Abstract
A synergistic enhancement of activities has been described for the amphipathic cationic antimicrobial peptides magainin 2 and PGLa when tested in antimicrobial assays or in biophysical experiments using model membranes. In the presence of magainin 2, PGLa changes from an in-planar alignment parallel to the membrane surface to a more transmembrane orientation when investigated in membranes made from fully saturated PC or PC/PG, but not when one of the fatty acyl chains is unsaturated. Such lipid-mediated changes in the membrane topology of polypeptide domains could provide an interesting mechanism for the regulation of membrane proteins. Here we investigated the PGLa topology in a wide variety of membranes made of saturated or unsaturated PE, PC, and/or PG using 15N solid-state NMR spectroscopy. In contrast to predictions made by previous models the data show that membrane curvature has only a minor effect on PGLa realignment. Furthermore, using 2H solid-state NMR spectroscopy of deuterated phospholipid fatty acyl chains the order parameters of the lipids were investigated in the presence of PGLa, magainin, or equimolar peptide mixtures. Both peptides cause a pronounced decrease in the order parameters when oriented parallel to the membrane surface, an effect that reverts when PGLa flips into transmembrane alignments. Taken together, these data are suggestive that the magainin-induced disordering of fatty acyl chains provides an important driving force for PGLa realignment.
Introduction
During the past three decades, the widespread use of conventional antibiotics has led to a drastic reduction in their therapeutic efficacy with over 70% of hospital bacterial infections being resistant (1). Furthermore, the number of new antibiotics approved by the US Food and Drug Administration has steadily declined over the last four decades (2). This discovery void is impacting the therapeutic options to treat infections caused by multidrug-resistant bacteria. Cationic antimicrobial peptides have shown some promise as potential antibiotics and several of them are undergoing clinical trials (3, 4). Antimicrobial peptides are sentinels of the innate immune system of most species and are usually the first line of defense against any infection. Although most of the conventional antibiotics act by targeting the metabolic pathways of bacteria, antimicrobial peptides, and their mimics are thought to primarily interact with the cell membrane. Consequently, unlike in the case of conventional antibiotics, where even point mutations can render them inactive, bacteria find it difficult to develop resistance against antimicrobial peptides. Whereas predominantly hydrophobic antimicrobial peptides such as alamethicin and other peptaibols can fully insert across lipid membranes (5), magainins, and related cationic amphipathic peptides were found to align and act parallel to the bilayer surface (6, 7). Membrane surface association has been rationalized by a variety of approaches as an essential early step in the functional mechanism of antimicrobial peptides, leading to massive membrane poration and bacterial cell death (6, 8).
Magainin 2 and PGLa were among the first discovered cationic amphipathic antimicrobial peptides with good experimental evidence that these compounds selectively impair bacterial plasma membranes (6, 9). It is worthwhile mentioning that naturally these peptides are stored together with other sequences in the skin granules of the African clawed frog Xenopus laevis, in the form of mixtures/cocktails for immediate release upon infection (10). Moreover, magainin 2/PGLa mixtures show intriguing synergistic effects both in antimicrobial assays as well as in fluorescent dye-release experiments using lipid-only model membranes (11, 12, 13). The mechanistic basis for the ∼10-fold enhancement of membrane disruption and antibacterial activities of the mixture when compared to the individual peptides remains poorly understood (12, 14, 15) and has recently been reviewed and investigated in (16, 17).
Magainin 2 and related cationic amphipathic peptides adopt very stable alignments parallel to the membrane surface under all conditions tested so far (18, 19, 20). In contrast, the topological alignment of PGLa with respect to the membrane surface has been shown to be affected by the detailed composition of the phospholipid membrane, its peptide-to-lipid ratio, the hydration level or the presence of the other peptide (19, 20, 21). For example, PGLa adopts various membrane topological states in PC and PC/PG mixtures with desaturated hydrocarbons, where the angle varies with the fatty acyl chain length composition and with the peptide-to-lipid ratio (20, 21). Furthermore, in the presence of magainin, PGLa switches to even smaller tilt angles, which suggests a transmembrane configuration, but this transition only occurs in fully saturated membranes and not in POPC, POPC/POPG, or Escherichia coli lipid extracts (13, 19, 20).
Investigating the lipid interactions of PGLa, magainin, and PGLa/magainin mixtures is of twofold interest. On the one hand, scrutinizing lipid/peptide interactions will aid to unravel the fundamental biophysics of antimicrobial peptide activities including synergism. Both peptides remain oriented along the membrane surface when studied in natural or closely related saturated-unsaturated lipid model membranes strongly suggesting that a mechanism explaining synergism should be based on such peptide topologies (13, 14). On the other hand, the PGLa realignment in the presence of magainin observed in DMPC and DMPC/DMPG membranes is not only of academic interest but can also be important for other polypeptides where such lipid-mediated topological changes may be involved in the regulation of membrane protein function (20).
To explain the magainin-induced differential alignment of PGLa in saturated (transmembrane) and unsaturated lipids (in-plane), it has been proposed that hydrophobic mismatch contributions and the inherent shape of the lipids provide a driving force to push the topological equilibrium from an in-plane orientation along the bilayer surface toward a membrane inserted state (19, 20). Previously, the data were rationalized by the intrinsic curvatures of the lipids where phospholipids with zero or positive intrinsic curvature such as fully saturated dimyristoyl- and dipalmitoyl-PC or -PG allow transmembrane insertion of PGLa (19). In contrast, it was stated that the lipid-induced interfacial strain from the more negative curvature of POPC and POPG stabilizes the in-planar orientation of the PGLa amphipathic helix and prevents the peptides from inserting deeply into the bilayer (19). However, quantitative data show that the difference in thickness and in molecular shape between POPC and DMPC are quite small with the curvature of POPC being −0.02, that of DMPC +0.04 (20, 22, 23).
To further explore the role of curvature strain here, we investigate membranes made up from PE lipids. The small head group of PE induces a strong negative curvature (∼ −0.32) contrasting the much smaller changes that occur when the palmitoyl-oleoyl fatty acids of zwitterionic lipids are exchanged by myristoyls (22, 23). In fact, the strong negative curvature of PE lipids at higher temperatures results in hexagonal macroscopic phases of the pure lipid (24). Therefore, if molecular shape is the main driving force for magainin 2-induced PGLa realignment, the effect of PE lipids should be quite pronounced. Thus, from the inherent negative curvature of DMPE or POPE, one would predict that in bilayers made from either of these lipids, PGLa is oriented parallel to the surface regardless of the fatty acyl chain composition and/or the presence of magainin 2.
Furthermore, we investigated the order parameter profiles of the fatty acyl chains in the presence of the peptides individually as well as in equimolar mixtures. Whereas this approach reveals important details about the lipid perturbing activities of the peptides alone, comparing with the order parameter profiles in the presence of equimolar mixtures of these peptides, which is known to possess synergistically enhanced activity (12), allows the evaluation on how and whether this synergistic interaction correlates with changes in the lipid packing and thereby membrane thickness. Finally, the potential energetic contributions of the in-plane to transmembrane transition of PGLa are discussed (25, 26).
Materials and Methods
Amino acid derivatives and other reagents for peptide synthesis were from Novabiochem-Merck (Darmstadt, Germany). Water (high-performance liquid chromatography (HPLC) grade) and deuterium-depleted water were from Sigma-Aldrich (St. Quentin Fallavier, France). All lipids were purchased from Avanti Polar Lipids (Alabaster, AL):
DLPE: 1,2-dilauroyl-sn-glycero-3-phosphoethanolamine (di-C12:0-PE).
DMPC: 1,2-dimyristoyl-sn-glycero-3-phosphocholine (di-C14:0-PC).
DMPC-2H54: 1,2-dimyristoyl-d54-sn-glycero-3-phosphocholine.
DMPG: 1,2-dimyristoyl-sn-glycero-3-phospho-(1’-rac-glycerol) sodium salt.
DMPG-2H54: 1,2-dimyristoyl-d54-sn-glycero-3-phospho-(1’-rac-glycerol) sodium salt.
DMPE: 1,2-dimyristoyl-sn-glycero-3-phospholethanolamine.
DMPE-2H54: 1,2-dimyristoyl-d54-sn-glycero-3-phosphoethanolamine.
POPC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (C16:0, C18:1-PC).
POPC-2H31: 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-phosphocholine.
POPE: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine.
POPE-2H31: 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-phosphoethanolamine.
POPG: 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) sodium salt.
