Abstract
Central retinal artery occlusion is visually devastating and has no proven treatments. The therapeutic interval between symptom onset and potentially sight-saving intervention is narrow. Traditional conservative approaches include digital massage, administration of systemic vasodilators and diuretics, and lowering of intraocular pressure. Systemic and targeted fibrinolytic therapy is under investigation but is associated with significant adverse reactions. We report a case in which hyperbaric oxygen therapy restored retinal perfusion, and the patient’s vision was improved.
Introduction
One of the most devastating eye diseases that ophthalmologists face is central retinal artery occlusion (CRAO). Within minutes, vision can go from crystal clear 20/20 vision to seeing only hand motions. CRAO typically presents with painless, sudden, and profound unilateral vision loss. Animal models of CRAO suggest that the retina can make a full recovery when the occlusion lasts less than 90 minutes. Occlusions for longer than 100 minutes tend to leave varying degrees of retinal damage. When the blood flow is interrupted for longer than 4 hours, retinal damage is usually massive and vision loss is irreversible.1 The tissue that is potentially salvageable before this stage is often referred to as the “ischemic penumbra.”2 Penumbral tissue has been described as hypoperfused tissue that is destined for infarction unless normal perfusion is promptly restored.
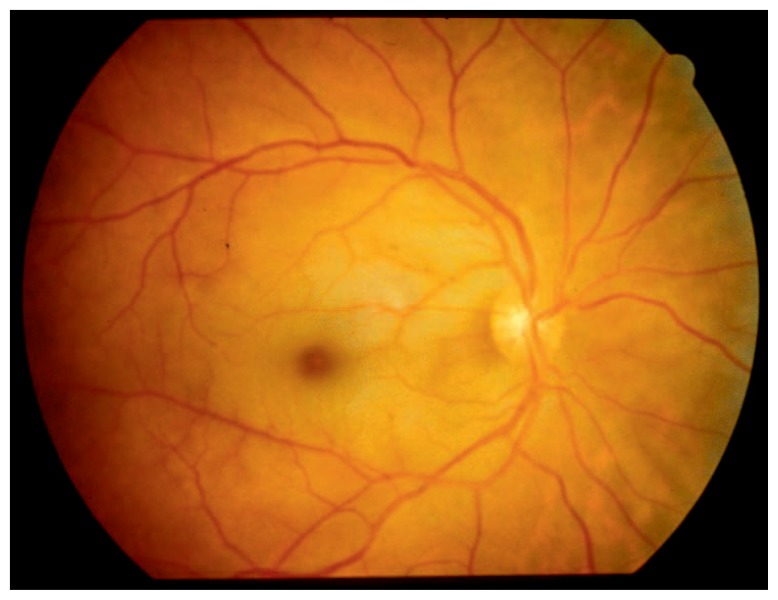
The retina is one of the most metabolically active tissues, consuming more oxygen per gram of tissue than many other tissues, including the brain.3 The retina has a dual blood supply, with the inner layers nourished by the central retinal artery and the outer retinal layers supported by the choroidal circulation. Although the average diameter of the central retinal artery is only 0.16 mm,4 few blood vessels have the sole responsibility of nourishing more important real estate. In contrast to the solitary central retinal artery that supplies the inner retinal layers, around 20 ciliary arteries work together (called the choroidal circulation) to nourish the outer layers of the retina. They also supply the central fovea. It is the preservation of this blood supply that leads to the pathognomonic CRAO “cherry red spot” against the background of diffuse retinal whitening (see Figure 1).
Figure 1.
Funduscopic appearance of the classic cherry red spot against the background of retinal whitening in central retinal artery occlusion.
