Abstract
Purpose
Prospective and longitudinal neuroimaging studies of posterior fossa tumors are scarce. Here we evaluate the early changes in white matter and intellectual outcome up to 3 years after diagnosis.
Patients and methods
Twenty-two children with posterior fossa tumors and 24 similarly-aged healthy children participated. Patients included: (a) 12 individuals who received surgery, cranial-spinal radiation (CSR), and focal radiation to the tumor bed (CSR group) and (b) 10 individuals who received local therapy, either surgery only or surgery and focal radiation to the tumor bed (Local group). Diffusion tensor imaging (DTI) and intelligence measures were obtained an average of 3 months after diagnosis and then at 12, 24, and 36 months later. DTI tractography and voxel-wise approaches were employed. The Neurological Predictor Scale was used to summarize the type and amount of treatment for PF tumor patients. Linear mixed modelling was used to evaluate group differences at baseline and changes over time in DTI metrics for both the specific white matter tracts and voxel-wise, as well as for intelligence measures.
Results
Based on tractography, patients treated with CSR had significantly higher Axial and Mean diffusivity in the cortical-spinal tracts (CST) 3 month after diagnosis – particularly on the right side, p < .003, compared to healthy children. Mean diffusivity in right CST decreased over time in this group of patients, p = .001. No differences compared to controls were evident in specific tracts for the Local group, p > .10. Voxel-wise analyses revealed multiple areas of white matter compromise in both patients groups. Notably, both patient groups had lower scores on intelligence measures compared to the Control group: The CSR group displayed lower performance 3 months following diagnosis, ps < 0.001, and their performance remained stable over time ps > 0.10, whereas the Local group displayed no differences at 3 months, ps> 0.10, but their performance declined over time, ps < 0.01. At baseline, higher MD in right CST predicted lower Perceptual Reasoning scores across all participants, p = .001. Furthermore, lower FA in left IFOF at baseline predicted decline in Processing Speed over time, p = .001. In patients, more aggressive treatment protocols and presence of mutism were related to lower performance on intelligence measures at baseline, ps < 0.04.
Conclusions
Children treated with CSR displayed diffuse white matter compromise and poor intellectual outcome shortly after radiation treatment. There was evidence of subsequent growth of white matter structure, but stable intellectual insult. Conversely, in children treated with either surgery only or surgery and focal radiation to the tumor bed we observed less compromise of white matter early following treatment and no intellectual insult compared to healthy children. However, declines in intellectual function were evident for these children, though their performance remained within the average normative range. Overall, results suggest that early intervention is necessary to circumvent these deficits.
Keywords: Brain tumor, Children, Cognition, White matter, Diffusion tensor imaging, Longitudinal
Highlights
-
•
There are early deficits to intellect and white matter shortly after treatment
-
•
Early deficits were observed only after cranial-spinal radiation
-
•
Intellectual deficits are generally stable over time
-
•
White matter indices, mutism, and treatment predicted intellectual outcome
1. Introduction
Pediatric brain tumors are most frequently located in the posterior fossa (PF), including medulloblastoma, ependymoma, and astrocytoma (Pollack, 1994). With advances in therapy, survival rates have increased dramatically, but these tumors and their treatment can lead to injury to white matter as well as poor intellectual outcome (Lassaletta et al., 2015; Mabbott et al., 2006; Reddick et al., 2005; Riggs et al., 2014; Rueckriegel et al., 2010; Scantlebury et al., 2016). Cranial-spinal radiation (CSR) is related to white matter insult and cognitive impairment (Copeland et al., 1999; Hoppe-Hirsch et al., 1995; Reddick et al., 1998). Previous studies show that younger age at diagnosis and larger radiation dose and field consistently predict the poorest outcomes (Chapman et al., 1995; Grill et al., 1999; Khong et al., 2003; Moxon-Emre et al., 2014; Mulhern et al., 1998; Silber et al., 1992). However, the time course of when these deficits appear is less clear, including whether it starts immediately following a diagnosis of PF tumor or several years after treatment completion. This knowledge is crucial for monitoring the emergence of and potentially mitigating the impact of these adverse effects, including informing the implementation of neuro-rehabilitation strategies.