POPG-2H31: 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)].
Peptide synthesis
Magainin 2-COOH (GIGKFLHSAKKFGKAFVGEIMNS) and [15N-Ala10, 2H3-Ala8]-PGLa (GMASKAGAIAGKIAKVALKAL-NH2) were obtained by solid-phase peptide synthesis using a Millipore 9050 (Millipore, Burlington, MA) automated peptide synthesizer, the Fmoc (9-fluorenylmethyloxycarbonyl) chemistry and purified by semipreparative HPLC as described previously for other antimicrobial peptides (27). The underlined alanine-10 of PGLa and the underlined alanine-8 positions were labeled with 15N and 2H3, respectively, by incorporating the isotopically labeled fmoc-precursors (Eurisotop, Paris, France or Isotec Sigma-Aldrich). The identity and high purity of the product (>90%) was confirmed by reversed-phase HPLC matrix-assisted laser desorption ionization time of flight mass spectrometry. After lyophilisation, the trifluoroacetic acid counter ions were exchanged in 5% acetate (v/v) (28).
Sample preparation for mechanically oriented membranes
Oriented NMR samples of homogeneous mixtures of the appropriate lipids and peptides were prepared by co-dissolving the lipid and peptide in suitable organic solvents, spreading the mixture on glass plates, removal of the organic solvent, and subsequent hydration (28). Usually 2.5 mg of the 15N-labeled PGLa peptide was used. In samples with both magainin 2 and PGLa present, the PGLa: magainin 2: lipid molar ratio was 1:1:100, giving a total P/L ratio of 1:50. The peptides were suspended in water (100 μL). Lipids were co-dissolved in chloroform and methanol (1:1) and added to the peptide solution. The lipid vessel was rinsed with additional chloroform (50 μL), which was also added to the peptide solution. The resulting clear solution was spread onto 20 ultrathin cover glasses (00, 8 × 12 mm; Marienfeld, Lauda-Königshofen, Germany) and dried, first in air and thereafter in high vacuum overnight to remove all organic solvent. Thereafter, the glass plates samples were hydrated for 24 h at 37°C in a chamber where the air is in contact with a saturated solution of K2SO4, giving 96% relative humidity in the chamber. Once equilibrated, the glass plates were stacked on top of each other, and the hydrated stack was wrapped with Teflon tape and sealed in plastic wrapping to keep the sample fully hydrated during the NMR measurements (28). Liquid crystalline DMPE samples, measured at 60°C, showed a higher tendency to dry out. Therefore, 2μL of mQ water was added on top of each glass plate to promote stacking. The sample quality was checked by 31P-NMR and showed a dominant peak around 30 ppm (Fig. S1).
Sample preparation of nonoriented membranes
The nonoriented samples used to analyze the deuterium order parameters were prepared in an analogous manner as described in (29). Hydration was set to 80% (h = 0.8 with h = mass of water over the total mass of the system (phospholipids and water)). The molar ratio between protonated and deuterated lipids was 3:1.
Solid-state NMR spectroscopy
Solid-state NMR spectroscopy was performed on a Bruker Avance wide-bore solid-state NMR spectrometers (Rheinstetten, Germany). Proton-decoupled 15N cross polarization (CP) spectra were acquired at 50.7 MHz (11.7 T) using a commercial flat-coil NMR probe (30). A cross polarization pulse sequence was used, with a spectral width, acquisition time, CP contact time, π/2 pulse width, and recycle delay of 50 kHz, 10 ms, 0.8 ms, 10 μs and 3 s, respectively (28). Typically, 65,000 scans were accumulated. The spectra were recorded at temperatures above the gel to liquid phase transition. A 200 Hz exponential line broadening was applied before Fourier transformation. The spectra were referenced relative to ammonium chloride (39 ppm (31)).
31P NMR spectra were acquired at 202.4 MHz (11.7 T) using a phase-cycled Hahn-echo pulse sequence with gated broadband proton decoupling (32). Typical acquisition parameters were as follows: spectral window, π/2 pulse width and recycle delay of 100 kHz, 6 μs and 5 s, respectively, for mechanically aligned samples. Typically, 1024 scans were recorded. A line broadening of 50 Hz was usually applied prior to Fourier transformation. Phosphorus chemical shifts were calibrated relative to H3PO4 (85% in H2O, 0 ppm).
Deuterium quadrupole echo experiments (33) were acquired with a spectral width of 100 kHz, a recycle delay of 1 s, 25 μs echo delay, 30 ms acquisition time, π/2 pulses of 3.4 μs, and 50–80 k scans. All samples were heated to temperatures at least 10 K above the phase transition of the individual lipids used in the lipid mixture. A line broadening of 50 Hz was usually applied prior to Fourier transformation. The spectra were referenced relative to D2O.
For further analysis, the 2H solid-state NMR powder spectra were further processed by de-Pake-ing (34). The de-Pake-ing procedure was performed according to the fast Fourier transform-based fast deconvolution algorithm (35) and first spectral moments were calculated using NMR-Depaker software (available from the Launchpad Web site). Individual C–2H bond order parameters (SCDi) were calculated from quadrupolar splittings as described previously (36), SCD = , where AQ is 167 KHz (Eq. 1). From the quadrupolar splittings the acyl chain length can be estimated by considering the average projection of each C-C bond along the bilayer normal (37) and described in detail in (29).
Statistical considerations
In this article, we focus on comparing order parameter profiles of lipids in the absence and presence of peptides. It can be estimated that the quadrupolar splitting can be determined with ∼1 kHz accuracy, which translates to 0.01 for the order parameters and 0.14 for the relative order parameters. However, we did not attempt to compare the effects of the peptides on individual fatty acyl chain positions but rather compare order parameter profiles encompassing up to n = 13 data points. Student’s t can be calculated from the sample average and the SD of the pair-wise differences between corresponding points of two data sets according to , where n is the number of data points/differences (38). By comparing with tabulated reference t-values, it can thus be estimated that relative order parameters profiles that are at least 0.02 units apart are different with high probability. In this article, we only discuss much larger differences (≥0.05).
Results
In order to better understand the effect of lipid composition on peptide topology, the PGLa sequence was prepared with a single 15N label at the Ala-14 position, reconstituted into uniaxially oriented lipid bilayers and investigated by proton-decoupled 15N solid-state NMR spectroscopy. When the membrane normal is aligned parallel to the magnetic field of the NMR spectrometer, the 15N chemical shift is a direct indicator of the approximate orientation of helical domains relative to the lipid bilayer and provides valuable information about the peptide topology and the peptide-lipid interactions (39). Whereas a 15N chemical shift <100 ppm is indicative of a helix alignment approximately parallel to the membrane surface (in-plane state) 15N chemical shifts around 200 ppm correlate with transmembrane helices (transmembrane state). For intermediate “tilted” orientations, intermediate chemical shifts are observed (39). With more than one label exact tilt angles have been determined (40, 41, 42, 43).
In a first step, phospholipid membranes made from mixtures of zwitterionic and anionic phospholipids were studied, thereby including PE/PG 3/1 mol/mole, a mimetic of bacterial membranes (Fig. 1), whereas in a second step, membranes made of single lipids were investigated (Fig. 2). Although the PGLa alignment is sensitive to numerous environmental factors (19, 20, 21), the orientation of magainin 2 has previously been shown to remain parallel to the membrane surface in many different lipid mixtures and conditions and was not investigated here any further (18, 20).
Figure 1.
Proton-decoupled 15N solid-state NMR spectra of [15N-Ala14]-PGLa in the presence of equimolar magainin 2 (total peptide concentration 2 mol%) reconstituted into oriented (A) DMPC: DMPG 3:1 (mole/mole) membranes at 310 K, (B) DMPE: DMPG (3:1) (mole/mole) at 333 K, (C) POPC / POPG 3:1 (mole/mole) at 310K, and (D) POPE: POPG 3:1 (mole/mole) at 310 K. Peak intensities around 30 ppm have previously been observed from oriented membranes containing PE (13, 63) and are probably from the natural abundant 15N amines of this lipid head group. Samples were equilibrated at 95–96% r.h. The sketches to the right of the 15N NMR spectra schematically illustrate the corresponding PGLa and magainin 2 topologies in lipid bilayers, where the stable alignment of the latter approximately parallel to the membrane has been demonstrated previously (18, 19, 20). To see this figure in color, go online.
Figure 2.
