Abstract
Annually, there are nearly 3000 new cases and 500 deaths from prostate cancer in Missouri. When treatment is appropriate and necessary, radiotherapy offers similar cure rates to prostatectomy, with fewer long-term sexual side effects and little effect on urinary continence. Radiotherapy is delivered with external beam or implanted radioactive sources (brachytherapy). In high-risk disease, combinations of external beam and brachytherapy offers improved biochemical control. Following prostatectomy, salvage radiotherapy should be initiated as soon as possible.
Introduction
In 2017 the estimated number of new cases and deaths from prostate cancer (PCa) in the United States are 161,360 and 26,730, respectively. For the state of Missouri, the estimated new cases and deaths from PCa are 2990 and 500, respectively.1 From birth to death, the probability of developing PCa is 1 in 8 men. There has been a sharp reduction in PCa incidence of more than 10% annually from 2010 to 2013. This drop is attributed to decreased prostate-specific antigen (PSA) testing following the US Preventive Services Task Force recommendations against the routine use of PSA testing because of growing concerns about overdiagnosis and overtreatment.2 PCa is the third leading cause of cancer death in patients 60 to 79 years old and second in the ≥ 80 year old group.
The National Comprehensive Cancer Network (NCCN) PCa guidelines include a variety of radiation therapy modalities as part of the standard of care for the definitive treatment of PCa:
Very low risk patients (T1c, Gleason score ≤ 6, PSA < 10 ng/mL, fewer than 3 prostate biopsy cores positive, ≤ 50% cancer in each core, PSA density <0.15 ng/mL/g) with a life expectancy ≥ 20 years, external beam radiation therapy (EBRT) or brachytherapy (BT);
Low risk patients (T1–T2a, Gleason score ≤ 6, PSA < 10 ng/mL) with a life expectancy ≥ 10 years, EBRT or BT;
Intermediate risk patients (T2b–T2c or Gleason score 7 or PSA 10 – 20 ng/mL), EBRT ± 4 to 6 months of androgen deprivation therapy (ADT) ± BT or BT alone;
High-risk patients (T3a or Gleason score 8 – 10 or PSA > 20 ng/mL) EBRT + 2 to 3 years of ADT, or EBRT + BT ± 2 to 3 years of ADT.3
Indications for adjuvant EBRT following prostatectomy are: tumor extension through the prostate capsule or invasion into the seminal vesicles (pT3), positive margins, Gleason score 8–10, seminal vesicle involvement, or detectable PSA. Patients who have an undetectable PSA after prostatectomy with a subsequent detectable PSA that increases on two or more occasions without detectable distant metastases should be offered salvage EBRT.3 All PCa patients with localized PCa should have a consultation with both a surgeon and a radiation oncologist to make an informed decision regarding their care.
Which Approach Is Better: Active Surveillance, Surgery, or Radiotherapy?
The 10 year outcomes of the Prostate Testing for Cancer and Treatment (ProtecT) trial from the United Kingdom has provided valuable insights into the management of localized PCa.4 The trial recruited 1643 men 50 to 69 years old. Of these 545 men underwent active surveillance, 553 surgery, and 545 radiotherapy. For the participants, the median follow-up was 10 years, the median age was 62 years, the median PSA was 4.6 (range, 3.0 to 19.9), 77% were Gleason 6 and 21% were Gleason 7, and 76 % were T1c and the remaining T2. There were 17 prostate-cancer–specific deaths overall: 8 in the active surveillance group, 5 in the surgery group, and 4 in the radiotherapy group. The difference was not statistically significant among groups.
