Abstract
Background
Inhalational injury is a major cause of morbidity and mortality in burns patients. This study aims to analyse the clinical outcomes, complications and bacteriology of inhalational burn patients.
Methods
A prospective study was done on consecutive admissions to Burn Department, Singapore General Hospital over 15 months from January 2015 to March 2016. Presence of inhalational injury, demographics, complications and outcomes was recorded. Diagnosis of inhalational injury was based on history, symptoms and nasoendoscopy. Diagnosis of acute respiratory distress syndrome (ARDS), acute kidney injury (AKI) and infective complications were according to the Berlin criteria, acute kidney injury network (AKIN) classification stage 2 and above and the American Burns Association guidelines.
Results
Thirty-five patients (17.3%) had inhalational burns out of 202 patients (63.4% male, 57.4% Chinese population). The average age was 43 ± 16.7 years (range 16–86), and percentage of total body surface area (%TBSA) was 12.1 ± 18.0 (range 0–88). In patients with inhalational injury, age was 38.9 ± 17.2 years and %TBSA was 30.3 ± 32.3. In patients without inhalational injury, age was 44.1 ± 12.8 years and %TBSA was 8.3 ± 9.59. Compared to patients with cutaneous injury alone, patients with inhalational burns had more surgeries (3 ± 7.07 vs 1 ± 1.54, p = 0.003), increased length of stay (21 days vs 8 days, p = 0.004) and higher in-hospital mortality rate (17.1% vs 0.6%, p < 0.001). Incidence of ARDS and AKI was 48.6% and 37.1%, respectively, compared to 0.6% and 1.2% in the patients without inhalational injury (p < 0.001). Patients with inhalational injury had increased incidence of bacteraemia (31.4% vs 2.4%, p < 0.001), pneumonia (37.1% vs 1.2%, p < 0.001) and burn wound infection (51.4% vs 25.1%, p = 0.004). Inhalational injury predicted AKI with an adjusted odds ratio (OR) of 17.43 (95% confidence interval (CI) 3.07–98.87, p < 0.001); ARDS, OR = 106.71 (95% CI 12.73–894.53, p < 0.001) and pneumonia, OR = 13.87 (95% CI 2.32–82.94, p = 0.004). Acinetobacter baumannii was the most frequently cultured bacteria in sputum, blood and tissue cultures with inhalational injury. Gram-negative bacteria were predominantly cultured from tissue in patients with inhalational injury, whereas gram-positive bacteria were predominantly cultured from tissue in patients without inhalational injury.
Conclusions
Inhalational injury accompanying burns significantly increases the length of stay, mortality and complications including AKI, ARDS, infection and sepsis.
Keywords: Inhalational, Burns, Injury, Acute kidney injury, Acute respiratory distress syndrome, Pneumonia
Background
The importance of inhalational injury as a predictor of mortality is widely recognised [1–4]. Few prospective studies, especially in a tropical burns setting, compared clinical outcomes in patients with inhalational burns and those with cutaneous burns only [5]. This study aims to analyse the effect of inhalational injury on inpatient mortality, length of stay and complications.
Methods
A prospective observational study was done on consecutive admissions to Burn Department, Singapore General Hospital from January 2015 to March 2016. Patients were excluded if they are deceased within 24 h of admission or transferred (Fig. 1). Data was collected on our Research Electronic Data Capture (REDCap) database. The study was approved by the Singapore General Hospital Institutional Review Board.
Fig. 1.
Patient recruitment flowchat
In-hospital mortality, number of surgeries, length of stay and percentage of total body surface area (%TBSA) were recorded. Complications analysed were acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), pneumonia and culture-positive burns wound infection and bacteraemia.
Statistical analysis
Data was analysed using IBM SPSS Statistics with institutional statistician’s help. χ2 and Mann-Whitney U tests were used. Binary logistic regression was applied to determine if inhalational injury was an independent predictor of complications, removing effect of %TBSA and age. p < 0.05 was considered to indicate a statistically significant result. Dependent variables were the outcome/complication, e.g., AKI. Covariates were inhalational injury, age and %TBSA.
Inhalational injury
Inhalational injury was diagnosed by attending doctors in patients with history of smoke/fumes inhalation/fires in an enclosed space and three or more symptoms or signs and one positive finding on nasoendoscopy (Table 1).
Table 1.
Symptoms and signs suggestive of inhalational injury
| Symptoms and signs of inhalational injury | Nasoendoscopy |
|---|---|
| • Hoarseness of voice • Sore throat • Dyspnea • Painful respirations • Carbonaceous sputum • Tachypnea • Stridor • Facial burns/mucosal burns of lips and mouth • Singed facial hair/nasal hair • Soot in nostrils or mouth |
• Airway erythema/edema • Soot in airways • Abnormal mobility and appearance of vocal cords |
Bronchoscopy was performed only in patients with mucus plugging and was not part of diagnostic criteria. Patients referred from overseas with prior diagnosis were included.
Treatment principles
Patients were treated according to Advanced Burn Life Support (ABLS) [6] principles and modified Parkland’s regime (2–3 ml/kg/TBSA). In particular, if there was inhalational injury based on clinical findings or nasoendoscopy, patients with inhalational burns were monitored with hourly oxygen saturations and given a heparinised saline nebuliser (50 U heparin in 5 ml normal saline). Patients were intubated if findings on nasoendoscopy suggested severe airway injury with airway or vocal cord edema and if they had stridor or had an inappropriate PaO2 /FiO2 (P/F) ratio. Other factors taken into account include age—patients more than 60 years old with risk factors for poor cough reflex were intubated.