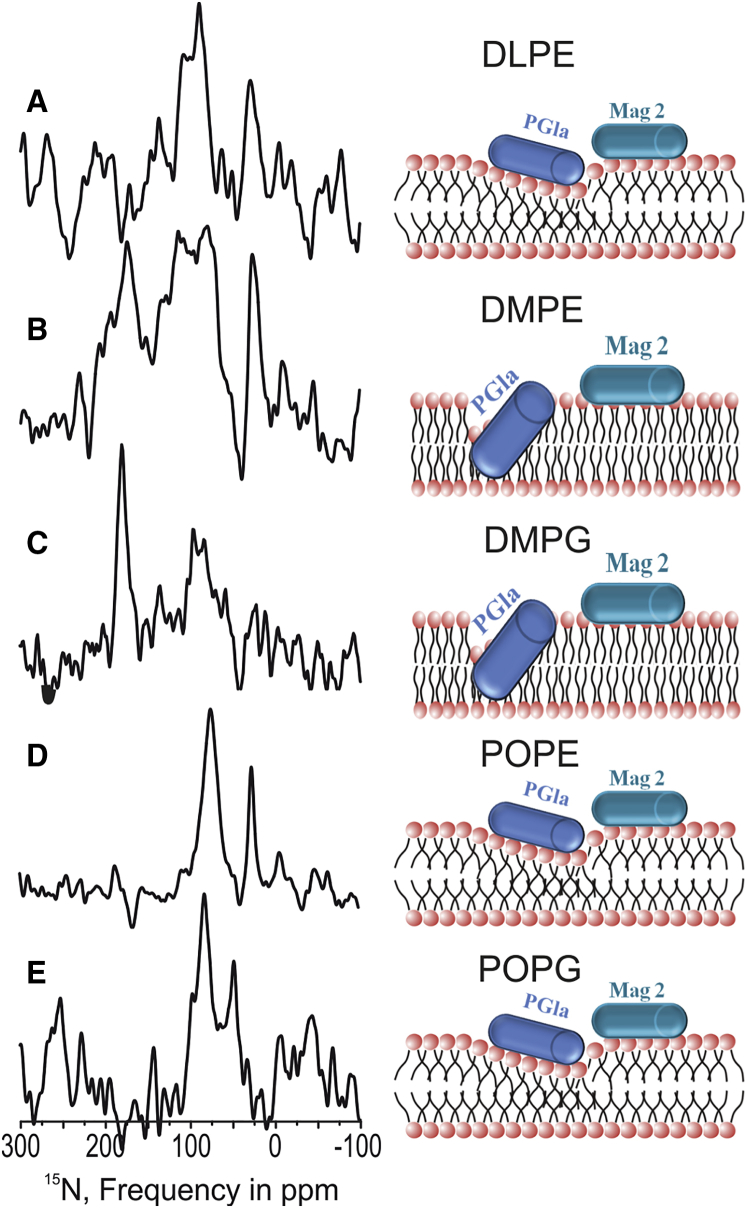
Proton-decoupled 15N solid-state NMR spectra of [15N-Ala14]-PGLa in the presence of equimolar magainin 2 (total peptide concentration 2 mol%) reconstituted into oriented (A) DLPE membranes at 320 K, (B) DMPE at 333 K, (C) DMPG at 310K, (D) POPE at 310 K, and (E) POPG at 310 K. Peak intensities around 30 ppm are probably from the natural abundant 15N amines of the PE head group. Samples were equilibrated at 95–96% r.h. The sketches to the right of the 15N NMR spectra schematically illustrate the corresponding PGLa and magainin 2 topologies in lipid bilayers, where the stable alignment of the latter approximately parallel to the membrane has been demonstrated previously (18, 19, 20). The broad intensities <100 ppm in (B) and (C) can represent in-plane alignments in equilibrium with transmembrane orientations, powder pattern contributions, or a combination of both (cf. text for details). To see this figure in color, go online.
PGLa alignment in membranes of mixed lipid composition
The 15N solid-state NMR spectra of [15N-Ala14]-PGLa indicate a 15N chemical shift of 178 ppm in DMPC:DMPG (3:1 mol/mole) membranes (Fig. 1 A). A closely related chemical shift of 182 ppm is observed in DMPE:DMPG (3:1) (Fig. 1 B), indicating transmembrane alignments of its helix in both the DMPC and the DMPE mixed membranes.
In contrast, in POPC:POPG (3:1 mol/mole), the PGLa chemical shift of 88 ppm (Fig. 1 C) confirms previous studies that showed that in such mixtures both PGLa and magainin 2 remain surface oriented (20). In oriented POPE:POPG (3:1), the chemical shift is 78 ppm (Fig. 1 D) indicating also in this case that closely related topologies are observed when the phosphatidylcholine head group is exchanged with phosphatidylethanolamine.
Thus, this data contradict previous predictions that the inverted cone shape of PE lipids should favor an in-plane alignment of PGLa (44). In contrast, the data underline the importance of lipid saturation of both myristoyl chains for a transition to the transmembrane topology.
PGLa alignment in membranes made of a single type of lipid
In order to gain further insight into the interactions that determine the PGLa membrane topology we also investigated the alignments of this peptide in membranes made from a single phospholipid. In the presence of magainin 2, [15N-Ala14]-PGLa exhibits a chemical shift of 84 ppm in POPG (Fig. 2 E) and of 75 ppm in POPE membranes (Fig. 2 D), indicating alignments of its helix long axis perpendicular to the membrane normal. In contrast, in DMPG bilayers, a chemical shift of 178 ppm is observed (Fig. 2 C), indicative of a PGLa transmembrane orientation. In this sample, additional contributions <100 ppm are visible that can arise from other membrane topologies and/or from nonoriented sample contributions. Notably, a sensitive equilibrium between in-plane and transmembrane alignments that shifted upon replacement of DMPC by DMPG was previously observed for the LAH4 amphipathic peptide (Fig. 4, A, C, and D in (45)). In DMPE, a broad chemical shift distribution ranging from 60 to 200 ppm is observed with maximal peak intensities at 175 and 100 ppm, indicative of an equilibrium of transmembrane and tilted orientations of the PGLa 15N-H vector when mixed with magainin 2 (Fig. 2 B). To get more insight into the preferred membrane alignment of PGLa in the presence of magainin 2, the peptides were also investigated in the fully saturated DLPE lipid (di-C12:0; Fig. 2 A, with a gel-to-liquid crystalline phase transition temperature Tm = 302 K). In this “thin” membrane, PGLa exhibits a chemical shift of 90 ppm, indicating an alignment parallel to the membrane surface. Notably, the differences in signal-to-noise ratio of the cross-polarized 15N solid-state NMR spectra arise from shorter acquisition times (Fig. 2 A) and spreading of the total intensity over a wider chemical shift range (e.g., Fig. 2 B) but are also affected by motions and exchange processes interfering with efficient polarization transfer.
The 31P solid-state NMR spectra, in particular those of the single lipids (Fig. S1, D–G), exhibit considerable amounts of phospholipid orientations at different angles relative to the sample normal suggesting that the 15N intensities <150 ppm in particular of the DMPE and DMPG sample (Fig. 2, B and C) at least partially arise from nonoriented/powder pattern contributions. However, it should be noted that the alignment of the 31P and 15N solid-state spectra do not always correlate as becomes obvious when comparing the spectra obtained from the POPE samples (Figs. 2 D; Fig. S1 G). Whereas the 31P solid-state NMR spectrum shows similar amounts of “nonoriented” signals (<25 ppm; alignments of the lipid different from parallel to the membrane normal), the 15N solid-state NMR spectrum is indicative of a well-defined orientation perpendicular to the sample normal. Similar combinations of 31P and 15N solid-state NMR spectra have previously been observed in the presence of other amphipathic peptides (27, 46).
Lipid order parameters in the presence of magainin, PGLa, and equimolar mixtures
Lipid order parameters provide valuable information about the packing, hydrophobic thickness, and surface area per lipid. To gain quantitative insight how the peptides and mixtures thereof change the packing order and the geometrical arrangement of the lipid matrix 2H solid-state NMR spectra of phospholipids carrying either a single palmitoyl or two myristoyl chains that have been deuterated throughout were recorded. To assess how the lipid chain mobility and the conformational distribution of the methylene segments is influenced by the peptides, the quadrupolar splittings of the 2H-NMR spectra were converted into an order parameter profile. In most cases, the chain order was found to be slightly reduced compared to the lipid sample without peptide (Figs. 3 and 4, filled squares). Upon addition of peptide the order parameters changed across the entire chain, but the amplitude of change varies substantially among the different lipid and peptide compositions.