Metastases developed in more men in the active-monitoring group (33 men) than in the surgery group (13 men) or the radiotherapy group (16 men) (P=0.004). Higher rates of disease progression were seen in the active-monitoring group (112 men) than in the surgery group (46 men) or the radiotherapy group (46 men) (P<0.001). In summary, at a median of 10 years, prostate-cancer–specific mortality was low irrespective of the treatment assigned, with no significant difference among treatments. Surgery and radiotherapy were associated with lower incidences of disease progression and metastases than was active monitoring, while 44% of the patients who were assigned to active monitoring did not receive radical treatment and avoided side effects.5
Of the three treatments, prostatectomy had the greatest negative effect on sexual function and urinary continence, and although there was some recovery, these outcomes remained worse in the prostatectomy group than in the other groups throughout the trial. By year 6, the percentage of men reporting erections firm enough for intercourse were: 17 % in the prostatectomy group, 27 % in the radiotherapy group, and 30% in the active surveillance group. By year 6, 17% of men in the prostatectomy group were using pads, as compared with 8% in the active-monitoring group, and 4% in the radiotherapy group.
Bowel function was worse in the radiotherapy group at 6 months than in the other groups but then recovered somewhat, except for the increasing frequency of bloody stools; bowel function was unchanged in the other groups. Urinary voiding and nocturia were worse in the radiotherapy group at 6 months but then mostly recovered and were similar to the other groups after 12 months. No significant differences were observed among the groups in measures of anxiety, depression, or general health-related or cancer-related quality of life.
These results are very important and relevant to our patients. From a radiation oncology perspective, we can inform low and intermediate risk PCa patients that prostatectomy, active surveillance, and radiotherapy offer a similar PCa-specific mortality. More importantly, for these low and intermediate risk PCa patients, undergoing radiotherapy can avoid long term incontinence from prostatectomy, and provide better sexual function compared to surgery.
Radiation Therapy Innovations for Prostate Cancer
Over the past decade radiation techniques have improved to allow better coverage of tumor volumes with better sparing of adjacent normal structures. In PCa, this means a lower incidence of bowel, urinary and sexual side effects.
Image-Guided Intensity Modulated Radiation Therapy (IG-IMRT)
The approach to radiation therapy has been available for more than a decade. It employs multiple radiation beams that intersect within the target volume. The dose of radiation from an individual beam is modulated to maximize energy deposition within the tumor. In addition, we have employed implanted fiducial markers that allow daily localization of the prostate using diagnostic quality x-rays. This limits the margin traditional utilized for setup uncertainties to less than 0.5 cm. A smaller margin around the target means less radiation dose to the rectum, bladder and penile structures.
With the use of IG-IMRT, we have been able to conduct radiation dose escalation studies that have demonstrated an improved biochemical control compared to lower, conventional doses of radiation therapy. Men treated with dose escalated radiation therapy have a significantly lower rate of treatment failure and subsequent salvage therapy. In addition, we have gained considerable knowledge about the radiation factors that may contribute to urinary and bowel side effects. This allows radiation oncologists to tailor the radiation plan to individual patients and optimize their expected outcomes.
Proton Beam Radiati on Therapy
Proton beam radiation therapy capitalizes on a unique physical property of high energy protons generated from a cyclotron. The accelerated charged particles travel through tissue until reaching a depth determined by their energy. Once they reach that depth, the remainder of the radiation dose is deposited in a sharp Bragg peak with no dose going beyond that point. When multiple proton beams are used, a very sharp and tight radiation dose distribution is created. This modality is especially attractive when tumors are in close proximity to sensitive organs. PCa is one of the more common indications in which proton therapy is utilized.
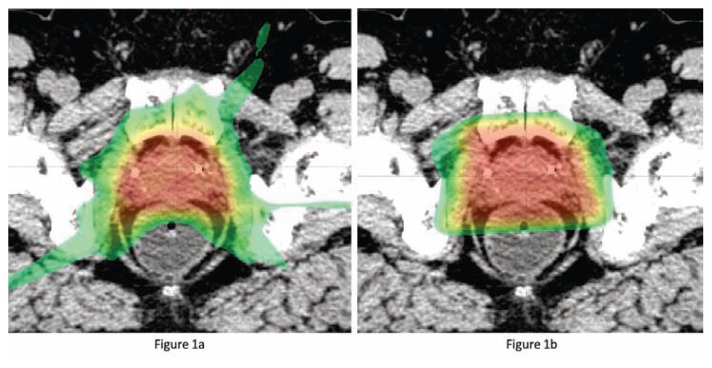
At Washington University in St. Louis we are collaborating with investigators from Massachusetts General Hospital and the Harvard Medical School conducting a randomized clinical trial of IG-IMRT versus proton beam radiation in men with low and intermediate risk PCa. The PARTIQoL™ trial is seeking to measure and compare relative the impact of the two modalities on patient quality of life after treatment. Figure 1 provides a comparison of these two treatment modalities.
