Abstract
In 2011, the Division of Allergy/Immunology at Children’s Mercy published a themed mini-series1 including an article on food allergies that recommended allergen avoidance and prevention/treatment of anaphylaxis as the key pillars of management.2 Since then, the escalating food allergy “epidemic” has stimulated diagnostic and therapeutic advances, as well as coordinated multidisciplinary approaches to treat nutritional imbalances and psychosocial issues. We aim to highlight the team approach to food allergy care in this article.
Introduction
Food allergies have dramatically increased in prevalence, with some hailing it “the new epidemic.” Recent studies suggest a near 50% increase in the prevalence since 2007.3 It is estimated that about 15 million individuals in the U.S., including ethnic populations4, have food allergies. Review of patient encounter data at Children’s Mercy Allergy/Immunology (A/I) clinics over the past five years mirror the upward trend with one in six patients presenting for evaluation of food allergies. While speculations abound regarding the reasons for this spike, the “hygiene hypothesis” is currently the most favored explanation. Muted exposure to microorganisms in an environment that emphasizes germ-free cleanliness prompts the cytokine milieu to tilt towards a predominantly allergic phenotype. This concept can be encapsulated as “The 5 Ds”: dry skin, diet, dogs, dribble (shared microbial exposure), and vitamin D.5
Diagnosis of Food Allergies
The top eight allergens that trigger 90% of food allergic reactions are milk, egg, soy, wheat, fish, shellfish, peanut and tree nuts.6 With the enhancement of the repertoire of the western diet, a wide variety of food allergens ranging from fresh fruits and vegetables causing mild oropharyngeal symptoms (i.e., Oral Allergy Syndrome), to seeds such as sesame causing severe allergic reactions, are increasingly implicated. Oral Allergy Syndrome, also known as Pollen-Food Syndrome, is caused by cross-reacting allergens found in both pollen and foods such as raw fruits, vegetables, and some tree nuts. The immune system recognizes the cross-reacting epitopes in the pollen/food and directs an allergic response to it. Interestingly, the same fruits or vegetables can be ingested in cooked form because the heat-labile proteins get distorted during the heating process, rendering them unrecognizable by the immune system. The typical cross-reacting pollens are birch, ragweed, or grass allergens.
The diagnosis of food allergies pivots around a meticulous medical history looking for the presence of immediate onset of consistent and reproducible symptoms with ingestion of trace-small amounts of a particular food.6 Improvement of the symptoms with elimination of the food, and recurrence with reintroduction are also helpful historical features. If the history is suspicious of an IgE mediated reaction, percutaneous (prick) skin testing or blood testing for specific IgE (ImmunoCAP) to the food allergen, may be considered as the next step in the diagnostic evaluation. In particular, the tests are near-confirmatory when negative since they have a 95% negative predictive value.7 In other words, if the history is unclear or not suggestive of an allergic reaction (e.g., gassiness/fussiness on drinking milk-based formula) and the skin/blood test to milk is negative, IgE mediated allergy to milk can be ruled out with a 95% certainty. Unfortunately, the high false positive rate of 50% causes a large number of individuals to be incorrectly labeled as having food allergies.8 Use of 95% positive predictive cut off values may help differentiate sensitization (presence of positive IgE alone) from true food allergy (associated clinical symptoms).
Fortunately, recent advances in testing methods, such as the emergence of Component Testing, are enabling delineation of cross-reactivity to shared proteins (such as described in the Oral Allergy Syndrome), from allergic sensitization to the anaphylactic components, though this is still a work in progress. For instance, component testing to peanut enables identification of individuals who may have a severe reaction to peanut ingestion (those with elevated ara h2 greater than 2 kUA/L), versus those who may have mild oropharyngeal symptoms secondary to cross-reactivity with plant proteins/pollens (elevated ara h8 or ara h9).9 Similarly, the absence or low level of specific IgE to ovomucoid, helps predict the likelihood of an individual with egg allergy tolerating baked egg products.10 With the same rationale, the absence or low levels of specific IgE to casein helps delineate patients who may tolerate baked milk products.10 (See Table 1). This knowledge not only facilitates safe introduction of baked egg and baked milk products into the child’s diet, but also has prognostic value in delineating sub-groups of patients who may outgrow these allergies. Research into component analysis for soy, wheat, and other food allergens is ongoing, and promises to open up clinical management options for individuals with these allergies in the future.