Figure 3.
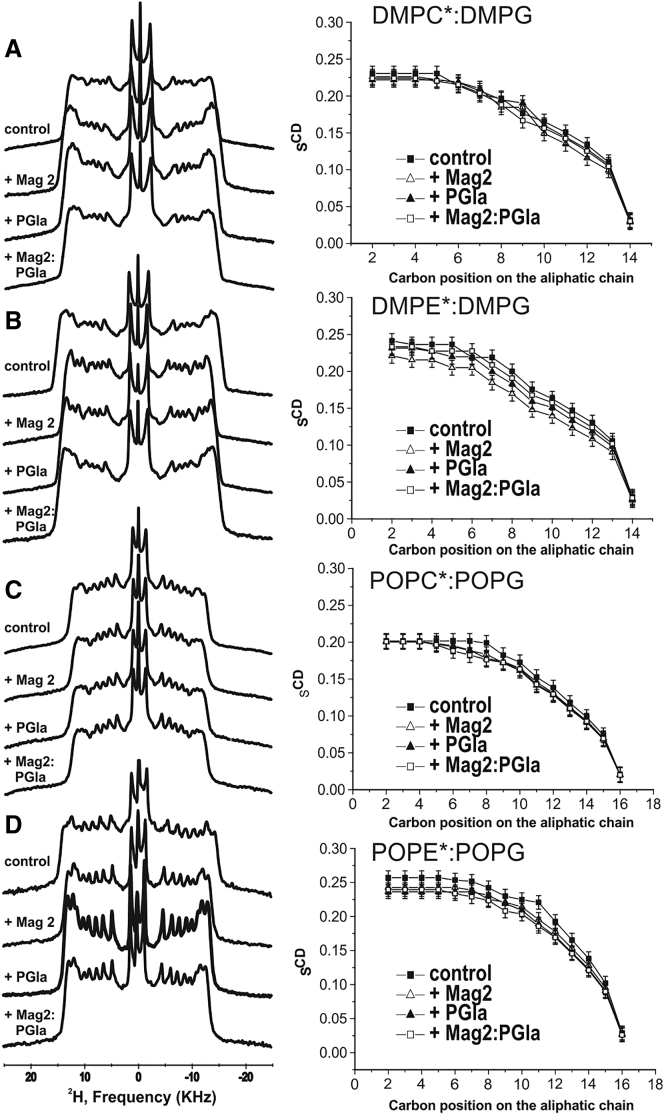
2H solid-state NMR spectra and the corresponding SCD order parameters profiles of mixed membranes encompassing zwitterionic lipids (PE or PC) carrying deuterated fatty acyl chains. (A) DMPC: DMPC-2H54: DMPG, (B) DMPE: DMPE-2H54: DMPG, (C) POPC: POPC-2H31: POPG, and (D) POPE: POPE-2H31: POPG recorded at T = 310, 340, 310, and 320 K, respectively, are shown. The lipid composition was adjusted so that the molar ratio of zwitterionic lipid/PG is 3/1, and the total deuterated lipid represents 25 mol%. The order parameters are obtained from quadrupolar splittings, according to Eq. 1, in the absence (closed squares) or presence of 2 mol% PGLa (closed triangles), 2 mol% magainin 2 (open triangles), and PGLa: magainin 2 (1 mol % each; open squares), with an estimated uncertainty of 0.01 (corresponding to 1 kHz in measuring the quadrupolar splittings).
Figure 4.
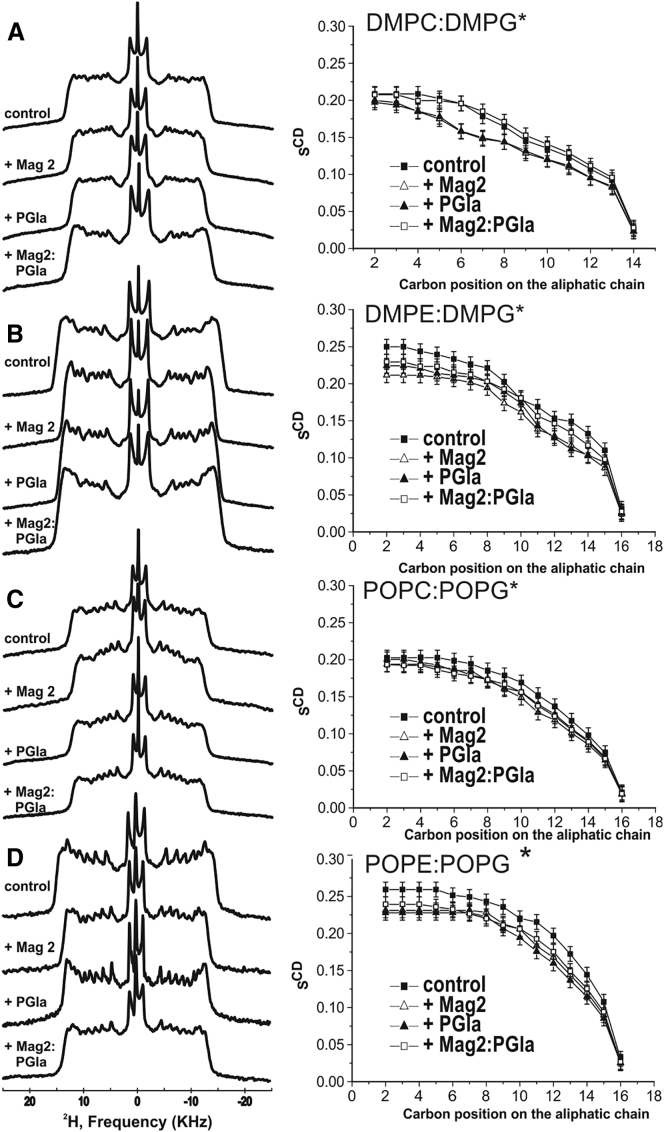
2H solid-state NMR spectra and the corresponding SCD order parameters profiles of mixed membranes encompassing PG lipids carrying deuterated fatty acyl chains. (A) DMPC: DMPG-2H54, (B) DMPE: DMPG-2H54, (C) POPC: POPG-2H31, and (D) POPE: POPG-2H31, recorded at T = 310, 340, 310, and 320 K, respectively, are shown. The lipid composition was adjusted so that the molar ratio of zwitterionic lipid/PG is 3/1 and the total deuterated lipid represents 25 mol%. The order parameters are obtained from quadrupolar splittings, according to Eq. 1, in the absence (closed squares) or presence of 2 mol% PGLa (closed triangles), 2 mol% magainin 2 (open triangles), and PGLa: magainin 2 (1 mol % each; open squares), with an estimated uncertainty of 0.01 (corresponding to 1 kHz in measuring the quadrupolar splittings).
In a first step, mixed membranes made from one of the zwitterionic lipids, PE or PC, and the anionic PG were investigated. Either the zwitterionic or the anionic lipid was deuterated such that the effect of the peptides is selectively evaluated for each of the lipids. The corresponding order parameter profiles are shown in Figs. 3 and 4 for the eight lipid mixtures and display the characteristic signatures of bilayer packing, with a plateau of |SCD| values extending toward the 6th carbon position with an average value of 0.202 for POPC:POPG (T−Tm = 29 K relative to Tm of POPC), 0.233 for POPE:POPG (T−Tm = 15 K, relative to Tm of POPE (47)), 0.245 DMPE:DMPG (T−Tm = 15 K, relative to Tm of DMPE) as well as 0.230 and 0.209 for DMPC:DMPG (T−Tm = 15 K, relative to Tm of DMPC).
In the presence of 2 mol% magainin 2 or PGLa, the order parameters of the plateau region decrease by up to 15%, albeit the variations are quite large between peptides, lipid components and lipid mixtures (Figs. 3 and 4, open and closed triangles, respectively). Whereas the absolute order parameters reported above provide valuable insight into the lipid dynamics, changes upon addition of peptides are best visible when the relative order parameters are shown (Figs. 5 and 6). In this representation, the pure lipid in the absence of peptide serves as a reference with a set value of 1. When compared to PGLa (closed circles), magainin 2 (closed squares) tends to have a somewhat more pronounced disordering activity, although this is not systematically observed. Notably, the cationic peptides have a tendency to exhibit a more pronounced effect on the PG lipid (typically a 10–20% reduction; Fig. 6) when compared to the zwitterionic lipid in the same mixture (typically a 5–10% reduction; Fig. 5) or when compared to membranes made of a single lipid (cf. below, Fig. 7), a notable exception being DMPE/DMPG where the changes in the DMPE quadrupolar splittings are quite high (Fig. 5 B). Furthermore, in the mixed membranes, the PE lipids (Fig. 5, B and D) tend to be more affected by the peptides than the PC lipids in the analogous mixture (Fig. 5, A and C).
