ABSTRACT
The survival of people living with human immunodeficiency virus (HIV) has increased markedly since the advent of antiretroviral therapy (ART). However, other morbidities have emerged, including osteoporosis. The estimated incidence of fractures at any site in people living with HIV ranges from 0.1 per 1000 person‐years to 8.4 per 1000 person‐years: at least twice that of people without HIV. This increased risk seems to be related to HIV itself and its treatment. Risk factors for bone disease in HIV‐positive (HIV+) subjects include both classical risk factors for osteoporosis and fracture and factors linked to HIV itself, such as inflammation, reconstitution syndrome, low CD4, ART, and co‐infection with hepatitis B and C viruses. The risk of fractures in these individuals can be at least partially assessed by measurement of BMD and the Fracture Risk Assessment Tool (FRAX™). Only alendronate and zoledronic acid have been studied in HIV+ individuals; both show beneficial effects on BMD, although data on fracture reduction are not available. © 2018 The Authors. JBMR Plus Published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Keywords: Fracture, HIV, AIDS, Osteoporosis
Introduction
The first cases of acquired immunodeficiency syndrome (AIDS) were described at the beginning of the 1980s. Infection with the immunodeficiency virus (HIV) presented, although varying in some cases, a typical evolution. In the early days, primary infection was characterized by a cellular and humoral immune response against the virus, followed by a prolonged period of clinical latency—in which the patient remained virtually asymptomatic—and subsequently by the appearance of clinical signs and symptoms of the disease. The mean time between the clinical onset of the disease and death was approximately 2 years.1 With the advent of antiretroviral therapy (ART) in the mid‐1990s, there was a dramatic decline in morbidity and mortality2, 3; currently, the life expectancy of HIV‐positive (HIV+) subjects who are diagnosed and treated promptly is close to that of HIV‐negative (HIV−) subjects.4, 5
As life expectancy in people living with HIV has increased, a number of comorbidities have become apparent; many of these are common in the general population, but develop prematurely in HIV‐infected individuals. The skeleton is one of many tissues affected by HIV infection and its treatment. Although the etiology of bone changes remains incompletely understood, prospective and cross‐sectional studies demonstrate that HIV+ patients have lower BMD and increased fracture risk compared with the general population.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The aim of this review is to describe what is known about the epidemiology, pathogenesis, pathophysiology, and management of bone disease in people living with HIV.
The Hidden Burden of Fractures in HIV‐Positive Subjects
An increase in the incidence of fractures in HIV+ individuals was initially reported at the end of the last decade. Since then, several studies have described the incidence of fractures in people living with HIV (Table 1).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
Table 1.
Characteristics of the Studies That Evaluated Fracture Frequency and Fracture Risk in People Living With HIV
| Author | Year | Study design | Site | Age (years) | Male gender (%) | HAART (%) | Outcome |
|---|---|---|---|---|---|---|---|
| Arnsten et al.6 | 2007 | Cohort | USA | 55 | 100 | – | Fracture incidence |
| Battalora et al.7 | 2016 | Cohort | USA | 43 (36–49) | 83.2 | 96.1 | Fracture incidence |
| Bedimo et al.8 | 2012 | Cohort | USA | 18–70+ | 98 | 69.4 | Fracture incidence |
| Borges et al.9 | 2017 | Cohort | Europe, Argentina, Israel | 41 | 75 | 90 | Fracture incidence |
| Collin et al.10 | 2009 | Cohort | France | 36 | 77.2 | 100 | Fracture incidence |
| Gallant et al.11 | 2004 | RCT | South America, Europe, USA | 36 | 73.9 | 100 | Fracture incidence |
| Gedmintas et al.12 | 2017 | Cohort | USA | 43 | 72 | 100 | Fracture incidence |
| Guaraldi et al.13 | 2011 | Case‐control | Italy | 46 | 63 | – | Risk of fracture |
| Güerri‐Fernandez et al.14 | 2013 | Cohort | Spain | 50 | 75.3 | – | Risk of fracture |
| Hansen et al.15 | 2012 | Cohort | Denmark | 37(31–45) | 76 | 78 | Risk of fracture |
| Hasse et al.16 | 2011 | Cohort | Swiss | 45 (39–51) | 70.8 | 85.1 | Fracture incidence |
| Kurita et al.17 | 2014 | Cohort | Japan | 15–81 | 92.8 | 65.9 | Fracture incidence |
| Mazzotta et al.19 | 2015 | Cross‐sectional | Italy | 44 | 70.6 | 79.7 | Fracture prevalence |
| Martin et al.18 | 2009 | RCT | Australia | 45 | 97.5 | 100 | Major fracture incidence |
| Mundy et al.20 | 2012 | Case‐control | USA | 40 | 71 | 50 | Risk of fractures in HIV‐treated people |
| Peters et al.21 | 2013 | Case‐control | UK | 46 | 60 | 85 | Risk of fractures |
| Prieto‐Alhambra et al.22 | 2014 | Case‐control | Denmark | 43 | 48.2 | – | Risk of fractures |
| Prior et al.23 | 2007 | Case‐control | Canada | 38 | 100 | 72.5 | Risk of fractures |
| Sharma et al.24 | 2015 | Cohort | USA | 40 (34–46) | 0 | 63 | Risk of fractures |
| Short et al.25 | 2014 | Cross‐sectional | UK | 45 (38–51) | 100 | 78 | Fracture prevalence |
| Triant et al.26 | 2008 | Case‐control | USA | 20–79 | 65.2 | – | Risk of fractures |
| Womack et al.27 | 2011 | Cohort | USA | 53 (48–61) | 100 | 75 | Increased risk of fractures |
| Yang et al.28 | 2012 | Cohort | Taiwan | <20–>60 | 76.9–90.1 | – | Orthopedic injury incidence |
| Yin et al.29 | 2012 | Cohort | USA | 39 (33–45) | 83 | 99.7 | Fracture incidence |
| Yin et al.30 | 2010 | Cross‐sectional | USA | 56 | 0 | 79.3 | Prevalence of fractures |
| Yong et al.31 | 2011 | Case‐control | Australia | 49.8 | 88.5 | 80.3 | Fracture incidence |
| Young et al.32 | 2011 | Cohort | USA | 40 (34–46) | 79 | 72.7 | Fracture incidence |
HIV = human immunodeficiency virus; HAART = highly active antiretroviral therapy; – = information not given; RCT = randomized clinical trial; ICD = International Code of Diseases.
The reported incidence of fractures at any skeletal site in people living with HIV ranges from 0.1 fractures per 1000 person‐years to 8.4 fractures per 1000 person‐years (Table 2).6, 7, 8, 9, 10, 11, 12, 14, 15, 16, 17, 29, 31, 32 However, it is important to note that most of the studies were carried out in men with a mean age of between 36 and 56 years (Table 1).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 Men in this age group usually have a low frequency of fractures, so comparison with an age and sex‐matched population is important. Shiau and colleagues conducted a systematic review and meta‐analysis in 2012.33 They found a crude incidence ratio of 1.58 (95% CI, 1.25 to 2.00) for fracture at any site in HIV+ individuals when compared with HIV− controls.33 As the aim of Shiau et al.'s meta‐analysis was to evaluate the incidence of fractures and not the risk of fractures, they included only cohort studies. Furthermore, after this meta‐analysis,33 more studies have been published.14, 21, 22 Our group has also performed a new meta‐analysis to evaluate the risk of fractures in people living with HIV for this review (see Supporting Information online for protocol), which included 10 studies. The odds ratio (OR) for fracture in people living with HIV was 2.17 (95% CI, 1.29 to 3.66; Fig. 1).
