Abstract
Prostate cancer is common, and recent efforts in clinical management have focused on identifying patients who could be candidates from less aggressive management or who could benefit from more aggressive therapy. As prostate cancer histology, especially Gleason score, plays a critical role in predicting patient outcomes, attempts have been made to refine histologic classification and reporting in prostate cancer to facilitate patient risk stratification. This review discusses recent updates in prostate cancer grading and reporting.
Introduction
Gleason grading of acinar adenocarcinoma of the prostate represents one of the earliest and most successful applications of evidence-based medicine in routine clinical practice. For several decades, the original Gleason system was used to grade acinar adenocarcinomas based on architectural features, with excellent correlation with clinical outcomes. However, in the past ten to fifteen years, it has become apparent that further refinement of the original Gleason grades is possible, with improved correlation with patient outcomes. Indeed, much of the recent prostate pathology literature has focused on attempts to optimize pathologic grading and reporting of prostatic adenocarcinoma in support of ongoing clinical efforts to select optimal treatment for prostate cancer (PCa) patients by minimizing treatment-related morbidity while maximizing therapeutic benefit and quality of life. The most recent revision of the Gleason grading system put forth by the International Society of Urological Pathology (ISUP) and adopted in the World Health Organization (WHO) 2016 classification of prostate tumors is discussed herein, including the motivation for the revised grading and associated grade grouping, as well as controversies regarding their application and use in routine practice. (In this article, Gleason grade or Gleason pattern refers to the current ISUP consensus on Gleason grading,1 unless otherwise specified.) Diagnosis and clinical implications of intraductal carcinoma also are covered, and general recommendations for use of immunohistochemistry in the differential diagnosis of PCa are briefly discussed. Variant histologic patterns of prostatic adenocarcinoma and rare prostate tumors such as stromal neoplasms and lymphomas are not discussed.
Prostate Adenocarcinoma Grading
In 2014, the ISUP convened a panel of expert urologic pathologists and clinicians to address issues related to PCa grading;1 the recommendations of the panel were adopted by the WHO in the 2016 classification of prostate tumors.2 The primary issues addressed were histologic patterns of Gleason patterns 4 and 5 and adoption of a reporting system to group tumors according to Gleason score and associated prognosis.
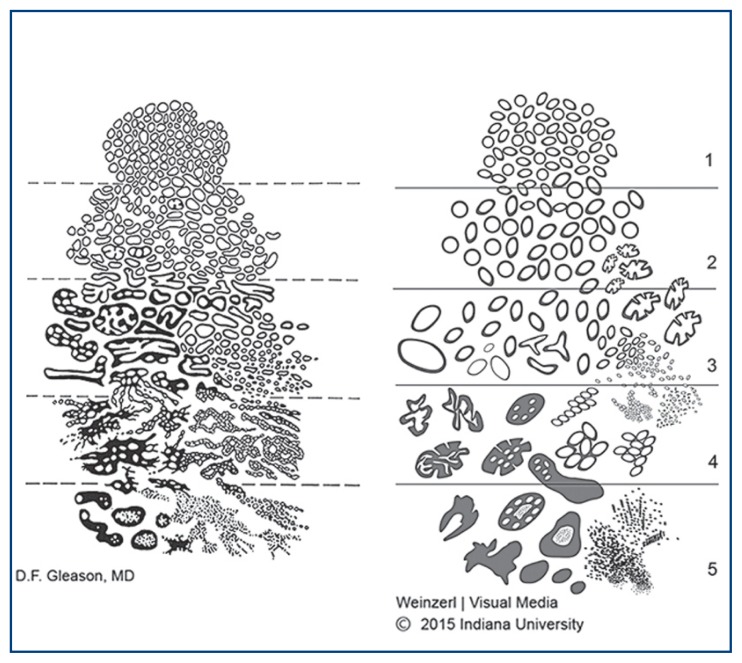