In most longitudinal studies, relatively intact intellectual function is observed immediately after acute stages of diagnosis and treatment, along with subsequent decline with increasing time from diagnosis (Copeland et al., 1999; Mulhern et al., 1998) (Mulhern et al., 2005; Palmer et al., 2001; Radcliffe et al., 1994; Ris et al., 2001). However, some studies show immediate deficit, stability over time, or even improvement in cognition according to each patient's trajectory (Kun and Mulhern Jr., 1983; Moxon-Emre et al., 2014; Moxon-Emre et al., 2016b). The majority of studies have focused on children treated with CSR and have not included children treated with local therapies or healthy control children.
Similarly, there is some evidence of decreased white matter organization and volume with increased time from treatment (Glass et al., 2017; Palmer et al., 2002; Reddick et al., 2000). However, the majority of imaging studies are retrospective, cross sectional, and conducted many years following diagnosis. Only recent studies have focused on changes in white matter immediately following diagnosis and treatment; these studies provide emerging evidence that there is the presence of early insult in white matter (Glass et al., 2017; Nieman et al., 2015; Perreault et al., 2014).
There is a dearth of prospective longitudinal studies examining the trajectory of white matter change in children with PF tumors and relating such changes to cognitive function. Furthermore, previous longitudinal imaging studies have not examined changes in white matter for both specific tracts and in a voxel-wise manner, nor have they included children treated with CSR, those treated with local therapy, and healthy children. A comprehensive approach to evaluating white matter across multiple groups is critical to determine the areas of white matter most affected by disease or treatment. Here we evaluate changes in white matter and intellectual outcome in children treated for PF tumors in the first 3 years after diagnosis, compared to healthy children. Our specific research objectives are to: 1) evaluate differences between patients and controls in white matter microstructure (using tractography and voxel-wise methods) and in intelligence scores close to diagnosis and over time, and 2) examine whether white matter indices or clinical information predict cognitive outcomes. We hypothesize that declines over time in white matter metrics and intellectual outcome will be observed in children with brain tumors, and this decline will be greatest in children treated with CSR.
2. Methods
2.1. Participants
Following REB approval at each site, 61 participants were recruited from 3 pediatric hospitals in Canada between 2007 and 2011 (The Hospital for Sick Children, Alberta Children's Hospital, and British Columbia Children's Hospital). Fifteen children (n = 6 healthy control; n = 9 brain tumor) were excluded due to having only one data point or poor imaging quality. The final cohort included 22 children diagnosed with PF tumors and 24 similarly-aged healthy children (Control group). Patients were placed into 2 groups. The first patient group (CSR group) included 12 patients who received surgery, CSR, and focal radiation to the tumor bed:(2 patients on the St. Jude Medulloblastoma (SJMB) -96 protocol (Gajjar et al., 2006); 10 patients on SJMB-03 protocol (Green et al., 2015). The second patient group (Local group) included 10 patients who received local therapy, including surgery only (n = 7) or surgery and focal radiation to the tumor bed (n = 3). Demographic details are provided in Table 1. The 3 groups did not differ for sex, χ2 (2) = 0.25, p = .884, handedness, χ2 (2) = 0.23, p = .893, or age at first testing, F(2, 43) = 0.47, p = .631. Parent education was significantly higher in the Control group compared to the CSR and Local group (mother's education: F(2, 40) = 7.40, p = .002; father's education: F(2, 40) = 6.05, p = .005). Medical information is provided in Table 2.There were 17 children diagnosed with PF tumors at The Hospital for Sick Children within the same time frame and who did not participate in our study; those patients had similar diagnostic and treatment information to those included in the current study.
Table 1.
Demographic information for all groups.
| Variable | Control group (n = 24) |
Local group (n = 10) |
CSR group (n = 12) |
|---|---|---|---|
| Sex (male: female) | 12: 12 | 5: 5 | 7: 5 |
| Handedness (left: right) | 3: 21 | 1: 9 | 2: 10 |
| Age at first testing (years) | |||
| Mean (SD) | 10.51 (2.55) | 9.88 (3.65) | 9.59 (2.66) |
| Range | 5.81–14.93 | 6.01–16.07 | 6.27–15.41 |
| Mother's education (years)⁎ | |||
| Mean (SD) | 19.04 (4.81) | 14.30 (3.30) | 14.00 (2.60) |
| Range | 12–30 | 7–18 | 11–18 |
| Father's education (years)⁎ | |||
| Mean (SD) | 18.17 (3.81) | 14.50 (1.90) | 14.67 (3.24) |
| Range | 12–25 | 12–17 | 10–20 |
Indicates significantly higher values for the Control group in comparison to the brain tumor groups (p < .05).
Table 2.
