Abstract
Patients and physicians in the 21st century require new tools to manage the growing burden of chronic illness. For providers responsible for the care of diabetic patients, developments in information management, real-time health education and feedback, and new approaches to self-monitoring and insulin delivery hold great promise to improve the quality and safety of diabetes care. This article will briefly highlight some of the major developments in the field, and the ways these technologies can be integrated into a typical practice.
A 52-year-old man with type 2 diabetes, hyperlipidemia, and hypertension returns to your office. A glucometer and a smart phone sit on the table at his side. Since his last visit, you’ve received a few messages from him through the patient portal about elevated fasting blood sugars, asking for your advice. His blood pressure is elevated today, and as you log into his chart the EMR reminds you that he is overdue for a foot exam and a colonoscopy referral. He needs refills on three medications and wants to ask you about a rash. Your next patient will be roomed in 12 minutes.
As clinicians are being asked to manage and prevent chronic illnesses, the scene described above is becoming all too common. For the care of diabetic patients, new approaches are needed that allow interaction and education outside of the traditional office visit. These tools will need to be easy to access and not overburden the provider or the patient. Ideally, these technologies should also improve patient safety and health outcomes. This article presents a number of tools, organized from low-tech solutions to higher tech solutions, which can improve care for diabetic patients in the primary care setting.
Collecting and Using Self-Monitored Blood Glucose Data
How can the health care provider make use of the self-monitored blood glucose (SMBG) data that the patient brings to clinic? How often should patients be asked to check their blood sugars, and how should the clinician access that data and modify treatment plans? Self-monitored blood glucose is clearly important to improve outcomes and decrease complications in patients with type 1 diabetes (T1D) and intensively treated type 2 diabetes (T2D). However, in adults with T2D who are not treated with intensive medication regimens, meta-analyses of trials using SMBG have not shown improvement in clinically meaningful endpoints, and the SMBG is associated with higher financial costs and patient dissatisfaction.1,2 This is probably due to the somewhat random collection of SMBG data by patients, and the failure to develop a treatment plan that uses the collected information.
Several studies have shown that the most effective use of SMBG is to link that data into a structured clinical action plan involving the patient and health care provider. The STeP trial was a cluster randomized control trial of 483 insulin naive adults with poorly controlled T2D. The intervention was collection of blood glucose monitoring in a standardized pattern for three days prior to clinic visits, and review of the data with the clinician. Over 12 months of follow-up, there was a small but significant improvement in HbA1c in the intervention arm compared to the control group, and a much higher likelihood of medication change (new diabetes medication or adjustment in dose of existing medication).3 Patients also reported higher levels of self-confidence and autonomy in diabetes management in the structured self-monitoring arm of the trial.4 This is just one of many examples of structured glucose collection plans that could be integrated into an office workflow to maximize the utility of information and utilize scarce resources including time and money for both patients and providers. (See Figure 1.)
Figure 1.
