Abstract
Raynaud’s phenomenon is a relatively common but often unrecognized clinical syndrome causing characteristic color changes in the digits as a result of vasospasm. This may occur after exposure to a cold environment, emotional stress, or from other physical or medication exposures. Differentiating between primary and secondary Raynaud’s is important as secondary Raynaud’s can be complicated by digital ischemia and gangrene whereas primary Raynaud’s is generally a benign condition. Referral to a rheumatologist is recommended to help evaluate for an underlying rheumatologic condition and to guide future therapy.
Epidemiology
Community-based surveys estimate Raynaud’s may be present in 5–20% of women and in 4–14% of men. The prevalence in individuals over age 60 is 0.1–1%.1 Primary Raynaud’s is generally considered a benign condition and typical age of onset is from 15 to 30 years of age. This tends to be more common in young females and can be familial.2 Primary Raynaud’s may remit over time. A prospective study over seven years in a middle age Caucasian white population found remission occurred in 64% of women and men although some symptoms remained in about 20% of those labeled as remission.3
Clinical Presentation
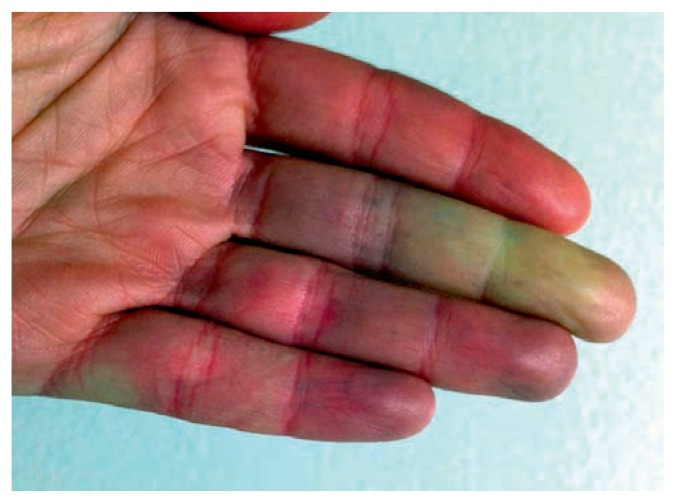
Raynaud’s phenomenon is classically described with a triphasic color change of the digits with initial white or pallor (ischemic phase), then blue or cyanosis (deoxygenation phase), followed by red or erythema (reperfusion phase). Screening questions should include:
Are your fingers unusually sensitive to the cold?
Do your fingers change color when they are exposed to cold temperatures?
Do they turn white, blue, or both?
Generally, the diagnosis of Raynaud’s is confirmed if there is a positive response to all three questions.4 Then, if positive for the diagnosis of Raynaud’s, further evaluation should occur to differentiate between primary and secondary Raynaud’s. It is typically present in the hands but can also affect the toes, nose, earlobes, or nipples. It may begin in one finger and spread symmetrically to other digits, often sparing the thumb.
A typical attack may last less than an hour but can also persist for hours. In primary Raynaud’s, attacks are more likely symmetric, episodic, and without evidence of peripheral vascular disease. Patients more commonly have a negative ANA and normal inflammatory markers. There should be no evidence of tissue gangrene, digital pitting, or tissue injury in primary Raynaud’s. In contrast, patients with secondary Raynaud’s will describe attacks that are more frequent, painful, often asymmetric and may lead to digital ulcerations. These ulcerations can resolve to leave scars or digital pits, or may progress to more severe digital necrosis, gangrene, or auto-amputation. Digital ulcerations can become secondarily infected, leading to osteomyelitis, and can require surgical intervention. Clues that may raise concern for secondary Raynaud’s include age of onset greater than age 40, male gender, digital ulcerations, asymmetric attacks, ischemic signs proximal to the fingers and toes, and abnormal nailfold capillaroscopy. Nailfold capillaroscopy is an inexpensive, quick, and non-invasive exam technique that can help differentiate primary from secondary Raynaud’s. Lubricant is placed on the nailfolds and the nailbed capillaries can be viewed using an ophthalmoscope set at 10 to 40 diopters. The fourth and fifth digits have the greatest translucency of the skin but all fingers should be visualized.
