Abstract
This data article investigates cadmium removal efficiency using garbage ash as a cheap and effective adsorbent. Influence of different parameters, such as initial cadmium (II) concentration (mg/L), contact time (min), adsorbent dose (gr/L), pH and temperature (°C) were investigated. The characterization data of the garbage ash was determined using SEM analysis. The experimental data indicated that the adsorption of cadmium on garbage ash follows pseudo second order model and Langmuir isotherm model with R2 = 0.99. Also, the maximum adsorption capacity of adsorbent was 100.25 mg/g. Thermodynamic data showed that cadmium adsorption on garbage ash was a spontaneous and endothermic process. Based on acquired data, garbage ash could be proposed as an efficient and low-cost adsorbent for the removal of cadmium from aqueous solution.
Keywords: Cadmium, Adsorption, Garbage ash, Aqueous solution
Specifications Table
| Subject area | Chemical Engineering |
| More specific subject area | Adsorption |
| Type of data | Table, figure |
| How data was acquired | The uptake of cadmium (II) by the adsorbent (qe) was determined based on the subtraction of the initial and final concentration of adsorbate |
| Atomic Absorption Spectrophotometer (Shimadzu, AA-7000) was used for determination of cadmium (II) concentration | |
| Data format | Raw, analyzed |
| Experimental factors | For the preparation of adsorbent, garbage was placed in a furnace at 550 °C for 4.5 h to produce ash |
| Experimental features | Cadmium (II) adsorption from aqueous solution using garbage ash |