Table 2.
Incidence of Fracture at Any Site by Cohort
| Cohort | Incident fracture a | 95% CI |
|---|---|---|
| Arnsten et al., 20076 | 3.1 | 1.9–4.6 |
| Battalora et al., 20167 | 8.4 | 6.8–10.3 |
| Bedimo et al., 20128 | 0.3 | 0.3–0.3 |
| Borges et al., 20179 | 4.2 | 3.8–4.6 |
| Collin et al., 200910 | 0.3 | 0.1–0.9 |
| Gallant et al., 200411 | 1 | 0.6–1.7 |
| Gedmintas et al., 201712 | 2.2 | 1.9–2.5 |
| Guerri‐Fernandez et al., 201314 | 0.8 | 0.3–1.6 |
| Hansen et al., 201215 | 2.1 | 2.0–2.2 |
| Hasse et al., 201116 | 0.7 | 0.6–0.8 |
| Kurita et al., 201417 | 0.1 | 0.0–0.3 |
| Yin et al., 201229 | 0.1 | 0.1–0.1 |
| Yong et al., 201131 | 0.5 | 0.4–1.6 |
| Young et al., 201132 | 0.3 | 0.2–0.3 |
Per 1000 persons/years.
Figure 1.
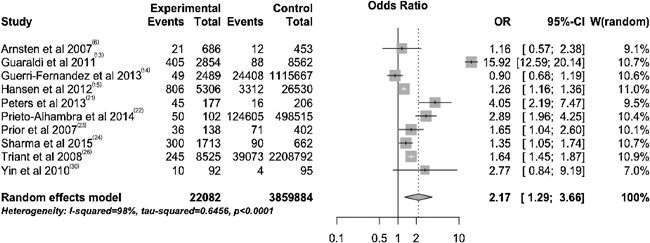
Forest plot of the odds ratio of total fractures in HIV‐positive subjects.
Recent studies have also reported an increased risk of vertebral fractures in HIV+ subjects.24, 27, 30, 31, 32, 34 In a systematic review and meta‐analysis, Ilha et al. reported that the prevalence of vertebral fractures was 11.1% (95% CI, 4.5 to 25.0).35 As in HIV− individuals, the prevalence of clinical vertebral fractures was lower, 3.9% (95% CI, 0.9 to 15.8) than the prevalence of morphometric vertebral fractures, 20.2% (95% CI, 15.7 to 25.6), when evaluated by X‐ray.35 These findings suggest that, as in the general population, most spine fractures in people living with HIV do not come to clinical attention. The risk of vertebral fractures in these individuals was 2.30 times greater than the corresponding risk in the general population (OR = 2.30; 95% CI, 1.37 to 3.85).35
Bone Mineral Density and Bone Quality in HIV‐Positive Subjects
Low BMD in people living with HIV has been consistently reported.36, 37, 38 The prevalence of osteoporosis in both the lumbar spine and the hip is twice as high as in people living without HIV.38 Furthermore, in HIV+ individuals treated with ART, this frequency increases by threefold when compared with HIV− controls.38
Although low BMD is well documented, little is known about the effects of HIV on bone microstructure and bone quality. Changes in trabecular and cortical bone structure have been described in premenopausal39 and postmenopausal women,40 as well as in young41 and middle‐aged men.42 Calmi and colleagues found a decrease in the trabecular number and trabecular density in the tibial bone of HIV+ women using ART when compared with HIV− women.39 They also found a decrease in the cortical density of the radius of HIV+ women when compared with controls.39 In postmenopausal African American and Hispanic women, the cortical area and thickness of the tibia were approximately 12% lower in HIV+ than in HIV− individuals.40 Another study in HIV+ young men infected in childhood or adolescence found similar results, with a decrease in cortical and trabecular thickness of the radius and tibia when compared with HIV− men of the same age.41 Similarly, Tan and colleagues described a decrease in cortical thickness of the tibia and radius in HIV+ middle‐aged men.42
Bone quality is difficult to assess in clinical studies; therefore, such data are scarce. Guerri‐Fernandez and colleagues used the microindentation technique to evaluate the material properties of bone at the tissue level in 50 HIV+ individuals with a mean age of approximately 37 years.43 These individuals had a lower bone material strength index (BMSi) when compared with matched controls.43 Yin and colleagues evaluated bone strength in young men using finite element analysis41 and reported a 14% to 17% decrease in bone stiffness in HIV+ subjects.41
Bone and Antiretroviral Therapy
The initiation of ART is associated with a loss of 2% to 6% of BMD at the hip and the spine.44, 45, 46, 47, 48, 49 This bone loss appears to stabilize at 24 months after antiretroviral initiation,44, 45, 48 with a subsequent plateau or even increase after this period.44, 45, 48 The longer‐term effects of ART are unknown because the longest follow‐up extends to only 72 months.38 Different forms of ART vary in their effects on bone. Regimens, including a nucleotide reverse transcriptase inhibitor (TDF) and protease inhibitors, have been described as the most deleterious.8, 9 On the other hand, the new integrase inhibitors seem to have a better bone profile.45, 50, 51, 52 Most studies describe a low rate of bone loss, or even bone gain, with raltegravir or dolutegravir.45, 50, 51, 52
Even within the same class of antiretroviral drugs the effect on bone may vary. A newer form of tenofovir, tenofovir alafenamide, has been associated with lower BMD loss than with TDF.53 Furthermore, in the ASSERT study (Study of Once‐Daily Abacavir/Lamivudine Versus Tenofovir/Emtricitabine, Administered With Efavirenz in Antiretroviral‐Naive, HIV‐1 Infected Adult Subjects) a greater decrease in BMD was shown in subjects treated with tenofovir–emtricitabine when compared with abacavir–lamivudine after 96 weeks of treatment.44 It is important to note, however, that both ART regimens were associated with bone loss.44 Recently, Guerri‐Fernandez and colleagues reported lower BMD in long‐term users of tenofovir compared with long‐term users of abacavir in a small cross‐sectional study.54 Nonetheless, they found no differences in trabecular bone score (TBS) or microindentation‐derived BMSi between the two groups.54
At present, the only associations reported between specific forms of ART and fracture risk are for TDF and protease inhibitors.8, 9 It is important to note that most of the studies that evaluated the incidence of fractures in people living with HIV had insufficient power to evaluate the association between specific antiretrovirals and fractures.