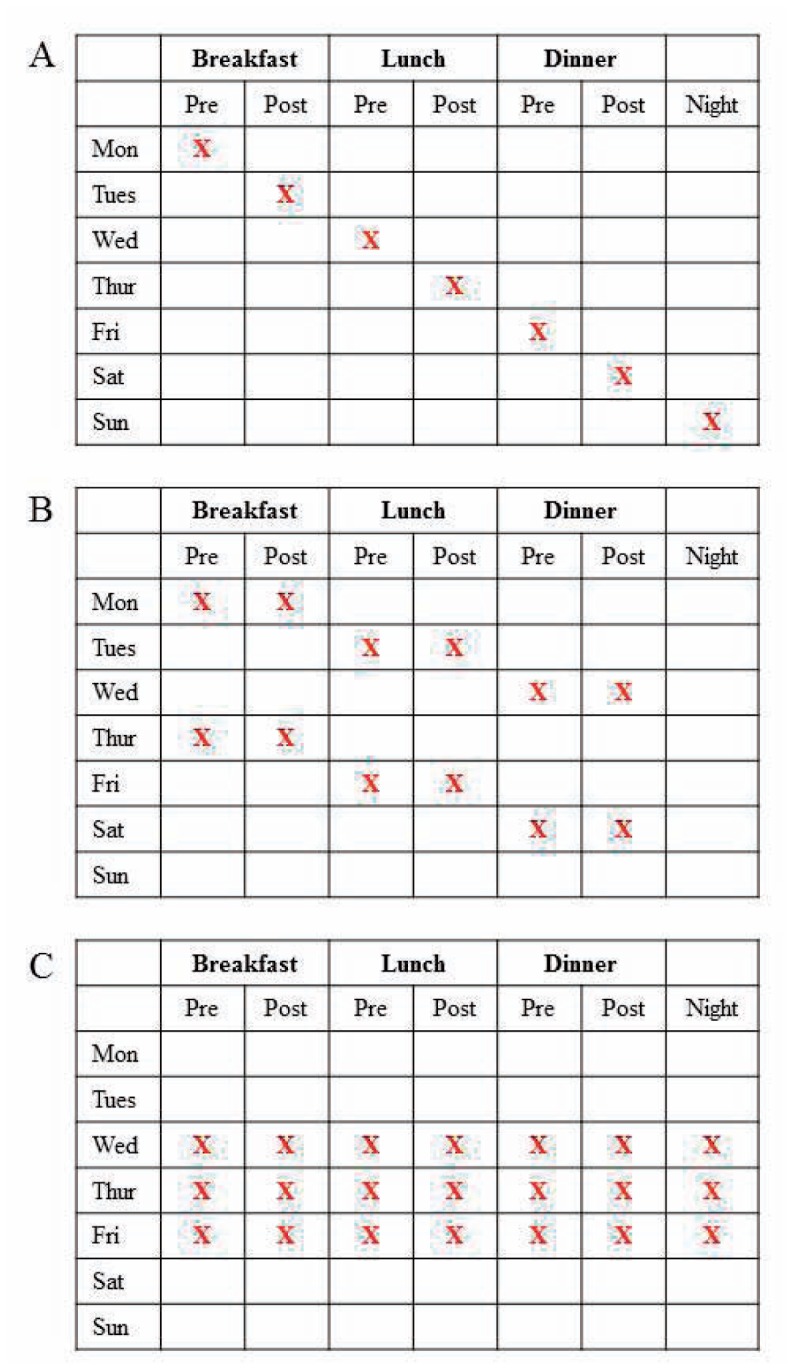
Examples of structured self-monitoring of blood glucose plans for patients not treated with multiple daily injections of insulin.
A) Staggered testing plan.
B) Testing in Pairs plan. Assesses the impact of prandial medications, dietary choices, and activity.
C) 7-point Self-Monitoring plan. Comprehensive monitoring for over short period of time, as used in the STeP trial.
In the simplest implementation, patients would be asked to bring paper diaries of glucose readings to the office visit. Alternatively, most glucometer manufacturers have readily available software that can be downloaded from the web or installed via CD on an office PC to enable providers to print summarized data from the patient’s glucometer. The provider would also need an inexpensive transfer cable, obtained from glucometer manufacturers or an electronics store. This is an easy technological upgrade that can be provided at minimal cost and training, and should assist in improving the quality of the actionable data available at an office visit. Similarly, point of care HbA1c testing is an easy upgrade to provide immediate results during a clinic visit.