| Data source location | Gonabad, Khorasan Razavi province, Iran |
| Data accessibility | Data are included in this article. |
Value of the data
-
•
The application of adsorbent of garbage ash due to cost-effectiveness and good potential is a suitable option for the removal of Cd2+ from aqueous solution.
-
•
The isotherm, thermodynamic and kinetic data will be useful for predicting the adsorption capacity, modeling and mechanism of Cd2+removal by garbage ash.
-
•
These data can be important for removal of Cd2+ from aqueous solution.
1. Data
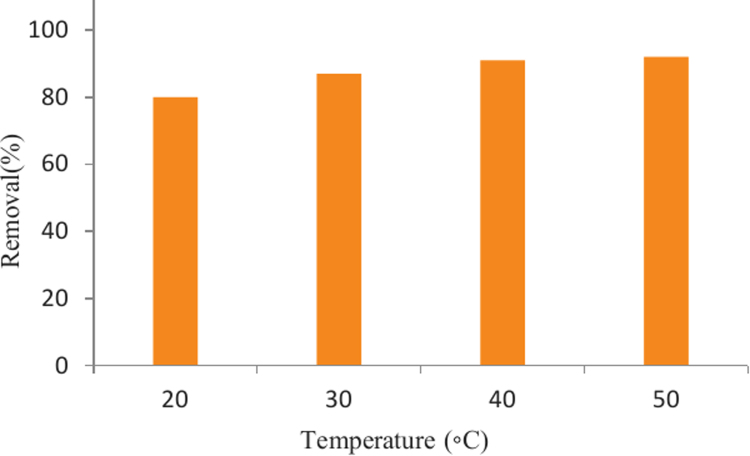
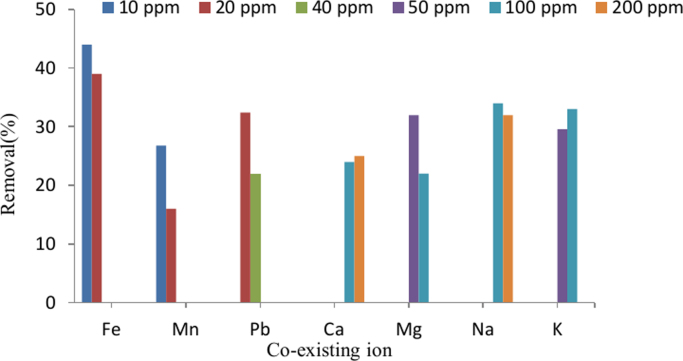
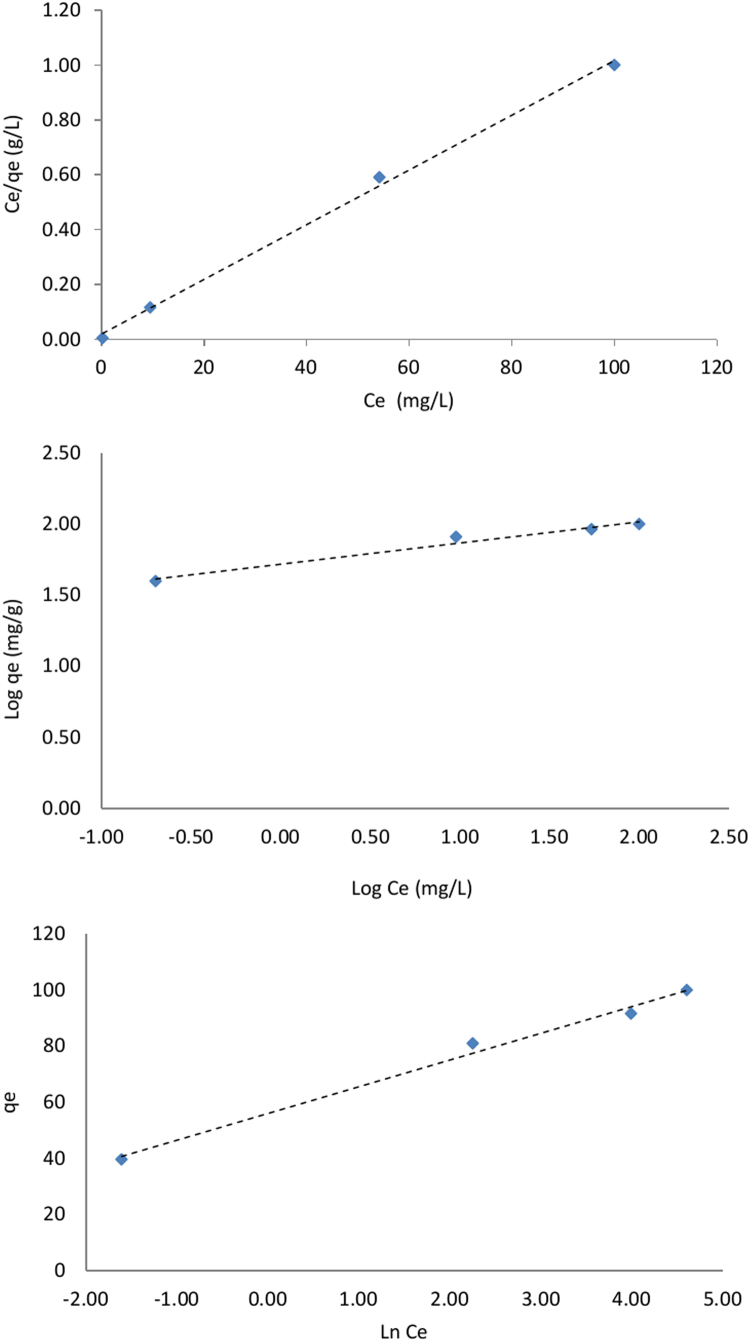
The SEM image of garbage ash is shown in Fig. 1. The effect of adsorbent dosage on the removal efficiency of Cd2+ is presented in Fig. 2. Also, Fig. 3, Fig. 4 depict the effect of initial Cd2+concentration on the removal efficiency and adsorption capacity. The effect of pH on Cd2+ removal efficiency is shown in Fig. 5. The effect of temperature on Cd2+ removal efficiency is also depicted in Fig. 6. The effect of coexisting ions on Cd2+ removal efficiency under optimized conditions is shown in Fig. 9. The plots of the kinetics and adsorption isotherms are shown in Fig. 7, Fig. 8. The kinetic equations are listed in Table 1. Kinetic parameters and correlation coefficient for Cd2+ adsorption by garbage ash are given in Table 2. Equations and parameters related to adsorption isotherms are summarized in Table 3. Thermodynamic parameters for Cd2+ removal by garbage ash are given in Table 4.
