Abstract
Background
Since the majority of morphological changes evaluation of myocardium in ischaemic heart disease was in animal model, we detected the importance to evaluate such changes in human patients to gain insights into the targets of cellular damage and to reconcile or refine those experiments.
Methods
Tissue sections from left atrial appendage of the heart were carefully dissected from seventy five patients underwent conventional coronary artery bypass grafting at the cardiothoracic surgical department, Manchester Royal Infirmary. Tissue was fixed, sectioned, stained and six random sections were photographed and the images were assessed and quantified using Image Analyser Pro-Plus software, version 4.1. Arterioles, venules, intermediate sized vessels, and capillaries were directly counted within the highlighted area of myocardium under LM. Ultra-thin sections were imaged in a Tecnai 12 Biotwin transmission electron microscope at a magnification of ×4200 and photographed by a camera with a black and white film to quantify different structures of myocardium.
Results
The arteriole wall to lumen ratio was significantly increased in ischaemic heart disease patients 18.57 ± 2.89 compared to controls 8.3 ± 1.57, (P < 0.01). The regression analysis between vascular density and cardiomyocyte size demonstrated a significant inverse correlation between transverse cardiomyocyte diameter and arteriole, capillary and total vessel density (P < 0.01, 0.04, 0.02), respectively. Lumen area of the distal myocardial capillary was significantly reduced in IHD patients compared to controls (P < 0.01).
Conclusion
These results elucidate the morphological changes in the myocardial microvasculature of patients with ischaemic heart disease and its pathological magnitudes.
Keywords: ischaemic heart disease, Left atrial appendage, Cardiomyocyte, Myocardial capillaries
At a glance commentary
Scientific background of the subject
The majority of studies have evaluated the morphological changes of myocardium in ischaemic heart disease in animal model. It is therefore of considerable importance to study the morphological changes of myocardium from patients to reconcile or refine experimental animal models of ischaemic heart disease.
What this study adds to the field
Several morphological changes were observed in the myocardial microvasculature and cardiomyocyte of ischaemic heart disease patients. Such changes elucidate the pathological magnitude of the disease.
ischaemic heart disease (IHD) is one of the commonest chronic diseases with high morbidity and mortality, (115.8/100,000 people) 12.6% of total disease death [1]. Only few pathomorphological studies define the changes occurring in the myocardium of patients with IHD are available in the literature [2], [3], [4], [5], [6]. Most of these studies have either used samples from autopsy cases, infarcted left ventricle or in myocardium from animal models. To the best of our knowledge none of the studies have used human tissue to quantify the capillary changes in IHD. It is therefore of considerable importance to define morphological changes of myocardium from patients with IHD to gain insights into the targets of cellular damage in patients, and to reconcile or refine experimental animal models of IHD. In the present study we have studied the Left Atrial Appendage (LAA) from patients with IHD.
The LAA or left auricle is a small projection from the upper border of left atrium curving around the root of pulmonary trunk to the front [7]. It is a long, tubular, hooked structure of different shapes and sizes [8]. It is trabeculated with muscular ridges called pectinate muscles running parallel to each other giving a comb like appearance. Its blood supply comes from left coronary artery running in atrio-ventricular sulcus [9]. Because of its higher distensibility as compared to left atrium proper, it may augment hemodynamic function by modulating relationship between left atrial pressure and volume [9], [10]. It is an important source for the release of atrial natriuretic peptides (ANP) [10], [11]. It may play a role in mediating thirst during hypovolemia [12].
The objectives of the current study were to employ light (LM) and electron microscopy (EM) to quantify the changes of myocardial microvasculature and cardiomyocytes in the myocardial tissue and to relate pathology to clinical variables in patients with IHD.
Materials and methods
Patients: seventy five patients underwent conventional coronary artery bypass grafting (CABG) with cardiopulmonary bypass, mitral valve replacement (MVR) or aortic valve replacement (AVR), or concomitant MVR and AVR with CABG at the cardiothoracic surgical department, Manchester Royal Infirmary were involved. Before surgery, all patients underwent cardiac catheterization and coronary angiography. This study was approved by the local Ethics Committee, and all patients gave their written informed consent before participation. Patients were classified into two clinical groups:
-
•
Patients undergoing CABG alone (IHD group) (n = 53).
-
•
Patients undergoing cardiac valve replacement with normal coronary angiography (control group) (n = 22).