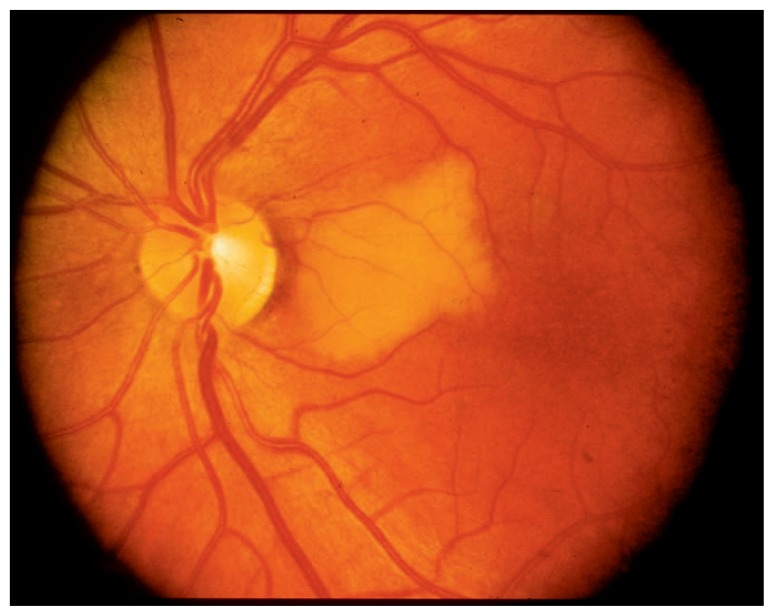
In the vast majority of the population, the central retinal artery is the only artery that feeds the inner retina. Only 15–30% of the population have a cilioretinal artery that also feeds the inner retina, serving as a back-up blood supply that can provide adequate blood flow to the macula in the case of an acute CRAO. Patients with a cilioretinal artery have a much better visual prognosis than those with a central retinal artery only.5 In the 70–85% of the population who do not have a cilioretinal artery, CRAO typically results in permanent vision loss in the range of light perception only to counting-fingers vision. Spontaneous visual recovery after CRAO is extremely rare. When a cilioretinal artery becomes occluded (see Figure 2), however, the visual prognosis depends on how much of the macular blood supply is dependent on this vessel.
Figure 2.
Cilioretinal artery occlusion on funduscopy.
The incidence of CRAO is around 1 in 10,000 to 100,000.6 Given the rarity of CRAO and the unlikely event that it is diagnosed within the first several hours of symptom onset, no well-established treatment guidelines have been developed. A variety of therapeutic modalities have been attempted but with relatively limited success. Although interventions have little proven benefit, most ophthalmologists and their patients with CRAO are willing and desperate to try anything that will do no harm in the hope that something might help. The reality is that most of the time such efforts end up being futile and the retina is declared “dead.” Following CRAO, patients are typically monitored for long-term complications such as neovascular glaucoma.
Risk factors for CRAO include giant cell arteritis, carotid artery atherosclerosis, cardiogenic emboli, hypertension, smoking, diabetes, and thromboembolic disease. The possibility of such etiologies is addressed in the work-up on an individualized basis. Systemic steroids are administered when giant cell arteritis is suspected clinically. Smoking cessation and blood pressure control are important in all patients. Patients with significant carotid disease are usually treated with carotid endarterectomy, and those with cardiogenic emboli are usually treated with long-term anticoagulation.
Conservative Therapies for CRAO
For many decades, ophthalmologists have held onto the hope that an intervention of some type would be identified to consistently improve the dire prognosis of CRAO. One of the most frequently attempted interventions is ocular massage, as it can be readily performed with no special equipment. Intermittent digital pressure is applied on the eye to help facilitate the outflow of aqueous fluid. The release of digital pressure, theoretically, causes a sudden lowering of the intraocular pressure, which should improve the retinal perfusion pressure.7 This maneuver is not without risk, because it could theoretically cause a retinal embolus to go downstream.
Intraocular pressure can also be rapidly decreased by administering topical glaucoma drops, performing an anterior chamber paracentesis, or administering diuretics such as acetazolamide or mannitol.8,9 A limitation of anterior chamber paracentesis is that it only takes 1–2 hours for a deflated anterior chamber to return to a normotensive range. Sometimes the central retinal artery is noted on funduscopy to open back up completely following anterior chamber paracentesis. However, the blockage has a tendency to recur as soon as the anterior chamber reinflates. A recent retrospective review of more than 50 patients who underwent anterior chamber paracentesis following CRAO showed that there was no added gain in visual acuity with this intervention.10
Others have postulated that administration of a potent vasodilator such as nitroglycerin or pentoxifylline can be used to improve ocular blood flow during the ischemic insult.11 An alternative method is to have the patient breathe into a brown paper bag in order to cause a hypercapnic vasodilatory response.
Despite many anecdotal case series describing successful outcomes with any or all of the above measures, major review articles have concluded that there is not sufficient evidence from prospective trials to state that any intervention makes a statistical difference in clinical outcomes.12,13
Revascularization Through Fibrinolysis
Some authors have described the use of systemic or targeted administration of tissue plasminogen activator (t-PA) for CRAO, since CRAO is the ocular analogue of a cerebral stroke. The results have been mixed. It is more challenging to deliver the ideal concentration of t-PA to a smaller caliber vessel like the central retinal artery in comparison to a larger vessel that may be affected in acute ischemic stroke. As a result, the potential risk-benefit ratio with t-PA may be higher with CRAO than with stroke-related occlusion.