Fig. 1.
SEM micrograph of garbage ash.
Fig. 2.
Effect of adsorbent dosage on removal efficiency (Cd2+ concentration: 100 mg/L, contact time: 60 min and pH: 6).
Fig. 3.
Effect of initial Cd2+ concentration on removal efficiency (adsorbent dosage: 0.5 g/L and pH: 6).
Fig. 4.
Effect of initial Cd2+ concentration on adsorption capacity (adsorbent dosage: 0.5 g/L and pH: 6).
Fig. 5.
Effect of pH on Cd2+ removal efficiency (adsorbent dosage: 0.5 g/L, contact time: 60 min and Cd2+ concentration: 50 mg/L).
Fig. 6.
Effect of temperature on Cd2+ removal efficiency (adsorbent dosage: 0.5 g/L, contact time: 60 min and Cd2+concentration: 50 mg/L).
Fig. 9.
The effect of coexisting ions on Cd2+ removal efficiency. (adsorbent dosage: 0.5 g/L, contact time: 60 min, pH: 6 and Cd2+ concentration: 50 mg/L).
Fig. 7.
Plots of kinetic models: pseudofirstorder (a), pseudosecondorder (b) and intraparticle diffusion (C).
Fig. 8.
Plots of Langmuir, Freundlich, Temkin isotherms for the adsorption of Cd2+ by garbage ash.
Table 1.
Adsorption kinetics for Cd2+ removal by garbage ash.
| Kinetic model | Formula | Plot |
|---|---|---|
| Pseudo first order kinetic model | log(qe− qt) vs. t | |
| Pseudo second order kinetic model | vs. t | |
| Intra-particle diffusion kinetic model | qt vs. t0.5 |
Table 2.
Kinetic parameters and correlation coefficient for Cd2+ adsorption by garbage ash.
| Ce | qe, exp [mg/g] | Pseudo first order |
Pseudo second order |
Intraparticle diffusion |
|||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | K1 (min–1) | qe (mg/g) | K2 (min−1) | Kp [mg/g min−0.5] | R2 | ||||
| 20 | 40 | 28.77 | −0.010 | 0.94 | 36.33 | 0.002 | 0.96 | 5.47 | 0.95 |
| 50 | 80 | 41.28 | −0.003 | 0.91 | 47.19 | 0.010 | 0.99 | 1.35 | 0.94 |
| 100 | 128 | 9.72 | −0.020 | 0.81 | 128.37 | 0.015 | 1 | 1.73 | 0.65 |
| 150 | 221 | 11.38 | −0.020 | 0.94 | 220.89 | 0.012 | 0.99 | 1.26 | 0.96 |
Table 3.
Adsorption isotherms and obtained parameters for Cd2+ removal by garbage ash.
| Isotherm | Linear form | Plot | Parameter | |
|---|---|---|---|---|
| Langmuir | qmax (mg/g) | 100.2490 | ||
| KL (L/mg) | 0.5031 | |||
| R2 | 0.9975 | |||
| Freundlich | KF(mg/g(L/mg)1/n) | 52.1889 | ||
| n | 6.7140 | |||
| R2 | 0.9729 | |||
| Temkin | A (L/mg) | 0.1701 | ||
| B | 9.5318 | |||
| R2 | 0.9908 | |||
Table 4.
Thermodynamic parameters for Cd2+ removal by garbage ash.
| Temperature (K) | |||
|---|---|---|---|
| 298 | −0.183 | 21.206 | 7.391 |
| 308 | −2.251 | ||
| 318 | −2.813 | ||
| 328 | −2.221 |
2. Experimental design, materials and methods
2.1. Preparation of garbage ash
The sampling of garbage was performed according to physical and chemical sampling methodology proposed by the Iranian National Standard Organization. The waste samples were collected from the garbage separated for composting in Mashhad solid waste management organization located in Mashhad, Iran. In order to prepare the adsorbent, the samples were placed in oven to remove any moisture. For the preparation of ash, the sample was placed in a furnace at 550 °C for 4.5 h and was kept in desiccator after cooling.