Figure 1.
(a) Axial CT slice showing conformality of intensity modulated radiation therapy. Radio-opaque markers are apparent in the anterior aspect of the prostate gland. (b) Axial CT slice showing conformality of proton beam radiation therapy in the same patient as panel a. More sparing of the anterior-lateral rectal wall and less dose to peripheral tissues is apparent.
Image-Guided Brachytherapy
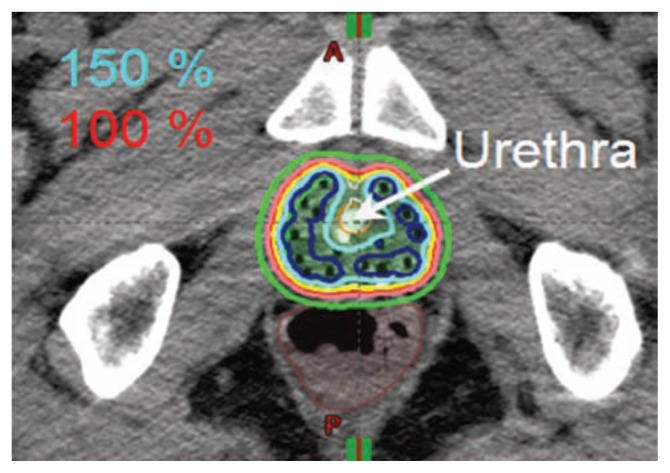
An alternative to external beam radiation therapy with either x-rays or protons is the use of implanted radioactive sources (brachytherapy) directly into the prostate gland. This technique was introduced in the St. Louis region by physicians at Washington University. There are two general approaches to prostate brachytherapy, low-dose rate (LDR) permanent radioactive seed implant and high-dose rate (HDR) temporary radioactive seed implant. Figure 2 demonstrates the radiation dose distribution for HDR. Both approaches utilize real time ultrasound image guidance to assure accurate placement of sources into the prostate while avoiding delivering high doses to the rectum, urethra and bladder. In addition, CT or MR imaging of the implant following the procedure. This imaging allows quality assessment of the implant and adjustment of the implant if areas of underdosing are identified.
Figure 2.
HDR implant dose distribution, allow demonstrating HDR sparing the urethra.
In some cases of high risk PCa, a combination of external beam radiation therapy and brachytherapy will be recommended. A recent Canadian study, the ASCENDERT trial, has reported superior biochemical control compared to external beam radiation alone. Of the 398 participants, 200 were assigned to the EBRT boost and 198 to the LDR boost. Compared with the 78 Gy EBRT boost, men randomized to the LDR boost were twice as likely to be free of biochemical failure at a median followup of 6.5 years (P=.004). The 5-, 7-, and 9-year Kaplan- Meier biochemical progression-free survival estimates were 89%, 86%, and 83% for the LDR boost versus 84%, 75%, and 62% for the EBRT boost (P<.001). The LDR boost benefited both intermediate- and high-risk patients. From a toxicity standpoint, the LDR boost increased the risk of needing temporary catheterization and/or requiring incontinence pads. At 5 years the cumulative incidence of grade 3 genitourinary events was 18.4% for the LDR boost, versus 5.2% for the EBRT boost (P<.001). The 5-year cumulative incidence of grade 3 gastrointestinal events was 8.1% for the LDR boost, versus 3.2% for the EBRT boost (p=0.124). The 5-year prevalence of grade 3 gastrointestinal toxicity was lower than the cumulative incidence for both arms (1.0% vs 2.2%, respectively).6
Because of the improved biochemical progression-free survival, there is an increased interest in the radiotherapy community to boost intermediate and high risk patients with brachytherapy. Brachytherapy also has the advantage of shortening the treatment duration. At Washington University, a brachytherapy boost will reduce the EBRT treatments from 44 to 25 for the intermediate and high risk patients.