Figure 5.
2H order parameter profiles relative to peptide-free vesicles of mixed membranes encompassing zwitterionic lipids (PE or PC) carrying deuterated fatty acyl chains. (A) DMPC: DMPC-2H54: DMPG, (B) DMPE: DMPE-2H54: DMPG, (C) POPC: POPC-2H31: POPG, and (D) POPE: POPE-2H31: POPG recorded at T = 310, 340, 310, and 320K, respectively, in the presence of 2 mol% PGLa (closed circles), 2 mol% magainin 2 (closed squares), and PGLa: magainin 2 (1 mol % each; open triangles) are shown. The lipid composition was adjusted so that the molar ratio of zwitterionic lipid/PG is 3/1 and the total deuterated lipid represents 25 mol%. To see this figure in color, go online.
Figure 6.
2H order parameter profiles shown relative to peptide-free vesicles of mixed membranes encompassing PG lipids carrying deuterated fatty acyl chains (A) DMPC: DMPG-2H54, (B) DMPE: DMPG-2H54, (C) POPC: POPG-2H31, and (D) POPE: POPG-2H31 recorded at T = 310, 340, 310, and 320 K, respectively, in the presence of 2 mol% PGLa (closed circles), 2 mol% magainin 2 (closed squares) and PGLa: magainin 2 (1 mol% each; open triangles). The lipid composition was adjusted so that the molar ratio of zwitterionic lipid/PG is 3/1 and the total deuterated lipid represents 25 mol%. To see this figure in color, go online.
Figure 7.
2H order parameter profiles shown relative to peptide-free vesicles of (A) DMPC-2H54, (B) DMPE-2H54, and (C) DMPG-2H54 carrying two deuterated dimyristoyl chains, (D) POPC-2H31, (E) POPE-2H31, and (F) POPG-2H31 carrying a deuterated palmitoyl chain, in the presence of 2 mol% PGLa (closed circles), 2 mol% magainin 2 (closed squares), and PGLa: magainin 2 (1 mol% each; open triangles). The temperatures were T = 310 K (Tm = 297), 340 K (Tm = 323), 310 K (Tm = 296), 310 K (Tm = 271), 320 K (Tm = 298), and 310 K (Tm = 271) for the DMPC, DMPE, DMPG, POPC, POPE, POPG membranes, respectively (i.e., > 13 K above the phase transition temperature Tm). To see this figure in color, go online.
In a second step, membranes made up of only one lipid component were investigated (Fig. S2). For all membranes investigated, PC, PE, and PG with either 1,2-dimyristoyl or 1-palmitoyl-2-oleoyl fatty acyl chain composition, the bilayers contained 25% chain-deuterated lipid. It is noteworthy that the order parameters in pure PE lipids are considerably increased when compared to PC lipids (at temperatures that are 20 K above the gel-liquid crystalline phase transition) in agreement with previous investigations (24). For example, the plateau region of DMPE and POPE exhibit order parameters of 0.259 and 0.242, respectively (Fig. S2, B and E; splittings of 64.94 and 60.34 kHz, respectively), whereas that of DMPC and POPC are at 0.210 and 0.195, respectively (Fig. S2, A and D; splittings of 52.57 and 48.95 kHz, respectively). This reflects the increased hydrogen bonding capacity, more negative intrinsic curvature Jo, and the tighter packing of PE when compared to PC.
Furthermore, the zwitterionic lipids have quite different order parameters in the mixture with PG or when studied alone (Figs. 3 and S2). Whereas those of PE alone are higher, those of PC alone are lower when compared to the mixture. This reflects the higher phase transition temperature of PE (Tm = 298 K) when compared to the PE/PG mixture, the latter exhibiting a phase transition temperature representing an average of the two components (Tm < 283 K (47)). As a result, PE alone, being investigated closer to its phase transition temperature, is more ordered.
The decrease in order parameter upon addition of either 2 mol% PGLa or magainin 2 is around 5% (Fig. 7) and in average less pronounced when compared to the effects these peptides exert on the mixed membranes (reductions up to 25%, Figs. 5 and 6).
In a third step, both peptides were added in equimolar amounts (1 mol% each). For the mixed membranes, we focused our analysis on the effect on the PG fatty acyl chain where the effects of the peptides are stronger. In cases where both peptides remain aligned along the membrane surface (POPC/POPG, POPE/POPG, POPC, POPE and POPG; Figs. 1, C and D and 2, D and E; (20)), the effect of the peptide mixture on the deuterium order parameters approximates the average effects observed with 2 mol% of the individual peptides (Figs. 6, C and D and 7, D–F, open triangles). Interestingly, when the PGLa peptide shows a predominant transmembrane alignment (DMPC/DMPG, DMPE/DMPG and DMPC; Fig. 1, A and B; (20)), the disordering effects of the mixture are reduced when compared to the effects with 2 mol% of the individual peptides (Figs. 6, A and B and 7 A). Because the alignment of the peptides remains heterogeneous in DMPE and DMPG (Fig. 2, B and C), the relatively small changes in order parameters were not compared any further (Fig. 7, B and C).
Discussion
Fatty acyl chain saturation is the main driving force for PGLa realignment in the presence of magainin 2
Previously, it has been demonstrated that in the presence of equimolar amounts of magainin, the PGLa alignment is parallel to the surface of membranes made of partially unsaturated 1-palmitoyl-2-oleoyl phospholipids and transmembrane in dimyristoyl phospholipids (19, 20). In a related manner, when PGLa is studied in the absence of other peptides, its tilt angle in PC membranes made of saturated lipids changes as a function of the fatty acyl chain composition as well as the peptide-to-lipid ratio (21), whereas magainin adopts more stable in-plane configurations (18, 19, 20). These previous studies have focused on PC and PC/PG 3/1 membranes, and the results are confirmed here (Fig. 1, A and C). In order to explain this observation, it has been suggested that the cylindrical shape of the DMPC and DMPG lipids, when compared to the more inverted cone shape of the POPC and POPG lipids, favors the transmembrane insertion (19). Indeed, POPC tends to impose a small negative curvature strain on the membrane (J = −0.02), which is not the case for DMPC (J = +0.04) (22, 23). In the work presented here, this hypothesis was further tested by using PE lipids where this negative curvature strain is much increased (J = −0.316) (22). For completeness, it should be noted that the POPG lipid has an intrinsic curvature close to zero (16). Notably, all measurements were conducted at least 15 K above the gel-liquid crystalline phase transition temperature of the pure lipid, ensuring a homogenous liquid crystalline phase.
When PE/PG membranes are investigated, the same behavior is observed as with the corresponding PC/PG bilayers, clearly indicating that in these mixed membranes the overall shape of the lipids has a negligible effect on the PGLa alignment. Instead, other properties that distinguish the dimyristoyl from the palmitoyl-oleoyl fatty acyl chains must strongly influence the membrane alignment of PGLa.
Other interactions contribute to the membrane topology of amphipathic peptides
The predominantly in-planar 15N solid-state NMR spectra of PGLa in POPC (20), POPG, and POPE (Fig. 2, D and E), and the strong transmembrane contributions in DMPE and DMPG bilayers all confirm the importance of fatty acyl chain saturation over molecular shape. However, matters become somewhat more complex when the PGLa alignment in DMPE, DLPE, and DMPG membranes are considered in more detail. The 15N solid-state NMR spectra of PGLa in DMPG shows mixed alignments where in-plane (or powder pattern) and transmembrane orientations coexist (Fig. 2 C). In a related manner, DMPE exhibits spectral contributions in the transmembrane and in-plane (or powder pattern) range. However, because of the high transition temperature of this lipid, with concomitant difficulties to maintain the sample in an intact and equilibrated state during preparation and NMR measurements (taking several hours in the spectrometer), it is difficult to draw unambiguous conclusions from this data. In contrast to other saturated PE membranes (Figs. 1 B and 2 B), the DLPE bilayer results in a higher proportion of in-planar alignments (Fig. 2 A).
