Abstract
Major adverse cardiac events (MACE) are some of the most common complications occurring in the perioperative period. Even though traditionally more focus has been on the patient with ischemic disease other cardiac conditions pose a greater risk. The risk is related to both patient-specific and surgical factors. Patients with significant cardiac disease undergoing vascular or major surgery may have up to a 10% risk of major morbidity and mortality. Identifying modifiable risks and intervening pre-, intra- and postoperatively can improve outcomes.
Heart Failure
Heart failure (HF) increases perioperative mortality three- to fivefold and is associated with a substantially higher risk than coronary artery disease (CAD) with a 30-day mortality rate 9.3% versus 2.9% risk with ischemic disease.2 HF may be present in up to 20% of elderly surgical patients. HF is also an independent predictor for ischemic heart disease and perioperative myocardial infarction risk.2 Patients with HF are twofold more likely to require readmission compared to those with CAD. HF is a strong predictor of pulmonary complications, and at least as important as a diagnosis of chronic obstructive pulmonary disease (COPD) in predicting perioperative respiratory adverse events.3 Many studies have shown that HF is among the most significant risk factors for perioperative complications. Patients with compensated HF have a 5 to 7% risk of cardiac complications; those with decompensated HF have a 20 to 30% incidence. Patients with active HF symptoms such as orthopnea, paroxysmal nocturnal dyspnea, or findings on examination such as rales, jugular venous distention, peripheral edema, or pulmonary edema on chest radiography have a very high risk of perioperative complications. Optimization of HF is important before proceeding with elective procedures. Waiting until B-type natriuretic peptide (BNP) levels have returned to baseline may assist with timing of procedures after a HF exacerbation.
Determination of ejection fraction (EF) with echocardiography can assist with intra- and postoperative care. Systolic HF with EF less than 30% is predictive of the highest risk followed by systolic HF with EF greater than 30%, then patients with diastolic HF who are higher risk than patients with normal EF. Elevated preoperative levels of C-reactive protein (CRP) and BNP are independent and strong predictors of serious postoperative cardiovascular events in patients having non-cardiac surgery.4 A normal BNP predicts a low risk of perioperative MACE. Ischemic disease is the most common cause of systolic HF, and hypertension is the most common cause of diastolic dysfunction. Much less information is known about the perioperative consequences of diastolic dysfunction compared to systolic dysfunction.
Valvular Disease
Aortic stenosis (AS) is the most common valvular lesion in the U.S. (2%–4% of adults older than 65 years); severe AS is associated with a high risk for perioperative complications. Aortic sclerosis, present in 25% of people 65–74 years old and 50% of those older than 84 years, causes a systolic ejection murmur similar to that of AS, but does not compromise hemodynamics. Aortic sclerosis can progress to stenosis, especially with aging or unmodified risk factors. The same risk factors that predispose to CAD are associated with development of AS. Any patient with AS should be considered at risk of ischemia due to a high likelihood of concomitant CAD, and even in the absence of CAD due to left ventricular hypertrophy secondary to the increased resistance from a stenotic valve.
Echocardiography is beneficial, especially if more than sedation is planned for a procedure. Patients with mild AS need echocardiography every three to five years; those with moderate stenosis benefit from evaluation every two to three years; and severe AS should be evaluated yearly with echocardiography. Patients with severe AS or mitral stenosis should not have anesthesia (unless emergency and life-saving) without a cardiology evaluation. It is reasonable to proceed with elective procedures in patients with severe AS if they are asymptomatic, and appropriate resources are available intra- and postoperatively. Additional hemodynamic monitoring may be necessary to avoid hypotension and tachycardia which can decrease coronary perfusion and cardiac output with untoward development of arrhythmias, ischemia, HF, or death. If patients meet independent criteria for valve replacement but are considered too high risk consideration should be given to balloon valvuloplasty, or transcatheter aortic valve replacement (TAVR).
Severe mitral stenosis poses significant risk. Patients with symptoms such as syncope, dyspnea, or HF associated with mitral stenosis should not undergo elective procedures. Patients with regurgitant lesions tolerate anesthesia and surgery if volume status is managed appropriately.