Bone Turnover Markers in HIV‐Positive Subjects
Changes in bone markers have been described in several studies in HIV+ individuals.44, 45, 52, 55 The initiation of ART is associated with an increase in markers of both bone resorption and bone formation44, 45, 52; in prospective studies, this increase has been associated with a decrease in BMD.44, 45
The magnitude of the rise in markers of bone formation and resorption varies according to the ART used45, 52 and is greater in combinations that include TDF.45 The increase in turnover markers peaks at 48 weeks after initiation of ART,44, 52 decreasing or stabilizing afterwards.52 This time course is similar to that seen for immune reconstitution, as described by Ofotokun and colleagues.55
The SMART Body Composition substudy, an arm of the Strategies for Management of Antiretroviral Therapy (SMART) trial, reported lower serum levels of bone‐specific alkaline phosphatase (bALP), osteocalcin, N‐terminal propeptide of type 1 procollagen (P1NP), and C‐terminal telopeptide of type 1 collagen (CTx) in individuals that received intermittent ART compared with those who received continuous ART at the end of 12 months.56
Although current evidence suggests that the effect of ART on bone turnover markers may become attenuated with longer duration of therapy,52 little is known about longer‐term changes. In the study by Yin and colleagues, which included African American and Hispanic postmenopausal women, elevated levels of bone markers were present even in those who had taken ART for more than 4 years.30
Because of the proven efficacy of ART in increasing the survival of HIV+ individuals, there have been few studies in treatment‐naive individuals. Moreover, the studies that have been performed in untreated subjects have mainly included those with a low or undetectable viral load, making it difficult to assess the impact of HIV per se on bone metabolism. In a study carried out in 1995, a histomorphometric analysis of iliac crest biopsy samples from 22 untreated HIV+ adults showed a significant reduction in bone turnover.57 In another small study of treatment‐naïve young subjects, values for bone resorption and formation markers were within those expected for the general population.58 Likewise, in postmenopausal HIV+ women without treatment in the Yin et al. study, bone markers were at levels similar to those in HIV− controls.30
Factors Associated With Bone Metabolic Disease in HIV Subjects
Many risk factors for osteoporosis and fracture have been identified in HIV+ subjects. Some of these are common to the general population, whereas others are related to HIV itself (Table 3).
Table 3.
Factors Associated With Bone Disease and Fracture in People Living With HIV
| Factors in common with the general population | Factors related to the HIV |
|---|---|
| Aging6, 32, 38 | Chronic inflammation55 |
| Previous fractures38, 59 | Reconstitution syndrome55 |
| Low BMI32, 37, 60, 61 | ART use8, 15, 20, 27 |
| Tobacco use38, 62 | Co‐infection with hepatitis B79 |
| Alcohol abuse63 | Co‐infection with hepatitis C33, 80, 81 |
| Glucocorticoid use63 | Low CD431, 32, 38, 81 |
| Anticonvulsant use59 | AIDS‐defining disease81 |
| Postmenopausal status6, 24, 26 | |
| Hypogonadism38, 64 | |
| Vitamin D deficiency24, 65, 66, 67 | |
| White race8, 27 | |
| Diabetes mellitus59 | |
| Frailty69 | |
| Sarcopenia38 | |
| Selective serotonin reuptake inhibitors70 | |
| Comorbidities72 | |
| Falls59 | |
| Renal disease59 |
HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome; ART = antiretroviral therapy.
The presence of previous fractures,38, 59 a low BMI,32, 37, 60, 61 tobacco38, 62 and alcohol abuse,63 the use of glucocorticoids,63 the use of anticonvulsants,59 postmenopausal status,6, 24, 26 hypogonadism,38, 64 vitamin D deficiency,24, 65, 66, 67 white race,8, 27 renal disease,59, 68 falls,59 diabetes mellitus (DM),59 and aging6, 32, 38 are factors classically associated with fragility fracture and have also been described in HIV+ subjects. Other factors most recently associated with an increased risk of fractures in non‐HIV subjects such as frailty,69 sarcopenia,38 selective serotonin reuptake inhibitors (SSRIs),70 cardiovascular disease,68 cancer,68 liver disease,68 neurocognitive impairment,71 and other comorbidities72 have also been reported in HIV+ individuals.
Some of the risk factors commonly present in the general population appear to have increased frequency in people living with HIV. The incidence of type 2 DM is at least 1.4 times higher in this population, and it seems to occur at an earlier age when compared with the general population.73, 74 In addition, HIV+ men may have low testosterone levels,75 and the frequency of hypogonadism is increased in people living with HIV.64, 76 There is also an increased prevalence of vitamin D deficiency when compared to the general population,65, 66 possibly due to the presence of several risk factors for vitamin D deficiency, including the use of efavirenz (a non‐nucleoside reverse transcriptase inhibitor)77 or protease inhibitors,65, 78 anticonvulsant therapy, kidney disease, and liver disease.66
Factors specifically associated with HIV are the presence of chronic inflammation,55 reconstitution syndrome,55 the use of ART,8, 15, 20, 27 co‐infection with hepatitis B79 or C,33, 80, 81 low CD4,31, 32, 38, 81 or an AIDS‐defining disease.81 Co‐infection with hepatitis C virus is associated with a one‐ to twofold increase in the risk of fractures when compared with monoinfection with HIV.80 Similarly, treatment for co‐infection with hepatitis B virus appears to increase the risk of fractures in these individuals.79 CD4 counts below 200 cells/mm3 have been associated with a higher incidence of fractures in HIV+ subjects in cohort studies.31, 32
Other factors, such as cocaine use, injectable drug abuse, the use of opioids, methadone maintenance therapy, hyperparathyroidism, and growth hormone deficiency, have also been associated with fractures in HIV+ individuals.59, 63, 72
People living with HIV have an increased risk both of fragility fractures and traumatic fractures. The latter may be associated with factors such as substance abuse, high‐risk behavior increasing the risk of physical injury, and neurocognitive dysfunction, among others.68, 71
Mechanisms of Bone Disease in HIV‐Positive Subjects
The factors responsible for bone disease in HIV+ individuals have not been fully established. Chronic inflammation activates inflammatory cytokines and TNF alpha, resulting in stimulation of RANKL production and increased bone resorption. Hilleman and colleagues suggest that the dysregulation of B cells present in these individuals may also contribute to bone loss,72, 82 postulating an imbalance between B‐lymphocytic expression of RANKL and osteoprotegerin. Furthermore, the imbalance that occurs in the immune reconstitution syndrome may also contribute to bone loss in HIV subjects soon after ART initiation.72, 82
Little is known about drug‐specific mechanisms of antiretroviral bone loss. TDF is associated with the increased renal loss of phosphate and secondary hyperparathyroidism.70, 83, 84 In extreme cases, Fanconi's syndrome has been described.85 As mentioned earlier, efavirenz and protease inhibitors affect vitamin D metabolism.(78,86, 87, 88) Efavirenz acts on cytochrome P450 enzymes, decreasing the expression of CYP2R1 enzyme and increasing the expression of CYP24, which plays a role in the conversion of vitamin D to 25‐ hydroxyvitamin D [25(OH)D] and to inactive metabolites.86, 87, 88 In addition, protease inhibitors inhibit the action of 25‐hydroxylase and 1‐alpha‐hydroxylase enzymes.78, 86