Diagnostic and treatment details for brain tumor groups.
| Variable | Local group (n = 10) |
CSR group (n = 12) |
|---|---|---|
| Age at diagnosis (years) | ||
| Mean (SD) | 9.59 (3.62) | 9.32 (2.69) |
| Range | 5.77–15.63 | 5.96–15.26 |
| Time between diagnosis and first testing (months) | ||
| Mean (SD) | 3.55 (1.07) | 3.19 (2.38) |
| Range | 2.17–5.29 | 0.79–8.28 |
| Tumor type (%) | ||
| Medulloblastoma | 0 (0%) | 12 (100%) |
| Astrocytoma | 6 (60%) | 0 (0%) |
| Ependymoma | 3 (30%) | 0 (0%) |
| Choroid plexus papilloma (4th ventricle) | 1 (10%) | 0 (0%) |
| Tumor location | ||
| Vermis | 3 (30%) | 6 (50%) |
| 4th Ventricle | 2 (20%) | 3 (25%) |
| Cerebellar hemisphere | 2 (20%) | 2 (17%) |
| Posterior fossa – unspecifieda | 3 (30%) | 1 (8%) |
| Tumor size (mm2)b | ||
| Mean (SD) | 1946 (1021) | 1667 (940) |
| Range | 700–3360 | 12–2800 |
| Number of surgeries (%) | ||
| One | 8 (80%) | 10 (83%) |
| Two | 2 (20%) | 1 (8%) |
| Three | 0 (0%) | 1 (8%) |
| Surgery outcomes (%) | ||
| >95% of tumor resected | 7 (70%) | 8 (73%) |
| 50–95% of tumor resected | 1 (10%) | 1 (9%) |
| Biopsy only | 2 (20%) | 2 (18%) |
| Radiation dose, cGy | ||
| Mean cranial-spinal dose (SD) | – | 2550 (490) |
| Range | – | 2340–3600 |
| Mean cranial-spinal dose plus PF boost (SD) | – | 5563 (54) |
| Range | – | 5400–5580 |
| Mean focal dose (SD) | 5940 (0) | 3240 (0) |
| Range | 5940 | 3240 |
| Chemotherapy (%) | ||
| Yes | 1 (11%) | 12 (100%) |
| No | 8 (89%) | 0 (0%) |
| Hydrocephalus (%) | ||
| No hydrocephalus | 1 (17%) | 3 (27%) |
| Hydrocephalus with no treatment | 2 (33%) | 1 (9%) |
| Hydrocephalus treated with external ventricular drain, shunt, or ventriculostomy | 3 (50%) | 7 (64%) |
| Number of post-operative complications (%) | ||
| No complications | 3 (33%) | 1 (10%) |
| One complication | 2 (22%) | 1 (10%) |
| Two or more complications | 4 (44%) | 8 (80%) |
| Type of post-operative complications (%) | ||
| Visual | 4 (44%) | 5 (50%) |
| Motor | 4 (44%) | 7 (70%) |
| Speech | 1 (11%) | 0 (0%) |
| Cerebellar mutism syndrome | 0 (0%) | 4 (40%) |
Note. Sample sizes vary as details were not available for all patients. Children with astrocytoma or choroid plexus papilloma were treated with surgery only, 2 ependymoma patients received focal radiation, and one ependymoma patient received focal radiation and chemotherapy. Children with medulloblastoma were treated with chemotherapy and cranial-spinal radiation, including a reduced radiation dose to the head and spine and a focal boost to the tumor bed. Chemotherapy agents included cisplatin, vincristine, and cyclophosphamide.
The specific location of these PF tumors was not identified in medical records.
Tumor size was defined by the largest size (in mm) in 2 dimensions of the MRI scans; this presents the number as mm2.
2.2. Study design
Patients completed baseline cognitive testing and MRI scans approximately 3 months after diagnosis (i.e., during or immediately after radiation for those receiving this therapy) and then 12, 24, and 36 months later; the Control group completed testing at corresponding intervals. All participants (except one at the first time point) completed cognitive testing and MRI scans on the same day across all time points. Children were included in the analyses if they had participated for at least 2 time points (median of 3 assessments per participant, total = 127).