One multicenter (five centers) randomized trial compared local intra-arterial fibrinolysis with conservative treatment in 84 patients with nonarteritic CRAO.14 This five-year study demonstrated identical visual outcomes in each treatment group. However, the study was stopped prematurely because adverse reactions were much more prevalent in the local intra-arterial fibrinolysis group: 37% versus 4% in the control group.
Additional prospective trials are underway. In the meantime, there is insufficient evidence to recommend the use of systemic or targeted fibrinolytics for CRAO. The use of thrombolytics should be limited to centers participating in ongoing clinical trials.
Laser or Surgical Arteriotomy
In the event that the offending clot or embolus is visible on the optic disc surface, some ophthalmologists have had successful outcomes with laser or surgical arteriotomy. The Nd-YAG laser is accessible in most ophthalmology clinics and has been used in selected cases of CRAO and branch retinal artery occlusion (BRAO).15,16 The laser beam is focused directly on the clot in an attempt to dislodge or fragment it in order to revascularize the ischemic tissue.
A surgical technique has been described for physically removing the obstruction. During vitreoretinal surgery, a stylet is used to directly puncture and remove the offending embolus or clot.17 As would be expected, vitreous hemorrhage is a common complication of both surgical and laser arteriotomy.
Hyperbaric Oxygen for CRAO
Hyperbaric oxygen (HBO) therapy may have a more favorable side effect profile than more invasive approaches like fibrinolysis or surgical arteriotomy.18 The rationale behind HBO therapy lies in the aforementioned fact that the retina has a dual blood supply. If the oxygen levels in the choroidal circulation are increased to a sufficient degree, then enough oxygen can diffuse downstream to the inner retinal layers to allow them to maintain some degree of viability. This theory is supported by CRAO animal models in which preservation of the normal inner retinal layer architecture has been demonstrated when choroidal oxygen levels have been high enough to allow for diffusion to the inner layers.19
Numerous case series support the idea that hyperbaric oxygen can improve outcomes following CRAO.20–25 Aisenbrey et al. reported a mean increase in visual acuity of 2 lines in a series of eight patients treated with HBO.20 Beiran et al. found that over 80% of patients in their series reported subjective improvement in vision with HBO therapy compared to less than 30% in the control group.21,22 In a series of 21 patients, 19 noted subjective improvement of vision, which was confirmed in 13 patients by visual acuity testing.23 Cope et al. pooled data from their case series of 11 patients with data from two other case series of HBO therapy, giving a combined total of 51 patients, and found that more than half of patients experienced visual acuity improvement of greater than 2 lines with HBO therapy.24 Menzel-Severing et al. compared the visual acuity outcome in 51 HBO-treated patients with that in a control group of 29 patients and found an average improvement of 3 lines of vision in the HBO group compared to only 1 line in the control group.25
While these studies vary in their selection criteria and treatment algorithms, the vast majority recruited only patients who presented within 24 hours of symptom onset. These studies show hope that early HBO may improve outcomes following CRAO. Prospective randomized trials are necessary to demonstrate a proven benefit. Hyperbaric centers that treat these patients should consider forming a central registry of standardized data to prospectively study the results of HBO therapy.24
Case Report
A 67-year-old African-American man presented to the ophthalmology clinic with complaints of sudden, painless loss of vision in his left eye. He had a history of hypertension, previous placement of two cardiac stents and a 50 pack-year history of tobacco abuse. More than 25 years prior to presentation, he lost the vision in his right eye from a central retinal vein occlusion (CRVO). As is not uncommon following a CRVO, he went on to develop neovascular glaucoma, with severely elevated intraocular pressures (IOPs) that reached up to the 50s (normal range: 10–22 mm Hg). Despite aggressive treatment with maximal glaucoma drops and multiple palliative laser procedures, his right eye became completely blind, with no light perception. He ultimately decided to undergo an enucleation procedure to remove the right eye because it had no visual potential and was causing intolerable pain.