Tissue processing for pathomorphology: Tissue sections from left atrial appendage of the heart were carefully dissected, divided into small sections and fixed in 2.5% glutaraldehyde in 1% cacodylate buffer (pH 7.4).
They were then secondarily fixed in 1% Osmium tetroxide. After that embedded in epon blocks and sectioned using Reichert mechanical advance ultra-microtome. O.7 μm sections were obtained for light microscopy and 0.08–0.09 μm sections for electron microscopy. Semi-thin sections were stained with 1% Toluidine blue while ultra-thin sections were stained with Methanolic Uranyl acetate and Lead citrate, the metallic and slightly radioactive dyes.
For LM 6 random areas from each section were visualized and photographed using Kodak image software. Sections were photographed at 2 magnifications (×20 and ×40). The ×20 lens was used to photograph most of the parameters while the ×40 was used to photograph the venules and the arterioles. Images were assessed and quantified using Image Analyser Pro-Plus software, version 4.1, with a standard grid bar to calibrate the images.
Arterioles, venules, intermediate sized vessels, and capillaries were directly counted within the highlighted area of myocardium under LM. From each section at least 6 random images were analyzed enabling the assessment of mean density (no./mm2) for each subtype of blood vessel. Wall to lumen ratio (WLR) was also calculated by dividing the wall area by the corresponding luminal area of each arteriole or venule.
The transverse diameters of cardiomyocytes were measured. Cardiomyocytes with central nuclei were selected to ensure uniform selection. Approximately 100 cardiomyocytes were selected from each section. Independent assessment for the first few samples was carried out by expert pathologist to insure reading accuracy.
Ultra-thin sections were imaged in a Tecnai 12 Biotwin transmission EM at a magnification of ×4200 and photographed by a camera with a black and white film.
By EM following parameters were quantified:
-
-
Luminal area (μm2) (×4200) was measured by tracing the inner endothelial membrane.
-
-
Endothelial outer membrane area (μm2) (×4200) was measured by tracing along the outer endothelial membrane.
-
-
Endothelial area (μm2) (×4200) was calculated by subtracting the luminal area from endothelial outer membrane area.
-
-
Vessel area (μm2) (×4200) was calculated by tracing along the outer line of the basement membrane.
-
-
Pericyte area (μm2) (×4200) was calculated by tracing around each pericyte profile and the total/capillary assessed.
-
-
Basement membrane area (μm2) (×4200) was calculated by subtracting endothelial outer membrane area and pericyte area from the vessel area.
-
-
Endothelial cell profile (number) (×4200) was derived by counting the number of intercellular junctions.
-
-
Number of endothelial nuclei (number) (×4200) was counted directly from the electron micrographs as the number of endothelial nuclei/capillary.
-
-
Number of pericyte nuclei (number) (×4200) was calculated directly from the electron micrographs as the number of pericyte nuclei/capillary.
Statistical analysis: It was performed using SPSS for Windows 14.0. All data are expressed as the Mean and standard error of mean (SEM). Clinical data were compared between two groups by non-parametric Mann–Whitney U-test. Linear regression correlation coefficient was performed to determine correlation between various pathological changes and angiogenic factor expression. Statistical significance was accepted for P < 0.05 (two-tailed).
Results
Myocardial microvasculature and cardiomyocyte pathology: LM [Fig. 1]
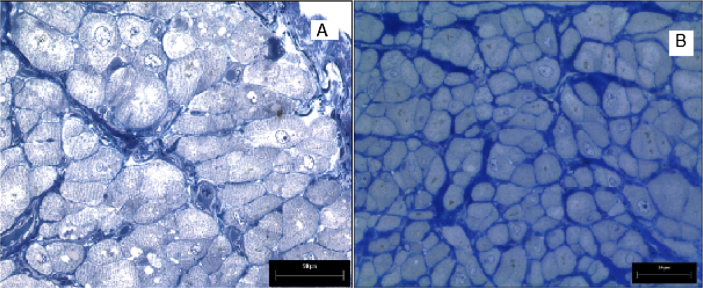
Fig. 1.
A- LM section of LAA from a control subject (×20). B- LM section of LAA from a patient with IHD (×20).
-
-
Vascular density (no.mm2): There were no significant difference in arteriole (P = 0.58), venule (P = 0.41), intermediate vessel (P = 0.49), and total vessel (P = 0.18) density between IHD patients compared to controls. However, there was a non-significant trend towards an increase in capillary density in IHD patients (376.31 ± 9.96) compared to controls (341.72 ± 17.11), (P = 0.06) [Table 1].