Table 1.
Values that may help in Diagnosis of Food Allergy7
| Age Group | Food | Specific IgE Levels Associated with 95% Risk of Reaction Serum IgE (kU/L) | Component Testing |
|---|---|---|---|
| Child | Egg | ≥ 7 | |
| <2 years | Egg | ≥ 2 | Absent or trace Ovomucoid Can tolerate baked egg |
| Child | Cow Milk | ≥ 15 | |
| <2 years | Cow Milk | ≥ 5 | Absent or trace Casein Can tolerate baked milk |
| Child | Peanut | ≥ 14 | Ara h2 >2 Predictive of systemic reactions |
| Child | Fish | ≥ 20 | Elevated Ara h8 and ara h9 (predictive of cross-reactive minor symptoms) |
Oral Food Challenges
Since a combination of history, blood/skin test, elimination/reintroduction of the food is only able to correctly confirm or rule out the presence of food allergies in up to 50–60% of patients, the Oral Food Challenge (OFC) remains the gold standard for confirming a food allergy.6 This is a process where the suspected food is ingested by the patient using a graded protocol, while being closely monitored by a trained specialist in a setting appropriately equipped to handle anaphylaxis. This can be performed in a double-blind placebo controlled manner) or, more commonly, as an “open challenge” where the suspected food is introduced in a preparation that is normally consumed (e.g., peanut butter, scrambled eggs).11 At Children’s Mercy, we prefer the latter approach since the family will then be comfortable using the same preparation/recipe at home. If there are issues with texture or special needs (e.g. G tube feeds) or the family needs help, then our on-site Chef helps to create a customized recipe/dish. We have been performing OFCs at our Food Allergy Center for over 19 years and have not had any significant adverse safety outcomes to date.
Proximity Food Challenges
Food allergies cause significant impairment in the quality of life for affected individuals and their families, with many of them worried about accidental contact with the trigger allergen. Published literature is conflicting, with some earlier reports suggesting a heightened risk of reaction on contact or inhalation of food allergens (e.g., peanuts) in public places such as airplanes and baseball games.12 Contrary to those reports, some recent studies13,14 including those from our center15,16 suggest that the risk of a severe reaction is largely related to ingestion or contact of the allergen with mucus membranes, and not with casual contact on intact skin. Therefore, in order to address the concerns regarding the possibility of airborne and contact food sensitivity in individual patients, we developed the Proximity Food Challenge test at our center. This test helps them understand what to expect with similar exposure in a real world environment in the future.
The Proximity Food Challenge procedure is conducted in an office setting equipped to handle anaphylaxis. After obtaining verbal consent, the provider opens a jar containing the suspect allergen, often peanut (since it is the most feared) in the room. While conversing, the peanut butter jar is then brought increasingly closer to the child. If there is no reaction, a dab is applied on the patient’s arm, and the patient monitored. Five minutes later, the food allergen is washed off with soap and water. After an initial period of anxiety, we typically notice visible calming and relief. In situations where there is heightened anxiety secondary to the perception of occurrence of a previous reaction, an attempt to mimic the scenario may be undertaken in a double-blind, placebo controlled fashion. In our experience, the Proximity Food Challenge has helped alleviate fears of the families and enabled the child to integrate in the social network at school and in the community.