Ventilation was interchanged between conventional ventilation and airway pressure release ventilation (APRV) depending on patient response. Tidal volume was 6–8 ml/kg and positive end expiratory pressure (PEEP) was titrated to P/F ratio as in the ARDSNet study/protocol [7]. Prone positioning and neuromuscular paralysis are weak recommendations in the Surviving Sepsis Guidelines [8] and also not practical (in cases of facial swelling and abdominal distention). In refractory hypoxaemia, APRV is used as recruitment and haemodynamics are superior [9–11] with improved compliance, reduced duration of ventilation and lower mortality.
Indications for antibiotic therapy was for prophylaxis for all major burns > 20%, infected burns and 5 days post graft or biobrane. Intravenous cefazolin is the usual first line.
Surgical treatment strategy was for early biobrane (epidermal skin dressing) or excision within 48 h. Biobrane as the first surgery has dramatically reduced the need for subsequent excision in partial thickness burns by reducing burns conversion and providing ‘coverage’. Subsequent surgeries were for further excision and grafting if burns conversions, infection or biobrane non-take occurred.
Non-infective complications
Berlin definition for ARDS was used [12]. Arterial blood gas was obtained in patients with inhalational injury, respiratory distress or abnormal chest radiographs. AKI was defined as ≥ acute kidney injury network (AKIN) classification stage 2 [13].
Infective complications
The American Burns Association recommendations for diagnosis of infectious complications in patients with thermal trauma were followed [14]. In patients with clinical suspicion of pneumonia/infection, appropriate investigations were ordered and bacteria cultures were recorded.
Sputum cultures in patients with pneumonia were obtained via induced sputum with nebulised saline. Bronchoalveolar lavage (BAL) in patients with bronchoscopy was done in patients with desaturations refractory to mechanical ventilation.
Results
Characteristics of patients included in this study were listed in Table 2. The most common mechanisms of injury were fire and scalds. Thirty-five (17.3%) patients had inhalational burns out of 202 patients (63.4% male, 57.4% Chinese population). The average age was 43 ± 16.7 years (range 16–86), and %TBSA was 12.1 ± 18.0 (range 0–88). In patients with inhalational injury, age was 38.9 ± 17.2 years and %TBSA was 30.3 ± 32.3. In patients without inhalational injury, age was 44.1 ± 12.8 years and %TBSA was 8.3 ± 9.59.
Table 2.
Patient characteristics included in the study
| Demographics | All patients n (%) |
Patients with inhalational injury n (%) | Patients without inhalational injury n (%) | p value, U |
|---|---|---|---|---|
| Number | 202 (100) | 35 (17.3) | 167 (82.7) | |
| Age (years) | ||||
| Mean (SD) | 43.1 (16.7) | 38.8 (17.25) | 44.1 (12.78) | |
| Range | 16–86 | 18–69 | 16–86 | |
| Median (IQR) | 42 (29–56) | 43(29–58) | 35 (30–48) | 0.176, U = 2451 |
| %TBSA, median (IQR) | 5 (2.38–13.0) | 5 (2.5–12) | 9 (0–66) | 0.036, U = 3582 |
| Facial burns | 72 (35.6) | 18 (51.4) | 54 (32.3) | 0.032 |
| Upper body burns | 162 (80.2) | 21 (60.0) | 141 (84.5) | 0.001 |
| Sex | ||||
| Male | 128 (63.4) | 26 (74.2) | 102 (61.1) | |
| Female | 74 (36.6) | 9 (25.7) | 65 (38.9) | |
| Race | ||||
| Chinese | 116 (57.4) | 15 (42.8) | 101(60.5) | |
| Indian | 19 (9.4) | 3 (8.6) | 16 (9.6) | |
| Malay | 22 (10.9) | 4 (11.4) | 18 (10.8) | |
| Others | 45 (22.2) | 13 (37.1) | 32 (19.2) | |
SD standard deviation, IQR interquartile range, %TBSA percentage of total body surface area
Compared to patients with cutaneous injury alone, patients with inhalational burns had more surgeries (3 ± 7.07 vs 1 ± 1.54, p = 0.003), increased length of stay (21 days vs 8 days, p = 0.004) and higher in-hospital mortality rate (17.1% vs 0.6%, p < 0.001) (Table 3).
Table 3.
