Abstract
Acute aortic syndromes are disorders of the thoracic and abdominal aorta that are usually symptomatic and require urgent evaluation and treatment. They include acute aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer. Knowledge of the natural history of these conditions, prompt diagnosis, and surgical intervention, when indicated, are the keys to successful outcomes.
Background
Acute aortic syndromes are disorders of the thoracic and abdominal aorta that are usually symptomatic and require urgent evaluation and consideration for surgical intervention. They include acute aortic dissection and two variants: intramural hematoma, and penetrating atherosclerotic ulcer. Physicians will encounter patients with these conditions across a spectrum of specialties, including family and internal medicine, emergency medicine, critical care, radiology, cardiology and cardiac surgery.
Acute Aortic Dissection
Acute aortic dissection, (AAD), is the most common and most lethal of the acute syndromes, and requires urgent diagnosis and treatment. Untreated, it is associated with mortality rates of 1% to 2% per hour after the onset of symptoms, and 90% at three months. 1,2 Aortic dissection results from a tear in the aortic wall, and a column of blood enters the medial layer of the aorta creating a “hydraulic endarterectomy.”3 This divides the aorta into true and false lumens that are separated by a septum consisting of medial and intimal aortic tissue (See Figure 1). The medial tissue is often abnormal and weakened as the result of cystic medial degeneration. The separated outer layer, comprised of aortic medial tissue and adventitia, may be strong enough in some patients to prevent death from rupture of the aorta into the pericardial cavity and fatal cardiac tamponade, or rupture into the pleural cavities resulting in fatal exsanguination. The extent of the dissecting hematoma determines outcome.3 This hematoma, which most commonly originates from a tear in the proximal ascending aorta, extends antegradely (or distally) for a variable distance, and may cause obstruction of critical aortic branches, including those to the brain, kidneys, abdominal viscera, and extremities. Occlusion of intercostal and lumbar arteries can result in paraplegia. If the dissecting hematoma extends proximally (retrogradely), it can occlude the ostia of the coronary arteries, resulting in myocardial infarction, and can disrupt the aortic valve, creating severe aortic regurgitation.
Figure 1.
Risk Factors
Hypertension is the most common risk factor associated with AAD.1 Cystic medial degeneration of severe degree is present in about 20% of patients. Other risk factors include the presence of genetically mediated syndromes (Marfan, Loeys-Dietz, Turner, Noonan, Ehlers-Danlos), a bicuspid aortic valve, dilatation of the ascending aorta and aortic root, and coarctation of the aorta (See Table 1). A genetic basis for nonsyndromic familial thoracic aortic aneurysm and dissection, another risk factor, has recently been identified.4 Additional risk factors are aortic inflammatory disorders (aortitis), pregnancy, and extreme isometric exertion (such as weight lifting or other activity that results in marked and rapid elevation of systolic blood pressure). Extreme emotional upset associated with severe hypertension has resulted in AAD. We treated a patient who sustained an aortic dissection while berating the referees at his daughter’s basketball game. Cocaine use is a risk factor. Catheter-based interventions on the aorta and the coronary arteries and manipulations during open cardiac surgical procedures can result in aortic dissection
Table 1.
| Risk Factors for Acute Aortic Dissection | |
|---|---|
| Hypertension | |
| Cystic medial degeneration | |
| Genetically mediated syndromes | Marfan Loeys-Dietz Turner Noonan Ehlers-Danlos |
| Bicuspid aortic valve | |
| Dilatation of ascending aorta and aortic root | |
| Coarctation of the aorta | |
| Non-syndromic familial thoracic aneurysm | |
| Aortic Inflammatory disorders (Aortitis) | Takayasu Giant Cell Arteritis Behcet's |
| Pregnancy | |
| Extreme Isometric exertion | |
| Cocaine use | |
| Aortic Intervention | Cardiac Catheterization Cardiac Surgery |
Classification
Two classifications of aortic dissection are widely used (See Figure 2).1,2 The Stanford classification is the most popular. Stanford type A includes any dissection that involves the ascending aorta, with or without involvement of the aortic arch or the distal aorta. Stanford type B includes any dissection that originates in the descending thoracic aorta, and the dissection can occasionally extend proximally into the aortic arch. In the DeBakey classification, type I is analogous to the Stanford type A, and type III is analogous to the Stanford type B. The DeBakey type II is a dissection that originates in the ascending aorta, is confined to it, and is the least common.
Figure 2.