Treatment of Food Allergies
The staple treatment of food allergies over the past decade has been strict avoidance of the triggering allergen.6 Patients and families are educated on how to read labels and are provided written food/anaphylaxis plans and epinephrine autoinjector delivery device demonstration using the “teach-back method.” Since some food allergies are outgrown (e.g., milk, egg, soy, wheat), periodic evaluation of the serum IgE to check for decline suggestive of onset of tolerance, followed by diagnostic oral food challenges when levels fall to predictive cut-off values, is standard of care. In instances where the food allergy is thought to be life-long (e.g., peanut, fish), the patients are advised to continue to avoid the food as the only recourse available to them. To assist with the challenges of keeping up with the ever-changing dietary products available in the market, patients and families are referred to resources such as “Food Allergy Education and Research” (FARE) for credible and updated information (www.foodallergy.org). Newer Options for Treatment of Food Allergies Recent emergence of promising options to patients with unremitting food allergies are generating excitement and hope. Research using techniques geared towards inducing tolerance such as oral immunotherapy, sublingual immunotherapy and epicutaneous immunotherapy (e.g. topical peanut patch application) have shown promise17 and may be commercially available to patients and families with food allergic disorders in the future.
Food Allergy Center of Excellence/Clinical Trial Network Member
The Children’s Mercy Food Allergy Center was recently recognized as a Food Allergy Center of Excellence and a member of the Food Allergy Clinical Network by FARE.18 The Clinical Network will strive to accelerate the development of new therapeutics and best practices for patients with food allergies.
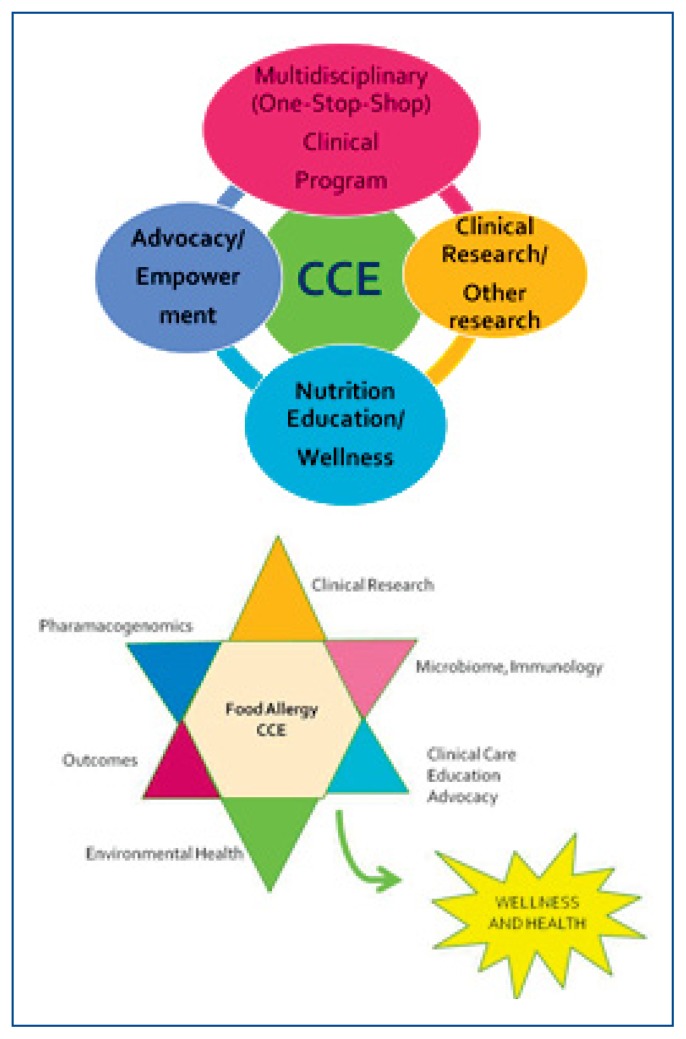
The Children’s Mercy Food Allergy Center of Excellence (CM_FACE) offers comprehensive clinical care and cutting edge opportunities to participate in clinical and translational research for individuals with food allergic disorders. The four key pillars and the additional unique strengths of CM_FACE are depicted in Figure 1.