The patient was quite frustrated with the sudden onset of vision loss in his remaining eye. Baseline visual acuity in this eye was 20/25. The evening prior to presentation, he noticed an abrupt onset of vision loss with a peculiar “kaleidoscope of colors” that developed over several minutes. He denied any weakness, slurred speech, balance issues, nausea/vomiting, or other neurologic symptoms. His blood pressure was 150/80 mm Hg. Although he had never had any episodes like this before, he went to bed hoping that his vision would be back to normal after a good night’s rest.
His vision remained poor the following morning, which prompted a visit to the ophthalmology clinic. He was able to count fingers from 5 feet away but could not identify the 20/400 line of the Snellen eye chart. His intraocular pressure was within normal limits. Fluorescein angiography demonstrated delayed filling of the retinal circulation, consistent with acute CRAO.
The hyperbaric medicine team was consulted and expediently started HBO therapy, based on a 2012 treatment algorithm published in the Journal of the Undersea and Hyperbaric Medical Society.18 The patient underwent a total of six treatments (one every 12 hours) over a 72-hour period. Each treatment consisted of three 30-minute sessions, with a 5-minute break between each session. The pressure level was set between 2.4 and 2.5 atmospheres. After each treatment, visual acuity was measured to assess for any interval changes. He tolerated the sessions without any adverse side effects.
Following the first hyperbaric treatment, the patient noticed an abrupt recovery in his sight. His vision improved to 20/70. After the second treatment, his vision improved an additional line, to 20/60. Over the next four treatments, his vision continued to trend upward until it stabilized at 20/40. The following week, he underwent a second fluorescein angiography study. This time, there was no delay in the transit of the fluorescein dye into the retinal circulation. The previously obstructed central retinal artery had completely recanalized. After two years of follow-up, the patient’s vision has remained stable at 20/40.
Conclusion
When it comes to CRAO, “time is vision.” CRAO is a true ophthalmic emergency. If any intervention is capable of reversing or limiting the associated vision loss from CRAO, it must be done in a timely fashion while there is still hope to save the ischemic penumbra. When patients present longer than 24 hours after symptom onset, the damage has already been done and there is little hope for spontaneous visual improvement. The shorter the time between onset of symptoms and presentation, the more hope there is that intervention may be beneficial to some degree.
Because of the relative infrequency of CRAO in comparison to other ischemic events, it is challenging to perform large-scale randomized controlled trials. The few randomized trials that have been published have failed to demonstrate any proven intervention that is more likely to work better than doing nothing.13 Well-designed prospective trials are still required to establish the most effective treatment for CRAO. Hopefully, future studies will demonstrate interventions that can improve the poor visual prognosis associated with CRAO. In the meantime, ophthalmologists will, within reason, continue to try anything and everything to improve oxygen delivery to the ischemic retina, with the hopes of salvaging as much vision as possible. When patients present within 24 hours of the onset of CRAO symptoms, HBO therapy is another possible intervention. It has the advantages of a relatively low-risk profile and can be attempted to help improve visual outcome.
Biography
Evan A. Olson, MD, (left), is currently a comprehensive ophthalmologist in private practice in Vancouver, Wash. In June 2015 Dr. Olson completed his ophthalmology residency at the Department of Ophthalmology, Mason Eye Institute. Kathy Lentz, MD, (right), MSMA member since 1985, is a clinical assistant professor in the Department of Ophthalmology, Mason Eye Institute, University of Missouri.
Contact: lentzk@health.missouri.edu


Footnotes
Disclosure
None reported.