2.3. Image acquisition and processing
MRI scans were obtained on a 1.5 T GE Signa Excite LX scanner with an 8-channel head coil at The Hospital for Sick Children (SickKids) or 1.5 T Siemens Avanto scanners with a 12-channel head coil at Alberta Children's Hospital (ACH) or British Columbia Children's Hospital (BCCH). An axial 3D-T1 anatomical scan using a rapid acquisition gradient echo sequence with inversion recovery preparation and a diffusion tensor imaging (DTI) scan using echo-planar imaging with a single-shot spin echo sequence were acquired. Imaging signal-to-noise ratios differed between the GE (SickKids) and Siemens (ACH and BCCH) scanners for our cohort (Law et al., 2011). Consequently, scanner type was included as a covariate in all analyses of imaging data.
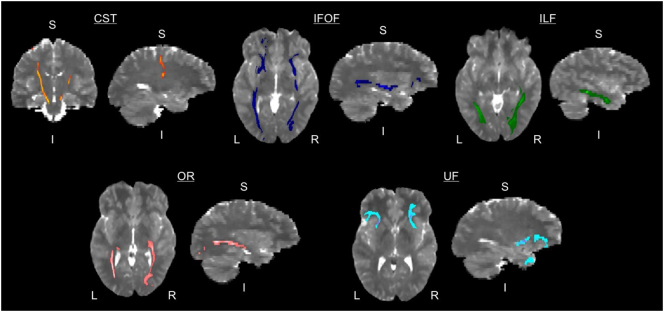
DTI images were corrected for motion and eddy current distortions (Smith et al., 2004). Probabilistic tractography (Behrens et al., 2007; Behrens et al., 2003) was used to localize tracts for bilateral cortical spinal tract (CST), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), optic radiations (OR), and uncinate fasciculus (UF) (Fig. 1) using a standardized template described previously (Scantlebury et al., 2016). Mean diffusion indices of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) were calculated.
Fig. 1.
White matter tracts used in analyses. The following bilateral white matter tracts were delineated and used in the analyses: cortico-spinal tract (CST), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), optic radiations (OR), and uncinate fasciculus (UF). Tracts are shown on one healthy control participant. Left = L; Right = R; Superior = S; Inferior = I.
Voxel-wise analyses were conducted using Tract Based Spatial Statistics (TBSS)(Smith et al., 2006) following an established longitudinal protocol (Engvig et al., 2012) to examine changes in DTI metrics over time. Each participant's DTI image was aligned to their first scan and then TBSS was used to create a white matter skeleton across all participants and time points. Subsequent analyses were conducted on only those voxels within the white matter skeleton mask. (See Supplementary Materials for MRI scan parameters and post-processing details).
2.4. Intelligence measures
Either the Wechsler Intelligence Scale for Children, 4th Edition (WISC-IV) or the Wechsler Adult Intelligence Scale, 4th Edition (WAIS-IV) (D, 2008) was employed according to the participant's age. The primary indices included in the analyses were Full Scale IQ (FSIQ), Perceptual Reasoning Index (PRI), Verbal Comprehension Index (VCI), Working Memory Index (WMI), and Processing Speed Index (PSI).
2.5. Medical and treatment related variables
The Neurological Predictor Scale (NPS) was used to summarize the type and amount of treatment for PF tumor patients (Micklewright et al., 2008). Information for 3 of 4 domains was obtained as our records did not have information on the fourth domain for all patients (i.e., seizure medication or hormone deficiency). A summative NPS score for the 3 treatment domains of surgery, chemotherapy, and radiation was calculated for each patient. Age at diagnosis and post-surgical details (presence of hydrocephalus or cerebellar mutism) were also recorded.
2.6. Analytic plan
All analyses were conducted in R (version 3.3.2). We used linear mixed modelling to evaluate group differences at baseline (intercept) and changes over time (slope) in FA, MD, RD, and AD for both the specific white matter tracts and voxel-wise metrics within the skeleton mask. Models included fixed effects for the 3 groups and time between assessments, random effects of repeated measures within participants, and covariates of MRI scanner type and mean parental education. Similar linear mixed models were used to examine group differences in IQ scores, with the exception that MRI scanner type was not included as a random effect. A false discovery rate (FDR) of 10% was used to correct for multiple comparisons (Benjamini et al., 2006).
We then conducted a series of uncorrected analyses to examine predictors of IQ at baseline and change over time. First, we used linear mixed models to test if DTI indices from the specific tracts predicted the intercepts and slopes of the IQ scores; these were controlled for parental education and MRI scanner type across the entire sample. Only tracts with the largest group effects were carried forward as independent variables to reduce the number of comparisons. Then for the brain tumor patients only, Pearson and Spearman correlations were used to evaluate relationships between the medical/treatment related variables and IQ.