Infective endocarditis prophylaxis
Antibiotic prophylaxis to prevent endocarditis has never been evaluated with a randomized controlled trial. Over the last several decades the recommendations by the American College of Cardiology/American Heart Association (ACC/AHA) have drastically reduced the numbers of patients for whom prophylaxis is recommended. Adverse effects from antibiotics exceed the benefit from prophylactic therapy for most patients for whom past recommendations applied. It is felt that only an extremely small number of endocarditis resulting from bacteria can be prevented by taking antibiotics. Infective endocarditis is more likely to result from daily activities than from bacteremia caused by dental procedures, or those involving the gastrointestinal (GI) or genitourinary (GU) tracts. It is likely that educating patients to maintain optimal oral hygiene is most important.
Antibiotic prophylaxis to prevent infective endocarditis (IE) is only recommended for patients having dental procedures with manipulation of gingival tissue or the periapical region of teeth, or perforation of the oral mucosa. Prophylaxis is no longer indicated for procedures that involve the respiratory tract unless the procedure involves incision of the mucosa, such as tonsillectomy or adenoidectomy. Prophylaxis is no longer recommended for patients undergoing GI or GU procedures. Patients with enterococci should receive antibiotics to eradicate the bacteria from the urine before elective cystoscopy or other urinary tract manipulation if the presence of cardiac disease warrants prophylaxis. Only patients with the following cardiac conditions need IE prophylaxis: prosthetic valves, previous endocarditis, unrepaired cyanotic congenital heart disease (CHD), for 6 months after repair of CHD with prosthetic material or device, repaired CHD with residual defects at the site of prosthetic material, or transplanted hearts with valvular regurgition.5
Ischemic Disease
Patients with risk factors for CAD experience cardiovascular (CV) complications after non-cardiac surgery. CAD varies from mild, stable disease with little impact on perioperative outcome to severe disease accounting for serious complications during anesthesia. The basis of cardiac assessment is the history and the physical examination. Review of records and previous studies, especially stress tests and catheterization results, is necessary. Although commonly ordered, routine preoperative electrocardiograms (ECG) rarely add value to the care of surgical patients, particularly if ordered for advanced age.2 Recommendations for age-based testing were derived from the frequent incidence of abnormalities found on ECGs of elderly patients. Several studies have shown that clinical risk factors predict perioperative MACE better than ECG abnormalities. The ACC/AHA guidelines do not include ECG findings in the algorithm for evaluation of ischemic risk. A preoperative ECG should be ordered only to evaluate for rhythm disturbances or an acute coronary event. It is equally misguided to order a preoperative ECG to establish a baseline for comparison to postoperative ECG for the detection of ischemia or myocardial infarction (MI). Troponins levels detect far more events (40%) than ECG (6%) postoperatively.2 Troponin elevation predicted death at 1 year, but ECG changes did not. The ACC/AHA guidelines state “The current use of ECGs may have developed as a method to screen for MI when little else was routinely available.”
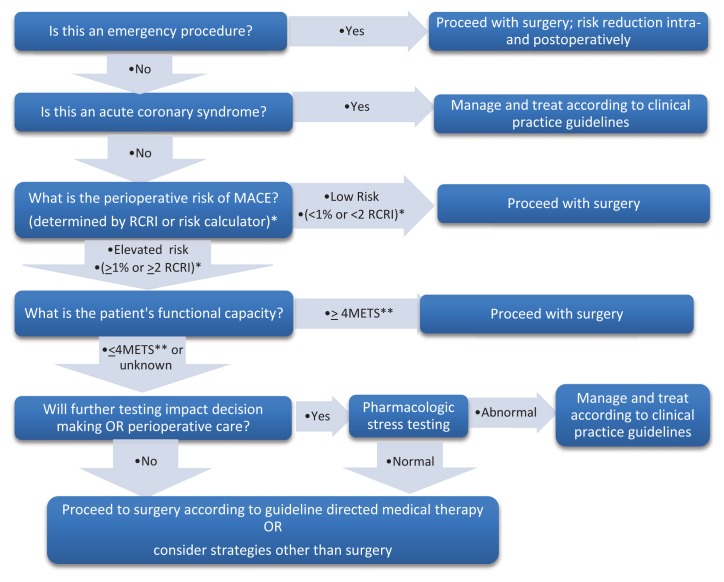
The ACC/AHA guidelines for CV evaluation for non-cardiac surgery have decreased recommendations for testing or revascularization.2 The guidelines have an algorithm to be followed in stepwise fashion, stopping at the first point that applies to the patient (See Figure 1). For emergency surgery, the focus is perioperative surveillance (e.g., troponins, hemodynamic monitoring) and risk reduction (e.g., beta blockers, statins, aggressive pain management). Active cardiac conditions (acute MI, unstable or severe angina, decompensated HF, severe valvular disease, or significant arrhythmias) warrant postponement for all except life-saving emergency procedures. Step 3, is the most complicated and requires calculation of the risk of a MACE. Using either a risk calculator (http://www.riskcalculator.facs.org or surgicalriskcalculator.com/miorcardiacarrest.com) or the Revised Cardiac Risk Index (RCRI) to define MACE rates determines the next steps. (See Table 1.) If