Clinical outcomes, complications and mortality of the burn patients
| Non-inhalational burns n = 167 | Inhalational burns n = 35 | p value, U | |
|---|---|---|---|
| Number of surgery sessions, median (IQR) | 1 (1–1) | 3 (0–10) | 0.003, U = 3552 |
| Length of stay (days), median (IQR) | 8 (5–12) | 21 (4–43) | 0.004, U = 3582 |
| Average length of stay/%TBSA (sum of length of stay/sum of %TBSA) | 1.307 | 0.976 | NA |
| Length of ICU stay (days), median (IQR) | 0 (0–0) | 3 (0–10) | < 0.001, U = 4785 |
| Average length of ICU stay/%TBSA (sum of length of ICU stay/sum of %TBSA) | 0.020 | 0.313 | NA |
| Days on IV antibiotics, median (IQR) | 3 (1–6) | 9.5 (0–28.25) | 0.014, U = 3552 |
| Average days on IV antibiotics/%TBSA (Sum of days of IV antibiotics/sum of %TBSA) | 0.580 | 0.474 | NA |
| Days of mechanical ventilation, median (IQR) | 0 (0–0) | 2 (0–10) | < 0.001 |
| Initial P/F ratio, median (IQR) | 532.6 (316.8–678.9) | 370.4 (291.8–554.2) | 0.191 |
| Lowest P/F ratio in 2 days from admission, median (IQR) | 518.5 (372.0–689.5) | 253.6 (202.7–408.4) | 0.026 |
| Lowest P/F ratio in 7 days from admission, median (IQR) | 518.5 (372.0–689.5) | 246.8 (181.5–389.4) | 0.018 |
| Admission to HD, n (%) | 46 (27.5) | 31(88.6) | < 0.001 |
| Admission to ICU, n (%) | 10 (6.0) | 22 (62.9) | < 0.001 |
| Intubation, n (%) | 4 (2.4) | 22 (62.8) | < 0.001 |
| Mortality, n (%) | 1 (0.6) | 6 (17.1) | < 0.001 |
| AKI, n (%) | 2 (1.2) | 13 (37.1) | < 0.001 |
| ARDS, n (%) | 1 (0.6) | 17 (48.6) | < 0.001 |
| Bacteraemia, n (%) | 4 (2.4) | 11 (31.4) | < 0.001 |
| Pneumonia, n (%) | 2 (1.2) | 13 (37.1) | < 0.001 |
| Burn wound infection, n (%) | 42 (25.1) | 18 (51.4) | 0.004 |
%TBSA percentage of total body surface area, ARDS acute respiratory distress syndrome, AKI acute kidney injury, IV intravenous, ICU intensive care unit, NA not applicable, HD high dependency unit, IQR interquartile range, P/F PaO2 /FiO2
Incidence of ARDS and AKI was 48.6% and 37.1%, respectively, compared to 0.6% and 1.2% in the patients without inhalational injury (p < 0.001). Patients with inhalational injury had increased incidence of bacteraemia (31.4% vs 2.4%, p < 0.001), pneumonia (37.1% vs 1.2%, p < 0.001) and burn wound infection (51.4% vs 25.1%, p = 0.004). Inhalational injury predicted AKI with an adjusted odds ratio (OR) of 17.43 (95% confidence interval (CI) 3.07–98.87, p < 0.001); ARDS, OR of 106.71 (95% CI 12.73–894.53, p < 0.001) and pneumonia, OR of 13.87 (%95 CI 2.32–82.94, p = 0.004) (Table 4).
Table 4.
Adjusted odds ratios (OR) of getting poorer clinical outcomes compared to the median, adjusted for age and %TBSA (except age—adjusted for %TBSA, %TBSA—adjusted for age only)
| Non-inhalational burns n (%) | Inhalational burns n (%) | p value | Adjusted OR* | 95% CI for OR | p value | |
|---|---|---|---|---|---|---|
| %TBSA > median,5 | 92 (55.1%) | 24 (68.6%) | 0.142 | 1.66 | 0.76–3.64 | 0.204 |
| Age > median, 42 (years) | 88 (52.7%) | 14 (40.0%) | 0.172 | 0.71 | 0.31–1.62 | 0.414 |
| Number of surgery sessions > median, 1 | 131 (78.4%) | 23 (65.7%) | 0.108 | 0.21 | 0.07–0.62 | 0.004 |
| Length of stay > median, 8.5 (days) | 79 (47.3%) | 22 (62.9%) | 0.094 | 1.12 | 0.47–2.67 | 0.797 |
| Days on IV antibiotics > median, 4 | 83 (49.7%) | 22 (62.9%) | 0.157 | 0.86 | 0.36–2.11 | 0.749 |
| Days of mechanical ventilation > median, 2.5 | 3 (1.8%) | 15 (42.9%) | < 0.001 | 18.38 | 2.97–113.68 | 0.002 |
| Initial P/F ratio < median, 394.02 | 3 (1.8%) | 14 (40.0%) | < 0.001 | 44.85 | 9.10–221.13 | < 0.001 |
| Lowest P/F ratio < median, 343.88 | 1 (0.6%) | 15 (42.9%) | < 0.001 | 140.58 | 13.14–1504.25 | < 0.001 |
| Lowest P/F ratio < 300 | 1 (0.6%) | 17 (48.6%) | < 0.001 | 480.70 | 28.47–8115.89 | < 0.001 |
| Mortality | 1 (0.6%) | 6 (17.1%) P/F < 300:5/17(29.4%) P/F > 300:1/18 (5.6%) |
< 0.001 | 0.604 | 0.01–44.53 | 0.818 |
*Odds ratio adjusted for %TBSA and age
%TBSA percentage of total body surface area, CI confidence interval, P/F PaO2 /FiO2, IV intravenous
Patients with inhalational burns had a higher %TBSA. Seventeen out of 35 patients fitted the Berlin criteria (P/F ratio < 300 and within 7 days). This is reflected in Table 4 (lowest P/F ratio < 300). Within the group of PIB, those with P/F ratios < 300 in the first week had a higher in-hospital mortality (29.4%) compared to those with P/F ratios > 300 in the first week (5.56%) (p = 0.08). We could not get statistical significance when adjusted for %TBSA and for both age and %TBSA (Table 5).
Table 5.