3. Results
3.1. White matter tracts
3.1.1. CSR vs. control
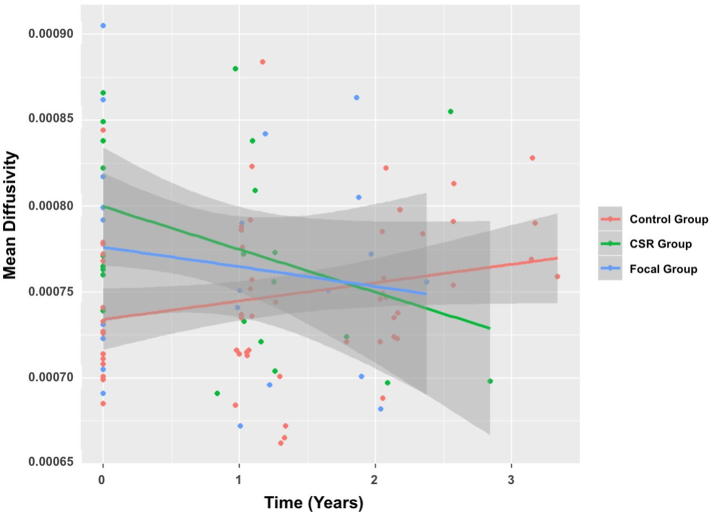
At baseline, higher AD in bilateral CST (left: t (37.85) = 3.04, p = .004, right: t(49.37) = 2.73, p = .009), and higher MD in right CST (t(43.54) = 3.14, p = .003) was observed for the CSR group versus the Control group (qs < 0.10) (Fig. 2). MD in right CST decreased over time (t (64.44) = −3.28, p = .001, q = 0.03) (Fig. 2).
Fig. 2.
Mean diffusivity (MD) in right cortical-spinal tract (CST) at baseline and over time.
There were other baseline and slope differences between the CSR and the Control group; however, these did not survive FDR correction. At baseline, there was higher FA in left IFOF (t(36.69) = 2.11, p = .04), higher MD in left CST (t(32.58) = 2.18, p = .04), and higher RD in right CST (t(41.89) = 2.68, p = .01) for the CSR group compared to the Control Group. There were also decreases over time in the CSR group in RD in right CST, (t(63.33) = −2.20, p = .03), MD in right ILF (t(43.40) = −2.30, p = .03), and RD in right ILF (t(67.11) = −2.23, p = .03).
3.1.2. Local vs. control
No differences between the Local group and Control group survived FDR correction. The following results were found for uncorrected effects. At baseline, the Local group had lower FA and higher RD in right OR (FA: t(47.35) = −2.24, p = .03; RD: t(47.02) = 2.24, p = .03) and right UF (FA: t(51.48) = −2.13, p = .04, RD: t(50.93) = 2.22, p = .03) when compared to the Control group.
There were changes over time for the Local group when compared to the Control group. Specifically, AD and MD decreased over time in the CST bilaterally (left CST AD: t(56.68) = −2.14, p = .04, right CST MD: t(55.87) = −2.07, p = .04) with indices approaching those of the Control group.
3.2. Voxel-wise analyses
3.2.1. CSR vs. control
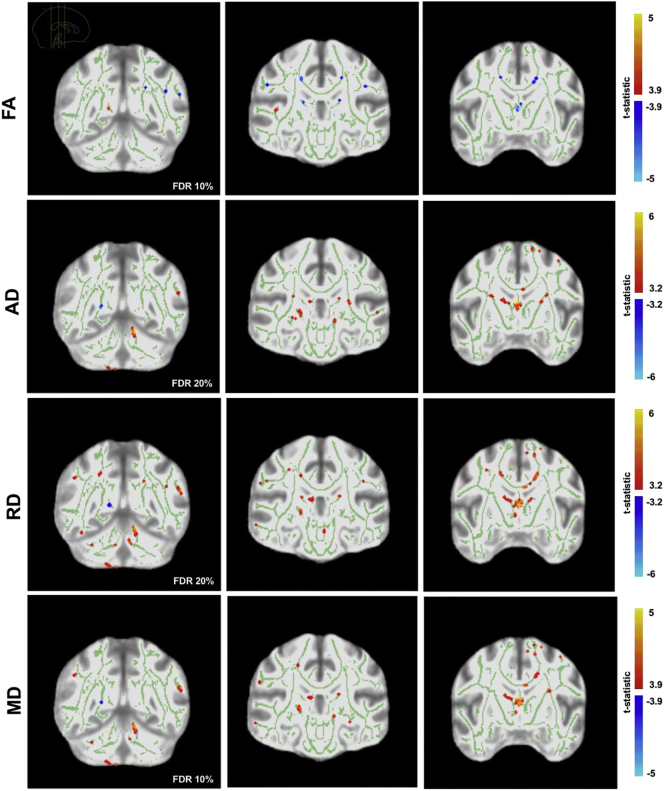
Compared to the Control group, the CSR group displayed lower FA (t(42.07) = 3.87, p < .001, q = 0.10) and higher MD (t(17.82) = 5.04, p < .001, q = 0.01), AD (t(21.77 = 5.61, p < .001, q = 0.05), and RD (t(17.64) = 4.70, p < .001, q = 0.10), in multiple areas of the white matter skeleton at baseline (Fig. 3).