Early abnormal findings will include few capillary hemorrhages and few enlarged capillaries. A more active pattern may show moderate loss of capillaries, frequent giant capillaries, and disorganized vascular architecture. A late pattern may show “drop-out” with severe loss of capillaries with extensive avascular areas and ramified or bushy capillaries. These abnormalities are found in patients with underlying connective tissue diseases such as systemic sclerosis, dermatomyositis, or undifferentiated connective tissue disease. In a study of 586 patients with Raynaud’s followed for 3,197 patient years, systemic sclerosis developed in 25.8% of patients with abnormal capillary pattern, in 35.4% of those with a specific autoantibody, and in 79.5% of those with both abnormal nailfold capillaries and a specific autoantibody. Patients with both predictors were 60 times more likely to develop systemic sclerosis than in those with neither.5
Disease Associations
Raynaud’s is often the presenting symptom for numerous connective tissue diseases such as scleroderma, lupus, or mixed connective tissue disease. Thus, early on, many patients are thought to have primary Raynaud’s; however, a meta-analysis of 640 patients with primary Raynaud’s found that 13% eventually developed into a connective tissue disease.6 Another study showed 15– 20% of patients with Raynaud’s who had autoantibodies, abnormalities of nail fold capillaries or both and who did not initially meet criteria for connective tissue disease developed into a defined connective tissue disease typically within two years.7 Further investigation is warranted to look for an underlying cause. Etiologies may be due to vasospastic, structural, or hematologic causes. Vasospastic causes may be drug-induced (ergot, methysergide, beta-blockers, dextroamphetamine, methylphenidate, bleomycin, cisplatin, clonidine, cocaine, cyclosporine, interferon-alpha, nicotine, and vinblastine). Other vasospastic causes include pheochromocytoma, carcinoid syndrome, or thyroid disease. Structural causes are numerous and may include thoracic outlet syndrome, atherosclerosis, brachiocephalic trunk disease as in Takayasu’s arteritis, Buerger’s disease or thromboangiitis obliterans, cold injury, connective tissue diseases, polyvinyl chloride, use of vibratory tools such as jackhammers, or angiocentric lymphoma. Hematologic causes include cryoglobulinemia, cryofibrinogenemia, paraproteinemia, or polycythemia. Mimickers of Raynaud’s include other vascular disorders such as acrocyanosis and erythromelalgia. Acrocyanosis typically lacks the triphasic color change but can cause a more persistent blue-red discoloration of the skin of the hands and feet after cold exposure. It is more common in females but is painless. Erythromelalgia also occurs more commonly in females but is exacerbated by heat exposure and leads to pain in the feet and lower limbs, and less commonly, the hands.
Pathophysiology
Raynaud’s phenomenon was first described in 1862 by Maurice Raynaud and was described as “local asphyxia of the extremities” which was a result of “increased irritability of the central parts of the cord presiding over vascular innervation.” Over a century later the pathophysiology is still not perfectly understood. There are likely numerous factors involved in the pathogenesis of Raynaud’s, such as vascular, intravascular, and neural abnormalities, and these factors may differ among those with primary versus secondary Raynaud’s. Digital arteries constrict more strongly with people with Raynaud’s compared with those without Raynaud’s. Capillaries provide nutrients to the skin but there is also a rich concentration of arteriovenous anastomoses in the fingers and toes that function in thermoregulation. In two small studies (12 patients with primary Raynaud’s, 12 with secondary Raynaud’s associated with scleroderma and 10 controls, and eight patients with scleroderma and nine controls)8, 9 finger capillary blood flow was not significantly affected during moderate systemic cooling in controls but finger capillary blood flow was slightly reduced in primary Raynaud’s and almost abolished in secondary Raynaud’s patients.