Provider and Patient Responses to Blood Glucose Monitoring
How can the patient and primary care provider use blood glucose monitoring to improve glycemic control? Linking the data to (i) real time decision support for the patient, and (ii) an aggregated summary of patient data for the clinician, are promising strategies. Several software approaches have been developed to achieve these goals. The ABACUS trial was a small, randomized controlled trial of adults with poorly controlled DM using multiple dose insulin therapy. The intervention was to provide a bolus dose calculator on the patient’s glucometer that automatically recommends an insulin dose based on blood sugar reading and carbohydrate intake. The trial documented improved glycemic control and patient satisfaction.5 Glucometers that provide this bolus calculation are commercially available (e.g. Accu-Chek Aviva Expert). Many patients are using one of numerous free or inexpensive apps for iPhone or Android that help to record medication administration, glucose logs and diets. Notably, while health behavior applications are popular with patients and frequently recommended by physicians, more than 80% of diabetes applications available for download have no privacy policies. Many actively transmit individual patient data to third parties without obtaining permission from the patient, suggesting against carte blanch recommendations of these tools.6
At the level of the health care delivery system, several companies are developing platforms for data collection and analysis. One such commercial platform (WellDoc) was tested in a cluster randomized trial that connected patient generated glucose monitoring with automated text message based responses to blood glucose values, telephonic and web based health coaching, and quarterly panel management reports for the provider. The study demonstrated significant improvement in glycemic control compared to usual standard of care without worsening distress or diabetes symptoms.7 The technology is now an FDA licensed medical device, delivered to patients as a prescription mobile phone application. Another company, Glooko, markets a unified population management system to health plans and clinics. This includes hardware for downloading a wide range of glucometers, remote monitoring and telemedicine interventions for glycemic control, and a provider population management application.
Behavioral Modification and Real Time Feedback
The effective treatment of diabetes begins with patient education. In-person visits with certified diabetes educators have been documented to improve diabetes self-education and glycemic control, but not all patients have access to these sessions. Unfortunately, CDC analysis of insurance claims found that less than 10% of newly diagnosed diabetic patients participated in a diabetes self-management education class within the first year of diagnosis.8 This unmet need has prompted study about the use of electronic delivery systems to deliver diabetes education. Most of these trials are small, and not surprisingly there is a great deal of heterogeneity in study design and patient population. A Cochran meta-analysis of 16 trials of 3,578 participants using computer-based interventions for diabetes education and self-care documented a small decrease in HgbA1c with computer only interventions, with larger effect in the group using text-messaging.9 Text-based health coaching has also been studied in pediatric populations; the technology was well received by the participations and led to higher self-reported rates of medication adherence and self-efficacy, but no clear improvement in glycemic control.10 Not surprisingly, dozens of trials are currently registered in the NIH Clinical trial database testing text message based strategies for behavioral intervention in general and selected populations of diabetics. As an example, the recently completed Mobile Insulin Titration Intervention (MITI) trial showed that patient reported fasting blood sugars with RN review and protocolled weekly adjustment of insulin glargine doses led to a significantly greater number of patients achieving optimal basal insulin doses during the trial with fewer clinic visits and increased patient satisfaction.11 As discussed above, some of these services will be incorporated into health care systems with added fees covered by the patient and health plans.
Continuous Glucose Monitoring
The recent development of continuous glucose monitoring (CGM) devices has expanded the capacity for self-monitoring. CGM’s measure interstitial glucose levels in the subcutaneous tissue via a small implanted catheter that is connected to an electrochemical recorder. The hardware can be discretely worn under clothing. Several challenges persist with the technology: 1) the CGM must be calibrated using a glucometer several times daily, 2) the glucose reading is not as accurate as capillary glucose measurements, and 3) the interstitial fluid lags behind the plasma compartment. Despite these limitations, the use of CGM has been shown to improve glycemic control and to avoid of hypoglycemia more effectively than SMBG alone in adults with T1D. Meta-analyses suggest that benefit from CGM is most pronounced in patients who wear the sensor for more days each week, and for patients who have higher HbA1c levels. (See Figure 2.)12
Figure 2.
Continuous glucose monitoring.
A) Medtronic Dexcom G5 Receiver. Image used with permission of the company.
B) Sample data download from a CGM.