It is speculated that HIV itself might be associated with some bone toxicity. Although there is no evidence of infection of bone cells by the virus, some viral proteins appear to have a negative interaction with bone metabolism.89, 90, 91 The HIV‐1 gp 120, and the Tat, Nef, Rev, and Vpr proteins have been shown to have some adverse effects on bone in experimental studies.89, 90, 91, 92, 93 The HIV gp 120 protein appears to interact negatively with osteoblasts, stimulating their apoptosis.89, 90, 91, 92, 94 This protein also appears to reduce the activity of alkaline phosphatase, reduce bone mineralization, and interfere with the expression of runt‐related transcription factor 2 (RUNX‐2).91 Tat and Nef proteins decrease the number of progenitor cells in the bone marrow with the potential to differentiate into osteoblasts.93 Vpr protein increases RANKL expression in peripheral blood monocytes,91 whereas Tat and Rev proteins divert the differentiation of these monocytes towards osteoclasts.94
Fracture Risk Assessment in HIV‐Positive Subjects
As in the general population, the risk of fractures in people living with HIV increases as BMD decreases. There is a threefold increase in the risk of fractures in middle‐aged HIV+ men with a BMD T‐score ≤1.6 In an analysis of the HIV Outpatient Study (HOPS) and Study to Understand the Natural History of HIV/AIDS (SUN Study), Batalora and colleagues found that HIV+ subjects with osteoporosis had a HR of 2.4 (95% CI, 2:02 to 8:01) of suffering a fracture compared with HIV− subjects with normal BMD.7
The estimation of fracture probability in people living with HIV using the FRAX (Fracture Risk Assessment Tool) algorithm has been evaluated in several studies. This tool appears to underestimate the risk of fracture in HIV+ subjects. In the Veterans Aging Study Virtual Cohort (VACS‐VC), which included 24,451 men over 50 years of age, FRAX was less accurate in predicting fractures in HIV+ individuals when compared with HIV− individuals.95 In this study, the FRAX algorithm was modified and the variables secondary osteoporosis and family history of hip fracture were not included, which may have contributed to the underperformance of FRAX. However, when the HIV+ variable was included in the calculation as a cause of secondary osteoporosis, the accuracy of the instrument was increased.95
The utility of TBS in fracture prediction in people living with HIV has been assessed in one study. Ciullini and colleagues34 studied the association between vertebral fractures and TBS in 141 HIV+ individuals. The subjects in the lowest quartile of TBS had a higher prevalence of vertebral fractures.34 This observation is interesting because BMD appears to underestimate the incidence of vertebral fractures in the same way as in non‐HIV subjects.61
Based on the currently available evidence, both the European AIDS Clinical Society (EACS)96 and the Osteo Renal Exchange Program (OREP)97 recommend that fracture risk assessment should be performed in all HIV‐infected individuals over 40 years of age using the FRAX algorithm. According to the EACS, those individuals with an estimated 10‐year probability of major osteoporotic fractures ≥20% should undergo BMD measurement. On the other hand, OREP recommends measuring BMD in all HIV+ individuals with an estimated risk of major osteoporotic fractures ≥10%.96, 97 Furthermore, the EACS recommends that in individuals with other risk factors added to HIV—postmenopausal women, men older than 50 years, previous fracture, major risk of falls, clinical hypogonadism, and the use of more than 5 mg/day of oral glucocorticoids for more than 3 months—a DXA scan should be performed.96
Vertebral fracture assessment is recommended by the EACS in those subjects with low bone mass at the spine or osteoporosis at any site, loss of height, or kyphosis. It can be performed with a lateral X‐ray or a bone densitometry scan.96 Due to the recent data showing the increased risk of spine fractures in people living with HIV, vertebral fracture assessment should be considered in high‐risk subjects.
Morbidity and Mortality of Bone Disease in HIV Subjects
Little is known about the real impact of bone disease in people living with HIV. Fractures are associated with a poorer quality of life, loss of independence, hospitalization, and increased mortality in the general population. In a study conducted in Taiwan using data from the National Health Insurance (NHI) Program, which covers about 98% of the population, approximately 80% of fractures in HIV+ people required surgical intervention.28 There are no studies on mortality and quality of life in HIV+ individuals after a fracture. Nonetheless, just as in HIV− subjects, HIV+ individuals who suffer a fracture are at increased risk of a further fracture.38
Although there are no studies on the impact of vertebral fractures in people living with HIV, these are associated with worsening of quality of life, functional limitation, and reduction of lung capacity in the general population. Previous vertebral fractures are also a strong risk factor for future fractures.
Management
As in the general population, the management of osteoporosis and fracture prevention in individuals living with HIV should include a consideration of nonpharmacological and pharmacological measures. A healthy lifestyle should be recommended and a falls risk assessment should be performed and preventive measures taken when appropriate.
Although not studied in HIV+ individuals, an assessment of the risk of falls and measures to prevent them may be beneficial. Avoiding alcohol abuse decreases the risk of falls and also has beneficial effects on bone.98 Smoking cessation should be recommended in all individuals. A diet rich in calcium, fruits, and vegetables has been associated with a lower risk of fractures in the general population,99, 100 and should be recommended for people living with HIV. In addition to a higher fracture risk, these individuals are at increased risk of developing diabetes.73, 74 Therefore, the recommendation of a balanced diet can have multiple benefits in these subjects.
Studies addressing the effect of weight‐bearing exercise on HIV+ subjects are scarce. Santos and colleagues studied the effect of strength training in 20 HIV+ ART‐treated individuals with lipodystrophy.101 They found an increase in BMD after 12 weeks of training.101 These findings, in conjunction with the evidence found in the general population,98 corroborate the recommendation of physical exercise in these subjects.
People living with HIV are at increased risk of vitamin D deficiency. The measurement of serum 25‐hydroxyvitamin D [25(OH)D] levels should be performed in high‐risk HIV+ individuals. In subjects with serum levels below 20 ng/mL, the EACS recommends measurement of serum parathyroid hormone (PTH), calcium, phosphorus, and alkaline phosphatase levels.96 In vitamin D‐deficient individuals, vitamin D replacement with a loading dose of 10,000 IU/day for 8 to 10 weeks is recommended, followed by a maintenance dose of 800 to 2000 IU/day after achieving the goal of treatment.96 The recommended target serum level of 25(OH)D is over 20 ng/mL, with normalization of serum PTH levels. In addition, they recommend calcium supplementation in subjects with inadequate dietary intake.96
Other causes of secondary osteoporosis should be excluded.97 As the presence of hypogonadism is common in men living with HIV, it should be assessed with a thorough clinical history and careful physical examination. In those whom clinical evaluation suggests the presence of hypogonadism, further investigations should be performed and, if indicated, treatment established.
An early diagnosis of HIV with immediate initiation of ART may help to reduce bone disease, although this remains to be tested. In people at high risk of fracture, it may be prudent to avoid ART regimens containing TDF. Although switching to antiretroviral drugs, such as integrase inhibitors and tenofovir alafenamide, reduces adverse skeletal effects, the long‐term effects of this approach on viral resistance is not known.44, 45, 50, 51, 52, 53, 54 For this reason, any change in ART should be evaluated in conjunction with the patient's HIV treatment team.