the calculated MACE rate is 1% or less no further cardiac testing is recommended. The two risk calculators provide this number or if using the RCRI a patient with 0–1 RCRI predictors. The RCRI is calculated by assigning one point each for each of the following: a history of CAD, compensated HF, cerebrovascular disease, diabetes requiring insulin, a creatinine level ≥2 mg/dL, type of surgery (intrathoracic, intra-abdominal, or suprainguinal vascular surgery). For calculated risk greater than 1% or more than 2 RCRI predictors, Step 4 assesses functional capacity, defined by metabolic equivalents. Asymptomatic patients with average functional capacity (able to climb 2 flights of stairs without symptoms) can proceed to surgery. Step 5 considers patients with poor or indeterminate functional capacity, and a calculated risk of greater than 1%, or 2 or more RCRI predictors. However, additional testing is considered only if results will alter management.2, 6 The most benefit is derived from optimal medical interventions which can be instituted based on calculated risk usually without stress testing. The only utility of stress testing is determining patients with severe CAD with a large amount of myocardium at risk. These patients are typically those with 3 or more RCRI predictors who are symptomatic with exertion. Large areas of wall motion abnormalities on dobutamine stress echocardiography and poor exercise capacity on cardiopulmonary exercise testing (CPET) predict elevated risk.1, 6 Excess testing is associated with harm, especially for asymptomatic patients with no RCRI predictors.2,6
Figure 1.
Evaluation of Patients at Risk for Ischemic Complications for Noncardiac Surgery
*RCRI = Revised Cardiac Risk Index (see Table 1) or use risk calculator available at: surgicalriskcalculator.com/miorcardiacarrest.com
**MET= metabolic equivalents. 4 METs is the ability to walk 2–4 blocks or climb 1–2 flights of stairs.
Table 1.
Revised Cardiac Risk Index (RCRI)
|
Traditional risk factors for CAD such as smoking, hypertension (HTN), age, male gender, hypercholesterolemia, and family history do not predict perioperative risk. Surgical risk is no longer considered separately with the exception of the guidelines noting that cataract and plastic surgery are very low risk, and one can assume that patients having these procedures need no further testing. Therefore, the algorithm does not apply.2 The risk of a postoperative MI is increased if surgery is performed soon after an acute MI. The ACC/AHA guidelines recommend delaying surgery for at least 60 days post-infarction. Additionally, an MI within 6 months of surgery increases the risk of a perioperative stroke with an 8-fold increase in mortality. 2
The benefits and risks of coronary revascularization before non-cardiac surgery are controversial. The only randomized prospective study of preoperative revascularization vs. medical management failed to show an outcome difference. Revascularization is not recommended simply to lower the risk of surgery. There should be long term benefits of revascularization independent of the planned noncardiac procedure. Percutaneous coronary intervention (PCI) before noncardiac surgery is indicated only for acute coronary syndromes, or for left main (LM) disease in patients who are too high risk for coronary artery bypass grafting. PCI has not been shown to lower the perioperative risk in patients with stable CAD (other than LM disease in non-operative patients).
For patients who have non-cardiac surgery soon after revascularization, rates of morbidity and mortality are high. Patients who have had a PCI, especially with a drug-eluting stent (DES) require months, if not a lifetime of anti-platelets to prevent restenosis or thrombosis. The type of stent, DES or bare metal (BMS), must be identified. A scientific advisory offers recommendations for managing patients with coronary stents.2 Elective procedures that require stopping dual antiplatelet therapy are delayed during the high-risk period (12 months for DES; 4–6 weeks for BMS; 2 weeks after angioplasty without stenting). Invasive procedures increase the risk of stent thrombosis with mortality rates more than 45%. Stent thrombosis is best treated with PCI, which can be safely performed postoperatively. High-risk patients should only have procedures in facilities with immediate access to interventional cardiology.2 Premature discontinuation of dual antiplatelet therapy can cause catastrophic stent thrombosis, MI, or death.