Insulin Pump Delivery and Closed Loop Delivery Systems
Technology also provides novel options to delivery of insulin to the patient. Syringes and pre-loaded pens work well for many patients using once daily or multiple daily injections (MDI), but some patients benefit from continuous subcutaneous insulin injection (CSII).13 Figure 3 shows several commercially available insulin pumps. The most commonly used insulin pumps on the market use push button control panels to program and direct basal and bolus delivery from an insulin reservoir (e.g. pumps from Medtronic, Animus). Increasingly, pumps are being designed with a touch screen control interface (e.g. Tandem t:slim), tubeless delivery systems (e.g. Insulet Omnipod), or wireless communication between the pump and the patient’s glucometer as well as the CGM device. At the opposite end of the complexity spectrum, a simple pump is being marketed for patients with T2D that delivers basal insulin at one of three pre-set constant basal rates, and allows the user to provide boluses on demand in two unit increments (Valeritas V-Go). Patients receiving MDI insulin therapy who might be candidates for an insulin pump should be referred to an Endocrinologist for full discussion of treatment options.
Figure 3.
Examples of insulin pumps in clinical use. All images used with permission of manufacturer. A) Medtronic MiniMed 530G. B) Insulet Omnipod. C) Valeritas V-Go. D) Tandem t:slim.
What is the evidence that insulin pumps improve outcomes compared to MDI? In patients with T1D, meta-analyses have shown better glycemic control (as measured by HbA1c) and lower rates of hypoglycemia with CSII in adults14 and children15 when compared with MDI. Whether or not the use of a pump will lead to improvements in long term clinical outcomes compared to optimized MDI is still uncertain. One recent study from Sweden showed that among adults with T1D followed for 6.8 years, patients using insulin pump therapy had a 27% decreased risk of all-cause mortality, a 45% reduction in fatal coronary heart disease, and a 42% decrease in fatal cardiovascular disease.16 The combination of a CGM device with CSII (“sensory augmented pump therapy”) has been shown to have greater efficacy at reducing HbA1c than MDI with SMBG alone (7.5% vs 8.1%), and with greater likelihood of achieving a HbA1c < 7%. These results were achieved without increasing the frequency or severity of hypoglycemic events.17
The most exciting technological development on the horizon is the development of closed loop insulin delivery devices. Known by a variety of names such as “artificial pancreas,” “bionic pancreas,” or “artificial beta cell,” these systems use computer algorithms to automatically modulate drug delivery through an insulin pump based on real time data from a continuous glucose monitor.18, 19 Some manufacturers are also integrating glucagon delivery systems to treat hypoglycemia in “bihormonal” delivery systems.20 No device has received FDA approval, but large national trials to establish safety of the devices and algorithms are ongoing. Washington University in St. Louis is one of nine centers expecting to begin enrollment of T1D patients in these trials.
Conclusion
The diabetic patient returns to your clinic for follow-up in six months. He has been collecting blood glucoses in a standardized pattern and uploading to your information management system through a secure website. The website analyzed his trends and suggested a dose increase in his basal insulin based on a pattern of fasting hyperglycemia. Today, his HbA1c today has fallen by 0.5%. Based on his answers to an intake survey, he’s been receiving customized text messages every week with links to reduced carbohydrate recipes and reminders to log his pedometer count. His weight is down five pounds, and his blood pressure is now controlled.
Management of diabetes requires a multidisciplinary team approach, centered on the patient, to achieve ideal outcomes. The use of technology and effective information management should be an integral part of the approach, applied in a personalized way to each patient’s particular needs. From low-tech structured self-monitoring of blood glucose to cutting edge closed loop insulin delivery systems, these approaches hold the potential for patients to achieve improved diabetes control with greater safety. These strategies may also help caregivers provide higher quality care more efficiently at relatively low cost.