Figure 1 illustrates the current state of Gleason grading in comparison with the original system proposed by Gleason himself. The main updates to Gleason grading in the 2014 ISUP consensus are related to diagnosis of Gleason pattern 4: all cribriform, glomeruloid, and fused or poorly formed glands visible at 10x magnification are considered Gleason pattern 4, though identification of only occasional poorly formed or fused glands between other wise well-formed glands does not warrant a diagnosis of pattern 4. Additionally, the ISUP consensus specifies that mucinous adenocarcinoma, in which extravasated pools of mucin are seen in the stroma around cancer glands, should be graded based on the architecture of the glands themselves rather than uniformly be assigned Gleason pattern 4. Pools of mucin associated with other wise well-formed tumor glands should be considered Gleason pattern 3 rather than pattern 4. The consensus points also include that any amount of comedonecrosis warrants a diagnosis of pattern 5, branched glands are permitted in Gleason pattern 3, and intraductal carcinoma (discussed later) should be reported but not graded.1 Prostate adenocarcinomas that are exempt from grading are tumors with morphologic changes secondary to androgen deprivation, chemotherapy, or radiation. Histologic features of treatment effect include luminal collapse, poorly formed glands, nuclear pyknosis, and overall decrease in the number of glands. Metastatic tumor deposits also are not assigned a Gleason score. Alpha-reductase inhibitors (e.g. finasteride), cryotherapy, and ultrasound ablation have not been shown to have significant effect on PCa morphology, and it is appropriate to grade carcinomas in patients who have received these treatments.3
Figure 1.
Illustrates the current state of Gleason grading in comparison with the original system proposed by Gleason himself.
Prostate Adenocarcinoma Grade Grouping
In order to facilitate better communication of the pathologic diagnosis to clinicians and patients, the ISUP consensus recommends adoption of so-called grade grouping, as proposed by Epstein et al.1,4 The grade groups (Table 1) correspond well with patient prognosis and are reasonably straightforward to adopt, since they are based on Gleason score.4 In essence, Gleason scores ≤6 are grouped into the grade group representing the best overall prognosis (grade group 1). Notably, Gleason scores <6 have all but ceased to exist in current clinical practice. Gleason score 7 is separated into Gleason 3+4 (grade group 2) and Gleason 4+3 (grade group 3), a move that is supported by well-established evidence showing a significantly worse prognosis in Gleason 4+3 tumors compared to Gleason 3+4. Gleason score 8 tumors comprise grade group 4, and Gleason score 9 or 10 falls into the worst prognostic group (grade group 5). Translation of Gleason scores 6–10 into grade groups 1–5 is designed, at least in part, to clearly communicate to patients that Gleason score 6 is a well-differentiated tumor with an overall excellent prognosis. In contrast, a patient receiving a diagnosis of Gleason score 6 on a scale of 2–10 may be understandably confused when told that his tumor has essentially the best prognosis for PCa. The hope is that a diagnosis of “grade group 1” can prevent overtreatment of Gleason 6 tumors by more clearly communicating the indolent nature of these tumors. It is currently recommended that the Gleason score be reported in combination with the grade group in prostate biopsies and resections.1,5
Table 1.
Grade group by corresponding Gleason score.