Both alendronate and zoledronic acid are effective in reducing bone loss in people living with HIV.102, 103, 104, 105, 106, 107, 108, 109, 110 In a meta‐analysis conducted by Pinzone and colleagues, there was less of a decrease in bone mass in both the hip and lumbar spine of HIV+ individuals treated with bisphosphonates when compared with HIV+ individuals receiving placebo.111 The mean difference between the groups after 96 months of treatment was 6.76% (95% CI, 4.98 to 8.54) in the lumbar spine and 3.2% (95% CI, 1.52 to 4.88) in the hip.111 Although there are no studies on the antifracture efficacy of these drugs, extrapolation from evidence for their effects in the general population appears reasonable. Some studies using zoledronic acid suggest that BMD gains are maintained with administration at approximately two yearly intervals.107, 110 In another study, Bolland and colleagues studied the effect of zoledronic acid on 35 HIV+ subjects using ART 5 years after the second dose of the drug. They found a persistence of beneficial effects on bone mass without evidence of significant adverse effects in these individuals.107
There are no studies evaluating the effects of denosumab in HIV+ people. Some authors report theoretical concerns about an increased risk of infections associated with this drug.112 However, concerns about the risk of serious infections associated with denosumab therapy have not been confirmed in the general population.113 Furthermore, denosumab has been used in other populations that are considered to be immunosuppressed, for example, subjects with rheumatoid arthritis or cancer, without an increase in risk of infections.114, 115 The use of bone‐forming medications in people living with HIV has also not been studied, although there is one case report describing the use of teriparatide in a 70‐year‐old HIV+ individual with a vertebral fracture.116 Thus, at present, bisphosphonates provide the first‐line treatment of HIV+ subjects at increased risk of fracture; in those with contraindications or intolerance, teriparatide and denosumab provide alternatives. Adherence to alendronate is poor, with up to 60% of women stopping this drug after one year117, 118; as zoledronic acid is administered once yearly by intravenous infusion, it may be the preferred option.
Conclusion
People living with HIV have a fracture risk twice that of people without HIV. The pathogenesis of increased bone fragility is multifactorial and includes both traditional and HIV‐specific risk factors. Fracture risk should be assessed in HIV+ individuals using clinical risk factors and, where indicated, measurement of BMD. Lifestyle measures to optimize bone health should be recommended in all people living with HIV; bisphosphonates are the first‐line treatment of those at increased fracture risk. Further studies are required to establish whether early identification of HIV+ individuals and prompt initiation of ART reduces the risk of bone disease.
Disclosures
All authors state that they have no conflict of interest regarding this manuscript. JEC has received advisory and speaking fees from Gilead and speaking fees from Amgen.
Supporting information
Supporting Appendix S1.
Acknowledgments
The Federal University of Santa Maria sponsored the meta‐analysis. The sponsor had no role in the design, conduct, or analysis of our study or in the decision to submit this manuscript for publication.
Authors’ roles: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Premaor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Pantaleo G, Graziosi C, Fauci AS. The immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. Feb 1993;328(5):327–35. [DOI] [PubMed] [Google Scholar]
- 2. Palella FJ, Jr. , Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. Mar 1998;338(13):853–60. [DOI] [PubMed] [Google Scholar]
- 3. Fang CT, Chang YY, Hsu HM, et al. Life expectancy of patients with newly‐diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM. Feb 2007;100(2):97–105. [DOI] [PubMed] [Google Scholar]
- 4. van Sighem AI, Gras LA, Reiss P, et al. Life expectancy of recently diagnosed asymptomatic HIV‐infected patients approaches that of uninfected individuals. AIDS. Jun 2010;24(10):1527–35. [DOI] [PubMed] [Google Scholar]
- 5.Collaboration of Observational HIVEREiE, Lewden C, Bouteloup V, et al. All‐cause mortality in treated HIV‐infected adults with CD4 >/=500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. Apr 2012;41(2):433–45. [DOI] [PubMed] [Google Scholar]
- 6. Arnsten JH, Freeman R, Howard AA, Floris‐Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21(5):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battalora L, Buchacz K, Armon C, et al. Low bone mineral density and risk of incident fracture in HIV‐infected adults. Antiviral Therapy. 2016;21(1):45–54. [DOI] [PubMed] [Google Scholar]
- 8. Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26(7):825 –31. [DOI] [PubMed] [Google Scholar]
- 9. Borges AH, Hoy J, Florence E, Sedlacek D, et al. Antiretrovirals, fractures, and osteonecrosis in a large international HIV cohort. Clin Infect Dis. May 2017;64(10):1413–21. [DOI] [PubMed] [Google Scholar]
- 10. Collin F, Duval X, Moing VL, et al. Ten‐year incidence and risk factors of bone fractures in a cohort of treated HIV1‐infected adults. AIDS. 2009;23(8):1021–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral‐naive patients: a 3‐year randomized trial. J Am Med Assoc. 2004;292(2):191–201. [DOI] [PubMed] [Google Scholar]
- 12. Gedmintas L, Wright EA, Dong Y, et al. Factors associated with fractures in HIV‐infected persons: which factors matter? Osteoporos Int. 2017. Jan; 28(1):239–244. DOI: . Epub 2016 Jul 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guaraldi G, Orlando G, Zona S, et al. Premature age‐related comorbidities among HIV‐infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–6. [DOI] [PubMed] [Google Scholar]
- 14. Güerri‐Fernandez R, Vestergaard P, Carbonell C, et al. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population‐based cohort study. J Bone Min Res. 2013;28(6):1259–63. [DOI] [PubMed] [Google Scholar]
- 15. Hansen ABE, Gerstoft J, Kronborg G, et al. Incidence of low and high‐energy fractures in persons with and without HIV infection: a Danish population‐based cohort study. AIDS. 2012;26(3):285–93. [DOI] [PubMed] [Google Scholar]
- 16. Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV‐infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 17. Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of Epzicom® (lamivudine/abacavir sulfate) in post‐marketing surveillance in Japan. Pharmacoepidemiol Drug Saf. 2014;23(4):372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir‐emtricitabine or abacavir‐lamivudine: a randomized, 96‐week trial. Clin Infect Dis. 2009;49(10):1591–601. [DOI] [PubMed] [Google Scholar]
- 19. Mazzotta E, Ursini T, Agostinone A, et al. Prevalence and predictors of low bone mineral density and fragility fractures among HIV‐infected patients at one Italian center after universal DXA screening: sensitivity and specificity of current guidelines on bone mineral density management. AIDS Patient Care STDS. Apr 2015;29(4):169–80. [DOI] [PubMed] [Google Scholar]