Vascular abnormalities lead to the balance between vasoconstriction and vasodilator to be tipped in favor of vasoconstriction. There may be a mismatch between the endothelium-derived vasoconstrictors such as endothelin-1 and vasodilators such as nitrous oxide and prostacyclin. Intravascular abnormalities promoting vasoconstriction may include platelet activation with increased thromboxane. There may be defective fibrinolysis and white blood cell activation. Red blood cells may have reduced deformability and increased viscosity may occur. Ultimately, ischemic reperfusion injury may lead to additional oxidative stress. Neural abnormalities including impaired vasodilation due to calcitonin gene related peptide deficiency also contribute. Arteriovenous anastomese are controlled by sympathetic adrenergic nerve activity and if increased stress occurs, there is increased vasospasm.
Sympathetic adrenergic vasoconstriction in those with Raynaud’s is also targeted to proximal arteries and this disruption of capillary blood flow is most profound in those with secondary Raynaud’s. Increased vasoconstriction mediated by activation of the alpha2-adrenoreceptors in the thermoregulatory process also occurs. Smooth muscle from cutaneous vessels is predominately innervated via the alpha2 adrenoreceptors. Cooling induces activation of Rho/Rho kinase signaling pathway which causes alpha-2c adrenoreceptors stored in the Golgi apparatus to translocate to the cell surface and causes increased sensitivity to calcium of contractile proteins.10 Other factors such as cigarette smoking, genetic factors, and hormonal influence are also significant. Several studies have shown that during the immediate pre-ovulatory period, healthy controls demonstrated digital vascular reactivity similar to that of patients with Raynaud’s.
Diagnostic Evaluation
Prompt recognition of Raynaud’s by clinical history is key and referral to a rheumatologist is encouraged. A detailed history and physical exam along with use of nail fold capillaroscopy will guide the rheumatologist in their laboratory evaluation. Typically work-up will include complete blood count, comprehensive metabolic panel, muscle enzymes, rheumatoid factor, anti-cyclic citrullinated peptide, thyroid studies, hepatitis screening, anti-neutrophil cytoplasmic antibodies, anti-phospholipid antibodies. Most important would be investigation for antibodies associated with connective tissue diseases as Raynaud’s is often the first presenting sign or symptom of several of these diseases such as scleroderma, mixed connective tissue disease, dermatomyositis, or lupus. Labs including anti-nuclear antibody by immunofluorescence (ANA), anti-double stranded DNA, anti-SSA, anti-SSB, anti-Smith, anti-ribonucleoprotein, anti-Scl-70, anti-centromere, anti-fibrillarin, anti-Pm-Scl, anti-RNA polymerase III, and possibly a myositis profile may be obtained. Further investigations may require serum protein electrophoresis, cryoglobulins, cold agglutinins, venous/arterial dopplers, or angiography depending on the clinical scenario.
Therapy
Patient education is the primary therapy recommended. Avoiding sudden cold and rapid changes in temperatures should be emphasized. Keeping the hands and feet warm as well as keeping the total body warm is important. Patients should be counseled to avoid tobacco and caffeine which may exacerbate symptoms. Avoidance of sympathomimetic drugs such as decongestants, amphetamines, and methylphenidate will help control symptoms. If Raynaud’s is thought to be vibration induced, discontinuation of vibratory tools such as jackhammers would be of benefit. As a better understanding of the pathophysiology arises, medical therapies will likely also improve. Currently, calcium channel blockers are the most common and practical treatment. These vasodilators have a direct effect on vascular smooth muscle and inhibit platelet aggregation. A meta-analysis of 18 randomized placebo controlled and double-blinded trials were evaluated and showed use was associated with a 33% reduction in severity and an average decrease of 2.8–5.0 attacks over a one-week period.11 A Cochrane review of calcium channel blockers for primary Raynaud’s also showed a decrease in frequency of attacks by around 1.7 attacks per person per week.12 Nifedipine is the most commonly used and most studied and can be dosed usually at 30–60mg per day, if the blood pressure permits its use. Other calcium channel blockers shown to help include nisoldipine, amlodipine, felodipine, and isradipine. Verapamil has not been shown to be effective. There also remains a concern that short-acting dihydropyridines may increase cardiovascular risk so these should be used with caution. If a patient is intolerant to calcium channel blockers, losartan, an angiotension II receptor blocker, may be considered.