| Grade group | Gleason score |
|---|---|
| 1 | ≤6 (usually 3+3) |
| 2 | 7 (3+4) |
| 3 | 7 (4+3) |
| 4 | 8 (4+4, 3+5, 5+3) |
| 5 | 9, 10 (4+5, 5+4, 5+5) |
Although the underlying motivation for changes in PCa grading and grade grouping are well intentioned, there are lingering issues related to diagnosis and reporting of high-grade adenocarcinoma (Gleason patterns 4 and 5) in particular. One critical point is that cases with a tertiary Gleason pattern were explicitly excluded in the description of the grade groups;4 as such, grade groups cannot be applied in cases with tertiary patterns. By convention, tertiary pattern is defined as involving <5% of the tumor volume and being of higher grade than either the primary (most common) or secondary (second most common) grade in a prostatectomy specimen. Tertiary patterns are not diagnosed in prostate biopsies; the highest grade is assigned as the secondary pattern (provided that it is not the most common pattern), even if it comprises less than 5% of the tumor volume.6
One of the primary goals for refinement of Gleason score 7 is identification of patients with Gleason 3+4 tumors who might be candidates for active surveillance. Unfortunately, grade grouping does not address the critical question of how much Gleason pattern 4 is too much for a patient to be considered for active surveillance, as the split between grade groups 2 (3+4) and 3 (4+3) is based on an arbitrary threshold of Gleason pattern 4 comprising more than half of the tumor volume. Recent studies to evaluate the prognostic impact of Gleason pattern 4 have focused on how best to quantitate Gleason pattern 4 (e.g., 10% increments, quartiles, etc.) to correlate with patient outcomes, and on the prognostic impact of specific architectural patterns within the Gleason pattern 4 category (e.g., cribriform versus glomeruloid versus poorly formed glands).7–9 In general, it appears as though cribriform architecture bears an overall worse prognosis compared with glomeruloid or poorly formed glands.7,8 Importantly, diagnosis of poorly formed glands (defined in one study as “glands with no or rare lumens, elongated compress glands, and elongated nests”) shows relatively poorly interobserver agreement, even among expert urologic pathologists (κ=0.34).10 Additionally, other histologic features besides gland architecture (particularly mucinous fibroplasia/collagenous micronodules) have been shown to confer a worse prognosis than would be expected based on Gleason score alone, though there are no clear recommendations at this time as to how that information should be included in the tumor grade or in the surgical pathology report.8 The practicing pathologist is encouraged to keep these points in mind, pending inevitable further refinement of classification and reporting.
As with Gleason pattern 4, grade grouping as currently recommended does not address complexities in the prognostic impact of varying amounts of Gleason pattern 5 in PCa. Mounting evidence shows that there are significant differences in prognosis for Gleason score 8 tumors (4+4, 3+5, 5+3), in particular related to the amount of Gleason pattern 5 that is present in the tumor.9,11,12 In fact, even focal Gleason pattern 5 (any amount, less than 20% of the tumor) has a significant prognostic impact, with tumors showing >20% pattern 5 having an even worse prognosis.9 Knowing that even focal (1–2%) pattern 5 confers a significantly worse prognosis than absence of pattern 5, and that the prognostic impact is similar for all percentages up to 20%, it is worrisome that there is relatively poor interobserver agreement in the diagnosis of Gleason pattern 5, even among urologic pathologists (κ=0.376).9,13
Quantification of High-Grade Adenocarcinoma
Although a consensus has not yet been reached on issues related to diagnosis and quantitation of Gleason patterns 4 and 5 (as discussed immediately above), the ISUP and College of American Pathologists (CAP) do currently recommend reporting the percent of tumor that is Gleason pattern 4 or 5.1,5 One can certainly imagine that it could be helpful to know that a tumor reported as Gleason score 3+4=7 is actually 90% Gleason pattern 3 with only 10% “poorly formed glands,” the diagnosis of which may be in the eye of the beholder. In contrast, one might pursue a more aggressive treatment plan in a patient with a Gleason 3+4=7 tumor if the report clearly indicated that the pattern 4 component comprised 40% of the tumor and showed predominantly cribriform architecture. The intent is to provide detailed information to treating clinicians such that patients may be optimally selected for more or less aggressive management, in keeping with long-standing efforts by urologic clinicians to tailor treatment based on predicted prognosis (e.g., through application of nomograms). Additionally, it is purported that routine reporting of the percent Gleason patterns 4 and 5 will be useful for future studies on outcomes.6
Intraductal Proliferations
Another primary point from the recent ISUP consensus is that intraductal carcinoma should be reported but not included in the Gleason score, qualified by a statement that intraductal carcinoma is almost invariably associated with aggressive invasive adenocarcinoma, even when the invasive component seen in a prostate biopsy is Gleason score 6.1,14 In the 2016 WHO Bluebook, intraductal carcinoma is defined as malignant prostatic epithelial cells filling prostatic ducts and large acini, with an intact basal cell layer, and having either A) a solid or dense cribriform (more solid areas than lumen formation) architecture or B) a loose cribriform or micropapillary pattern with associated comedonecrosis or marked nuclear atypia (nuclear size at least 6x normal).2 Some authors have highlighted limitations in the diagnosis and reporting of intraductal carcinoma.15 As intraductal carcinoma is a relatively new diagnosis, the primary differential diagnosis is discussed briefly here.