-
-
Arteriole and venule wall to lumen ratio: Arteriole wall to lumen ratio was significantly increased in IHD patients 18.57 ± 2.89 compared to controls 8.3 ± 1.57, (P < 0.01), but there was no difference in venule wall to lumen ratio (P = 0.43).
-
-
Transverse diameter of cardiomyocytes (μm2): There was no significant difference in transverse diameter of cardiomyocytes between the two groups (P = 0.45) [Table 1].
Table 1.
Light microscopic pathology of the myocardial microvasculature and cardiomyocyte in control subjects and patients with IHD.
| Parameters | Controls | IHD | P-value |
|---|---|---|---|
| Arteriole density | 30.8 ± 2.5 | 29.5 ± 1.3 | 0.58 |
| Venule density | 31.8 ± 2.1 | 30.0 ± 1.2 | 0.41 |
| Intermediate vessel density | 61.8 ± 3.8 | 58.0 ± 3.0 | 0.49 |
| Capillary density | 341.7 ± 17.1 | 376.3 ± 9.9 | 0.06 |
| Total vessel density | 446 ± 22.4 | 493.8 ± 12.9 | 0.18 |
| Arteriole W/L ratio | 8.3 ± 1.6 | 18.6 ± 2.9 | 0.000 |
| Venule W/L ratio | 1.4 ± 0.3 | 0.9 ± 0.1 | 0.43 |
| Cardiomyocyte diameter | 27.4 ± 0.8 | 26.8 ± 0.4 | 0.45 |
Mean ± standard error, vascular density (no.mm−2), W/L ratio = Wall to lumen ratio.
Correlations between vascular density and transverse cardiomyocyte diameter in IHD patients
Although there was no significant pathology of the cardiomyocyte, interestingly regression analysis between vascular density and cardiomyocyte size demonstrated a significant inverse correlation between transverse cardiomyocyte diameter and arteriole, capillary and total vessel density (P < 0.01, 0.04, 0.02), respectively [Table 2].
Table 2.
Correlation between vascular density and cardiomyocyte diameter in IHD patients.
| Art. density | Venule density | Inter- mediate vessel density | Capillary density | Total vessel density | |
|---|---|---|---|---|---|
| Cardio- myocyte diameter | R = −0.34 | R = −0.23 | R = −0.14 | R = −0.3 | R = −0.32 |
| P = 0.01 | P = 0.1 | P = 0.3 | P = 0.04 | P = 0.02 |
P = P-value, R = Correlation coefficient.
Pathology of distal myocardial capillaries: electron microscopy [Fig. 2]
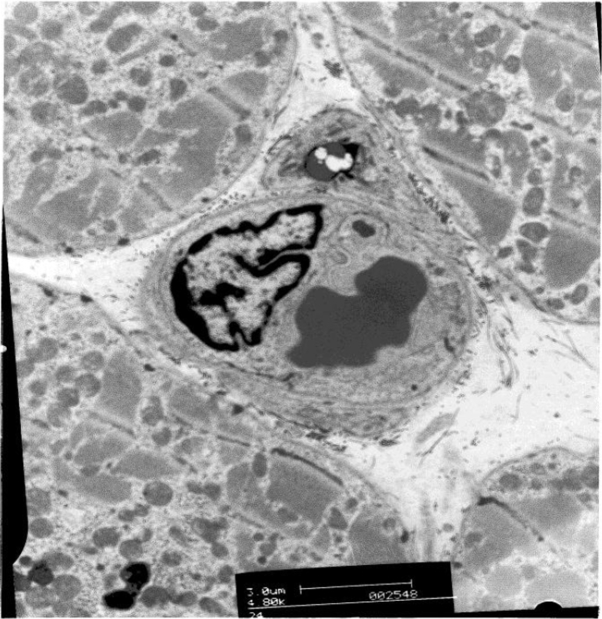
Fig. 2.
Electronmicrograph of a myocardial capillary in the LAA of a patient with IHD.
-
-
Lumen area (μm2): Lumen area of the distal myocardial capillary was significantly reduced in IHD patients compared to controls (P < 0.01) [Table 3].
-
-
Basement membrane area (μm2): No significant difference was found for basement membrane area of distal myocardial capillary between the two groups (P = 0.39) [Table 3].