2.2. Experimental procedures
Adsorption of Cd2+ from synthetic aqueous solution using garbage ash was performed in batch experiments. A stock solution of Cd2+ with a concentration 1000 ppm was prepared by dissolving appropriate quantity of Cd(NO3)2 in 1 L of deionized water. The required concentrations of Cd2+ solution were prepared by dilution of stock solution. The pH of solution was adjusted by 0.1 M HCl or 0.1 M NaOH. The Cd2+ solution containing different adsorbent dosages were placed in shaker incubator at 150 rpm at various time intervals. Finally, samples were filtered through Whatman papers No. 0.45 µm and the residual concentrations of Cd2+ were analyzed by an Atomic Absorption Spectrophotometer (AAS).The effect of key variables, such as initial cadmium (II) concentration (20, 50, 100, 150, 200 mg/L), contact time (2, 5, 15, 30, 45, 60 min), adsorbent dose (0.2, 0.5, 1, 2, 3 gr/L), pH (2–12) and temperature (20, 30, 40, 50 °C) were investigated.The experiments were conducted in duplicate and the results were reported as averages. The removal efficiency of Cd2+ ion (%R) and the adsorption capacity qe(mg/g) of the Cd2+ ion adsorbed per unit mass of adsorbent was calculated by the following equation [1]:
| (1) |
| (2) |
where, C0 and Ce is the initial concentration of Cd2+ and the equilibrium concentration of Cd2+ in solution in mg/L, respectively, V is the volume of the solution in L, and m is mass of the garbage ash in g.
2.3. Kinetic modeling
The experimental data were analyzed using kinetic models like pseudo firstorder, pseudo secondorder and intraparticle diffusion [2]. The kinetic equations are presented in Table 1. The kinetic study was performed by placing 0.5 g of adsorbent dosage in 1 L solution in concentration range 20–150 mg/L at an optimum pH of 6 under varying time intervals (5–60 min) at 25 °C and 150 rpm. In this equation, qe and qt is the adsorption capacity of Cd2+(mg/g) at equilibrium and at time t, respectively; k1 (min−1) is the rate constant of pseudo firstorder which can be computed from the slope of the linear plot of log (qe−qt) versus time, (min−1) is the pseudo second order rate constant. Slope of the plot of t/qt against t yield k2 value. In the intraparticle diffusion model, Kp and C is the intraparticle diffusion constant and intercept, respectively. The value of Kp was calculated from slope of the plot of qt against t0.5 [3], [4], [5].
2.4. Isotherm modeling
In order to describe the adsorption mechanism of Cd2+on the garbage ash, isothermal studies were used. The obtained data were evaluated using the isotherm models including the Langmuir, Freundlich and Temkin [6]. Batch adsorption isotherm tests were carried out at different initial concentrations from 20 to 200 mg/L under optimized conditions at pH around six and temperature of 25 °C.The linear forms of the isotherm equations are given in Table 2. According to isotherm equations, Ce and qe is the equilibrium concentration of Cd2+ (mg/L) and the amount of Cd2+ adsorbed per unit weight of adsorbents at equilibrium (mg/g),respectively. qm is the maximum adsorption capacity for the Langmuir isotherm (mg/g), is the Langmuir isotherm constant (L/mg). KF and n is Freundlich adsorption constants related to adsorption capacity and adsorption intensity, respectively, and were determined from slope and intercept of the plot of ln (qe) versus ln (Ce). In Temkin equation, A and B are the binding constant (L/mg) and constant corresponding to the heat of adsorption [7], [8], [9], [10].
2.5. Thermodynamic modeling
Thermodynamic parameters of the adsorption process such as enthalpy change (ΔH°), entropy change (ΔS°) and Gibbs free energy change (ΔG°) at temperatures 20, 30, 40 and 50 °C were estimated using the following equations:
| (3) |
| (4) |
where, ΔG° is Gibbs free energy change (J/mol), ΔS° is entropy change (J/mol K), ΔH° is enthalpy change (J/mol), R is the ideal universal gas constant (8.314 J/K mol), and T is the temperature (Kelvin). (ΔH°) and (ΔS°) is determined using the plot of ln KL versus 1/T [11], [12].