The main differential for intraductal proliferations are high-grade prostatic intraepithelial neoplasia (HGPIN), intraductal prostatic carcinoma (IDC), and extension of urothelial carcinoma into prostatic ducts.16 All of these lesions have a retained basal cell layer, which can be demonstrated immunohistochemically with antibodies such as p63 and/or cytokeratin 34βE12 (often used in a cocktail along with alpha-methyl acyl coenzyme A racemase, AMACR), differentiating these lesions from invasive carcinoma. HGPIN-like adenocarcinomas have been described, but these also lack basal cells.17 Immunostaining for basal cells is primarily recommended when the differential diagnosis is IDC versus invasive carcinoma and the distinction would have an impact on Gleason scoring (e.g., cribriform IDC versus cribriform invasive carcinoma in a background of otherwise well-formed invasive tumor glands). Use of basal cell markers is probably not useful in cases that also harbor frank high-grade invasive carcinoma, as the presence of IDC is not especially important in this setting.16
Differentiation of HGPIN from IDC can be difficult on morphologic grounds alone, and some have proposed use of PTEN immunostaining (with or without the slightly less helpful ERG immunostaining) to differentiate these lesions: IDC frequently shows loss of PTEN and reactivity for ERG, with the opposite findings in HGPIN. However, atypical intraductal proliferations equivocal for IDC versus HGPIN by morphology are associated with invasive carcinoma in 64% of re-biopsy cases when PTEN is lost, compared with 50% when PTEN staining is retained.18 Hence, some experts simply recommend rebiopsy in these “in-between” cases that exceed the degree of atypia typically seen in HGPIN but do not meet the criteria for IDC, without further evaluation by immunohistochemistry.16
Immunostaining is particularly useful in differentiation of IDC versus extension of urothelial carcinoma into prostatic ducts. A panel of prostate versus urothelial markers can be applied, including PSA, PSAP, prostein and/or NKX3.1 for prostate origin versus GATA3, 34βE12, and/or p63 for urothelial origin.
It is important to note that ductal carcinoma of the prostate (formerly referred to as endometrioid variant in reference to its columnar epithelial cell morphology) is a separate entity from what is diagnosed as intraductal carcinoma, despite having a similar name.2
Prostate Immunohistochemistry: Potential Pitfalls
As many laboratories utilize a prostate cocktail immunostain (usually including p63 and 34βE12 as basal cell markers and AMACR for staining of epithelial cells in HGPIN and/or carcinoma), it is worth briefly considering a few possible pitfalls in interpreting these stains. Stains useful in differentiating PCa from urothelial carcinoma are mentioned in the discussion of intraductal carcinoma above.
One consideration is the presence or absence of basal cells in focal areas of small glands. The basal cell layer may be incomplete or even only focally retained in foci of partial atrophy, adenosis, or in outpouchings of HGPIN. Careful scrutiny is required to determine whether the basal cell layer in focal small glands is truly lost or merely incomplete. All of these lesions may also show some degree of reactivity for AMACR. A diagnosis of focal glandular atypia or atypical small acinar proliferation may be warranted, if histologic and immunophenotypic features are not definitive for carcinoma. Nephrogenic adenoma, a mimicker of PCa in transurethral resection specimens, is negative for p63 and positive for AMACR; PAX8 can be used to differentiate nephrogenic adenoma (PAX8+) from PCa (PAX8−).
AMACR is not useful in diagnosis of metastatic PCa, as it is positive in a wide variety of tumor types. More specific prostate markers including PSA, PSAP, prostein and NKX3.1 are recommended instead.19 Any collection of foamy-appearing cells in a lesional biopsy (e.g., bone lesion) from a man should be evaluated with prostate markers, as metastatic PCa often takes on a deceptively bland, foamy appearance in metastatic sites.
Conclusions
Significant changes to PCa grading and recommended reporting have occurred in the past several years, with the underlying motivation of facilitating tailored treatment based on accurately predicted prognosis. The recommended grade grouping is readily adopted, as it is based directly on Gleason grading. However, issues remain regarding diagnosis and quantification of high-grade patterns in particular. Pathologists continue to play an essential role in guiding care in PCa patients.