These data show that equilibria exist where both head group and fatty acyl chains have an influence on the Gibbs free energy. During the transition of a peptide from an in-planar to a transmembrane alignment changes in the Gibbs free energy ΔG occur due to hydrophobic interactions (ΔGh), energy contributions from placing polar side-chains into the hydrophobic interior (ΔGp), where applicable a pH-dependent energy of discharge (ΔGd, e.g. histidines), and hydrophobic mismatch energy (ΔGm) ((48) and references cited therein). In addition, conformational changes may occur and influence the equilibrium (ΔGc). For example, it has been measured that changes from random coil to helical add −0.5 kJ/mole per residue during membrane partitioning of the peptide (49).
Furthermore, it has been stipulated that direct interactions between magainin 2 and PGLa occur (ΔGi) (50). Indeed, the negative charge of the magainin glutamate-19 has been identified as an important residue (12), and a cross linking studied revealed the preferential formation of parallel heterodimers in membranes (51). However, no experimental evidence for a long-lived, stable, direct association into hetero-oligomeric structures could be found so far (14). Therefore, electrostatic and/or dipole interactions may relate to this glutamate.
In addition, H-bonding and electrostatic interactions between the peptides and the lipids, or the peptide and other molecules, may have an impact on the in-plane to transmembrane transition (ΔGj). For example, the topology of the amphipathic peptide LAH4 is strongly affected by the PG content of the membrane as well as the buffer composition (45).
Importantly, changes in entropic (lipophobic) (48, 52) and van der Waals interactions (ΔGl + ΔGW) may derive from changes in the lipid curvature strain, lipid structure, and/or peptide-lipid interactions ((48) and references cited therein). Hydrophobic mismatch and inherent curvature strain/lipid shape have indeed been shown to have an effect (19, 20). Therefore, ΔG = ΔGh + ΔGp + ΔGd + ΔGm + ΔGl + ΔGW + ΔGc +ΔGi +ΔGj.
The most important conclusion from the 15N solid-state NMR topological analysis is that the dominant driving force for PGLa transmembrane alignment is not curvature and molecular shape. If this were the case, the peptide should be stabilized in an in-planar alignment at the PE membrane interface regardless of fatty acyl chain composition (22, 23). It should not exhibit the experimentally observed transmembrane alignments in DMPE-rich membranes (Figs. 1 B and 2 B) but rather resemble the predominantly in-planar line shapes observed with POPC- or POPE-rich lipid bilayers (Figs. 1, C and D and 2 D). These data, together with the pronounced disordering effects of the in-planar oriented amphipathic peptides, point to a more important role of the fatty acyl chain saturation.
Amphipathic peptides at the membrane interface cause strong membrane disordering of saturated fatty acyl chains
Indeed, the profound decrease in the 2H order parameters provides evidence that the lipid contributions ΔGl + ΔGW play an important role in the process of PGLa realignment. Interestingly, it was recently suggested that synergistic activity is related to PGLa lowering the membrane intrinsic curvature-mediated free energy barrier of PE-rich membranes for magainin 2 insertion, a mechanism that does not necessarily require a pairwise interaction of the peptides (16).
A peptide that is partitioning into the membrane interface pushes apart the lipid head groups without completely filling the membrane hydrophobic phase, a situation that has been treated by a number of theoretical approaches (25, 53, 54, 55). Such a situation results in considerable positive curvature strain, an increased disorder of the lipid fatty acyl chains, and membrane thinning. Profound changes in order parameters are indeed observed for PGLa and magainin 2 as well as mixtures of both peptides when they both remain oriented parallel to the membrane surface (Figs. 5, 6, and 7) concomitant with membrane thinning (56). The strain is considerably released when PGLa adopts a transmembrane alignment to the extent that the order parameters closely resemble the ones of the pure lipid membrane (e.g., Figs. 6, A and B and 7 A). Therefore, the unfavorable packing of the lipid fatty acyl chains at in-planar alignments has been recognized as an important driving force for transmembrane alignments (25). It is tempting to speculate that the disordering effect due to in-planar peptide insertion is particularly pronounced in saturated membranes, whereas the cis double bond of an unsaturated chain induces a kink and packing disorder already in the case of pure lipid bilayers. It seems likely that in the direct neighborhood of the kinked oleoyl chain, the palmitoyl chains are also more disordered. Therefore, the additional disorder by the peptide and thus the driving force for realignment is less pronounced for POPX (one saturated chain) when compared to DMPX lipids (two saturated chains) or for the much shorter DLPE fatty acyl chains.
To further substantiate the lipophobic effect as a driving force, the order parameters that are calculated from the deuterium solid-state NMR quadrupolar splittings of the palmitoyl or myristoyl chains reveal the following about the interactions of PGLa and magainin 2 with the membranes investigated:
First, when 2 mol% peptide are added, the order parameter decreases (locally) by up to 25% (Fig. 6 B). For many but not all membranes, the most pronounced relative changes occur in the membrane interior, as expected from an in-planar partitioning into the membrane interfaces. As a consequence, the interface is densely packed (and remains relatively well ordered), whereas the fatty acyl chains kink to fill the “void” underneath the peptide. Interestingly, many order parameter profiles exhibit pronounced discontinuities around the central segments of the fatty acyl chains regardless of the presence or absence of a neighboring kinked oleoyl chain at position 8–9 (cf. Figs. 5, 6, and 7).
Second, the effects are more pronounced for the mixed membranes made from a zwitterionic lipid and PG when compared to the bilayers made from a single lipid. For the mixed membranes, the typical decreases are 5–10% (Fig. 5) and 10–20% (Fig. 6) for the zwitterionic and anionic components, respectively, but only around 5% for the pure lipid membranes (Fig. 7). This difference is probably due to electrostatic attraction between the cationic peptide and the anionic lipids, which results in a higher peptide density in the mixed membranes, and within the mixed bilayers preferential association with the PG lipids (57, 58, 59). It has been shown previously that such electrostatic effects can be reduced by increasing the salt concentration (60).
Third, in general, magainin 2 has a more pronounced effect than PGLa, which could be due to the bigger polar angle of the former when compared to PGLa (61) (a helical wheel and hydrophobic moment analysis are shown in (14); Fig. 5 A). Thus, it seems likely that magainin 2 partitions less deeply into the membrane interface, leaving more space at the level of the fatty acyl chain, thereby causing additional “disorder” at the lipids (61). Indeed, 2 mol% magainin 2 causes a massive decrease of the order parameters of DMPG (−20%), DMPE (−15%), and DMPC (−10%) (Figs. 5, A and B and 6, A and B). These large changes occur at the lipid segments >10, except for DMPG in combination with DMPC, where a dip (−20%) is observed at position 6 with considerable disordering up to position 14 (−12%).
Fourth, and most strikingly, related changes in the order parameters are observed when the individual peptides are compared to the PGLa/magainin mixtures provided that the in-planar alignment of PGLa is maintained (i.e., in the presence of the oleoyl unsaturation). However, the lipid order in the presence of both peptides is considerably increased when compared to the individual in-planar peptides when a transmembrane alignment of PGLa is predominant (when at the same time magainin 2 remains surface bound (19, 20)). This is clearly the case for DMPC/DMPG and DMPE/DMPG (Figs. 5 B and 6, A and B), and also for DMPC (Fig. 7 A), where globally the changes are less pronounced.
Finally, it should be mentioned that whereas in PC, PE/PG 3/1, and PC/PG 3/1 membranes, the saturation of the fatty acyl chains has been found the determining factor for the PGLa alignment (Fig. 1; (19, 20)), the pure DMPG membranes exhibit a somewhat different behavior (Fig. 2 C). Possibly, interactions of the cationic residues of PGLa with the high surface charge density of 100% DMPG stabilizes the in-planar alignments (thereby contributing to ΔGj) similar to the cationic amphipathic designer peptide LAH4 (45). In the latter case, it has also been speculated that the reduction of membrane pore-forming activity in POPG is correlated with a different interfacial penetration depth (53, 62).
Conclusions
The magainin-induced realignment of PGLa also in DMPE/DMPG membranes (Fig. 1 B) clearly indicates that the fatty acyl chain saturation rather than the molecular shape plays a dominant role governing the in-plane transmembrane equilibrium of this amphipathic peptide. The peptide undergoes a clear fatty acyl chain saturation-dependent transition in most lipids investigated here and previously but intermediate situations are probably observed in some samples (e.g., Fig. 2, B and C). Theoretical analysis has shown that such disordering is a strong driving force for topological changes, oligomerization and pore formation (25, 55). Indeed, a strong disordering of the deuterated myristoyl and palmitoyl chains by magainin 2 and PGLa has been demonstrated here.