-
-
Vessel Area (μm2): Vessel area of the distal myocardial capillary did not show a significant difference between the two groups (P = 0.2) [Table 3].
-
-
Endothelial area (μm2): No significant difference was found in capillary endothelial area between the two groups (P = 0.1) [Table 3].
-
-
Endothelial cell profile (no): Endothelial cell profile did not show a significant difference between the two groups (P = 0.14) [Table 3].
-
-
Number of endothelial nuclei (no): Number of endothelial nuclei did not show a significant difference between the two groups (P = 0.43) [Table 3].
-
-
Pericyte area (μm2): There was no significant difference in capillary pericyte area between the two groups (P = 0.45) [Table 3].
-
-
Number of pericyte nuclei (no): There was a non-significant trend toward a reduction in the number of pericyte nuclei in IHD patients compared to controls (P = 0.07) [Fig. 3].
Table 3.
Electron microscopic pathology of myocardial capillaries in control subjects and IHD patients.
| Parameter | Controls | IHD | P-value |
|---|---|---|---|
| Lumen area | 20.9 ± 1.2 | 14.9 ± 0.1 | 0.00 |
| Basement membrane area | 3.7 ± 0.2 | 4.0 ± 0.1 | 0.39 |
| Vessel area | 49.7 ± 2.2 | 46.3 ± 1.5 | 0.2 |
| Endothelial area | 18.2 ± 1.1 | 21.4 ± 1.2 | 0.1 |
| Endothelial cell profile no. | 4.1 ± 0.1 | 3.9 ± 0.1 | 0.14 |
| Endothelial nuclear no. | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.43 |
| Pericyte area | 6.8 ± 0.7 | 6.1 ± 0.5 | 0.45 |
| Pericyte nuclear no. | 0.24 ± 0.1 | 0.13 ± 0.06 | 0.07 |
Mean ± standard error.
Fig. 3.
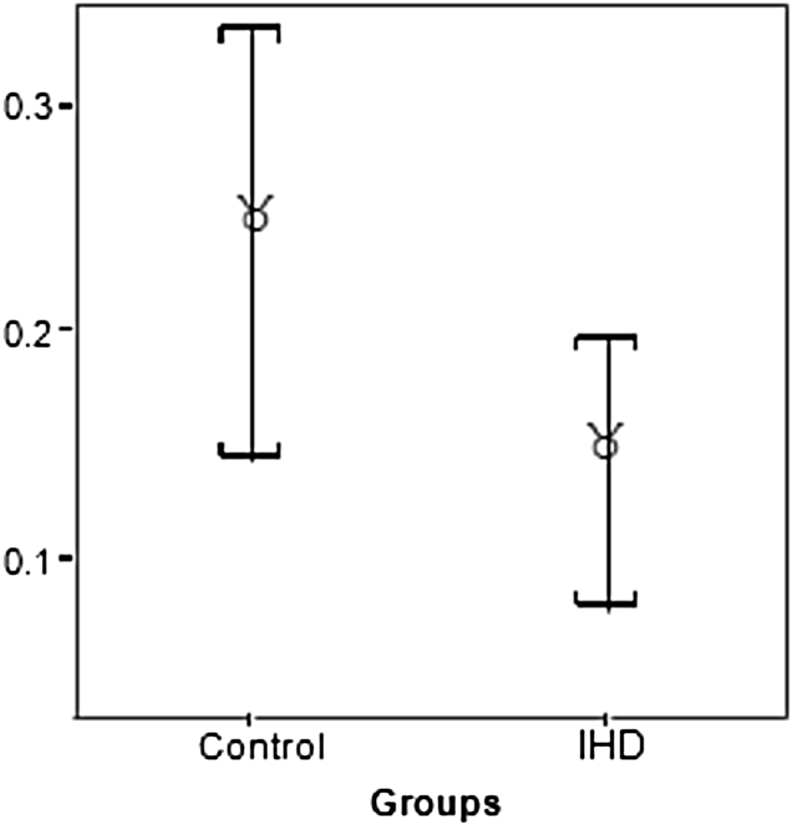
Changes in myocardial capillary pericyte area and number of pericyte nuclei between control subjects and IHD patients.
Discussion
IHD is a leading cause of mortality and morbidity worldwide and remains one of the major health issues in clinical cardiovascular practice. Myocardial ischemia may result in differing pathology and clinical outcomes. In the current study pathological changes of cardiomyocytes and the microvasculature have been quantified in the LAA of patients with and without IHD.