Adjusted odds ratios of complications in patients with inhalational injury using logistic regression analysis
| Inhalational injury | Inhalational injury, adjusted for %TBSA | Inhalational injury, adjusted for %TBSA and age (except %TBSA, adjusted for age only) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | Adjusted OR | 95% CI | p value | Adjusted OR | 95% CI | p value | |
| %TBSA > median, 5 | 1.78 | 0.82–3.87 | 0.146 | – | – | – | 1.66 | 0.76–3.64 | 0.204 |
| Age > median, 42 (years) | 0.60 | 0.29–1.26 | 0.175 | 0.71 | 0.31–1.62 | 0.414 | – | – | – |
| All complications | 8.33 | 3.62–19.17 | < 0.001 | 5.31 | 2.17–12.98 | < 0.001 | 6.27 | 2.49–15.77 | < 0.001 |
| Intubation | 68.96 | 20.65–230.3 | < 0.001 | 67.19 | 12.47–361.89 | < 0.001 | 86.99 | 14.22–544.43 | < 0.001 |
| Pneumonia | 48.75 | 10.30–230.55 | < 0.001 | 13.87 | 2.32–82.94 | 0.004 | 35.51 | 3.90–323.07 | 0.002 |
| ARDS | 156.78 | 19.69–1248.17 | < 0.001 | 106.71 | 12.73–894.53 | < 0.001 | 480.70 | 28.47–8115.89 | < 0.001 |
| AKI | 48.75 | 10.31–230.55 | < 0.001 | 17.43 | 3.07–98.87 | < 0.001 | 31.71 | 4.38–229.7 | 0.001 |
| Bacteraemia | 18.68 | 5.50–63.39 | < 0.001 | 2.39 | 0.38–15.04 | NS | 2.56 | 0.40–16.57 | NS |
| Burn wound infection | 3.15 | 1.49–6.67 | 0.003 | 1.28 | 0.49–3.35 | NS | 1.35 | 0.52–3.54 | NS |
| Mortality | 34.35 | 3.99–295.86 | 0.001 | 0.38 | 0.01–11.29 | NS | 0.60 | 0.01–44.53 | NS |
| HD admission | 20.39 | 6.82–60.95 | < 0.001 | 15.70 | 4.87–50.62 | < 0.001 | 15.55 | 4.82–50.20 | < 0.001 |
| ICU admission | 26.57 | 10.41–67.90 | < 0.001 | 16.26 | 5.23–50.57 | < 0.001 | 16.63 | 5.28–52.34 | < 0.001 |
| Number of surgery sessions > median, 1 | 0.53 | 0.24–1.16 | 0.111 | 0.22 | 0.08–0.64 | 0.005 | 0.21 | 0.07–0.62 | 0.004 |
| Length of stay > median, 8.5 | 1.89 | 0.89–3.99 | 0.098 | 1.05 | 0.44–2.47 | 0.915 | 1.12 | 0.47–2.67 | 0.797 |
| Days on IV antibiotics > median, 4 | 1.71 | 0.81–3.63 | 0.160 | 0.84 | 0.35–2.04 | 0.699 | 0.86 | 0.36–2.11 | 0.749 |
| Days of mechanical ventilation > median 2.5 | 41.00 | 10.91–154.04 | < 0.001 | 11.94 | 2.34–60.8 | 0.003 | 18.38 | 2.97–113.682 | 0.002 |
| Initial P/F ratio < median 394.02 | 36.45 | 9.67–137.40 | < 0.001 | 28.89 | 7.02–118.89 | < 0.001 | 44.85 | 9.10–221.13 | < 0.001 |
| Lowest P/F ratio in 2 days < median, 343.88 | 124.50 | 15.61–993.29 | < 0.001 | 78.88 | 9.28–670.76 | < 0.001 | 140.58 | 13.14–1504.25 | < 0.001 |
| Lowest P/F ratio in 1 week < 300 | 156.78 | 19.69–1248.17 | < 0.001 | 106.72 | 12.73–894.54 | < 0.001 | 480.70 | 28.47–8115.89 | < 0.001 |
OR odds ratio, CI confidence interval, NS non-significant, ARDS acute respiratory distress syndrome, AKI acute kidney injury, IV intravenous, RF respiratory failure, ICU intensive care unit, HD high dependency unit, %TBSA percentage of total body surface area, SD standard deviation, CI confidence interval, P/F PaO2 /FiO2
Bacteriology
In patients with infective complications, cultures were taken and the number of positive cultures for a microbe was expressed as a percentage of all positive cultures (Fig. 2).
Fig. 2.