- 20. Mundy LM, Youk AO, McComsey GA, Bowlin SJ. Overall benefit of antiretroviral treatment on the risk of fracture in HIV: nested case‐control analysis in a health‐insured population. AIDS. 2012;26(9):1073–82. [DOI] [PubMed] [Google Scholar]
- 21. Peters BS, Perry M, Wierzbicki AS, et al. A cross‐sectional randomised study of fracture risk in people with HIV infection in the PROBONO 1 study. PLoS One. 2013;8(10):e78040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prieto‐Alhambra D, Güerri‐Fernández R, De Vries F, et al. HIV infection and its association with an excess risk of clinical fractures: a nationwide case‐control study. J Acq Immune Def Syn. 2014;66(1):90–5. [DOI] [PubMed] [Google Scholar]
- 23. Prior J, Burdge D, Maan E, et al. Fragility fractures and bone mineral density in HIV positive women: a case‐control population‐based study. Osteoporos Int. 2007;18(10):1345–53. [DOI] [PubMed] [Google Scholar]
- 24. Sharma A, Shi Q, Hoover DR, et al. Increased fracture incidence in middle‐aged HIV‐infected and HIV‐uninfected women: updated results from the women's interagency HIV study. J Acq Imm Def Syn. 2015;70(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Short CE, Shaw SG, Fisher MJ, Gilleece YC, Walker‐Bone K. Comparison of peripheral forearm DXA and clinical risk factor screening using FRAX® to assess the risk of HIV‐ associated low bone mass: a cross‐sectional study. Arch Osteoporos. 2014;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)‐infected versus non‐HIV‐infected patients in a large U.S. healthcare system. J Clin Endocrinol Metabol. 2008;93(9):3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6(2):e17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang NP, Lee YH, Chang NT, et al. Treatment incidence of orthopedic injuries among HIV‐infected subjects in Taiwan: a dynamic cohort survey, 2005–2008. HealthMED. 2012;6(8):2700–8. [Google Scholar]
- 29. Yin MT, Kendall MA, Wu X, et al. Fractures after antiretroviral initiation. AIDS. 2012;26(17):2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin MT, McMahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus‐infected women. J Clin Endocrinol Metab. 2010;95(2):620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yong MK, Elliott JH, Woolley IJ, Hoy JF. Low CD4 count is associated with an increased risk of fragility fracture in HIV‐infected patients. J Acquir Immune Defic Syndr. 2011;57(3):205–10. [DOI] [PubMed] [Google Scholar]
- 32. Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV‐infected persons in the HIV outpatient study (HOPS) compared with the US general population, 2000–2006. Clin Infec Dis. 2011;52(8):1061–8. [DOI] [PubMed] [Google Scholar]
- 33. Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV‐infected individuals: a systematic review and meta‐analysis. AIDS. Jul 2013;27(12):1949–57. Epub 2013 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ciullini L, Pennica A, Argento G, Novarini D, Teti E, Pugliese G, et al. Trabecular bone score (TBS) is associated with sub‐clinical vertebral fractures in HIV‐infected patients. J Bone Miner Metab. Jan 2018;36(1):111–8. [DOI] [PubMed] [Google Scholar]
- 35. Ilha TASH, Comin FV, Copes RM, Compston JE, Premaor MO. HIV and vertebral fractures: a systematic review and metanalysis. Scient Rep. 8: 838 DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta‐analytic review. AIDS. Nov 2006;20(17):2165–74. Epub 2006 Nov 2. [DOI] [PubMed] [Google Scholar]
- 37. Bolland MJ, Grey AB, Gamble GD, Reid IR. Low body weight mediates the relationship between HIV infection and low bone mineral density: a meta‐analysis. J Clin Endocrinol Metab. Dec 2007;92(12):4522–8. [DOI] [PubMed] [Google Scholar]
- 38. Goh SSL, Lai PSM, Tan ATB, Ponnampalavanar S. Reduced bone mineral density in human immunodeficiency virus‐infected individuals: a meta‐analysis of its prevalence and risk factors. Osteoporos Int. 2018. Mar; 29(3):595–613. DOI: . Epub 2017 Nov 20. [DOI] [PubMed] [Google Scholar]
- 39. Calmy A, Chevalley T, Delhumeau C, et al. Long‐term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos Int. Jun 2013;24(6):1843–52. Epub 2012 Nov 10. [DOI] [PubMed] [Google Scholar]
- 40. Yin MT, Shu A, Zhang CA, et al. Trabecular and cortical microarchitecture in postmenopausal HIV‐infected women. Calc Tiss Int. Jun 2013;92(6):557–65. Epub 2013 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS. Jan 2014;28(3):345–53. Epub 2013 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan DH, Raboud J, Szadkowski L, et al. Novel imaging modalities for the comparison of bone microarchitecture among HIV+ patients with and without fractures: a pilot study. HIV Clin Trials. Jan 2017;18(1):28–38. Epub 2016 Dec 14. [DOI] [PubMed] [Google Scholar]
- 43. Guerri‐Fernandez R, Molina D, Villar‐Garcia J, et al. Brief report: HIV infection is associated with worse bone material properties, independently of bone mineral density. J Acquir Immune Defic Syndr. Jul 2016;72(3):314 –8. Epub 2016 Feb 26. [DOI] [PubMed] [Google Scholar]
- 44. Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir‐lamivudine versus tenofovir‐emtricitabine in HIV‐infected adults: 48‐week results from the ASSERT study. Clin Infect Dis. Oct 2010;51(8):963 –72. Epub 2010 Sep 11. [DOI] [PubMed] [Google Scholar]
- 45. Brown TT, Moser C, Currier JS, et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis. Oct 2015;212(8):1241–9. Epub 2015 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grund B, Peng G, Gibert CL, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS. Jul 2009;23(12):1519–29. Epub 2009 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Assoumou L, Katlama C, Viard JP, et al. Changes in bone mineral density over a 2‐year period in HIV‐1‐infected men under combined antiretroviral therapy with osteopenia. AIDS. Sep 2013;27(15):2425 –30. Epub 2013 Sep 14. [DOI] [PubMed] [Google Scholar]
- 48. Haskelberg H, Hoy JF, Amin J, et al. Changes in bone turnover and bone loss in HIV‐infected patients changing treatment to tenofovir‐emtricitabine or abacavir‐lamivudine. PLoS One. 2012;7(6):e38377. Epub 2012 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral‐naive persons randomized to receive abacavir‐lamivudine or tenofovir disoproxil fumarate‐emtricitabine along with efavirenz or atazanavir‐ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. Jun 2011;203(12):1791–801. Epub 2011 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bedimo RJ, Drechsler H, Jain M, et al. The RADAR study: week 48 safety and efficacy of RAltegravir combined with boosted DARunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral‐naive patients. Impact on bone health. PLoS One. 2014;9(8):e106221. Epub 2014 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bernardino JI, Mocroft A, Mallon PW, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir‐ritonavir combined with either raltegravir or tenofovir‐emtricitabine in antiretroviral‐naive adults with HIV‐1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. Nov 2015;2(11):e464 –73. Epub 2015 Nov 2. [DOI] [PubMed] [Google Scholar]