Figure 1.
Multi-Disciplinary Clinical Care: A one-stop shop care for patients with complex food allergic disorders focused on providing outcomes-based quality care. Currently, the Comprehensive Food Allergy and Nutrition Clinic and the Eosinophilic Esophagitis Clinics include providers from the specialties of Allergy/Immunology, Nutrition and Gastroenterology.
Research: Therapy-oriented, outcomes-based clinical research with participation in local, national and international multi-centered clinical trials and establishment of a registry and bio-repository bank (including genetic material).
Education: Education of patients and families, providers, and the community on avoidance of food allergic triggers and selection of appropriate foods resulting in a well-balanced and nutritious diet.
Advocacy/Service: Active support of community initiatives (such as FARE, Allergy and Asthma Network, etc.) that champion the needs of patients and families with food allergic disorders.
Nutritional Imbalance and Multidisciplinary Team Approach
Children with multiple food allergies have been reported to have significantly decreased nutritional intake and growth and elevated systemic and gut inflammatory biomarkers, compared to children without food allergy.19, 20 It is therefore imperative to recognize these issues upfront and to provide resources for education, counseling and intervention. CM_FACE offers multi-disciplinary “Comprehensive Food Allergy and Nutrition Clinics” held both at the Missouri and Kansas locations. Each clinic is typically staffed by a board certified allergist, an advanced nurse practitioner trained in food allergy, and a registered dietitian. The role of the dietitian is to provide nutrition assessment, intervention, education, and follow-up services. The nutrition assessment includes anthropometric, biochemical, clinical and dietary analysis. Anthropometric measurements to monitor growth and development, and laboratory data pertinent to the assessment of nutritional status, such as Vitamin D insufficiency and anemia, are obtained. Clinical evaluation includes obtaining details of the food allergy, food related behavioral issues, and medication history along with the use of vitmans/minerals and nutrient/alternative supplements. Dietary assessment includes obtaining either a 24-hour or a three-day dietary record, as applicable. The feeding environment, cultural and ethnic practices that may impact dietary intake, feeding times, and food availability are assessed. Comprehensive nutrition education is provided including presentation of the CM_FACE food allergy folder containing age appropriate nutrition information, instructions on reading food labels, methods to avoid cross contamination, food safety, working with schools, and alternative food choices to prevent nutrient deficiencies that may occur secondary to food-restrictive diets (See Table 2). Innovations include collaboration with the Children’s Mercy Chef to create recipes for oral food challenges as well as assisting families with allergen-free home cooking.
Table 2.
Local and National Food Allergy Resources
|
www.childrensmercy.org/Allergy -> Clinical Services -> Evaluation of food allergies Children’s Mercy Allergy is an academic group of physicians and nurse practitioners who are dedicated to clinically caring for children with food allergies and advancing research in the field of food allergy. we are a FARE Center of Excellence/Clinical Trial Network member. we have dedicated Food Allergy and Nutrition Clinics at Children’s Mercy Broadway Clinics and at Children’s Mercy Kansas that occur monthly with a registered dietician. we also conduct research studies in food allergies. Contact studynurseallergy@cmh.edu or call 816-960-8905. |
|
Food Allergy Research & Education www.foodallergy.org Food Allergy Research & Education (FARE) is a 501(c)(3) nonprofit organization and the leading national organization working on behalf of the 15 million Americans with food allergies. |
|
Centerview Food Allergy www.centerviewfoodallergymanagement.com Centerview is a local non-profit in Kansas City that works to educate the community about food allergies. Centerview has 5 local support groups located throughout Kansas City to provide education and support to families dealing with food allergies. The web-site has handy links to local resources. |
|
Food Allergy Equality Initiative www.foodequalityinitiative.org Food Equality Initiative, Inc. (FEI) is a recognized non-profit, public benefit corporation established in 2015 by Emily Brown and Amy Goode, two food allergy moms, to support the low-income food allergy and Celiac communities by providing safe, healthy gluten free and allergy friendly food, nutrition education and advocacy. |