Fig. 3.
Voxelwise analyses of FA, AD, RD, and MD at baseline (CSR group vs. Control group). Figure shows areas of higher FA, AD, RD, MD (red) at baseline for the CSR group in comparison to the Control group, as well as areas of lower FA, AD, MD, RD (blue) at baseline.
Changes over time were observed for the CSR group. Decreases in AD (t(67.18) = 4.24, p < .001, q = 0.10) and MD (t(15.39) = 4.44, p < .001, q = 0.10) were observed in many areas of the white matter skeleton (not pictured). This included bilateral parietal, left temporal, left frontal white matter, as well as right cerebellar white matter, internal capsule, and fornix. Increases over time in AD and MD were present in right cerebellar white matter.
3.2.2. Local vs. control
Lower FA in small areas of bilateral parietal and left white matter skeleton were observed for the Local compared to the Control group at baseline (t(42.57) = 5.93, p < .001, q < 0.01). A single small area of higher FA was also observed in right temporal white matter for this group. There were no significant changes over time for the Local group.
3.3. Intellectual outcome
3.3.1. CSR vs. control
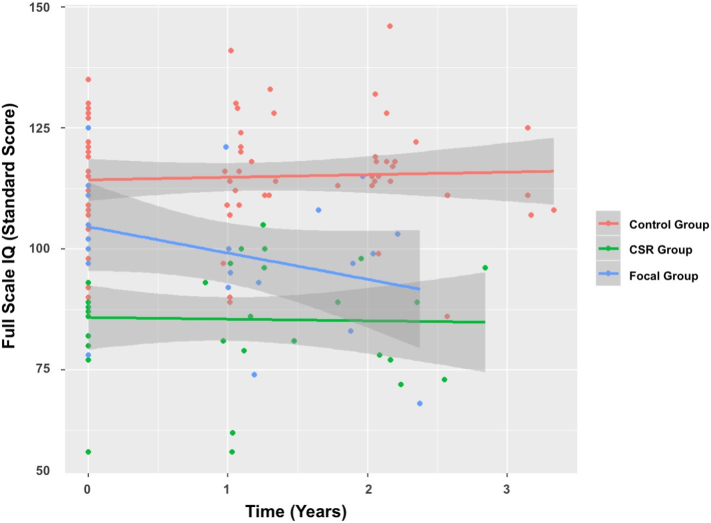
Lower intellectual scores for the CSR versus the Control group was observed at baseline (qs < 0.004). This included measures of: FSIQ (t(37.67) = −4.20, p < .001), PRI (t(37.50) = −3.53, p = .001), VCI (t(39.29) = −3.33, p = .002), and PSI (t(39.50) = −3.89, p < .001). There were no differences between the CSR and Control group on WMI (t(42.30) = −0.90, p = .374). There were no changes over time (p > .10) observed for the CSR group – with mean FSIQ, PRI, VCI, and PSI remaining more than one standard deviation below the normative mean across the first 3 years following diagnosis (Fig. 4).
Fig. 4.
Full scale IQ on Wechsler Intelligence measures as a function of time since diagnosis.
3.3.2. Local vs. control
Although the Local group had similar performance to the Control group at baseline (all ps > 0.10), there were significant declines across domains of intellectual functioning (qs < 0.10). This included measures of: FSIQ (t(48.11) = −3.83, p < .001), PRI (t(51.52) = −2.16, p = .04), VCI (t(39.87) = −2.66, p = .01), and WMI (t(37.39) = −2.66, p = .01).There was no significant change over time in the PSI (t(71.98) = −1.18, p = .239). Mean FSIQ, VCI, and WMI declined by ~ 6-points per year, however, performance remained within the normative average range throughout the time studied (Fig. 4).