- 52. Tebas P, Kumar P, Hicks C, et al. Greater change in bone turnover markers for efavirenz/emtricitabine/tenofovir disoproxil fumarate versus dolutegravir+ abacavir/lamivudine in antiretroviral therapy‐naive adults over 144 weeks. AIDS. Nov 2015;29(18):2459–64. Epub 2015 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV‐1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. Sep 2014;67(1):52 –8. Epub 2014 May 30. [DOI] [PubMed] [Google Scholar]
- 54. Guerri‐Fernandez R, Molina‐Morant D, Villar‐Garcia J, et al. Bone density, microarchitecture, and tissue quality after long‐term treatment with tenofovir/emtricitabine or abacavir/lamivudine. J Acquir Immune Defic Syndr. Jul 2017;75(3):322–7. Epub 2017 Apr 19. [DOI] [PubMed] [Google Scholar]
- 55. Ofotokun I, Titanji K, Vunnava A, et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS. Jan 2016;30(3):405–14. Epub 2016 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoy J, Grund B, Roediger M, et al. Interruption or deferral of antiretroviral therapy reduces markers of bone turnover compared with continuous therapy: The SMART body composition substudy. J Bone Min Res Jun 2013;28(6):1264–74. Epub 2013 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Serrano S, Marinoso ML, Soriano JC, et al. Bone remodelling in human immunodeficiency virus‐1‐infected patients. A histomorphometric study. Bone. Feb 1995;16(2):185–91. Epub 1995 Feb 1. [DOI] [PubMed] [Google Scholar]
- 58. Marques de Menezes EG, de Paula FJ, Machado AA, de Assis Pereira F, Barbosa Junior F, Navarro AM. Impact of antiretroviral therapy on bone metabolism markers in HIV‐ seropositive patients. Bone. Nov 2013;57(1):62–7. [DOI] [PubMed] [Google Scholar]
- 59. Compston J. HIV infection and bone disease. J Int Med. Oct 2016;280(4):350–8. Epub 2016 Jun 9. [DOI] [PubMed] [Google Scholar]
- 60. Grijsen ML, Vrouenraets SM, Wit FW, et al. Low bone mineral density, regardless of HIV status, in men who have sex with men. J Infect Dis. Feb 2013;207(3):386–91. Epub 2012 Nov 14. [DOI] [PubMed] [Google Scholar]
- 61. Stephens KI, Rubinsztain L, Payan J, Rentsch C, Rimland D, Tangpricha V. Dual‐energy X‐ray absorptiometry and calculated FRAX risk scores may underestimate osteoporotic fracture risk in vitamin D‐deficient veterans with HIV infection. Endocrine Pract. 2016;22(4):440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus‐infected women. J Clin Endocrinol Metab. Aug 2006;91(8):2938–45. Epub 2006 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stone B, Dockrell D, Bowman C, McCloskey E. HIV and bone disease. Arch Biochem Biophys. Nov 2010;503(1):66–77. Epub 2010 Aug 5. [DOI] [PubMed] [Google Scholar]
- 64. Wunder DM, Bersinger NA, Fux CA, et al. Hypogonadism in HIV‐1‐infected men is common and does not resolve during antiretroviral therapy. Antivir Ther. 2007;12(2):261–5. [PubMed] [Google Scholar]
- 65. Mueller NJ, Fux CA, Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy‐naive and successfully treated Swiss HIV patients. AIDS. May 2010;24(8):1127–34. Epub 2010 Feb 20. [DOI] [PubMed] [Google Scholar]
- 66. Hileman CO, Overton ET, McComsey GA. Vitamin D and bone loss in HIV. Curr Opin HIV AIDS. May 2016;11(3):277 –84. Epub 2016 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Atteritano M, Mirarchi L, Venanzi‐Rullo E, et al. Vitamin D status and the relationship with bone fragility fractures in HIV‐infected patients: a case control study. Int J Mol Sci. 2018. Jan 2; 19(1).pii: E119 DOI: 10.3390/ijms19010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non‐AIDS related morbidity. Brit Med J. Jan 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 69. Womack JA, Goulet JL, Gibert C, et al. Physiologic frailty and fragility fracture in HIV‐infected male veterans. Clin Infect Dis. May 2013;56(10):1498–504. Epub 2013 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peyriere H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr. Mar 2004;35(3):269–73. [DOI] [PubMed] [Google Scholar]
- 71. Rodriguez‐Penney AT, Iudicello JE, Riggs PK, et al. Co‐morbidities in persons infected with HIV: increased burden with older age and negative effects on health‐related quality of life. AIDS Patient Care STDS. Jan 2013;27(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hileman CO, Eckard AR, McComsey GA. Bone loss in HIV: a contemporary review. Curr Opin Endocrinol, Diabetes, Obes. Dec 2015;22(6):446–51. Epub 2015 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Samad F, Harris M, Puskas CM, et al. Incidence of diabetes mellitus and factors associated with its development in HIV‐positive patients over the age of 50. BMJ Open Diabetes Res Care. 2017;5(1):e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hernandez‐Romieu AC, Garg S, Rosenberg ES, Thompson‐Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV‐infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rochira V, Zirilli L, Orlando G, et al. Premature decline of serum total testosterone in HIV‐infected men in the HAART‐era. PLoS One. 2011;6(12):e28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rietschel P, Corcoran C, Stanley T, Basgoz N, Klibanski A, Grinspoon S. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin Infect Dis. Nov 2000;31(5):1240–4. [DOI] [PubMed] [Google Scholar]
- 77. Welz T, Childs K, Ibrahim F, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. Jul 2010;24(12):1923–8. Epub 2010 Jul 1. [DOI] [PubMed] [Google Scholar]
- 78. Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV‐protease inhibitors impair vitamin D bioactivation to 1,25‐dihydroxyvitamin D. AIDS. Mar 2003;17(4):513–20. [DOI] [PubMed] [Google Scholar]
- 79. Byrne DD, Newcomb CW, Carbonari DM, et al. Increased risk of hip fracture associated with dually treated HIV/hepatitis B virus coinfection. J Viral Hepat. Nov 2015;22(11):936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dong HV, Cortes YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: a systematic review and meta‐analysis. AIDS. Sep 2014;28(14):2119–31. Epub 2014 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gedmintas L, Wright EA, Dong Y, et al. Factors associated with fractures in HIV‐infected persons: which factors matter? Osteoporos Int. Jan 2017;28(1):239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hileman CO, Labbato DE, Storer NJ, Tangpricha V, McComsey GA. Is bone loss linked to chronic inflammation in antiretroviral‐naive HIV‐infected adults? A 48‐week matched cohort study. AIDS. Jul 2014;28(12):1759–67. Epub 2014 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Labarga P, Barreiro P, Martin‐Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. Mar 2009;23(6):689–96. [DOI] [PubMed] [Google Scholar]
- 84. Woodward CL, Hall AM, Williams IG, et al. Tenofovir‐ associated renal and bone toxicity. HIV Med. Sep 2009;10(8):482–7. [DOI] [PubMed] [Google Scholar]
- 85. Gupta SK. Tenofovir‐associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. Feb 2008;22(2):99–103. [DOI] [PubMed] [Google Scholar]