Symptoms and Signs
The most common symptom of AAD is sudden and severe chest pain.1 However, other variants of chest pain and back pain have been described, and AAD can also occur in the absence of pain. In type A dissection, the pain is usually located in the anterior chest, and may radiate into the neck, back or abdomen. In type B dissection, the pain usually occurs posteriorly in the chest and also in the abdomen. Other symptoms include syncope, caused by hypotension associated with cardiac tamponade or rupture of the aorta, and coma, due to occlusion or severe obstruction of the brachiocephalic arteries. Abdominal pain is caused by mesenteric and renal artery hypoperfusion. Renal failure in the setting of AAD is associated with a mortality rate of 50 – 70%, and mesenteric ischemia with a rate as high as 87%.1 Absence of pulses in the lower extremities results from occlusion of the aorta and iliofemoral arteries.
Diagnosis
Patients presenting to the emergency department with significant chest pain will have an electrocardiogram and a serum troponin determination to rule out myocardial infarction. It is important to note however, that the electrocardiogram can be abnormal in up to 70% of patients with an ascending aortic dissection. If the diagnosis of myocardial infarction is in question, other diagnostic studies should be performed promptly to rule out aortic dissection, so that agents that affect coagulation are not administered. A chest radiograph may show widening up the upper mediastinal shadow, but is often unremarkable. Computed tomographic scanning with contrast (CT) is currently the most widely used diagnostic study to detect AAD, and should be obtained in any patient with chest pain and an uncertain diagnosis (See Figure 1). It is highly sensitive and specific for dissection. Not infrequently, an aortic dissection is demonstrated on a CT study that is performed to rule out a pulmonary embolus, although enhancement of the thoracic aorta may be suboptimal using the pulmonary embolus protocol. Unfortunately, failure to diagnose AAD by not performing a CT study in patients presenting with chest pain of uncertain etiology is a not infrequent cause of law suits when these patients are discharged from the emergency department and subsequently die of their dissection.
Transesophageal echocardiography (TEE) is a highly sensitive and specific study to detect AAD involving the ascending aorta, and is distinctly superior to transthoracic echocardiography. It can be performed rapidly but requires sedation and a cardiologist to perform the study. In addition to identifying the dissecting membrane, TEE can identify pericardial tamponade, aortic regurgitation, and involvement of the coronary arteries in the dissection. It may also identify the site of the intimal tear, which is helpful to the surgeon (See Figure 3). Transthoracic echocardiography (TTE) is limited to examination of only the proximal ascending aorta, the aortic valve and the contents of the pericardial cavity. Magnetic resonance imaging (MRI) is also a highly sensitive and specific study to detect AAD (See Figure 4). It does not require use of contrast medium and thus can be useful in patients with impaired renal function. In some situations it can provide information similar to that which can be obtained from echocardiography, CT and aortography. Disadvantages of MRI compared with CT and TEE include higher cost, a longer time to complete the study, inaccessibility to patients who are connected to ventilators and monitoring devices, and limited availability.
Figure 3.
Figure 4.
Despite advances in noninvasive and minimally invasive techniques for diagnosis of AAD, aortography remains an important and highly accurate method for establishing the diagnosis, and remains the benchmark against which all other diagnostic studies are measured. However, it is used far less frequently, and most often in conjunction with coronary angiography in stable patients with AAD who are suspected of having significant coronary artery disease. Although some patients with AAD may have important coronary artery disease, the risk of rupture in hemodynamically unstable patients greatly exceeds the risk of a possible intraoperative myocardial infarction and consequently, the majority of patients with AAD do not undergo preoperative coronary angiography.
Treatment
The initial treatment of Type A AAD involves rapid control of blood pressure and heart rate thus reducing the velocity of contraction of the left ventricle (dP/dt), in an effort to prevent extension of the dissecting hematoma. Intravenous beta blocking agents are administered to reduce dP/dt and to induce bradycardia.3 Afterload reduction is achieved with administration of intravenous sodium nitroprusside, clevidipine, or nicardipine. In our institution, all patients with AAD involving the ascending aorta who are considered suitable candidates for operative treatment are taken directly to the operating room from the emergency department or when they are transferred from other hospitals. Patients with type B (DeBakey type III) dissection are initially managed similarly with beta blockade, followed by afterload reduction, and are transferred from the emergency department to an intensive care unit for invasive monitoring and control of blood pressure and pain.