A randomized, parallel-group, controlled trial demonstrated a reduction in the severity of Raynaud’s episodes and decreased episode frequency with use of losartan 50mg daily.13 Intravenous iloprost, a prostaglandin analogue, is a potent vasodilator and inhibitor of platelet aggregation, and remains a key medication for management of scleroderma patients with severe digital ulcerations. IV iloprost appears effective at decreasing frequency and severity of attacks. It also may prevent or heal digital ulcers with the effect prolonged after the intravenous infusion is given. 14 Oral prostaglandins show mixed results and more studies are needed to determine their efficacy. Endothelin 1 is the most potent endogenous vasoconstrictor. There are endothelin A and B receptors on vascular smooth muscle. In addition there are endothelin A receptors on cardiac myocytes and endothelin B receptors on endothelial cells. Bosentan is a nonselective endothelin A and B receptor antagonist. In a randomized, double-blind, placebo-controlled study of 188 patients with scleroderma with at least one active digital ulcer at baseline, treatment of bosentan reduced the number of new digital ulcers by 30% compared to placebo but did not increase the healing rate of existing ulcers.15 Phosphodiesterase inhibitors prevent the degradation of cGMP which mediates nitric oxide synthesis and release. Nitric oxide serves as a potent vasodilator, inhibitor of platelet activation, and inhibitor of vascular smooth muscle proliferation. Studies of sildenafil, vardenafil, and tadalafil have all shown benefit on Raynaud’s symptoms 16 17 18 with regards to decreased frequency of attacks and symptoms, although many studies are limited in number of patients and limited in follow-up time of less than 8 weeks. Topical nitrates can induce local vasodilation but have not been shown to be effective in limited studies and side effects of headaches and tachyphylaxis limit their use.
Statins have direct vasculoprotective effects as they increase endothelial nitric oxide synthase and nitric oxide production, decrease endothelin-1 expression, and promote microvascular growth but there is insufficient evidence for statins to be recommended as standard therapy for Raynaud’s; however, a study of use of atorvastatin at 40mg per day for 4 months did show a reduction in the overall number of new digital ulcers in scleroderma patients. 19 Small studies of gingko biloba and St. John’s Wort showed no clinical benefit over placebo in Raynaud’s. Botulinum toxin A is currently under investigation as a treatment for Raynaud’s as it may potentially have an effect on inhibiting sympathetic vasoconstriction.
Conclusion
Raynaud’s syndrome is a relatively common phenomenon and patients should be referred promptly to a rheumatologist to assist with differentiation between primary and secondary Raynaud’s. The extent of the work-up will be guided by a detailed history and physical exam but is important to pursue as Raynaud’s is often the earliest presentation of an underlying connective tissue disease. Patient education is a crucial part of the treatment. As the pathophysiology becomes better understood, treatments will also likely improve, but current therapies do exist to prevent new ulcer formation and to improve symptoms.
Figure 1.
Screening questions for Raynaud’s should include:
- Are your fingers unusually sensitive to the cold?
- Do your fingers change color when they are exposed to cold temperatures?
- Do they turn white, blue, or both?
Generally, the diagnosis of Raynaud’s is confirmed if there is a positive response to all three questions.
Source: http://www.nhlbi.nih.gov/health/health-topics/topics/raynaud/
Biography
Katherine K. Temprano, MD, is an Associate Professor of Internal Medicine, Rheumatology Fellowship Program Director, Division of Rheumatology, Saint Louis University Medical Center.