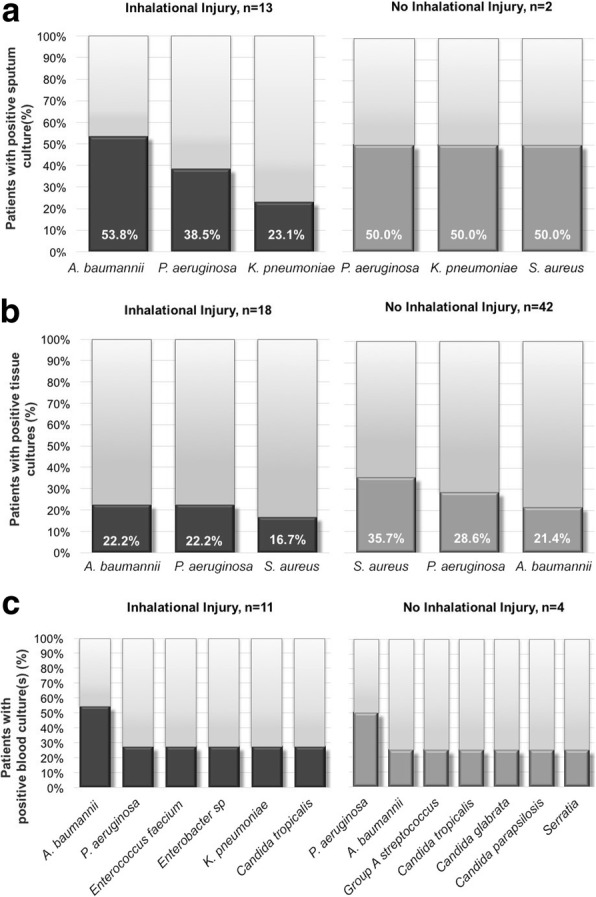
Chart showing most common bacteria grown in sputum (a), tissue (b) and blood (c) cultures in patients with inhalational burns and patients with cutaneous burns only
In patients with inhalational injury, Acinetobacter baumannii (A. baumannii) was the most frequently cultured in sputum, tissue and blood cultures.
Pneumonia was most frequently caused by A. baumannii followed by Pseudomonas aeruginosa (P. aeruginosa) and Klebsiella pneumoniae (K. pneumoniae).
These three species were also the most common in the patients with bacteraemia together with Candida tropicalis, Enterobacter and Enterococcus faecium. In tissue cultures, A. baumannii and P. aeruginosa were most common followed by Staphylococcus aureus (S. aureus).
A. baumannii was less common in patients without inhalational injury.
In patients without inhalational injury, burn wound infections were most commonly due to S. aureus followed by P. aeruginosa and A. baumannii. In those with inhalational injury, A. baumannii and P. aeruginosa were more common.
Discussion
Burn management has improved over the past decades with advances in fluid resuscitation, nutritional support, pulmonary care and infection control practices. This includes improvements in wound care/dressing, such as our previously described enhanced total body wrap technique using negative pressure wound therapy, which protects the wound from micro-organisms and provides an efficient channel to clear excessive exudate while keeping the wounds moist [15]. This reduces frequency of dressing changes and lowers infection rates through prevention of strikethrough [15]. Of note, incidence of burn wound infections in our centre have been reducing over the years, from 50.2% in a 2003–2005 study [5] to 29.7% in our study. Currently, infectious and respiratory complications remain a leading cause of morbidity. In burns cases with %TBSA of 40–75%, almost all deaths are due to sepsis from infectious complications and/or inhalation injury [16].
It is well-reported that inhalational injury results in longer stays and higher mortality which results in significant economic burden. In the current study, we prospectively followed patients in a burn unit and quantified the impact of inhalational injury on clinical outcomes and complications. All patients were managed uniformly using our institution’s burns protocol.
Mortality
Our study found that presence of inhalational injury was associated with increased mortality. The overall in-hospital mortality was 3.5%. In-hospital mortality in patients with inhalational injury was 17.1%, compared to those without inhalational injury of 0.6%.
The benchmark inhalational mortality is high and ranges from 9.5 to 46.6% internationally. Typically, patients with respiratory failure from inhalational burns have up to 50% risk of mortality. Our mortality rate of 17.1% (6 patients) was likely due to concomitant respiratory failure, large %TBSA burns (> 40%) and accompanying renal failure, accounting for their high mortality rate. The 6 patients were 23–48 years old, typically with severe Endorf-Gameli type 3 or 4 bronchoscopy findings with delayed resuscitation and debridement (72 h). The crux in further reduction of inhalational mortality we believe lies in early aggressive burns resuscitation, coverage and aggressive intensive care unit (ICU) respiratory care to modulate the systemic inflammatory response syndrome (SIRS) inflammatory response.
Statistical significance was not reached for mortality when adjusting for %TBSA, possibly due to the low mortality in the group without inhalational injury.
Inhalational injury has been shown to be a significant predictor of mortality in various multivariable retrospective studies on the prognostic factors in burn patients (Table 6).
Table 6.