Vascular density showed a significant trend towards an increase in patients with IHD compared to non IHD patients (controls). This increase in capillary growth may have occurred as a result of coronary angiogenic response mediated by ischemia. In a morphological study of a canine model of myocardial ischemia, Ren et al. demonstrated increased capillary density following 1 h of myocardial ischemia [13]. Coronary collateral formation or angiogenesis is an important adaptive process that might limit the size of myocardial infarction and improve clinical outcomes following coronary artery occlusion [14], [15].
Furthermore, studies showed that a period of at least 2 min of myocardial ischemia is essential to stimulate angiogenesis, as myocardial ischemia of less than 1 min failed to activate development of collaterals [16]. This suggests that epicardial coronary artery disease manifesting as IHD leads to a reduction in downstream blood supply and hence hypoxia to myocardial tissue which stimulates angiogenesis and formation of new blood vessels which increase capillary density in patients with IHD.
The arteriole wall to lumen ratio was significantly increased in IHD patients compared to controls. This measurement provides information regarding the wall area independent of vessel size. Many studies have demonstrated an increase in arteriole wall to lumen ratio, mainly in patients with essential hypertension [17], [18], [19], [20]. In our study, more than 60% of IHD patients had hypertension whilst only around 30% of controls had hypertension [Table 4], which might explain the increase in arteriole wall to lumen ratio in IHD patients. In addition, it has been shown that arteriole wall to lumen ratio is highly increased in hypertensive patients with IHD compared to hypertensive patients without IHD. Thus, the presence of IHD with hypertension may aggravate vascular remodeling [21]. An increased peripheral resistance is the only parameter that is consistently abnormal in patients with hypertension [22]. This is due to a general narrowing of resistance vessels as well as a reduction in the number of parallel-connected arterioles, a process known as rarefaction [23]. Studies have shown that the general narrowing of resistance vessels seen in hypertensive patients is due to structural changes with a reduction in lumen diameter and an increase in wall to lumen ratio, a process known as remodeling [24], [25], [26], [27]. These findings are consistent with our finding which shows a significant increase in myocardial arteriole wall to lumen ratio in hypertensive patients with IHD compared to normotensive patients with IHD.
Table 4.
Patients’ demographic data.
| Demographic data | Control | IHD |
|---|---|---|
| Number | 22 | 53 |
| Age | 61.6 ± 2.9 | 65.46 ± 1.4 |
| Gender (M:F) | 11:11 | 41:12 |
| Body Mass Index | 26.6 ± 1.2 | 27.2 ± 0.5 |
| Hypertension | 7 (31.8) | 32 (60.4) |
| Hyperlipidemia | 13 (59.1) | 45 (84.9) |
| Smoker | 15 (68.2) | 41 (77.4) |
| Atrial Fibrillation | 12 (54.5) | 20 (37.7) |
| Myocardial Infarction | 0 | 34 (64.2) |
| CVD 1 | 0 | 3 (5.7) |
| CVD 2 | 0 | 8 (15.1) |
| CVD 3 | 0 | 42 (79.3) |
| No Angina | 4 | 0 |
| Angina Symptoms G1 | 12 (54.5) | 9 (16.9) |
| Angina Symptoms G2 | 5 (22.7) | 17 (32.1) |
| Angina Symptoms G3 | 1 (4.6) | 27 (50.9) |
| Angina Symptoms G4 | 0 | 0 |
| β-blocker | 4 (18.2) | 43 (81.1) |
| Diabetes | 0 | 0 |
Mean ± standard error, No (%), G = Grade, CVD = Coronary Vessel Disease, IHD = ischaemic Heart Disease.
In the current study we showed an increase in the transverse diameter of cardiomyocytes in IHD patients with hypertension compared to IHD patients without hypertension. Chronic ischemia is associated with cardiomyocyte loss with corresponding hypertrophy of viable cardiomyocytes [28]. This hypertrophy may consist of lengthening or an increase of diameter, or a combination of both [29]. Rakusan et al. has also reported moderate hypertrophy of cardiomyocytes in patients with hypertrophic cardiomyopathy following acquired aortic stenosis [30]. This cardiomyocyte hypertrophy has been seen predominantly in ventricles rather than the atrium; this may reflect predominantly pressure related mechanisms which may not prevail in the atrium.