Type A Dissection
The goal of surgery for AAD involving the ascending aorta is to prevent death from pericardial tamponade or exsanguination. The surgical objective is to eliminate the site of the intimal tear by excision of the segment of aorta containing the tear and replacement with a synthetic graft, redirecting blood flow into the true lumen. The most common site of the intimal tear is in the ascending aorta just above the commissures of the aortic valve, and it is present in this location in approximately 70% of patients with Stanford type A and DeBakey types I and II dissections. The tear is present in the aortic arch in 10% of patients, and in the descending thoracic aorta in 20%.5 If the tear is present in the aortic arch, the aortic arch is replaced as well. If significant aortic regurgitation is present, the aortic valve is repaired or replaced. Operative treatment is associated with significant risk, and is adversely affected by the presence of severe hemodynamic instability, malperfusion of important organ systems as a result of the dissection, and severe central nervous system dysfunction. However, given the natural history of the condition without surgical intervention, few patients are refused operation. Early mortality in experienced centers has ranged from 15% to 35%.1
Type B Dissection
The majority of patients who sustain a type B (DeBakey III) dissection can be managed with non-operative therapy, which involves aggressive management of hypertension. For patients who do not require surgical intervention, one-year survival is approximately 90%.4 The indications for immediate surgical intervention include evidence for free or contained rupture, rapid expansion of the aortic diameter, organ or limb ischemia resulting from malperfusion, uncontrollable hypertension, and intractable pain. Presence of a genetically mediated syndrome such as Marfan or Loeys-Dietz may be an indication for intervention because of poorer results with medical therapy. Open surgical repair consists of resection of the segment of aorta containing the intimal tear and any aneurysmal aorta, and replacement with a synthetic graft, restoring flow to the true lumen Alternatively, and now more commonly, patients with acute type B dissection requiring intervention are treated with endovascular stent grafts which cover and exclude the intimal tear, and restore flow to the true lumen. These procedures have been associated with lower operative morbidity and mortality than open repair.
Intramural Hematoma
Intramural hematoma (IMH) results from rupture of vasa vasora (the blood vessels that supply the aorta), and a hematoma develops within the aortic wall (See Figures 5 and 6A and 6B).2 Less commonly it results from an intimal fracture of an atherosclerotic plaque. Patients can present with symptoms and signs identical to those seen in patients with AAD, and initial evaluation and management are similar to those for patients with AAD.
Figure 5.
Figure 6A.
Figure 6B.
The natural history of IMH is variable. Progression to frank aortic dissection occurs in from 28% to 47% of patients in the largest reported series.1 Larger aortic diameter (>5.5 cm) and hematoma thickness (>16mm) predict progression to AAD and a worse outcome.6 Treatment of patients with these findings in the ascending aorta, and of patients who have severe chest pain or hemodynamic instability is identical to that for patients with type A AAD. In most patients with ascending aortic involvement, there is no intimal tear. The ascending aorta is resected and replaced with a synthetic graft. This will eliminate the risk of rupture and tamponade. In our practice, the majority of patients with IMH involving the ascending aorta undergo aortic replacement.
Patients who present with IMH in the descending thoracic and abdominal aorta are initially managed conservatively with beta blockade and control of hypertension. Serial imaging is necessary to monitor changes in aortic diameter and evidence for extention of the hematoma. Resolution of the hematoma occurs commonly. If there is evidence for enlargement of the aorta or extension of the hematoma, open or endovascular repair is indicated.
Penetrating Atherosclerotic Ulcer
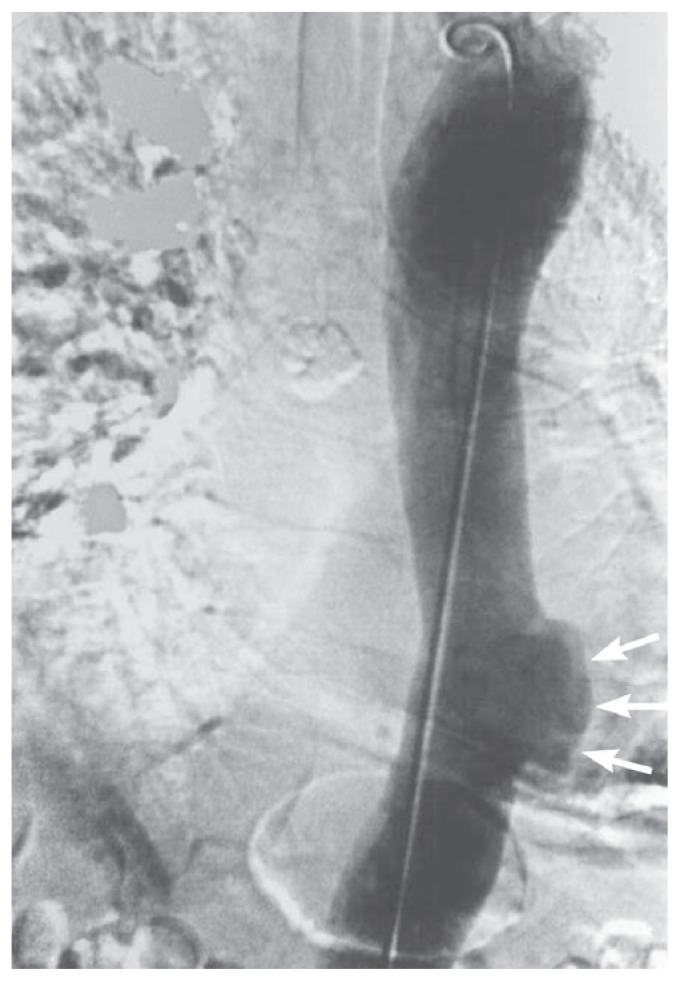
Penetrating atherosclerotic ulcer (PAU) is generally a less lethal condition than AAD and IMH (Figure 7).7 Patients can however, present with severe chest pain and require careful evaluation. More commonly however, PAUs are discovered incidentally on angiographic CT, or MRI imaging studies of the chest and abdomen that are performed for other indications (See Figure 8). They generally occur in densely calcified areas of atherosclerosis in the descending thoracic and abdominal aorta. A small percentage of the ulcers will progressively enlarge to form saccular aneurysms. They can also result in dissection and formation of an IMH. If surgical intervention is indicated for any of the above complications, endovascular repair is usually feasible if the ulcer is present in the ascending or abdominal aorta, and is preferred over open repair.
