative factors.2 Nevertheless, when the CY1 status is diagnosed at the time of staging laparoscopy, surgery could be postponed and chemotherapy delivered as an initial treatment, so that only patients who responded and were converted to CY0 status at the second staging laparoscopy could be selected to proceed to gastrectomy.37, 43 Treatments such as i.p. chemotherapy44 and hyperthermic i.p. effusion chemotherapy (HIPEC) could add further impact, and some clinical trials exploring preoperative HIPEC by the laparoscopic approach45 and HIPEC at the time of gastrectomy46 have been conducted for the CY1 population.
Gastrectomy is far less likely to be conducted for patients with macroscopic peritoneal metastasis. Gastrectomy accompanied by total peritonectomy followed by HIPEC has been theoretically proposed as the ideal mode of treatment.47, 48 Cytoreductive surgery and HIPEC remain to be widely explored for malignancies derived from the peritoneal mesothelioma, appendiceal mucinous neoplasms, and peritoneal metastases of colorectal and ovarian origin,49 but several researchers decided not to further pursue this approach for gastric cancer because of uncertainty in efficacy despite the high morbidity. It is not practical to expect further exposure to anticancer agents after the classical combination of total peritonectomy followed by HIPEC because of serious adhesions.
However, benefits of i.p. administration of anticancer drugs in terms of pharmacokinetic advantages of higher intratumoral concentrations and less systemic toxicity remain attractive. Randomized trials that explored i.p. chemotherapy in various settings are listed in Table 3. A single i.p. delivery of cisplatin on the day of surgery combined with further systemic chemotherapy did not confer any survival benefit over surgery alone when given in the postoperative adjuvant setting among serosa‐positive CY0 patients.50 Although this result was highly disappointing, repeated i.p. doses of paclitaxel in ovarian cancer53, 54 aroused interest in the subsequent generation of surgeons. An in vivo model of peritoneal dissemination implied efficacy of paclitaxel given i.p. compared with the same drug given i.v.55 Pharmacokinetic study of paclitaxel delivered i.p. to patients with malignant ascites showed that the concentration of paclitaxel in the ascites was >2000‐fold that of the plasma at 3 hours after the dosage.38 Safety of i.p. dosage of paclitaxel, once questioned in cases of ovarian resection with colorectal coresection and anastomosis,54 was confirmed in a trial in which i.p. paclitaxel was given immediately after gastrectomy.56 However, survival benefit of a short series of i.p. doses of paclitaxel prior to the evidence‐based systemic treatment was not proven for gastric cancer patients with risk factors for recurrence as peritoneal carcinomatosis (Table 3).51 This was considered attributable to the lack of systemic effect of single‐agent chemotherapy because of the extremely poor transition of i.p. delivered paclitaxel to the plasma.38
Table 3.
Outcome of patients registered for three prospective randomized trials exploring i.p. chemotherapy for gastric cancer and one randomized trial for ovarian cancer
| Study | Setting | No. of cases (IP vs others) | Eligibility | IP regimen | Control | No. of administrations | Primary endpoint (IP vs others) | Hazard ratio for OS | P‐value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gastric cancer | JCOG9206‐250 | Postoperative adjuvant | 135 vs 133 | cT4, CY0 | CDDP IP at surgery, 5FU + CDDP, UFT | Surgery alone | Once on the day of surgery | 5‐year OS 62.0% (53.7‐70.2) vs 60.9% (52.6‐69.2) | Not reported | .482 |
| INPACT51 | Advanced/metastatic | 39 vs 44 | Linitis plastica, CY1, P1 etc. | PTX IP | PTX IV | 7 times including the day of surgery | MST 42.3 mo vs 37.7 mo | 1.16 (0.64‐2.09) | .628 | |
| PHOENIX‐GC52 | Advanced/metastatic | 114 vs 50 | P1 | S‐1 + PTX IV + PTX IP | S‐1 + CDDP | Not limited | MST 17.7 mo vs 15.2 mo | 0.72 (0.49‐1.04) | .08 | |
| Ovarian cancer | GOG53 | Advanced/metastatic | 205 vs 210 | Stage III, with no residual mass >1 cm | PTX IV + PTX IP + CDDP IP | Paclitaxel IV + CDDP IV | 6 cycles | MST 65.6 mo vs 49.7 mo | 0.75 (0.58‐0.97 | .03 |
These trials suggest importance of repeated exposure and combination with systemic chemotherapy. In the PHOENIX‐GC trial,52 patients were allocated in a 2:1 method.
Figures in parentheses denote 95% confidence interval.
IP, intraperitoneal chemotherapy; mo, months; MST, median survival time; OS, overall survival; PTX, paclitaxel.