Retrospective studies on inhalational injury, mortality, length of stay and other clinical outcomes
| Our study SGH Chong | Texas Shirani 1987 [17] |
Utah Hollingsed 1993 [18] |
North Carolina Smith 1994 [19] |
Tokyo Suzuki 2005 [2] |
Egypt El-Helbawy 2011 [4] |
Taiwan Chen 2014 [1] |
USA Anand 2015 [3] |
|
|---|---|---|---|---|---|---|---|---|
| n | 202 | 1058 | 529 | 1447 | 5560 | 281 | 21,791 | 506,628 |
| Type of study | Prospective study | Retrospective single centre | Retrospective single centre | Retrospective single centre | Retrospective multicentre | Retrospective single centre | Retrospective multicentre | Retrospective multicentre |
| PIB (%) | 17.3 | 35 | 5.7 | 19.6 | 30.4 | 46.3 | 7.9 | 3.47 |
| Age (years) | (median) Overall: 42 PIB: 43 PCB: 35 |
PCB: 27 Abnormal xenography: 37 Abnormal bronchoscope: 39 |
(mean) PIB: 20.2 RF: 36.6 |
(mean) Overall: 30 (3 months–93 years) |
(mean (SD)) Overall: 40.1 (26.2) PIB: 49.0 (20.5) PCB: 36.2(27.4) |
– | (mean(SD)) Overall: 30.9 (22.6) |
(mean) Overall: 30 |
| %TBSA | (median) Overall: 5 PIB: 5 PCB: 9 |
PCB: 23 Abnormal xenography: 37 Abnormal bronchoscope:50 |
(mean) PIB: 16.2 RF: 40.4 |
(mean)18% (0.4–100%) | mean (SD)) Overall: PIB: 29.9 (30.3) PCB: 16.1 (17.3) |
– | mean Overall: 12.2% | – |
| Mortality (%) | Overall 3.5 PIB: 17.1 PCB: 0.6 |
Overall: 22.7 PIB: 46.6 PCB: 9.6 |
Overall: 6.2 PIB -without RF:0 - with RF: 27 PCB: - without RF: 3 - with RF: 50 |
Overall 9.5 PIB 31 PCB 4.3 |
Overall: 15.8 PIB: 33.6 PCB: 8.1 |
Overall: 23.1 PIB: 41.5 PCB: 7.2 |
Overall 2.1 PIB: 17.9 PCB: 0.76 |
Overall 3.73 PIB: 4× increase in mortality vs PCB |
| Length of stay (days) | PIB: 21 PCB: 8 |
– | PIB - without RF: 17.8 - with RF: 42.6 PCB - without RF: 15.4 -with RF: 43.2 |
– | – | – | – | PIB: 9 PCB: 6 |
| Other outcomes | Pneumonia ARDS, AKI infection IV antibiotics, ICU days |
Pneumonia PIB: 38% PCB: 8.8% |
ARDS PIB: 20% PCB: 2% |
– | – | – | Rate of dying due to pneumonia, sepsis and wound infection is higher in PIB | Hospital charges (median) PIB: US$32,070 PCB: US$ 17,600 |
PIB patients with inhalational burns, PCB patients with only cutaneous burns, ARDS acute respiratory distress syndrome, AKI acute kidney injury, IV intravenous, RF respiratory failure, ICU intensive care unit, %TBSA percentage of total body surface area, SD standard deviation
Shirani et al. estimated that the burn-related death rate is 20% higher in patients with combined inhalation injury and cutaneous burns than in those with cutaneous burns alone [17]. In a 2014 Taiwanese study, 21,791 burns patients from 44 hospitals were retrospectively reviewed. The overall mortality rate was 2.1%, and inhalation injuries were found in 7.9% of the patients. The mortality rate of inhalation and non-inhalation injury group was 17.9% and 0.7%, respectively, similar to our results [1]. In Suzuki et al.’s 17-year study, the mortality rate was 15.8% and multivariable analysis revealed inhalational injury to be the most important predictor of death [2]. In Smith et al's study, inhalational injury was a significant predictor for mortality, but the most important predictor of mortality was still %TBSA [18].
As in Table 6, the mortality ranges from 17% to 46.6% in inhalational burns. The reasons for high mortality in inhalational burns despite low %TBSA cutaneous burns is because of associated pneumonia, SIRS, sepsis and associated organ failure [1–3, 17].
ARDS
Patients with inhalational injury had > 100 times increase in developing ARDS. Incidence of ARDS in patients with inhalational injury was 48.6%, compared to 0.6% in the other group. A 4-year review of 529 burns patients reported a 20% incidence of ARDS, compared to 2% in patients without inhalational injury (p < 0.001) [19].
During inhalational injury, the inflammatory response forms airway casts and cause V/Q mismatch, pulmonary edema and cell death, promoting airway occlusion and pulmonary dysfunction [1]. In patients with large cutaneous burns, capillary hyper-permeability occurs not only at the injured site, but also in regions distant from the injury [20, 21] resulting in worsening of pulmonary edema. This systemic response is more severe when the thermal injury is associated with smoke inhalation [22].
AKI
Inhalational injury was also a predictor of AKI. In Coca’s retrospective study, inhalational injury was an independent predictor of AKI (OR = 3.6 (95% CI 1.76–7.34), p < 0.001) [23]. Other significant factors in multivariable analysis were catheter infections and sepsis. Both inhalational injury and AKI were independent predictors of mortality.
Pneumonia
In this study, inhalational injury was an independent risk factor for pneumonia. It was associated with 13 times higher likelihood of pneumonia, independent of %TBSA. Shirani et al.’s hallmark study quantitated that both inhalational injury and pneumonia have contributions to mortality that are independent and additive [17]. In that study, expected mortality increased by a maximum of 20% in the presence of inhalation injury alone, 40% in the presence of pneumonia alone and 60% when both inhalation injury and pneumonia were present.
Length of stay
After adjusting for %TBSA (Table 3), ICU stay and length of stay was longer for inhalational burns due to respiratory complications. Days of intravenous (IV) antibiotics were comparable. This is a result of more operative sessions, complications and longer duration of IV antibiotics. We postulate the increase in operative sessions to be due to increased wound infections requiring debridement and wound conversion due to systemic sepsis, cytokine alteration and inflammatory response. A study using the National Inpatient Sample database of 506,628 admissions found inhalational injury was a factor for length of stay (OR = 2.01 (95% CI 1.84–2.21), p < 0.001) [3].