3.3.3. Summary
Overall, our results indicate that both patient groups had lower IQ compared to the Control group. The CSR group started at a lower baseline, whereas the Local group declined over time.
3.4. Predictors of IQ at baseline and over time
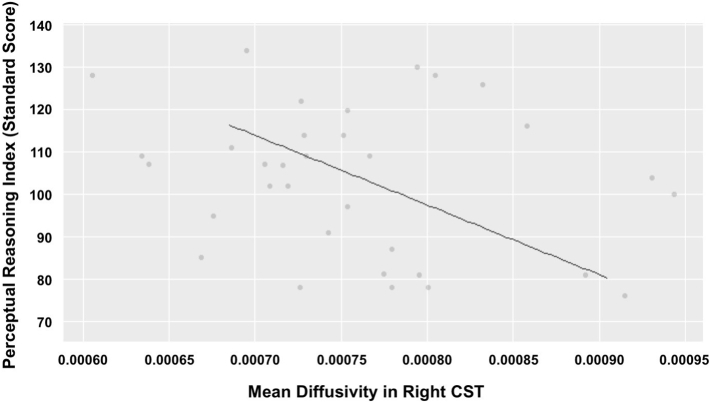
At baseline, higher MD in right CST predicted lower PRI across all participants (t(29.16) = −3.62, p = .001)(Fig. 5). Furthermore, lower FA in left IFOF at baseline predicted decline in PSI over time (t (35.00) = 3.50, p = .001).
Fig. 5.
Mean diffusivity within the Right CST predicts perceptual reasoning performance at baseline.
For the patients only (CSR and Local group), higher NPS score predicted lower intelligence scores at baseline. This included measures of: FSIQ (r(19) = −0.75, p < .001), PRI (r(19) = −0.54, p = .011), VCI (r(20) = −0.71, p < .001), WMI (r(20) = −0.49, p = .02), and PSI (r(19) = −0.51, p = .02). The presence of mutism predicted lower FSIQ (rho = −0.51, p = .04), and WMI (rho = −0.56, p = .04), scores at baseline. None of the medical or treatment variables predicted change in IQ scores over time (p > .10).
4. Discussion
Our findings challenge the long-held view of intact white matter and intelligence at baseline in PF tumor patients and then decline over time. We found that children treated with CSR displayed diffuse white matter compromise and poor intellectual outcome shortly after radiation treatment. There was evidence of subsequent growth of white matter structure, but stable intellectual insult. Children treated with either surgery only or surgery and focal radiation to the tumor bed display a different pattern. We observed less compromise of white matter early following treatment and no intellectual insult compared to healthy children. However, declines in intellectual function were evident for these children, though their performance remained within the average normative range. Greater attention must be paid to early white matter insult following radiation and intellectual compromise, particularly in patients treated with CSR.
DTI metrics provide indirect information about the microstructure of white matter (Basser et al., 2000; Song et al., 2003; Song et al., 2002). Lower FA reflects compromise of white matter microstructure(Assaf and Pasternak, 2008) and higher RD, AD, and MD values may reflect interrupted myelination, compromise of axonal thickness (Song et al., 2003), and decreased WM density, respectively(Barnea-Goraly et al., 2005; Mukherjee et al., 2001). Hence, the baseline differences in diffusion metrics that we observed for our whole PF brain tumor sample likely reflects early injury to white matter. Indeed, there is recent and emerging evidence of early compromise of white matter in PF tumor patients (Glass et al., 2017; Nieman et al., 2015; Perreault et al., 2014).
We found that early compromise was evident in areas distal to the tumor site in the Local group, suggesting broader impact in the brain in children treated with local therapy (i.e., surgery or focal radiation). This early insult may reflect the combined impact of the tumor, hydrocephalus, residual effects of surgery, and an acute injury associated with radiation on white matter. Notably, changes in MD and AD over time in children treated with CSR were reflective of white matter growth – providing evidence of some recovery following the acute phase of treatment. The only region of the brain showing decline in white matter structure was the cerebellum in children treated with CSR; a region that would have had the greatest adverse effect from the combined impact of the tumor and higher dose of radiation.