If aspirin is continued, there is a low risk of bleeding complications during most procedures.7 A meta-analysis involving almost 50,000 patients undergoing a variety of noncardiac surgeries (30% taking aspirin perioperatively) found that aspirin increased bleeding complications by a factor of 1.5, but not the severity of complications, except in patients undergoing intracranial surgery and possibly transurethral resection of the prostate.7 When surgeons were blinded to aspirin administration they could not identify patients taking or not taking aspirin based on bleeding. There is an increased risk of vascular events when aspirin is stopped in patients who take it regularly. A rebound hypercoagulable state may result. Acute coronary syndromes occur 8.5 ± 3.6 days and acute cerebral events 14.3 ± 11.3 days after aspirin cessation, the typical duration of cessation before surgery. Events are twice as common in patients who discontinued aspirin in the previous 3 weeks compared to those who have not. Discontinuing aspirin for 3–4 days should be sufficient, if aspirin is stopped at all. Dosing is resumed as soon as possible. New platelets, formed after aspirin is discontinued, will not be affected. Normally functioning platelets of at least 50,000/mm3 are adequate to control surgical bleeding. It is recommended to discontinue aspirin only if taken for primary prevention (no history of stents, strokes, MI, vascular disease) except for neurosurgical procedures. If taken for secondary prevention (history of vascular disease), continuing aspirin is recommended except for procedures with a risk of bleeding in closed spaces (e.g., intracranial or intraspinal procedures).
Non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) are associated with increased vascular events. Recently the Federal Drug Agency (FDA) placed a warning on over-the-counter NSAIDs highlighting this risk. NSAIDS can block the therapeutic antiplatelet effects of aspirin if ingested before aspirin. Caution is warranted in using NSAIDs in the perioperative period in patients at risk of ischemia, especially if aspirin is discontinued.
Statins and beta-blockers started ahead of the day of surgery lower risk.2,8 Beta-blockers should not be started the day of surgery merely to lower the risk of ischemic events. Titration of beta-blockers is necessary to avoid hypotension. Hypotension and anemia in conjunction with beta-blockers increase the risk of strokes and death. However, careful and appropriate use of beta-blockers in patients at risk of perioperative ischemia prevents MACE.8 Bisoprolol may be preferred over other beta-blockers.9 Statins have anti-inflammatory and plaque-stabilizing properties. All patients with atherosclerosis benefit from statins, regardless of cholesterol levels. Continuing statins perioperatively reduces risk, and withdrawal can result in more adverse events. Statins should be started before high risk procedures in patients with elevated risk of MACE. Angiotension receptor blockers (ARB) and angiotension converting enzyme inhibitors (ACEI) are commonly used to treat HF and hypertension. Continuation of these drugs is associated with hypotension during anesthesia. This has led many to advocate holding these medications before surgery. However, there is reasonable evidence from nonsurgical settings that discontinuing ACE inhibitors is associated with worse outcomes.
The ACC/AHA guidelines give a class IIa recommendation to continuing these drugs in the perioperative period. Failure to resume ACEI and ARBs within 48 hours of procedures is associated with a 50% increase in mortality. 10
Elevated troponin levels in the first 3 days after surgery are associated with 30-day mortality.11 There is evidence that patients who have silent ischemia postoperatively have higher mortality rates than those with overt myocardial infarction. Perhaps this result can be attributed to interventions such as aggressive medial management to reduce complications, which suggests that there may be opportunities to impact outcome. European guidelines recommend troponin screening postoperatively in higher risk patients (i.e., those with 1 or more RCRI predictors having intermediate-low risk surgeries).12
Cardiac Implantable Electronic Devices (CIEDs)
Pacemakers and implantable cardioverter-defibrillators (ICDs) can be affected by electromagnetic interference.13 Consultation with the manufacturer or cardiologist may be needed. Patients usually have a card with important designations and phone numbers. Patients with ICDs invariably have HF, ischemic or valvular disease, cardiomyopathies, or potentially lethal arrhythmias. Special features such as rate adaptive mechanisms in some pacemakers need to be disabled, or the device reprogrammed to asynchronous pacing to prevent interference. Some monitors, ventilators, vibrations, or chest prepping fool the sensors into increasing pacing, leading to ischemia or inappropriate treatment.