After some pilot studies, Ishigami et al57, 58 conducted successive phase I and phase II trials to establish a combination of S‐1, i.v. paclitaxel, and i.p. paclitaxel. In the phase II trial, they achieved an excellent 1‐year survival rate of 78% (95% confidence interval 65% to 90%) among gastric cancer patients mostly with macroscopic peritoneal metastasis.19 Although standardization of the technique to install and manage the i.p. reservoirs was recognized as mandatory for widespread use of the treatment throughout the country,54 toxicities in their series were mild and manageable. One of the key factors to achieve long‐term survival with i.p. chemotherapy was conversion surgery which Ishigami et al19 conducted in 64 of 100 patients, which included patients who were registered for the phase II trial. Of the 64 patients who underwent conversion surgery, R0 resection was achieved in 44 patients. In a classification of surgery for stage IV gastric cancer, Yoshida et al6 had classified patients with peritoneal disease into a group with the least possibility of proceeding to surgery. Intraperitoneal chemotherapy now challenges this commonly accepted opinion by a prospective phase III study, PHOENIX‐GC, in which survival benefit of the combination of S‐1, i.v. paclitaxel, and i.p. paclitaxel over S‐1/cisplatin, the standard treatment, was suggested after adjusting for relevant prognostic factors.52
Nevertheless, it is important to emphasize the need for R0 resection. Survival benefit of palliative resection prior to chemotherapy has been denied in a population of patients with unresectable distant metastases, including those with macroscopically evident peritoneal deposits.59 Intraperitoneal chemotherapy could achieve complete response in some patients with peritoneal deposits so that initially unresectable patients could become candidates for R0 resection and receive what has been referred to in this article as conversion surgery. Such responses could nowadays be diagnosed through secondary staging laparoscopy. However, candidates for such surgery should be carefully selected as, even in the successful series reported by Ishigami et al,19 long‐term survivors existed only among those who eventually received R0 resection. Five‐year survival rate was 18% (8 of 44) after R0 resection and 0% after R1/R2 resection (0 of 20).
The most recent progress in i.p. administration is pressurized intraperitoneal aerosol chemotherapy (PIPAC). This combines the pharmacokinetic advantages of i.p. administration and pressurized vaporization to ensure better drug distribution and penetration. In addition, all procedures are conducted by the minimally invasive laparoscopic approach to ensure repeated observation of the peritoneal cavity along with drug administration. There are some encouraging data showing low morbidity and short hospital stay with high histological response with no negative impact on the quality of life.60
5. JAPANESE GASTRIC CANCER TREATMENT GUIDELINES VERSION 5
The latest version of the Japanese gastric cancer treatment guidelines published in January 2018 contains a novel algorithm for gastric cancer. Treatment of stage IV gastric cancer has been depicted with greater detail compared with the previous version2 (Figure 2). Queries regarding patients with minimal numbers of metastases in various metastatic categories are led to relevant clinical questions that are answered in the Q&A section of the guidelines by referring to the currently available evidence, as has been described in this review.
Figure 2.
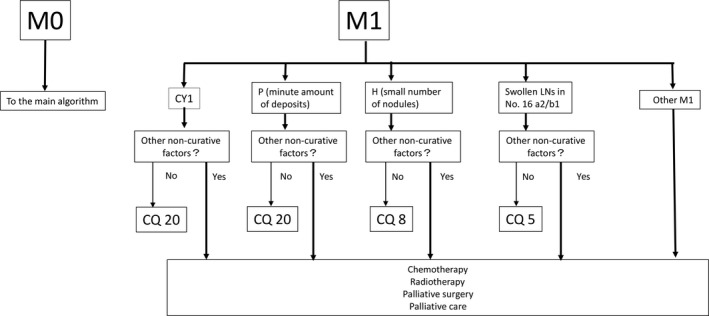
Proposal of the treatment for M1 disease in the algorithm of the Japanese gastric cancer treatment guidelines version 5. The proposal was granted and, for each category of distant metastases, relevant clinical questions were asked with answers provided as has been described in this review article
6. CONCLUSIONS
Surgery could play a part in multimodality treatment for stage IV gastric cancer. It may have a decisive role after neoadjuvant chemotherapy in highly selected cohorts of patients. In other cases, surgery could take on a role of adjuvant treatment in the form of conversion surgery when exceptional responses to chemotherapy are observed. Although the role of surgery cannot be denied, further prospective studies will be needed to establish more explicit indications for surgical treatments in each category of stage IV disease.
DISCLOSURE
Conflicts of interest: Yasuhiro Kodera was supported by grants or donations from Taiho Pharmaceutical, Chugai Pharmaceutical, Sanofi, Merck Serono, Yakult Honsha, Daiichi Sankyo, Otsuka Pharmaceutical Factory, Takeda Pharmaceutical, Johnson & Johnson, Eli Lilly Japan, ONO Pharmaceutical, Covidien Japan, Bristol Myers Squib, Novartis Pharmaceuticals Japan, and Olympus. The funding source had no role in the design, practice, or analysis of this study.