Bacteriology
Patients with inhalational burns were infected with gram-negative organisms, common agents for invasive infection. The prevalence of A. baumanni in our tropical burns unit has been previously reported [5, 24]. A. baumanni is endemic to the hospital because of the tropical climates and the particular high survivability of Acinetobacter even in adverse conditions [24] The most common agents were A. baumanni followed by methicillin-resistant Staphylococcus aureus (MRSA) then P. aeruginosa [24]. Interestingly, A. baumanni was the most common aetiologic agent in our patients with inhalational burns, but not in patients without inhalational burns.
Patients with burns are exposed to infections from loss of the skin barrier. In patients with inhalational burns, the respiratory tract is also damaged. The longer length of stay in hospital and duration of IV antibiotics may account for prevalence of resistant gram-negative nosocomial infections in inhalational burns patients. In an Indian study, gram-positive organisms were predominantly cultured during the first week of admission whereas gram-negative organisms with increasing levels of resistance were common from the second week onwards [16]. In patients with major burns, we may occasionally find multi-resistance organisms developing at 1–2 weeks of stay, which may be caused by antibiotic use.
Limitations
The generalizability of our results may be limited by the lack of bronchoscopy as an objective criterion for inhalational injury and different grades of severity. Our criterion for inhalational injury was a clinical diagnosis without taking into account bronchoscopy—a widely used technique [25–28] for diagnosing inhalational injury based on presence of hyperaemia, edema and soot [25]. A clinical criterion is readily applied in triage for quick stratification into patients with inhalational injury at high risks of the complications analysed in our paper. However, this diagnoses inhalational injury as being either present/absent instead of providing a scale of injury severity based on bronchoscopy proven lower tract injury. There is a lack of standard consensus in various clinical criteria, making inter-centre data comparisons difficult. Another limitation is that of inter-observer bias and reliability in each of the clinical criteria. We have optimised the variability by ensuring final diagnostic assessments are by attendings.
Bronchoscopy is an objective means of diagnosing and grading inhalational injury—with several grading systems proposed. This can potentially allow inter-centre comparisons should a standardised and validated grading system be accepted [25]. However, if bronchoscopy were to be done for diagnostic purposes in all patients in our study, some patients would be subjected to a bronchoscopy not otherwise indicated for diagnostic/therapeutic purposes. In patients with unlikely lower airway injury, it may not be necessary to pass the endoscope below the true vocal folds for negative diagnosis.
Bronchoscopy may be uncomfortable for the patient and requires careful anaesthesia of the larynx to avoid obstruction due to laryngospasm in a potentially inflamed airway. Furthermore, some institutions may not have the equipment or expertise for bronchoscopy. Fiberoptic laryngoscopy is better tolerated by the patients, requires minimal medication, and is often more readily available [26]. One study has addressed this with an objective decision tree that can be readily replicated in different centres, including bi-level endoscopic examination of the oro-rhino-pharynx and tracheobronchial tree and also clinical criteria [25]. Studies have discussed using bronchoscopy to evaluate severity of inhalational burns [27, 28]. Several scales of severity have been proposed; these have not been able to predict clinical outcomes [25, 27].
Further bacteriology studies can include analysis of time from injury to the date of culture for bacteriology and resistance of bacteria cultured.
Conclusion
Inhalational injury is associated with increased length of stay, morbidity and complications such as ARDS, AKI, pneumonia and infection. Mortality is higher (29.4% to 5.6%) if P/F is < 300. Counselling patients and their family regarding the associated morbidity is essential. Statistical significance was not reached when adjusted for %TBSA and age. Larger sample sizes are needed. Inhalational burn care is an important step towards advancing burn management, given its association with morbidity. Bronchoscopy classification is also encouraged as this was lacking in this study. This requires early recognition of inhalational burns, early lung recruitment protocol, early intubation, early ICU care, earlier lavage and earlier burns debridement to reduce the SIRS response.
Acknowledgements
The authors would like to thank Professor Tai Bee Choo for her statistical guidance.
Availability of data and materials
Database used during the current study is available from the corresponding author upon reasonable request.
Abbreviations
- AKI
Acute kidney injury
- APRV
Airway pressure release ventilation
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- ICU
Intensive care unit
- IV
Intravenous
- MRSA
Methicillin-resistant Staphylococcus aureus
- NS
Not significant
- OR
Odds ratio
- PCB
Patients with only cutaneous burns
- PEEP
Peak end expiratory pressure
- PIB
Patients with inhalational burns
- REDCap
Research Electronic Data Capture
- RF
Respiratory failure
- %TBSA
Percentage of total body surface area
Authors’ contributions
CSJ designed the research. KYO and RT carried out analysis of the data for this study with the database provided by KYO and CSJ. CSJ, KYO and RT participated in drafting the manuscript. All the authors read and approved the final manuscript. DSR participated in writing about the treatment principles—particularly the intubation and ventilation principles.
Ethics approval and consent to participate
Our research was done with the ethics approval from the SingHealth Centralised Institutional Review Board (CIRB) with CIRB reference number: 2014/2130D.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Si Jack Chong, Phone: +65 62223322, Email: chong_si_jack@hotmail.com.
Yee Onn Kok, Phone: +65 62223322, Email: yeeonn.kok@mohh.com.sg.
Rosanna Xiang Ying Tay, Phone: +65 88208757, Email: rosanna.tay@mohh.com.sg.
Desai Suneel Ramesh, Phone: +65 62223322, Email: suneel.ramesh.desai@singhealth.com.sg.