Timing of Introduction of High-Risk Allergenic Foods into the Infant Diet
With the increasing awareness of food allergies, providers are faced with questions from anxious families regarding the optimal timing of introduction of high-risk allergenic foods and its impact on the possible prevention of food allergies. In 2000, the American Academy of Pediatrics (AAP) made the recommendation to strictly avoid introduction of high risk foods such as peanuts until the age of two to three years. In 2008, the AAP rescinded the recommendations based on evidence based review of studies that failed to demonstrate that delayed introduction of highly allergenic foods prevented the onset of food allergies. In 2015, based on the findings of the Learning Early about Peanut Allergy (LEAP study), the AAP went one step further and recommended introduction of these foods between 4–11 months of age.21 In 2016, the National Institute of Inflammatory and Allergic Diseases (NIAID) proposed addenda to the 2010 NIAID Food Allergy Guidelines that are currently under public review, and are elaborated on below.22
The LEAP study enrolled 640 “high risk” U.K. infants at high risk for peanut allergy, a priori defined as those who had egg allergy, severe eczema, or both.23 The children, ranging from 4 to 11 months old, were split into two groups: a “consumption group” that was regularly fed peanut, and an “avoidance group” that ate no peanut protein. At age 5, the children were subjected to an Oral Food Challenge to peanut protein to determine their allergy status. Overall, feeding peanut to high-risk infants reduced the likelihood of developing peanut allergy in both groups of these infants by 70–80 percent.
The follow-up LEAP-ON study evaluated 550 of the original LEAP study participants to determine whether regular ongoing consumption of the peanut protein was essential to maintain the state of tolerance.24 The subjects in both groups were told to actively avoid peanut protein for 12 months. At the end of the year, they underwent an oral food challenge to peanut. The rate of peanut allergy was still significantly higher in the “avoidance” group (18.6 percent) compared to the “consumption” group (4.8 percent), providing reassurance that despite the lack of peanut protein in their diet, the rate of peanut allergy remained 74 percent lower in the consumption group than the avoidance group.
Based on data in the above studies, the NIAD is proposing the following recommendations that are currently under public review.22
NIAID Addendum Guideline 1–3 Recommendations
The NIAID Expert Panel (EP) recommends that infants with severe eczema and/or egg allergy be introduced to age-appropriate peanut-containing food as early as four to six months to reduce the risk of peanut allergy, following successful introduction of other solid food that demonstrates developmental readiness. They recommend that evaluation with peanut-specific IgE or skin prick testing be strongly considered before introduction of peanut in these high-risk infants, in order to determine whether peanut should be introduced, and if so, the preferred method of introduction. The document outlines an algorithmic management approach to facilitate the decision making process.
For infants with mild-moderate eczema, egg allergy or both, the EP recommends that dietary peanut be introduced at home without an in-office evaluation. If an in-office evaluation is desired, the decision points discussed above should apply. For infants without eczema or any food allergy, the EP recommends that age-appropriate peanut-containing foods be freely introduced in the diet, along with other solid foods, and in accordance with family preferences and cultural practices.
Introduction of Other High Risk Allergenic Solid Foods
Does introducing other allergenic foods, specifically cooked egg, cow’s milk, sesame, whitefish, or wheat, also protect against the development of allergy? In the Enquiring about Tolerance (EAT) study, 1,303 exclusively breastfed infants were randomized them into two groups.25 The “Standard introduction” group was exclusively breastfed until age six months, and then allowed to consume allergenic foods according to the parents’ discretion. The “Early-introduction” group was fed 2 grams of allergen protein twice weekly from three months of age. The prevalence of food allergy in the participants was assessed at one and three years of age. Overall, the trial did not demonstrate efficacy of early introduction of multiple allergenic foods.