Kodera Y. Surgery with curative intent for stage IV gastric cancer: Is it a reality of illusion. Ann Gastroenterol Surg. 2018;2:339–347. 10.1002/ags3.12191
REFERENCES
- 1. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Association Japanese Gastric Cancer . Japanese gastric cancer treatment guidelines (ver. 4). Gastric Cancer. 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Averbach AM, Jacquet P. Strategies to decrease the incidence of intra‐abdominal recurrence in resectable gastric cancer. Br J Surg. 1996;83:726–33. [DOI] [PubMed] [Google Scholar]
- 4. Kodera Y. The current state of stomach cancer surgery in the world. Jpn J Clin Oncol. 2016;46:1062–71. [DOI] [PubMed] [Google Scholar]
- 5. Kodera Y. Neoadjuvant chemotherapy for gastric adenocarcinoma in Japan. Surg Today. 2017;47:899–907. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma: where do we stand after the Dutch and British randomized trials? J Am Coll Surg. 2002;195:855–64. [DOI] [PubMed] [Google Scholar]
- 8. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15‐year follow‐up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. [DOI] [PubMed] [Google Scholar]
- 9. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, ESMO Guidelines Committee . Gastric Cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v38–49. [DOI] [PubMed] [Google Scholar]
- 10. Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone with para‐aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62. [DOI] [PubMed] [Google Scholar]
- 11. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- 12. Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–22. [DOI] [PubMed] [Google Scholar]
- 13. Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S‐1 and cisplatin followed by D2 gastrectomy with para‐aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60. [DOI] [PubMed] [Google Scholar]
- 14. Ito S, Sano T, Mizusawa J, et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S‐1 followed by gastrectomy with D2 plus para‐aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2017;20:322–31. [DOI] [PubMed] [Google Scholar]
- 15. Yamashita H, Seto Y, Sano T, et al. Results of a nation‐wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. 2017;20(Suppl 1):69–83. [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi K, Yoshida K, Tanahashi T, et al. The long‐term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22:6618–24. [DOI] [PubMed] [Google Scholar]
- 18. Sato Y, Ohnuma H, Nobuoka T, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S‐1 (DCS) chemotherapy: a multi‐institutional retrospective study. Gastric Cancer. 2017;20:517–26. [DOI] [PubMed] [Google Scholar]
- 19. Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20(Suppl 1):128–34. [DOI] [PubMed] [Google Scholar]
- 20. Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases. Analysis of 1452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kodera Y, Fujitani K, Fukushima N, et al. Surgical resection of liver metastasis from gastric cancer: a reviewer and new recommendation in the Japanese Gastric Cancer Treatment Guidelines. Gastric Cancer. 2014;17:206–12. [DOI] [PubMed] [Google Scholar]
- 22. Oki E, Tokunaga S, Emi Y, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer. 2016;19:968–76. [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita T, Kinoshita T, Saiura A, et al. Multicentre analysis of long‐term outcome after surgical resection of gastric cancer liver metastases. Br J Surg. 2015;102:102–7. [DOI] [PubMed] [Google Scholar]
- 24. Guner A, Son T, Cho I, et al. Liver‐directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer. 2016;19:951–60. [DOI] [PubMed] [Google Scholar]
- 25. Tatsubayashi T, Tanizawa Y, Miki Y, et al. Treatment outcomes of hepatectomy for liver metastases of gastric cancer diagnosed using contrast‐enhanced magnetic resonance imaging. Gastric Cancer. 2017;20:387–93. [DOI] [PubMed] [Google Scholar]
- 26. Tiberio GA, Ministrini S, Gardini A, et al. Factors influencing survival after hepatectomy for metastases from gastric cancer. Eur J Surg Oncol. 2016;42:1229–35. [DOI] [PubMed] [Google Scholar]
- 27. Markar SR, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. 2017;20:379–86. [DOI] [PubMed] [Google Scholar]
- 28. Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–7. [DOI] [PubMed] [Google Scholar]
- 29. Yoo CG, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–42. [DOI] [PubMed] [Google Scholar]
- 30. Kodera Y, Yamamura Y, Ito S, et al. Is Borrmann type IV gastric carcinoma a surgical disease? An old problem revisited with reference to the result of peritoneal washing cytology. J Surg Oncol. 2001;78:175–81. [DOI] [PubMed] [Google Scholar]
- 31. Kodera Y, Nakanishi H, Ito S, et al. Detection of disseminated cancer cells in linitis plastica‐type gastric carcinoma. Jpn J Clin Oncol. 2004;34:525–31. [DOI] [PubMed] [Google Scholar]
- 32. Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60–4. [DOI] [PubMed] [Google Scholar]