Shock functions are disabled before procedures if interference or unexpected movement is undesirable. During intracranial, spinal, or ocular procedures, an unexpected discharge with movement can be catastrophic. Central line placement can trigger cardioversion. ICDs are deactivated only after a patient’s arrival to a facility that has monitoring and external defibrillation devices. Many devices are complex and reliance on a magnet to disable them, except in emergencies, is not recommended. Some devices ignore magnet placement. A magnet typically suspends anti-shock therapies in ICDs only while it is in place. Magnets do not affect the pacing function of an ICD. If a patient is pacemaker-dependent with a combination ICD the pacemaker must be reprogrammed if interference is anticipated. Magnets put pacemakers in a fixed rate mode to prevent interference. Some manufacturers’ magnet rates may be too fast for many patients during surgery, and consideration should be to have pacemakers reprogrammed to an appropriate rate. For procedures below the umbilicus interference is very unlikely. If a pacemaker or ICD is reprogrammed the device must be re-interrogated and re-enabled before the patient leaves a monitored setting.13
Atrial Fibrillation
Patients with atrial fibrillation (AF) are twice as likely to die within 30 days of surgery as patients with CAD.2 AF is associated with age, hyperthyroidism and valvular disease. Patients with rapid ventricular rates require rate control before elective surgery. Patients with new onset AF should have echocardiography and thyroid function testing.
Summary
Cardiac disease is common in the surgical population. A large population of patients is at risk of MACE. The history and physical examination provide valuable information. Using risk predictors can identify patients who will benefit from optimization primarily with medical management preoperatively. High risk patients need specialized care intra-and postoperatively. Patients with heart failure, arrhythmias and valvular disease are at far greater risk than those with CAD. Anesthesiologists need to be aware of cardiac conditions that predispose patients to perioperative risk, and interventions to lower that risk.
Biography
BobbieJean Sweitzer, MD, FACP, is Professor of Anesthesiology, Director Preoperative Medicine at Northwestern University in Chicago. This paper is based on a presentation from the 2015 MSMA Annual Convention.
Contact: bobbie.sweitzer@nm.org

Footnotes
Disclosure
None reported.
References
- 1.Hennis PJ, Meale PM, Grocott MP. Cardiopulmonary exercise testing for the evaluation of perioperative risk in non-cardiopulmonary surgery. Postgrad Med J. 2011;87:550–7. doi: 10.1136/pgmj.2010.107185. [DOI] [PubMed] [Google Scholar]
- 2.2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. JACC. 2014;64:e77–137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: Results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1188–98. doi: 10.1016/j.jamcollsurg.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Cho DK, Song YB, et al. Preoperative NT-proBNP and CRP predict perioperative major cardiovascular events. Heart. 2010;96:56–62. doi: 10.1136/hrt.2009.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura RA, Carabello BA, Faxon DP, et al. 2008 Guideline Update on Valvular Heart Disease: Focused Update on Infective Endocarditis. JACC. 2008;52:676–85. doi: 10.1016/j.jacc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Wijeysundera DN, Beattie WS, Elliot RF, et al. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ. 2010;340:b5526. doi: 10.1136/bmj.b5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chassot P-G, Delabays A, Spahn DR. Perioperative antiplatelet therapy: the case for continuing therapy in patients at risk of myocardial infarction. Br J Anaesth. 2007;99:316–28. doi: 10.1093/bja/aem209. [DOI] [PubMed] [Google Scholar]
- 8.Wallace AW, Au S, Cason BA. Association of the pattern of use of perioperative B-blockade and postoperative mortality. Anesthesiology. 2010;113:794–805. doi: 10.1097/ALN.0b013e3181f1c061. [DOI] [PubMed] [Google Scholar]
- 9.Ashes C, Judelman S, Wijeysundera DN, et al. Selective 1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol. A single-center cohort study of 44,092 consecutive patients. Anesthesiology. 2013;119:777–87. doi: 10.1097/ALN.0b013e3182a17f12. [DOI] [PubMed] [Google Scholar]
- 10.Mudumbai SC, Takemoto S, Cason BA, et al. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: A retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9:289–296. doi: 10.1002/jhm.2182. [DOI] [PubMed] [Google Scholar]
- 11.Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J. 2014;35:2383–431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 13.Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/ American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management: Executive summary. Heart Rhythm. 2011;8:1114–54. doi: 10.1016/j.hrthm.2010.12.023. [DOI] [PubMed] [Google Scholar]