2.6. The effects of coexisting ions
In order to determine the effects of cations including Fe2+, Mn2+, Pb+, Ca2+, Mg2+, Na+ and K+ on the removal of Cd2+ by garbage ash, FeCl3, MnSO4, Pb(NO3)2, CaCl2, MgCl2, NaCl and KCl salts were used.
Acknowledgements
The authors would like to thank Gonabad University of Medical Sciences, Iran for financial support.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.08.163.
Transparency document. Supporting information
Supplementary material
References
- 1.Fazlzadeh M., Rahmani K., Zarei A., Abdoallahzadeh H., Nasiri F., Khosravi R. A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr (VI) from aqueous solutions. Adv. Powder Technol. 2017;28:122–130. [Google Scholar]
- 2.Shams M., Dehghani M.H., Nabizadeh R., Mesdaghinia A., Alimohammadi M., Najafpoor A.A. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: modeling, mechanical agitation versus sonication. J. Mol. Liq. 2016;224:151–157. [Google Scholar]
- 3.Dehghani M.H., Zarei A., Mesdaghinia A., Nabizadeh R., Alimohammadi M., Afsharnia M. Adsorption of Cr (VI) ions from aqueous systems using thermally sodium organo-bentonite biopolymer composite (TSOBC): response surface methodology, isotherm, kinetic and thermodynamic studies. Desalin. Water Treat. 2017;85:298–312. [Google Scholar]
- 4.Afsharnia M., Shams M., Sajjadi S., Qasemi M. Lead (Pb2+) removal from synthetic aqueous solution using food waste ash. Toloo-e-behdasht. 2016;15:12–21. [Google Scholar]
- 5.Shams M., Qasemi M., Afsharnia M., Hossein Mahvi A. Sulphate removal from aqueous solutions by granular ferric hydroxide. Desalin. Water Treat. 2016;57:23800–23807. [Google Scholar]
- 6.Ramavandi B., Asgari G. Comparative study of sun-dried and oven-dried Malva sylvestris biomass for high-rate Cu (II) removal from wastewater. Process Saf. Environ. Prot. 2018;116:61–73. [Google Scholar]
- 7.Dehghani M.H., Farhang M., Alimohammadi M., Afsharnia M., McKay G. Adsorptive removal of fluoride from water by activated carbon derived from CaCl2-modified Crocus sativus leaves: equilibrium adsorption isotherms, optimization, and influence of anions. Chem. Eng. Commun. 2018;205:955–965. [Google Scholar]
- 8.Leili M., Fazlzadeh M., Bhatnagar A. Green synthesis of nano-zero-valent iron from Nettle and Thyme leaf extracts and their application for the removal of cephalexin antibiotic from aqueous solutions. Environ. Technol. 2018;39:1158–1172. doi: 10.1080/09593330.2017.1323956. [DOI] [PubMed] [Google Scholar]
- 9.Shams M., Nabipour I., Dobaradaran S., Ramavandi B., Qasemi M., Afsharnia M. An environmental friendly and cheap adsorbent (municipal solid waste compost ash) with high efficiency in removal of phosphorus from aqueous solution. Fresenius Environ. Bull. 2013;22:723–727. [Google Scholar]
- 10.Ramavandi B., Rahbar A., Sahebi S. Effective removal of Hg2+ from aqueous solutions and seawater by Malva sylvestris. Desalin. Water Treat. 2016;57:23814–23826. [Google Scholar]
- 11.Khosravi R., Zarei A., Heidari M., Ahmadfazeli A., Vosughi M., Fazlzadeh M. Application of ZnO and TiO2 nanoparticles coated onto montmorillonite in the presence of H2O2 for efficient removal of cephalexin from aqueous solutions. Korean J. Chem. Eng. 2018;35:1000–1008. [Google Scholar]
- 12.Fazlzadeh M., Khosravi R., Zarei A. Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract, and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr(VI) from aqueous solution. Ecol. Eng. 2017;103:180–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material