Conclusion
The prevalence and impact of food allergies is considerable. Primary care providers are the first-line resource for individuals with food allergic disorders. Ongoing collaboration between primary care providers and A/I specialists is essential to ensure affected individuals lead productive and healthy lives.
Take-Home Messages
Food Allergies continue to increase in the U.S.
The clinical history is key to making an accurate food allergy diagnosis.
Oral food challenges are the gold standard to help confirm or rule out the presence of IgE mediated allergies.
Food diary, elimination/re-introduction of suspected foods, and evaluation via percutaneous skin/mmunoCap testing for evidence of significant IgE mediated sensitization have supplementary roles in diagnosis.
The “pitfalls” of food allergy diagnosis are an over-reliance on easily obtainable blood test panels, and under-reliance on corroboration with history and food challenges, that result in incorrect labels creating anxious “food allergic” families.
Avoidance, education, and preparation for emergencies continue as the pillars of current management.
Coordinated and collaborative co-management with allergists and trained dietitians is key to ensuring optimal nutritional intake and growth of children.
Some individuals with milk and egg allergies can consume them in baked form; these individuals are more likely to outgrow those allergies.
Component testing can help identify those individuals who tolerate baked milk/egg products, as well as facilitate determination of those who may have the more severe forms of peanut allergy.
Periodic re-challenges to monitor tolerance as indicated by history, food allergen, and serum IgE is a part of ongoing follow-up.
Delaying introduction of highly allergenic solid foods in infants is not supported by recent evidence.
There is increasing evidence that even infants at moderately higher risk of developing food allergies may be able to gain tolerance by consuming peanut products between four to eleven months of age.
Emerging therapeutic research options that may help individuals attain tolerance to some allergenic foods (such as peanut, milk and egg), may be commercially available in the near future.
The Food Allergy and Research Education (FARE) designated Children’s Mercy Food Allergy Center of Excellence offers state-of-the art food allergy care, including comprehensive, personalized food allergy and nutrition clinics, and opportunities to participate in potentially ground-breaking food allergy studies.
Biography
Chitra Dinakar, MD, (left), MSMA member since 2002, and Missouri Medicine Editorial Board Member, is Professor of Pediatrics, University of Missouri-Kansas City and Director, FARE Center of Excellence at Children’s Mercy, Division of Allergy/Immunology, Children’s Mercy Kansas City. Barbara warady, MS, RD, LD, (right), is Senior Clinical Nutrition Specialist in Nutrition Services, Children’s Mercy Kansas City.
Contact: cdinakar@cmh.edu


Footnotes
Disclosure
CD receives grant support from Food Allergy and Research Education (FARE).
References
- 1.Dinakar C. The changing climate of allergy/immunology disorders. Mo Med. 2011 Sep-Oct;108(5):335–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Dowling PJ. Food allergy: practical considerations for primary care. Mo Med. 2011 Sep-Oct;108(5):344–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011 Jul;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 4.Dinakar CKO, Yarbrough M, Gupta R. Asian Indian Food Allergy Survey: Unique Ethnic Food Allergens. Annals of Allergy, Asthma and Immunology. 2015;115(5) Suppl 1:F1 (p A3). Also P 240(p A107) [Google Scholar]
- 5.Allen KJ, Koplin JJ. Prospects for Prevention of Food Allergy. J Allergy Clin Immunol Pract. 2016 Mar-Apr;4(2):215–20. doi: 10.1016/j.jaip.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010 Dec;126(6):1105–18. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004 May;113(5):805–19. doi: 10.1016/j.jaci.2004.03.014. quiz 20. [DOI] [PubMed] [Google Scholar]