- 33. Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase‐polymerase chain reaction after 5 years of followup. J Am Coll Surg. 2006;202:231–6. [DOI] [PubMed] [Google Scholar]
- 34. Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17:3173–80. [DOI] [PubMed] [Google Scholar]
- 35. Fukagawa T, Katai H, Saka M, et al. Significance of lavage cytology in advanced gastric cancer patients. World J Surg. 2010;34:563–8. [DOI] [PubMed] [Google Scholar]
- 36. Leake PA, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2011;15:27–37. [DOI] [PubMed] [Google Scholar]
- 37. Badgwell B, Cormier J, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15:2684–91. [DOI] [PubMed] [Google Scholar]
- 38. Kodera Y, Ito T, Ito S, et al. Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology. 2007;54:960–3. [PubMed] [Google Scholar]
- 39. Sakuramoto S, Sasako M, Yamaguchi T, et al. ACTS‐GC Group. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. [DOI] [PubMed] [Google Scholar]
- 40. Kodera Y, Ito S, Mochizuki Y, et al. A phase II study of radical surgery followed by postoperative chemotherapy with S‐1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol. 2009;35:1158–63. [DOI] [PubMed] [Google Scholar]
- 41. Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S‐1 (1 M tegafur‐0.4 M gimestat‐1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–20. [DOI] [PubMed] [Google Scholar]
- 42. Mori T, Fujiwara Y, Yano M, et al. Prevention of peritoneal metastasis of human gastric cancer cells in nude mice by S‐1, a novel oral derivative of 5‐fluorouracil. Oncology. 2003;64:176–82. [DOI] [PubMed] [Google Scholar]
- 43. Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S‐1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227–36. [DOI] [PubMed] [Google Scholar]
- 44. Martharu G, Tucker O, Alderson D. Systematic review of intraperitoneal chemotherapy for gastric cancer. Br J Surg. 2011;98:1225–35. [DOI] [PubMed] [Google Scholar]
- 45. Badgwell B, Blum M, Das P, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis of positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24:3338–44. [DOI] [PubMed] [Google Scholar]
- 46. Van der Kaaij RT, Braam HJ, Boot H, et al. Treatment of peritoneal dissemination in stomach cancer patients with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC): rationale and design of the PERISCOPE study. JMIR Res Protoc. 2017;13:e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer. 1997;79:884–91. [DOI] [PubMed] [Google Scholar]
- 48. Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139:20–6. [DOI] [PubMed] [Google Scholar]
- 49. Sugarbaker PH. Management of peritoneal metastases‐Basic concepts. J BUON. 2015;20(Suppl 1):S1–11. [PubMed] [Google Scholar]
- 50. Miyashiro I, Furukawa H, Sasako M, et al. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa‐positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG 9206‐2. Gastric Cancer. 2011;14:212–8. [DOI] [PubMed] [Google Scholar]
- 51. Takahashi N, Kanda M, Yoshikawa T, et al. A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: final results of the INPACT trial. Gastric Cancer. 2018;1–10. 10.1007/s10120-018-0817-y [DOI] [PubMed] [Google Scholar]
- 52. Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S‐1 versus cisplatin plus S‐1 in gastric cancer patients with peritoneal metastasis: PHOENIX‐GC trial. J Clin Oncol. 2018;36:1922–9. [DOI] [PubMed] [Google Scholar]
- 53. Armstrong DK, Bundy B, Wenzel K, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. [DOI] [PubMed] [Google Scholar]
- 54. Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review with a focus on practical aspects of treatment. J Clin Oncol. 2006;24:988–94. [DOI] [PubMed] [Google Scholar]
- 55. Ohashi N, Kodera Y, Nakanishi H, et al. Efficacy of intraperitoneal chemotherapy with paclitaxel targeting peritoneal micrometastasis as revealed by GFP‐tagged human gastric cancer cell lines in nude mice. Int J Oncol. 2005;27:637–44. [PubMed] [Google Scholar]
- 56. Kodera Y, Takahashi N, Yoshikawa T, et al. Feasibility of weekly intraperitoneal versus intravenous paclitaxel therapy delivered from the day of radical surgery for gastric cancer: a preliminary safety analysis of the INPACT study, a randomized controlled trial. Gastrc Cancer. 2017;20:190–9. [DOI] [PubMed] [Google Scholar]
- 57. Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S‐1 for advanced gastric cancer. Oncology. 2009;76:311–4. [DOI] [PubMed] [Google Scholar]
- 58. Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S‐1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. [DOI] [PubMed] [Google Scholar]
- 59. Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single‐non‐curative factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18. [DOI] [PubMed] [Google Scholar]
- 60. Grass F, Vuagniaux A, Teixiera‐Farinha H, Lehmann K, Demartines N, Huebner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104:669–78. [DOI] [PubMed] [Google Scholar]