References
- 1.Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. IV: Retinal tolerance time to acute ischaemia. Br J Ophthalmol. 1980;64(11):818–825. doi: 10.1136/bjo.64.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrup J, Symon L, Siesjö BK. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 3.Ames A., III Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol. 1992;70:S158–S164. doi: 10.1139/y92-257. [DOI] [PubMed] [Google Scholar]
- 4.Lee KE, Klein BE, Klein R, Meuer SM. Association of retinal vessel caliber to optic disc and cup diameters. Invest Ophthalmol Vis Sci. 2007;48(1):63–67. doi: 10.1167/iovs.05-1203. [DOI] [PubMed] [Google Scholar]
- 5.Stoffelns BM, Laspas P. Cilioretinal artery occlusion. Klin Monbl Augenheilkd. 2015;232(4):519–524. doi: 10.1055/s-0034-1396327. [DOI] [PubMed] [Google Scholar]
- 6.Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820–823. doi: 10.1016/j.ajo.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen NV. Treatment of acute occlusion of the retinal arteries. Acta Ophthalmol (Copenh) 1979;57:1078–1081. doi: 10.1111/j.1755-3768.1979.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 8.Augsburger JJ, Magargal LE. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol. 1980;64(12):913–917. doi: 10.1136/bjo.64.12.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102:2029–2024. doi: 10.1016/s0161-6420(95)30758-0. [DOI] [PubMed] [Google Scholar]
- 10.Fieß A, Cal Ö, Kehrein S, Halstenberg S, Frisch L, Steinhorst UH. Anterior chamber paracentesis after central retinal artery occlusion: a tenable therapy? BMC Ophthalmol. 2014;14:28. doi: 10.1186/1471-2415-14-28. (7 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128(6):733–738. doi: 10.1016/s0002-9394(99)00359-1. [DOI] [PubMed] [Google Scholar]
- 12.Rudkin AK, Lee AW, Aldrich E, et al. Clinical characteristics and outcome of current standard management of central retinal artery occlusion. Clin Experiment Ophthalmol. 2010;38:496–501. doi: 10.1111/j.1442-9071.2010.02280.x. [DOI] [PubMed] [Google Scholar]
- 13.Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev. 2009:CD001989. doi: 10.1002/14651858.CD001989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumacher M, Schmidt D, Jurklies B, Gall C, Wanke I, Schmoor C, Maier-Lenz H, Solymosi L, Brueckmann H, Neubauer AS, Wolf A, Feltgen N EAGLE Study Group. Central retinal artery occlusion local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117(7):1367–1375. doi: 10.1016/j.ophtha.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 15.Akduman L, Currie M, Scanlon C, Grant A, Cetin EN. ND-yag laser arteriotomy for central retinal artery occlusion. Retin Cases Brief Rep. 2013;7(4):325–327. doi: 10.1097/ICB.0b013e31828ef0f2. [DOI] [PubMed] [Google Scholar]
- 16.Reynard M, Hanscom TA. Neodymium:yttrium-aluminum-garnet laser arteriotomy with embolectomy for central retinal artery occlusion. Am J Ophthalmol. 2004;137(1):196–198. doi: 10.1016/s0002-9394(03)00817-1. [DOI] [PubMed] [Google Scholar]
- 17.Tang WM, Topping TM. Vitreous surgery for central retinal artery occlusion. Arch Ophthalmol. 2000;118(11):1586–1587. [PubMed] [Google Scholar]
- 18.Murphy-Lavoie H, Butler F, Hagan C. Central retinal artery occlusion treated with oxygen: a literature review and treatment algorithm. Undersea Hyperb Med. 2012;39(5):943–953. [PubMed] [Google Scholar]
- 19.Patz A. Oxygen inhalation in retinal arterial occlusion: a preliminary rpeort. Am J Ophthalmol. 1955;40(6):789–795. doi: 10.1016/0002-9394(55)91107-7. [DOI] [PubMed] [Google Scholar]
- 20.Aisenbrey S, Knott R, Heller R, Krauss D, Rössler G, Heimann K. Hyperbaric oxygen therapy in retinal artery occlusion. Ophthalmologe. 2000;97(7):461–467. doi: 10.1007/s003470070075. [DOI] [PubMed] [Google Scholar]
- 21.Beiran I, Reissman P, Scharf J, Nahum Z, Miller B. Hyperbaric oxygenation combined with nifedipine treatment for recent-onset retinal artery occlusion. Eur J Ophthalmol. 1993;3(2):89–94. doi: 10.1177/112067219300300207. [DOI] [PubMed] [Google Scholar]
- 22.Beiran I, Goldenberg I, Adir Y, Tamir A, Shupak A, Miller B. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol. 2001;11(4):345–350. doi: 10.1177/112067210101100405. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger AW, Siekmann UP, Wolf S, Rossaint R, Kirchhof B, Schrage NF. Treatment of acute central retinal artery occlusion by hyperbaric oxygen therapy –pilot study with 21 patients. Klin Monbl Augenheilkd. 2002;219(10):728–734. doi: 10.1055/s-2002-35687. [DOI] [PubMed] [Google Scholar]
- 24.Cope A, Eggert JV, O’Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving Hyperb Med. 2011;41(3):135–138. [PubMed] [Google Scholar]
- 25.Menzel-Severing J, Siekmann U, Weinberger A, Roessler G, Walter P, Mazinani B. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol. 2012;153(3):454–459. doi: 10.1016/j.ajo.2011.08.009. [DOI] [PubMed] [Google Scholar]