Biography
Jennifer K. Sehn, MD, is Assistant Professor, Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri.
Contact: sehnj@wustl.edu

Footnotes
Disclosure
None reported.
References
- 1.Epstein JI, Egvad L, Amin MB, Delahunt B, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 2.Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors. WHO classification of tumors of the urinary system and male genital organs. 4th ed. IARC; Lyon, France: 2016. [DOI] [PubMed] [Google Scholar]
- 3.Srigley JR, Delahunt B, Evans AJ. Therapy-associated effects in the prostate gland. Histopathology. 2012;60:153–165. doi: 10.1111/j.1365-2559.2011.04079.x. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srigley JR, Zhou M, Amin MB, et al. College of American Pathologists protocol for the examination of specimens from patients with carcinoma of the prostate gland. [Accessed: 6/14/2017]. Version: Prostate 3.3.0.0, revised February 28, 2017 www.cap.org/cancerprotocols.
- 6.Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2017;41:e1–e7. doi: 10.1097/PAS.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 7.Choy B, Pearce SM, Anderson BB, et al. Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am J Surg Pathol. 40:1400–1406. doi: 10.1097/PAS.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 8.McKenney JK, Wei W, Hawley S, et al. Histologic grading of prostatic adenocarcinoma can be further optimized. Am J Surg Pathol. 2016;40:1439–1456. doi: 10.1097/PAS.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 9.Sauter G, Clauditz T, Steurer, et al. Integrating tertiary Gleason 5 patterns into quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.01.015. http://dx.dio.org/10.1016/j.eururo.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Li J, Cheng L, et al. Diagnosis of “poorly formed glands” Gleason pattern 4 prostatic adenocarcinoma on needle biopsy: an interobserver reproducibility study among urologic pathologists with recommendations. Am J Surg Pathol. 39:1331–1339. doi: 10.1097/PAS.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 11.Mahal BA, Muralidhar V, Chen YW, et al. Gleason score 5+3=8 prostate cancer: much more like Gleason score 9? BJUInt. 2016;118:95–101. doi: 10.1111/bju.13239. [DOI] [PubMed] [Google Scholar]
- 12.Huynh MA, Chen MH, Wu J, et al. Gleason score 3+5 or 5+3 versus 4+4 prostate cancer: the risk of death. Eur Urol. 2016;69:976–979. doi: 10.1016/j.eururo.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 13.Shah RB, Li J, Cheng L, et al. Diagnosis of Gleason pattern 5 prostate adenocarcinoma on core needle biopsy: an interobserver reproducibility study among urologic pathologists. Am J Surg Pathol. 2015;39:1242–1249. doi: 10.1097/PAS.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 14.Khani F, Epstein JI. Prostate biopsy specimens with Gleason 3+3=6 and intraductal carcinoma: radical prostatectomy findings and clinical outcomes. Am J Surg Pathol. 2015;39:1383–1389. doi: 10.1097/PAS.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 15.Varma M, Egevad L, Delahunt B, Kristiansen G. Reporting intraductal carcinoma of the prostate: a plea for greater standardization. Histopathology. 2016;70:504–507. doi: 10.1111/his.13081. [DOI] [PubMed] [Google Scholar]
- 16.Wobker SE, Epstein JI. Differential diagnosis of intraductal lesions of the prostate. Am J Surg Pathol. 2016;40:e67–e82. doi: 10.1097/PAS.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 17.Tavora F, Epstein JI. High-grade prostatic intraepithelial neoplasia-like adenocarcinoma of the prostate: a clinicopathologic study of 28 cases. Am J Surg Pathol. 2008;32:1060–1067. doi: 10.1097/PAS.0b013e318160edaf. [DOI] [PubMed] [Google Scholar]
- 18.Morais CL, Han JS, Gordetsky J, et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am J Surg Pathol. 39:16–178. doi: 10.1097/PAS.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin MB, Eptsein JI, Ulbright TM, et al. Best practices recommendations in the application of immunohistochemistry in urologic pathology. Am J Surg Pathol. 2014;38:1017–1022. doi: 10.1097/PAS.0000000000000254. [DOI] [PubMed] [Google Scholar]