Footnotes
Disclosure
None reported.
References
- 1.Ling SM, Wigley FM. Raynaud’s phenomenon in older adults: diagnostic considerations and management. Drugs Aging. 1999;15:183. doi: 10.2165/00002512-199915030-00002. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Medsger TA, et al. Raynaud’s phenomenon: A proposal for classification. Clin Exp Rheumatol. 1992;10:485. [PubMed] [Google Scholar]
- 3.Suter LG, Murabito JM, et al. The incidence and natural history of Raynaud’s phenomenon in the community. Arthritis Rheum. 2005;52:1259–1263. doi: 10.1002/art.20988. [DOI] [PubMed] [Google Scholar]
- 4.Wigley FM. Clinical practice Raynaud’s phenomenon. New Engl J Med. 2002 Sep 26;347:1001–1008. doi: 10.1056/NEJMcp013013. [DOI] [PubMed] [Google Scholar]
- 5.Koenig M, et al. Arthritis Rheum. 2008;58:3902–12. doi: 10.1002/art.24038. [DOI] [PubMed] [Google Scholar]
- 6.Spencer Green G. Outcomes in primary Raynaud’s phenomenon. Arch Intern Med. 1998;158:595–600. doi: 10.1001/archinte.158.6.595. [DOI] [PubMed] [Google Scholar]
- 7.Zufferey P, Depairon M, et al. Prognostic significance of nailfold capillary microscopy in patients with Raynaud’s phenomenon and scleroderma-pattern abnormalities: a six-year follow-up study. Clin Rheumatol. 1992;11:536–541. doi: 10.1007/BF02283115. [DOI] [PubMed] [Google Scholar]
- 8.Coffman JD, Dohen AS. Total and capillary fingertip blood flow in Raynaud’s phenomenon. N Engl J Med. 1971;285:259–263. doi: 10.1056/NEJM197107292850505. [DOI] [PubMed] [Google Scholar]
- 9.LeRoy EC, Downey JA, Cannon PJ. Skin capillary blood flow in scleroderma. J Clin Invest. 1971;50:930–939. doi: 10.1172/JCI106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey SR, Eid AH, et al. Rho kinase mediates cold-induced constriction of cutaneous arteries; role of a2c adrenoreceptor translocation. Circ Res. 2004;94:1367–74. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud’s phenomenon: meta-analysis. Rheumatology. 2005;44:145–150. doi: 10.1093/rheumatology/keh390. [DOI] [PubMed] [Google Scholar]
- 12.Ennis H, Anderson ME, Wilkinson J, Herrick AL. Calcium channel blockers for primary Raynaud’s phenomenon. Cochrane Library. 2014;1:1–47. doi: 10.1002/14651858.CD002069.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Dziadzio M, Denton C, et al. Losartan therapy for Raynaud’s phenomenon and scleroderma. Arthritis Rheum. 1999;42:2646–2655. doi: 10.1002/1529-0131(199912)42:12<2646::AID-ANR21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Pope J, Fenlon D, et al. Iloprost and cisaprost for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 2000;(2):CD000953. doi: 10.1002/14651858.CD000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matucci-Cerinic M, et al. Ann Rheum Dis. 2011;70:32–8. doi: 10.1136/ard.2010.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fries R, Shariat K, et al. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation. 2005 Nov 8;112(19):2980–5. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 17.Caglayan E, et al. Arch Intern Med. 2012;172:1182–4. doi: 10.1001/archinternmed.2012.2271. [DOI] [PubMed] [Google Scholar]
- 18.Shenoy PD, et al. Rheumatol. 2010;49:2420–8. doi: 10.1093/rheumatology/keq291. [DOI] [PubMed] [Google Scholar]
- 19.Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008 Sep;35(9):1810–8. [PubMed] [Google Scholar]