Our studies of the magainin 2-PGLa interactions pursue two different goals: namely, first, as discussed in this article, to understand what are the driving forces for PGLa transmembrane realignment in the presence of magainin 2, and second, how synergistic antimicrobial activity works. Notably, the magainin-driven topological change of PGLa is probably not involved in killing bacteria more efficiently because it does not occur when the peptides are investigated in E. coli lipid extracts or in membranes carrying unsaturated fatty acyl chains (as they occur in natural membranes). However, the topological changes that are stimulated by another peptide/protein domain through the lipophobic effect can have an important role in the regulation of membrane proteins and peptides.
Author Contributions
N.H. performed experiments, prepared figures, and helped in the writing sections of the manuscript. B.B. organized funding, designed the experiments, helped in the analysis and discussion, and wrote the article.
Acknowledgments
We are most grateful to Georg Pabst and Karl Lohner for discussion; Regina Leber, Michael Pachler from the University of Graz, and Daniela Nomura from Sao Paolo for providing spontaneous curvatures of lipids before publication; Christopher Aisenbrey, Evgeniy Salnikov, and Dennis Wilkins Juhl for many fruitful discussions on this subject; and to Delphine Hatey for her help with peptide synthesis. Christopher Aisenbrey also helped in preparing the figures.
The financial contributions of the Agence Nationale de la Recherche (projects membrane DNP 12-BSV5-0012, MemPepSyn 14-CE34-0001-01, and the LabEx Chemistry of Complex Systems 10-LABX-0026_CSC), the University of Strasbourg, the CNRS, the Région Alsace and the RTRA International Center of Frontier Research in Chemistry are gratefully acknowledged.
Editor: Michael Brown.
Footnotes
Two figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30936-6.
Supporting Material
References
- 1.Doi Y., Bonomo R.A., van Duin D., Gram-Negative Committee of the Antibacterial Resistance Leadership Group (ARLG)a Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the antibacterial resistance leadership group. Clin. Infect. Dis. 2017;64(Suppl 1):S30–S35. doi: 10.1093/cid/ciw829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohner K., editor. Development of Novel Antimicrobial Agents: Emerging Strategies. Horizon Scientific Press; Norfolk, UK: 2001. [Google Scholar]
- 3.Hancock R.E., Haney E.F., Gill E.E. The immunology of host defence peptides: beyond antimicrobial activity. Nat. Rev. Immunol. 2016;16:321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- 4.Domingues M.M., Santos N.C., Castanho M.A. Antimicrobial peptide rBPI21: a translational overview from bench to clinical studies. Curr. Protein Pept. Sci. 2012;13:611–619. doi: 10.2174/138920312804142101. [DOI] [PubMed] [Google Scholar]
- 5.Salnikov E.S., Raya J., Bechinger B. Alamethicin supramolecular organization in lipid membranes from 19F solid-state NMR. Biophys. J. 2016;111:2450–2459. doi: 10.1016/j.bpj.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechinger B. The SMART model: soft membranes adapt and respond, also transiently, in the presence of antimicrobial peptides. J. Pept. Sci. 2015;21:346–355. doi: 10.1002/psc.2729. [DOI] [PubMed] [Google Scholar]
- 7.Perrin B.S., Jr., Tian Y., Cotten M.L. High-resolution structures and orientations of antimicrobial peptides piscidin 1 and piscidin 3 in fluid bilayers reveal tilting, kinking, and bilayer immersion. J. Am. Chem. Soc. 2014;136:3491–3504. doi: 10.1021/ja411119m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oren Z., Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47:451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Lee D.-K., Bhunia A., Ramamoorthy A. Detergent-type membrane fragmentation by MSI-78, MSI-367, MSI-594, and MSI-843 antimicrobial peptides and inhibition by cholesterol: a solid-state nuclear magnetic resonance study. Biochemistry. 2015;54:1897–1907. doi: 10.1021/bi501418m. [DOI] [PubMed] [Google Scholar]
- 10.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 11.Westerhoff H.V., Zasloff M., Juretić D. Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor cells and liposomes. Eur. J. Biochem. 1995;228:257–264. [PubMed] [Google Scholar]
- 12.Matsuzaki K., Mitani Y., Miyajima K. Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry. 1998;37:15144–15153. doi: 10.1021/bi9811617. [DOI] [PubMed] [Google Scholar]
- 13.Glattard E., Salnikov E.S., Bechinger B. Investigations of the synergistic enhancement of antimicrobial activity in mixtures of magainin 2 and PGLa. Biophys. Chem. 2016;210:35–44. doi: 10.1016/j.bpc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Marquette A., Salnikov E.S., Bechinger B. Magainin 2-PGLa interactions in membranes - two peptides that exhibit synergistic enhancement of antimicrobial activity. Curr. Top. Med. Chem. 2016;16:65–75. doi: 10.2174/1568026615666150703115701. [DOI] [PubMed] [Google Scholar]
- 15.Zerweck J., Strandberg E., Ulrich A.S. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep. 2017;7:13153. doi: 10.1038/s41598-017-12599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leber R., Pachler M., Pabst G. Synergism of antimicrobial frog peptides couples to membrane intrinsic curvature Strain. Biophys. J. 2018;114:1945–1954. doi: 10.1016/j.bpj.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marquette A., Bechinger B. Biophysical investigations elucidating the mechanisms of action of antimicrobial peptides and their synergism. Biomolecules. 2018;8 doi: 10.3390/biom8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bechinger B. Insights into the mechanisms of action of host defence peptides from biophysical and structural investigations. J. Pept. Sci. 2011;17:306–314. doi: 10.1002/psc.1343. [DOI] [PubMed] [Google Scholar]
- 19.Strandberg E., Zerweck J., Ulrich A.S. Synergistic insertion of antimicrobial magainin-family peptides in membranes depends on the lipid spontaneous curvature. Biophys. J. 2013;104:L9–L11. doi: 10.1016/j.bpj.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salnikov E.S., Bechinger B. Lipid-controlled peptide topology and interactions in bilayers: structural insights into the synergistic enhancement of the antimicrobial activities of PGLa and magainin 2. Biophys. J. 2011;100:1473–1480. doi: 10.1016/j.bpj.2011.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremouilhac P., Strandberg E., Ulrich A.S. Conditions affecting the re-alignment of the antimicrobial peptide PGLa in membranes as monitored by solid state 2H-NMR. Biochim. Biophys. Acta. 2006;1758:1330–1342. doi: 10.1016/j.bbamem.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Kollmitzer B., Heftberger P., Pabst G. Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter. 2013;9:10877–10884. doi: 10.1039/C3SM51829A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negahbani N. Technical University of Graz; 2016. Intrinsic Curvature Value of DMPC. Master Thesis. [Google Scholar]
- 24.Perly B., Smith I.C., Jarrell H.C. Effects of replacement of a double bond by a cyclopropane ring in phosphatidylethanolamines: a 2H NMR study of phase transitions and molecular organization. Biochemistry. 1985;24:1055–1063. doi: 10.1021/bi00325a038. [DOI] [PubMed] [Google Scholar]
- 25.Zemel A., Ben-Shaul A., May S. Perturbation of a lipid membrane by amphipathic peptides and its role in pore formation. Eur. Biophys. J. 2005;34:230–242. doi: 10.1007/s00249-004-0445-9. [DOI] [PubMed] [Google Scholar]
- 26.Bechinger B. Understanding peptide interactions with the lipid bilayer: a guide to membrane protein engineering. Curr. Opin. Chem. Biol. 2000;4:639–644. doi: 10.1016/s1367-5931(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 27.Verly R.M., de Moraes C.M., Bechinger B. Structure and membrane interactions of the antibiotic peptide dermadistinctin k by multidimensional solution and oriented 15 N and 31 P solid-state NMR spectroscopy. Biophys. J. 2009;96:2194–2203. doi: 10.1016/j.bpj.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aisenbrey C., Michalek M., Bechinger B. Solid-state NMR approaches to study protein structure and protein-lipid interactions. In: Kleinschmidt J.H., editor. Lipid-Protein Interactions: Methods and Protocols. Springer; 2013. pp. 357–387. [DOI] [PubMed] [Google Scholar]