Kok Chai Tan, Phone: +65 62223322, Email: tan.kok.chai@sgh.com.sg.
Bien Keem Tan, Phone: +65 63214686, Email: bienkeem@gmail.com.
References
- 1.Chen MC, Chen MH, Wen BS, Lee MH, Ma H. The impact of inhalation injury in patients with small and moderate burns. Burns. 2014;40(8):1481–1486. doi: 10.1016/j.burns.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki M, Aikawa N, Kobayashi K, Higuchi R. Prognostic implications of inhalation injury in burn patients in Tokyo. Burns. 2005;31(3):331–336. doi: 10.1016/j.burns.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Veeravagu A, Yoon BC, Jiang B, Carvalho CM, Rincon F, Maltenfort M, et al. National trends in burn and inhalation injury in burn patients: results of analysis of the nationwide inpatient sample database. J Burn Care Res. 2015;36(2):258–265. doi: 10.1097/BCR.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 4.El-Helbawy RH, Ghareeb FM. Inhalation injury as a prognostic factor for mortality in burn patients. Ann Burns Fire Disasters. 2011;24(2):82–88. [PMC free article] [PubMed] [Google Scholar]
- 5.Chong SJ, Song C, Tan TW, Kusumawijaja G, Chew KY. Multi-variate analysis of burns patients in the Singapore General Hospital Burns Centre (2003-2005) Burns. 2009;35(2):215–220. doi: 10.1016/j.burns.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 6.American Burn Association . Advance Burn Life Support provider manual. 2011. [Google Scholar]
- 7.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Society of Critical Care Medicine . Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43(11):1648–1659. doi: 10.1007/s00134-017-4912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews PL, Shiber JR, Jaruga-Killeen E, Roy S, Sadowitz B, O’Toole RV, et al. Early application of airway pressure release ventilation may reduce mortality in high-risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641. doi: 10.1097/TA.0b013e31829d3504. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care. 2001;5(4):221–226. doi: 10.1186/cc1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenhalgh DG, Saffle JR, Holmes JH, 4th, Gamelli RL, Palmieri TL, Horton JW, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28(6):776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 15.Low OW, Chong SJ, Tan BK. The enhanced total body wrap—the new frontier in dressing care for burns. Burns. 2013;39(7):1420–1422. doi: 10.1016/j.burns.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Ghai S, Sachdeva A, Mahajan R, Dogra S, Soodan S, Mahajan B. Bacteriological and antibiotic susceptibility profile of aerobic burn wound isolates at a tertiary care institute in Northern India. J Appl Environ Microbiol. 2015;3(4):95–100. [Google Scholar]
- 17.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DL, Cairns BA, Ramadan F, Dalston JS, Fakhry SM, Rutledge R, et al. Effect of inhalation injury, burn size, and age on mortality: a study of 1447 consecutive burn patients. J Trauma. 1994;37(4):655–659. doi: 10.1097/00005373-199410000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Hollingsed Timothy C., Saffle Jeffrey R., Barton Richard G., Craft W. Bradley, Morris Stephen E. Etiology and consequences of respiratory failure in thermally injured patients. The American Journal of Surgery. 1993;166(6):592–597. doi: 10.1016/S0002-9610(05)80662-2. [DOI] [PubMed] [Google Scholar]
- 20.Traber D, Herndon D, Enkhbaatar P, et al. The pathophysiology of inhalation injury. D. Herndon (Ed.), Total burn care. 4. Edinburgh: Saunders Elsevier; 2012. pp. 219–228. [Google Scholar]
- 21.Jones SW, Williams FN, Cairns BA, Cartotto R. Inhalation injury: pathophysiology, diagnosis, and treatment. Clin Plast Surg. 2017;44:505–511. doi: 10.1016/j.cps.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soejima K, Schmalstieg FC, Sakurai H, Traber LD, Traber DL. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1233–L1241. doi: 10.1152/ajplung.2001.280.6.L1233. [DOI] [PubMed] [Google Scholar]
- 23.Coca SG, Bauling P, Schifftner T, Howard CS, Teitelbaum I, Parikh CR. Contribution of acute kidney injury toward morbidity and mortality in burns: a contemporary analysis. Am J Kidney Dis. 2007;49(4):517–523. doi: 10.1053/j.ajkd.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Chim H, Tan BH, Song C. Five-year review of infections in a burn intensive care unit: high incidence of acinetobacter baumannii in a tropical climate. Burns. 2007;33(8):1008–1014. doi: 10.1016/j.burns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Ikonomidis C, Lang F, Radu A, Berger MM. Standardizing the diagnosis of inhalation injury using a descriptibe score and mucosal injury criteria. Burns. 2011;38:513–519. doi: 10.1016/j.burns.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Muehlberger T, Kunar D, Munster A, Couch M. Efficacy of fiberoptic laryngoscopy in the diagnosis of inhalation injuries. Arch Otolaryngol Head Neck Surg. 1998;124(9):1003–1007. doi: 10.1001/archotol.124.9.1003. [DOI] [PubMed] [Google Scholar]
- 27.Spano S, Hanna S, Li Z, Wood D, Cartotto R. Does bronchoscopic evaluation of inhalation injury severity predict outcome? J Burn Care Res. 2016;37:1–11. doi: 10.1097/BCR.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 28.Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res. 2007;28:80–83. doi: 10.1097/BCR.0B013E31802C889F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Database used during the current study is available from the corresponding author upon reasonable request.