Stereotactic Body Radiation Therapy
Stereotactic body radiation therapy (SBRT) is a highly conformal and precise method of delivering ultra-high dose radiation therapy. Also called Stereotactic Ablative Radiation Therapy (SABR), this technique will ablate malignant tissue in just five treatments delivered over 1–2 weeks.7 This accelerated scheduling is appealing to patients due to its convenience to patients over the traditional course of radiation that takes 5 to 8 weeks of daily treatments.
Physicians at Washington University in St. Louis have participated in a phase II trial of SBRT and that has demonstrated excellent tolerance to the extremely short course of therapy. This year we will be participating in a national phase III trial comparing the standard long course of external beam radiation therapy to the short course of SBRT. Short term outcomes of this approach have been reported by several single institutional series with exceptional early results.
Injectable Hydrogel Rectal Spacer
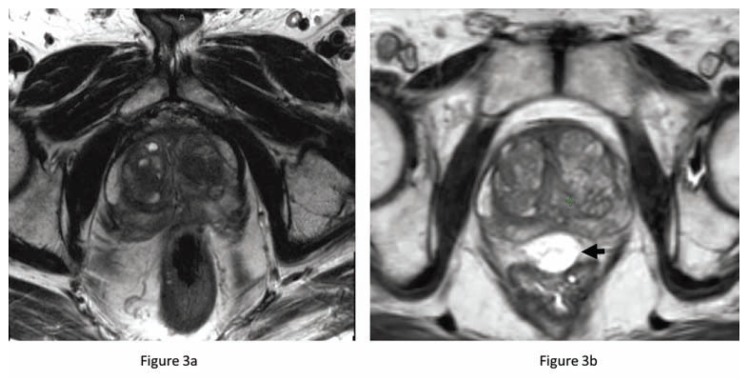
A new product has received FDA approval to further improve the outcomes of men receiving curative radiation therapy. A biodegradable hydrogel, SpaceOAR™, is injected transperineal into the prerectal space and increases the distance between the anterior rectum and the posterior border of the prostate (Figure 3). This increased distance of 9–11 mm reduces the volume of rectum receiving a high dose of radiation and has significantly improved the quality of life (rectal domain) in men treated with dose escalated IG-IMRT. At 15 months after treatment, only 12% of men treated with the rectal spacer had a 10 point decline in bowel symptom quality of life compared to 21% treated without it. Mature results from this randomized clinical trial suggest that this hydrogel not only improves rectal toxicity outcomes, it may also improve urinary and sexual function compared to men treated without it.8
Figure 3.
Prostate MRI (T2 sequence) before (a) and after (b) hydrogel injection. The rectum touches the prostate on the pre-injection MRI but is displaced away from the prostate by the T2 bright spacer (black arrow). This added space results in significantly less radiation dose to the adjacent rectum.
Conclusions
Radiotherapy is an integral part of the modern multidisciplinary management of PCa which is often underutilized or delayed. Greater efforts to educate our patients and the medical community are necessary so we can best serve our patients.
Biography
Hiram A. Gay, MD, and Jeff M. Michalski, MD, MBA, (above), is Professor and Vice Chair, Radiation Oncology, Washington University School of Medicine, St. Louis, Missouri.
Contact: jmichalski@wustl.edu

Footnotes
Disclosure
None reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA U.S.P.S.T. Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 3.Prostate Cancer. NCCN Clinical Practice Guidelines in Oncology. 2017. [cited 2017 5/11/2017]; Version 2.2017[Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [DOI] [PubMed]
- 4.Hamdy FC, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 5.Donovan JL, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodda S, et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):286–295. doi: 10.1016/j.ijrobp.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Kupelian P, et al. Stereotactic body radiation therapy for prostate cancer: Rational and reasonable. Practical radiation oncology. 2015;5(3):188–92. doi: 10.1016/j.prro.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Mariados N, et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. International journal of radiation oncology, biology, physics. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]