- 8.Sicherer SH, Wood RA American Academy of Pediatrics Section On A, Immunology. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012 Jan;129(1):193–7. doi: 10.1542/peds.2011-2382. [DOI] [PubMed] [Google Scholar]
- 9.Klemans RJ, Broekman HC, Knol EF, et al. Ara h 2 is the best predictor for peanut allergy in adults. J Allergy Clin Immunol Pract. 2013 Nov-Dec;1(6):632–8e1. doi: 10.1016/j.jaip.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Leonard SA, Caubet JC, Kim JS, Groetch M, Nowak-Wegrzyn A. Baked milk- and egg-containing diet in the management of milk and egg allergy. J Allergy Clin Immunol Pract. 2015 Jan-Feb;3(1):13–23. doi: 10.1016/j.jaip.2014.10.001. quiz 4. [DOI] [PubMed] [Google Scholar]
- 11.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, et al. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009 Jun;123(6 Suppl):S365–83. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Comstock SS, DeMera R, Vega LC, et al. Allergic reactions to peanuts, tree nuts, and seeds aboard commercial airliners. Ann Allergy Asthma Immunol. 2008 Jul;101(1):51–6. doi: 10.1016/S1081-1206(10)60835-6. [DOI] [PubMed] [Google Scholar]
- 13.Simonte SJ, Ma S, Mofidi S, Sicherer SH. Relevance of casual contact with peanut butter in children with peanut allergy. J Allergy Clin Immunol. 2003 Jul;112(1):180–2. doi: 10.1067/mai.2003.1486. [DOI] [PubMed] [Google Scholar]
- 14.Wainstein BK, Kashef S, Ziegler M, Jelley D, Ziegler JB. Frequency and significance of immediate contact reactions to peanut in peanut-sensitive children. Clin Exp Allergy. 2007 Jun;37(6):839–45. doi: 10.1111/j.1365-2222.2007.02726.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RM, Barnes CS. Airborne concentrations of peanut protein. Allergy Asthma Proc. 2013 Jan-Feb;34(1):59–64. doi: 10.2500/aap.2013.34.3622. [DOI] [PubMed] [Google Scholar]
- 16.Shroba JBC, Nanda M, Dinakar C, Ciaccio C. Comparison of Ara h2 in Household Dust of Peanut Allergic versus Nonallergic Individuals. Annals of Allergy, Asthma and Immunology. 2015 Nov;115(5) suppl 1:A5, A26. [Google Scholar]
- 17.Sindher S, Fleischer DM, Spergel JM. Advances in the Treatment of Food Allergy: Sublingual and Epicutaneous Immunotherapy. Immunol Allergy Clin North Am. 2016 Feb;36(1):39–54. doi: 10.1016/j.iac.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Available from: http://news.childrensmercy.org/food-allergy-research-amp-education-announces-launch-of-fare-clinical-network-names-22-centers-ofexcellence-as-inaugural-members/
- 19.Costa LC, Rezende ER, Segundo GR. Growth parameters impairment in patients with food allergies. J Allergy (Cairo) 2014;2014:980735. doi: 10.1155/2014/980735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanda MKDC. Food Allergy and its Impact on Growth: Missouri WIC2014-present. Annals of Allergy, Asthma and Immunology. 2015;115(5) Suppl 1:F4 (p A3). Also 35 (pA 27) [Google Scholar]
- 21.Primary c, Fleischer DM, Sicherer S, et al. Consensus Communication on Early Peanut Introduction and the Prevention of Peanut Allergy in High-risk Infants. Pediatrics. 2015 Aug 31; doi: 10.1186/s40413-015-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Available from: http://www.niaid.nih.gov/news/newsreleases/2016/Pages/Comment-Food-Allergy-Guidelines.aspx
- 23.Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015 Feb 26;372(9):803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Toit G, Sayre PH, Roberts G, et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med. 2016 Mar 4; doi: 10.1056/NEJMoa1514209. [DOI] [PubMed] [Google Scholar]
- 25.Perkin MR, Logan K, Tseng A, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med. 2016 Mar;:4. doi: 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]