- 29.Loffredo M.R., Ghosh A., Mangoni M.L. Membrane perturbing activities and structural properties of the frog-skin derived peptide esculentin-1a(1-21)NH2 and its diastereomer Esc(1-21)-1c: correlation with their antipseudomonal and cytotoxic activity. Biochim. Biophys. Acta. 2017;1859:2327–2339. doi: 10.1016/j.bbamem.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Bechinger B., Opella S.J. Flat-coil probe for NMR spectroscopy of oriented membrane samples. J. Magn. Reson. 1991;95:585–588. [Google Scholar]
- 31.Bertani P., Raya J., Bechinger B. 15N chemical shift referencing in solid state NMR. Solid State Nucl. Magn. Reson. 2014;61–62:15–18. doi: 10.1016/j.ssnmr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Rance M., Byrd R.A. Obtaining high-fidelity spin-1/2 powder spectra in anisotropic media: phase-cycled Hahn echo spectroscopy. J. Magn. Reson. 1983;52:221–240. [Google Scholar]
- 33.Davis J.H., Jeffrey K.R., Higgs T.P. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 1976;42:390–394. [Google Scholar]
- 34.Bloom M., Davis J.H., Mackay A.L. Direct determination of the oriented sample NMR spectrum from the powder spectrum for the systems with local axial symmetry. Chem. Phys. Lett. 1981;80:198–202. [Google Scholar]
- 35.McCabe M.A., Wassall S.R. Rapid deconvolution of NMR powder spectra by weighted fast Fourier transformation. Solid State Nucl. Magn. Reson. 1997;10:53–61. doi: 10.1016/s0926-2040(97)00024-6. [DOI] [PubMed] [Google Scholar]
- 36.Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q. Rev. Biophys. 1977;10:353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- 37.Douliez J.P., Léonard A., Dufourc E.J. Restatement of order parameters in biomembranes: calculation of C-C bond order parameters from C-D quadrupolar splittings. Biophys. J. 1995;68:1727–1739. doi: 10.1016/S0006-3495(95)80350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storm R. VEB Fachbuchverlag; Leipzig, Germany: 1967. Wahrscheinlichkeitsrechnung, Mathematische Statistik, Statistische Qualitätskontrolle. [Google Scholar]
- 39.Bechinger B., Sizun C. Alignment and structural analysis of membrane polypeptides by 15N and 31P solid-state NMR spectroscopy. Concepts Magn. Reson. 2003;18A:130–145. [Google Scholar]
- 40.Gopinath T., Mote K.R., Veglia G. Simultaneous acquisition of 2D and 3D solid-state NMR experiments for sequential assignment of oriented membrane protein samples. J. Biomol. NMR. 2015;62:53–61. doi: 10.1007/s10858-015-9916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dürr U.H., Gildenberg M., Ramamoorthy A. The magic of bicelles lights up membrane protein structure. Chem. Rev. 2012;112:6054–6074. doi: 10.1021/cr300061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resende J.M., Verly R.M., Bechinger B. Membrane interactions of phylloseptin-1, -2, and -3 peptides by oriented solid-state NMR spectroscopy. Biophys. J. 2014;107:901–911. doi: 10.1016/j.bpj.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michalek M., Salnikov E.S., Bechinger B. Structure and topology of the huntingtin 1-17 membrane anchor by a combined solution and solid-state NMR approach. Biophys. J. 2013;105:699–710. doi: 10.1016/j.bpj.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strandberg E., Tiltak D., Ulrich A.S. Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim. Biophys. Acta. 2012;1818:1764–1776. doi: 10.1016/j.bbamem.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Perrone B., Miles A.J., Bechinger B. Lipid interactions of LAH4, a peptide with antimicrobial and nucleic acid transfection activities. Eur. Biophys. J. 2014;43:499–507. doi: 10.1007/s00249-014-0980-y. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi S., Hong T., Hong M. Solid-state NMR investigations of peptide-lipid interaction and orientation of a beta-sheet antimicrobial peptide, protegrin. Biochemistry. 2002;41:9852–9862. doi: 10.1021/bi0257991. [DOI] [PubMed] [Google Scholar]
- 47.Pozo Navas B., Lohner K., Pabst G. Composition dependence of vesicle morphology and mixing properties in a bacterial model membrane system. Biochim. Biophys. Acta. 2005;1716:40–48. doi: 10.1016/j.bbamem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Bechinger B. Towards membrane protein design: pH-sensitive topology of histidine-containing polypeptides. J. Mol. Biol. 1996;263:768–775. doi: 10.1006/jmbi.1996.0614. [DOI] [PubMed] [Google Scholar]
- 49.Wieprecht T., Apostolov O., Seelig J. Thermodynamics of the alpha-helix-coil transition of amphipathic peptides in a membrane environment: implications for the peptide-membrane binding equilibrium. J. Mol. Biol. 1999;294:785–794. doi: 10.1006/jmbi.1999.3268. [DOI] [PubMed] [Google Scholar]
- 50.Strandberg E., Zerweck J., Ulrich A.S. Influence of hydrophobic residues on the activity of the antimicrobial peptide magainin 2 and its synergy with PGLa. J. Pept. Sci. 2015;21:436–445. doi: 10.1002/psc.2780. [DOI] [PubMed] [Google Scholar]
- 51.Hara T., Kodama H., Matsuzaki K. Effects of peptide dimerization on pore formation: antiparallel disulfide-dimerized magainin 2 analogue. Biopolymers. 2001;58:437–446. doi: 10.1002/1097-0282(20010405)58:4<437::AID-BIP1019>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 52.Duneau J.P., Sturgis J.N. Lateral organization of biological membranes: role of long-range interactions. Eur. Biophys. J. 2013;42:843–850. doi: 10.1007/s00249-013-0933-x. [DOI] [PubMed] [Google Scholar]
- 53.Zemel A., Ben-Shaul A., May S. Modulation of the spontaneous curvature and bending rigidity of lipid membranes by interfacially adsorbed amphipathic peptides. J. Phys. Chem. B. 2008;112:6988–6996. doi: 10.1021/jp711107y. [DOI] [PubMed] [Google Scholar]
- 54.Zuckermann M.J., Heimburg T. Insertion and pore formation driven by adsorption of proteins onto lipid bilayer membrane-water interfaces. Biophys. J. 2001;81:2458–2472. doi: 10.1016/S0006-3495(01)75892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aranda-Espinoza H., Berman A., Safran S. Interaction between inclusions embedded in membranes. Biophys. J. 1996;71:648–656. doi: 10.1016/S0006-3495(96)79265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ludtke S., He K., Huang H. Membrane thinning caused by magainin 2. Biochemistry. 1995;34:16764–16769. doi: 10.1021/bi00051a026. [DOI] [PubMed] [Google Scholar]
- 57.Mason A.J., Martinez A., Bechinger B. The antibiotic and DNA-transfecting peptide LAH4 selectively associates with, and disorders, anionic lipids in mixed membranes. FASEB J. 2006;20:320–322. doi: 10.1096/fj.05-4293fje. [DOI] [PubMed] [Google Scholar]
- 58.Wieprecht T., Apostolov O., Seelig J. Membrane binding and pore formation of the antibacterial peptide PGLa: thermodynamic and mechanistic aspects. Biochemistry. 2000;39:442–452. doi: 10.1021/bi992146k. [DOI] [PubMed] [Google Scholar]
- 59.Bechinger B. Structure and function of membrane-lytic peptides. Crit. Rev. Plant Sci. 2004;23:271–292. [Google Scholar]
- 60.Miyazaki Y., Aoki M., Matsuzaki K. Interaction of antimicrobial peptide magainin 2 with gangliosides as a target for human cell binding. Biochemistry. 2012;51:10229–10235. doi: 10.1021/bi301470h. [DOI] [PubMed] [Google Scholar]
- 61.Zemel A., Ben-Shaul A., May S. Membrane perturbation induced by interfacially adsorbed peptides. Biophys. J. 2004;86:3607–3619. doi: 10.1529/biophysj.103.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogt T.C., Bechinger B. The interactions of histidine-containing amphipathic helical peptide antibiotics with lipid bilayers. The effects of charges and pH. J. Biol. Chem. 1999;274:29115–29121. doi: 10.1074/jbc.274.41.29115. [DOI] [PubMed] [Google Scholar]
- 63.Michalek M., Salnikov E.S., Bechinger B. Membrane interactions of the amphipathic amino terminus of huntingtin. Biochemistry. 2013;52:847–858. doi: 10.1021/bi301325q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.