The present study shows that the lumen area of the distal myocardial capillary was significantly reduced in IHD patients compared to controls. Similar findings were observed in a study of twenty seven patients undergoing CABG surgery who showed a marked reduction in luminal area of right atrial coronary capillaries with a significant swelling of endothelial cytoplasm following ischemia [31]. The reduction in capillary lumen area could be due to compression of the vessels by external factors such as interstitial oedema or cardiomyocyte swelling [5] or alterations in contractile proteins (actin, myosin, tropomyosin and α-actinin) in the endothelial cells of the capillaries [32], [33]. Moreover, activated neutrophils may release vasoconstricting substances such as platelet activating factors and leukotrienes which may contribute to a further reduction in luminal size [31]. Thus, the up regulation of these endothelial contractile proteins may be responsible for the change in capillary shape and the reduction in capillary lumen area found in the present study, and clearly demand further study.
In conclusion, several morphological changes were observed by using LM and EM in the myocardial microvasculature and cardiomyocyte of IHD patients compared to other patients with normal coronary angiography. Such changes elucidate the pathological magnitude of IHD.
Conflicts of interest
The authors certify that there was no conflicts of interest.
Acknowledgments
The authors deeply thank the staff of the cardiothoracic surgical department, Manchester Royal Infirmary, patients and all who contributed to this study.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.World Health Organization . Annex Table 2: deaths by cause, sex and mortality stratum in WHO regions, estimates for 2002. 2004. [Google Scholar]
- 2.Connelly J.H., Clubb F.J., Vaughn W., Duncan M. Morphological changes in atrial appendages removed during the maze procedure: a comparison with autopsy controls. Cardiovasc Pathol. 2001;10:39–42. doi: 10.1016/s1054-8807(01)00057-6. [DOI] [PubMed] [Google Scholar]
- 3.Su X., Sekiguchi M., Endo M. An ultrastructural study of cardiac myocytes in postmyocardial infarction ventricular aneurysm representative of chronic ischemic myocardium using semiquantitative and quantitative assessment. Cardiovasc Pathol. 2000;9:1–8. doi: 10.1016/s1054-8807(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 4.Glyn M.C., Ward B.J. Contraction in cardiac endothelial cells contributes to changes in capillary dimensions following ischaemia and reperfusion. Cardiovasc Res. 2000;48:346–356. doi: 10.1016/s0008-6363(00)00173-5. [DOI] [PubMed] [Google Scholar]
- 5.Ward B.J., McCarthy A. Endothelial cell “swelling” in ischaemia and reperfusion. J Mol Cell Cardiol. 1995;27:1293–1300. doi: 10.1016/s0022-2828(05)82391-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira R., Milei J., Forcada P., Beigelman R., Molteni L., Cutrin J.C. The hypertrophied myocardium and coronary disease. Structural changes in patients submitted to aortocoronary bypass surgery. Int J Cardiol. 1992;36:203–212. doi: 10.1016/0167-5273(92)90008-q. [DOI] [PubMed] [Google Scholar]
- 7.Sinnatamby Chummy S. 11th ed. Churchill Livingstone; 2006. Last's anatomy: regional and applied. [Google Scholar]
- 8.Ernst G., Stollberger C., Abzieher F., Veit-Dirscherl W., Bonner E., Bibus B. Morphology of the left atrial appendage. Anat Rec. 1995;242:553–561. doi: 10.1002/ar.1092420411. [DOI] [PubMed] [Google Scholar]
- 9.Al-Saady N.M., Obel O.A., Camm A.J. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stollberger C., Schneider B., Finsterer J. Elimination of the left atrial appendage to prevent stroke or embolism? Anatomic, physiologic, and pathophysiologic considerations. Chest. 2003;124:2356–2362. doi: 10.1378/chest.124.6.2356. [DOI] [PubMed] [Google Scholar]
- 11.Rodeheffer R.J., Naruse M., Atkinson J.B., Naruse K., Burnett J.C., Merrill W.H. Molecular forms of atrial natriuretic factor in normal and failing human myocardium. Circulation. 1993;88:364–371. doi: 10.1161/01.cir.88.2.364. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman M.B., Blaine E.H., Stricker E.M. Water intake in hypovolemic sheep: effects of crushing the left atrial appendage. Science. 1981;211:489–491. doi: 10.1126/science.7455689. [DOI] [PubMed] [Google Scholar]