Biography
Brian D. Muegge, MD, PhD, (left), is a Clinical Fellow, and Garry S. Tobin, MD, (right), is a Professor, Division of Endocrinology, Metabolism, and Lipid Research, Department of Medicine, Washington University School of Medicine, St. Louis.
Contact: gtobin@dom.wustl.edu


Footnotes
Disclosures
BDM: Fellowhsip Trianing Grant 2T32DK007120-41.
References
- 1.Malanda ULL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. The Cochrane database of systematic reviews. 2012;1:CD005060. doi: 10.1002/14651858.CD005060.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farmer AJ, Perera R, Ward A, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ (Clinical research ed) 2012;344:e486. doi: 10.1136/bmj.e486. [DOI] [PubMed] [Google Scholar]
- 3.Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes care. 2011;34(2):262–7. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher L, Polonsky WH, Parkin CG, Jelsovsky Z, Petersen B, Wagner RS. The impact of structured blood glucose testing on attitudes toward self-management among poorly controlled, insulin-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96(2):149–55. doi: 10.1016/j.diabres.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler R, Cavan DA, Cranston I, et al. Use of an insulin bolus advisor improves glycemic control in multiple daily insulin injection (MDI) therapy patients with suboptimal glycemic control: first results from the ABACUS trial. Diabetes care. 2013;36(11):3613–9. doi: 10.2337/dc13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blenner S, Köllmer M, Rouse A, Daneshvar N, Williams C, Andrews L. Privacy Policies of Android Diabetes Apps and Sharing of Health Information. Jama. 2016;315(10):1051–1052. doi: 10.1001/jama.2015.19426. [DOI] [PubMed] [Google Scholar]
- 7.Quinn C, Shardell M, Terrin M, Barr E, Ballew S, Gruber-Baldini A. Cluster-Randomized Trial of a Mobile Phone Personalized Behavioral Intervention for Blood Glucose Control. Diabetes Care. 2011;34(9):1934–1942. doi: 10.2337/dc11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R, Shrestha SS, Lipman R, Burrows NR, Kolb LE, Rutledge S. Diabetes self-management education and training among privately insured persons with newly diagnosed diabetes--United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2014;63(46):1045–9. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6346a2.htm. [PMC free article] [PubMed] [Google Scholar]
- 9.Pal K, Eastwood SV, Michie S, et al. Computer-based diabetes self-management interventions for adults with type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2013;3:CD008776. doi: 10.1002/14651858.CD008776.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2007;23(12):1332–8. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy N, Moynihan V, Nilo A, et al. The Mobile Insulin Titration Intervention (MITI) for Insulin Adjustment in an Urban, Low-Income Population: Randomized Controlled Trial. J Med Internet Res. 2015;17(7):e180. doi: 10.2196/jmir.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ (Clinical research ed) 2011;343:d3805. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickup J. Insulin-Pump Therapy for Type 1 Diabetes Mellitus. New Engl J Medicine. 2012;366(17):1616–1624. doi: 10.1056/NEJMct1113948. [DOI] [PubMed] [Google Scholar]
- 14.Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941–51. doi: 10.1007/s00125-008-0974-3. [DOI] [PubMed] [Google Scholar]
- 15.Sherr JL, Hermann JM, Campbell F, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87–91. doi: 10.1007/s00125-015-3790-6. [DOI] [PubMed] [Google Scholar]
- 16.Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18, 168 people with type 1 diabetes: observational study. BMJ (Clinical research ed) 2015;350:h3234. doi: 10.1136/bmj.h3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. The New England journal of medicine. 2010;363(4):311–20. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 18.Thabit H, Tauschmann M, Allen J, et al. Home Use of an Artificial Beta Cell in Type 1 Diabetes. The New England Journal of Medicine. 2015;373(22):2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–33. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 20.Russell S, El-Khatib F, Sinha M, et al. Outpatient Glycemic Control with a Bionic Pancreas in Type 1 Diabetes. New Engl J Medicine. 2014;371(4):313–325. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]