- 86. Gutierrez F, Masia M. The role of HIV and antiretroviral therapy in bone disease. AIDS Rev. Apr–Jun 2011;13(2):109–18. Epub 2011 May 19. [PubMed] [Google Scholar]
- 87. Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose‐dependent induction by efavirenz in humans. Clin Pharmacol Ther. Jul 2002;72(1):1–9. [DOI] [PubMed] [Google Scholar]
- 88. Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25‐hydroxyvitamin D. Antivir Ther. 2010;15(3):425–9. [DOI] [PubMed] [Google Scholar]
- 89. Gibellini D, Borderi M, De Crignis E, et al. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV‐1‐positive men. J Med Virol. Oct 2007;79(10):1446–54. Epub 2007 Aug 21. [DOI] [PubMed] [Google Scholar]
- 90. Cotter EJ, Malizia AP, Chew N, Powderly WG, Doran PP. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Hum Retroviruses. Dec 2007;23(12):1521–30. [DOI] [PubMed] [Google Scholar]
- 91. Fakruddin JM, Laurence J. HIV envelope gp120‐mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon‐gamma/RANKL cross‐talk. J Biol Chem. Nov 2003;278(48):48251–8. [DOI] [PubMed] [Google Scholar]
- 92. Cotter AG, Mallon PW. The effects of untreated and treated HIV infection on bone disease. Curr Opin HIV AIDS. Jan 2014;9(1):17–26. Epub 2013 Nov 23. [DOI] [PubMed] [Google Scholar]
- 93. Beaupere C, Garcia M, Larghero J, Feve B, Capeau J, Lagathu C. The HIV proteins Tat and Nef promote human bone marrow mesenchymal stem cell senescence and alter osteoblastic differentiation. Aging Cell. Aug 2015;14(4):534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chew N, Tan E, Li L, Lim R. HIV‐1 tat and rev upregulates osteoclast bone resorption. J Int AIDS Soc. 2014;17(4 Suppl 3):19724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yin MT, Shiau S, Rimland D, et al. Fracture prediction with modified‐FRAX in older HIV‐infected and uninfected men. J Acquir Immune Defic Syndr. 2016;72(5):513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.European AIDS Clinical Society. European guidelines for treatment of HIV‐positive adults in Europe 2017. Available from: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
- 97. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. Apr 2015;60(8):1242 –51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Abrahamsen B, Brask‐Lindemann D, Rubin KH, Schwarz P. A review of lifestyle, smoking and other modifiable risk factors for osteoporotic fractures. Bonekey Rep. 2014;3:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sahni S, Mangano KM, McLean RR, Hannan MT, Kiel DP. Dietary approaches for bone health: lessons from the Framingham osteoporosis study. Curr Osteoporos Rep. Aug 2015;13(4):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Premaor MOB, Ebling J, Flores LM, Comim FV. Effects of fruit and vegetable intake on bones: systematic review and meta‐analysis. Endocrine Rev. Meeting abstract June 2017;38(3 Suppl). Abstract number SAT 331 https://www.endocrine.org/meetings/endo-annual-meetings/abstract-details?id=31394. [Google Scholar]
- 101. Santos WR, Santos WR, Paes PP, et al. Impact of strength training on bone mineral density in patients infected with HIV exhibiting lipodystrophy. J Strength Cond Res. Dec 2015;29(12):3466–71. [DOI] [PubMed] [Google Scholar]
- 102. Guaraldi G, Orlando G, Madeddu G, et al. Alendronate reduces bone resorption in HIV‐associated osteopenia/osteoporosis. HIV Clin Trials. Sep‐Oct 2004;5(5):269–77. Epub 2004 Nov 25. [DOI] [PubMed] [Google Scholar]
- 103. McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. Nov 2007;21(18):2473–82. Epub 2007 Nov 21. [DOI] [PubMed] [Google Scholar]
- 104. Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr. Apr 01 2005;38(4):426–31. Epub 2005 Mar 15. [DOI] [PubMed] [Google Scholar]
- 105. Negredo E, Martinez‐Lopez E, Paredes R, et al. Reversal of HIV‐1‐associated osteoporosis with once‐weekly alendronate. AIDS. Feb 2005;19(3):343–5. Epub 2005 Feb 19. [PubMed] [Google Scholar]
- 106. Rozenberg S, Lanoy E, Bentata M, et al. Effect of alendronate on HIV‐associated osteoporosis: a randomized, double‐blind, placebo‐controlled, 96‐week trial (ANRS 120). AIDS Res Hum Retroviruses. Sep 2012;28(9):972–80. Epub 2012 Feb 23. [DOI] [PubMed] [Google Scholar]
- 107. Bolland MJ, Grey A, Horne AM, et al. Effects of intravenous zoledronate on bone turnover and bone density persist for at least five years in HIV‐infected men. J Clin Endocrinol Metab. Jun 2012;97(6):1922–8. Epub 2012 Mar 16. [DOI] [PubMed] [Google Scholar]
- 108. Bolland MJ, Grey AB, Horne AM, et al. Annual zoledronate increases bone density in highly active antiretroviral therapy‐treated human immunodeficiency virus‐infected men: a randomized controlled trial. J Clin Endocrinol Metab. Apr 2007;92(4):1283–8. Epub 2007 Jan 18. [DOI] [PubMed] [Google Scholar]
- 109. Huang J, Meixner L, Fernandez S, McCutchan JA. A double‐blinded, randomized controlled trial of zoledronate therapy for HIV‐associated osteopenia and osteoporosis. AIDS. Jan 2009;23(1):51–7. Epub 2008 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Negredo E, Bonjoch A, Perez‐Alvarez N, et al. Comparison of two different strategies of treatment with zoledronate in HIV‐infected patients with low bone mineral density: single dose versus two doses in 2 years. HIV Med. Aug 2015;16(7):441–8. Epub 2015 May 7. [DOI] [PubMed] [Google Scholar]
- 111. Pinzone MR, Moreno S, Cacopardo B, Nunnari G. Is there enough evidence to use bisphosphonates in HIV‐infected patients? A systematic review and meta‐analysis. AIDS Rev. Oct–Dec 2014;16(4):213 –22. Epub 2014 Oct 11. [PubMed] [Google Scholar]
- 112. Negredo E, Warriner AH. Pharmacologic approaches to the prevention and management of low bone mineral density in HIV‐infected patients. Curr Opin HIV AIDS. May 2016;11(3):351–7. Epub 2016 Feb 19. [DOI] [PubMed] [Google Scholar]
- 113. Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. Dec 2015;26(12):2773 –83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Takeuchi T, Tanaka Y, Ishiguro N, et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose‐response study of AMG 162 (denosumab) in patients with rheumatoid arthritis on methotrexate to validate inhibitory effect on bone erosion (DRIVE)—a 12‐month, multicentre, randomised, double‐blind, placebo‐controlled, phase II clinical trial. Ann Rheum Dis. Jun 2016;75(6):983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stopeck AT, Fizazi K, Body JJ, et al. Safety of long‐term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Supp Care Canc. Jan 2016;24(1):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wheeler AL, Tien PC, Grunfeld C, Schafer AL. Teriparatide treatment of osteoporosis in an HIV‐infected man: a case report and literature review. AIDS. Jan 14 2015;29(2):245–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. Jun 2008;19(6):811–8. [DOI] [PubMed] [Google Scholar]
- 118. Landfeldt E, Strom O, Robbins S, Borgstrom F. Adherence to treatment of primary osteoporosis and its association to fractures‐‐the Swedish Adherence Register Analysis (SARA). Osteoporos Int. Feb 2012;23(2):433–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Appendix S1.