- 13.Ren G., Michael L.H., Entman M.L., Frangogiannis N.G. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–79. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 14.Cohen M., Rentrop K.P. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74:469–476. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- 15.Coggins M.P., Sklenar J., Le D.E., Wei K., Lindner J.R., Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–2477. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 16.Sasayama S., Fujita M. Recent insights into coronary collateral circulation. Circulation. 1992;85:1197–1204. doi: 10.1161/01.cir.85.3.1197. [DOI] [PubMed] [Google Scholar]
- 17.Korsgaard N., Aalkjaer C., Heagerty A.M., Izzard A.S., Mulvany M.J. Histology of subcutaneous small arteries from patients with essential hypertension. Hypertension. 1993;22:523–526. doi: 10.1161/01.hyp.22.4.523. [DOI] [PubMed] [Google Scholar]
- 18.Thybo N.K., Mulvany M.J., Jastrup B., Nielsen H., Aalkjaer C. Some pharmacological and elastic characteristics of isolated subcutaneous small arteries from patients with essential hypertension. J Hypertens. 1996;14:993–998. [PubMed] [Google Scholar]
- 19.Cooper A., Heagerty A.M. Blood pressure parameters as determinants of small artery structure in human essential hypertension. Clin Sci (Lond) 1997;92:551–557. doi: 10.1042/cs0920551. [DOI] [PubMed] [Google Scholar]
- 20.Rizzoni D., Agabiti Rosei E. Small artery remodeling in hypertension and diabetes. Curr Hypertens Rep. 2006;8:90–95. doi: 10.1007/s11906-006-0046-3. [DOI] [PubMed] [Google Scholar]
- 21.Li X.Y., Li R., Yu W., Shi H.Y., Wei L.X. Differences in coronary microvascular lesions in coronary heart disease and hypertension: an autopsy study of elderly patients. Chin Med J Engl. 2004;117:207–212. [PubMed] [Google Scholar]
- 22.Mulvany M.J. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002;17:105–109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- 23.Mulvany M.J. Small artery remodeling in hypertension. Curr Hypertens Rep. 2000;4:49–55. doi: 10.1007/s11906-002-0053-y. [DOI] [PubMed] [Google Scholar]
- 24.Aalkjaer C., Heagerty A.M., Petersen K.K., Swales J.D., Mulvany M.J. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation–contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res. 1987;61:181–186. doi: 10.1161/01.res.61.2.181. [DOI] [PubMed] [Google Scholar]
- 25.Schiffrin E.L., Deng L.Y., Larochelle P. Blunted effects of endothelin upon small subcutaneous resistance arteries of mild essential hypertensive patients. J Hypertens. 1992;10:437–444. doi: 10.1097/00004872-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Heagerty A.M., Aalkjaer C., Bund S.J., Korsgaard N., Mulvany M.J. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- 27.Falloon B.J., Heagerty A.M. In vitro perfusion studies of human resistance artery function in essential hypertension. Hypertension. 1994;24:16–23. doi: 10.1161/01.hyp.24.1.16. [DOI] [PubMed] [Google Scholar]
- 28.Anversa P., Kajstura J., Reiss K., Quaini F., Baldini A., Olivetti G. Ischemic cardiomyopathy: myocyte cell loss, myocyte cellular hypertrophy, and myocyte cellular hyperplasia. Ann N Y Acad Sci. 1995;752:47–64. doi: 10.1111/j.1749-6632.1995.tb17405.x. [DOI] [PubMed] [Google Scholar]
- 29.Anversa P., Sonnenblick E.H. Ischemic cardiomyopathy: pathophysiologic mechanisms. Prog Cardiovasc Dis. 1990;33:49–70. doi: 10.1016/0033-0620(90)90039-5. [DOI] [PubMed] [Google Scholar]
- 30.Rakusan K., Flanagan M.F., Geva T., Southern J., Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.F., Wu S.C., Huang C.H., Pan P.C., Lee C.S., Lin C.C. Morphometric identification of luminal narrowing of myocardial capillaries after cardioplegic arrest. Ann Thorac Surg. 2001;71:243–248. doi: 10.1016/s0003-4975(00)02028-2. [DOI] [PubMed] [Google Scholar]
- 32.Becker C.G., Nachman R.L. Contractile proteins of endothelial cells, platelets and smooth muscle. Am J Pathol. 1973;71:1–22. [PMC free article] [PubMed] [Google Scholar]
- 33.Hirschi K.K., D'Amore P.A. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]