Figure 7.
Figure 8.
Follow-Up
Patients who survive acute aortic syndromes especially AAD, require close follow-up after initial therapy to detect aortic aneurysm formation in the remaining aorta, extension of the dissection or intramural hematoma, and enlargement of a penetrating ulcer. CT or MR imaging and meticulous control of blood pressure with beta blocking agents or other antihypertensive medications are the mainstays of management. Follow-up at a dedicated aortic surgical center is advisable.
Any patient who is found to have an enlarged thoracic aorta with a diameter of 4 cm or greater should receive appropriate medical therapy, and be followed at regular intervals to determine the need for surgical intervention.8,9 Untreated hypertension is a risk factor for aortic enlargement, aneurysm formation and aortic rupture. Other risk factors, such as smoking and dyslipidemia should be addressed as well.
Currently, according to the American College of Cardiology/American Heart Association Guidelines, an asymptomatic patient with an ascending aorta measuring 5.5 cm or more in diameter, or who has evidence for aortic enlargement of more than 0.5 cm in a year should be considered for surgical intervention.10 The size threshold is somewhat greater for aneurysms involving the aortic arch and descending thoracic aorta. For patients with genetically mediated conditions, the threshold may be lower.
Aortic dissections, intramural hematomas, and penetrating atherosclerotic ulcers are all treatable conditions and rapid diagnosis and institution of appropriate medical and surgical therapy will result in satisfactory outcomes for the majority of patients.
Biography
Michael C. Murphy, MD, Catherine F. Castner, RN & Nicholas T. Kouchoukos, MD, MSMA member since 1986, are in the Division of Cardiovascular and Thoracic Surgery, Missouri Baptist Medical Center, BJC HealthCare, St. Louis, MO.
Contact: michael.murphy@bjc.org

Footnotes
Disclosure
None reported.
References
- 1.Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112:3802–3813. doi: 10.1161/CIRCULATIONAHA.105.534198. [DOI] [PubMed] [Google Scholar]
- 2.Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. NEJM. 1997;336:1876–1888. doi: 10.1056/NEJM199706263362606. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney MS, Lewis CTP, Murphy MC, Williams JP, Frazier OH. Cardiac Surgical Emergencies. In: Ewer MS, Naccarelli GV, editors. Critical Care Clinics. Philadelphia: W.B. Saunders Company; 1989. pp. 659–678. [PubMed] [Google Scholar]
- 4.Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2014;64:1725–39. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Roberts WC. Aortic dissection: anatomy, consequences, and causes. Am Heart J. 1981;101:1981. doi: 10.1016/0002-8703(81)90666-9. [DOI] [PubMed] [Google Scholar]
- 6.Evangelista A, Eagle KA. Is the optimal management of acute type A aortic intramural hematoma evolving? Circulation. 2009;120:2029–2032. doi: 10.1161/CIRCULATIONAHA.109.907246. [DOI] [PubMed] [Google Scholar]
- 7.Castillo-Sang M, Moon M. Indications for replacement of the thoracic aorta. MoMed. 2012;109:295–300. [PMC free article] [PubMed] [Google Scholar]
- 8.Gifford SM, Duncan AA, Greiten LE, Gloviczki P, Oderich GS, Kalra M, et al. The natural history and outcomes for thoracic and abdominal penetrating aortic ulcers. J Vasc Surg. 2016;63:1182–8. doi: 10.1016/j.jvs.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 9.Elefteriades JA, Tranquilli M, Darr U, Cardon J, Zhu BQ, Barret P. Symptoms plus family history trump size in thoracic aortic aneurysm. Ann Thorac Surg. 2005;80:1098–1100. doi: 10.1016/j.athoracsur.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 10.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for Diagnosis and Management of Patients With Thoracic Aortic Disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology American Stroke Association, Society of Cardiovascular Anesthesiologist, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]