Early compromise of intellectual function relative to healthy children was observed only following CSR. Poor early intellectual outcome was predicted by higher diffusivity in the right CST at baseline, presence of mutism, and more aggressive treatment indexed by a higher NPS score. This is consistent with recent reports suggesting that neurosurgery or chemotherapy regimen and whole brain radiation are associated with lower intellectual performance close to diagnosis (Glass et al., 2017; Ris et al., 2013). This early impairment may also reflect patients' diminished ability to perform neuropsychological testing due to fatigue and weakness related to chemotherapy, whole brain radiation toxicity and intercurrent infections, and organ dysfunction. We do note that patients showed no recovery in intellectual function past these acute stages, however. Declines over time in intelligence were observed only for Local group and this was predicted by baseline anisotropy in left IFOF.
The recent findings of early insult differ from previous longitudinal work showing continuous decline and may reflect the fact that newer treatment protocols have reduced the overall toxicity of therapy. For example, the relatively small changes over time we observed may be because most of our CSR group received a treatment protocol including a focal boost to the tumor bed (SJMB03 and SJMB96) as opposed to the whole PF, which spared hemispheric white matter from the highest doses of radiation (Moxon-Emre et al., 2016a). Previous studies from our lab have shown that limiting the PF boost is associated with less white matter compromise and minimal declines in intellectual function (Moxon-Emre et al., 2014; Moxon-Emre et al., 2016a; Moxon-Emre et al., 2016b).
4.1. Limitations
We sampled from a relatively short period of follow up (~ 3 years following diagnosis) and declines in white matter structure and IQ may become evident at longer intervals following diagnosis. Although we acknowledge that our sample size was relatively small, we note that we obtained a large number of imaging and intellectual function data points – and the robust nature of the baseline findings in our sample. Finally, we report on a limited number of white matter tracts: given the resource demands of tractography we limited our analyses to tracts studied previously. There may be other tracts where effects are observed. To mitigate this limitation, we included voxel-wise analyses. We think the combination of tractography and voxel-wise approaches yields a comprehensive evaluation of white matter in our sample. We acknowledge the presence of different variables that were confounded with treatment in this study. Our CSR group had greater post-operative complications than the Local group. Furthermore, tumor histology and treatment are confounded – which reflects the clinical practice that different tumor types require different therapy. We did combine tumor histology in the Local group. In future work with larger sample sizes it will be important to delineate the contribution of specific confounding variables – where possible - to cognitive and white matter outcomes. Finally, there were also differences between groups in parent education and we used different MRI scanners at our 3 testing sites; these effects were accounted for in the analyses and thus our results are likely not related to these differences.
4.2. Implications for practice and conclusions
We identified baseline deficits in children treated for PF tumors - this provides greater opportunity and rationale to act early in therapy to protect or mitigate against adverse effects, including initiatives to predict and reduce the incidence of mutism. Further, our findings support the need for neuro-rehabilitation for PF tumor patients (Gudrunardottir et al., 2016; Walker et al., 2014). Patients treated with CSR may benefit from intensive early intervention as they do not appear to “catch up” to their typically developing peers.
Acknowledgements
This work was supported by the Canadian Cancer Society.
Footnotes
Previous presentations: Some results in this manuscript were presented at the Organization for Human Brain Mapping 2017 meeting.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.09.005.
Appendix A. Supplementary data
References
- Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A., Dant C.C., Reiss A.L. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pajevic S., Pierpaoli C., Duda J., Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G., Clare S., Matthews P.M., Brady J.M., Smith S.M. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Krieger A.M., Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- Chapman C.A., Waber D.P., Bernstein J.H., Pomeroy S.L., Lavally B., Sallan S.E., Tarbell N. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J. Child Neurol. 1995;10:209–212. doi: 10.1177/088307389501000308. [DOI] [PubMed] [Google Scholar]
- Copeland D.R., Demoor C., Moore B.D., 3rd, Ater J.L. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J. Clin. Oncol. 1999;17:3476–3486. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- D, W . Pearson Education Inc; New York, NY: 2008. Wechsler Adult Intelligence Scale Fourth Edition (WAIS-IV) [Google Scholar]
- Engvig A., Fjell A.M., Westlye L.T., Moberget T., Sundseth O., Larsen V.A., Walhovd K.B. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajjar A., Chintagumpala M., Ashley D., Kellie S., Kun L.E., Merchant T.E., Woo S., Wheeler G., Ahern V., Krasin M.J., Fouladi M., Broniscer A., Krance R., Hale G.A., Stewart C.F., Dauser R., Sanford R.A., Fuller C., Lau C., Boyett J.M., Wallace D., Gilbertson R.J. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- Glass J.O., Ogg R.J., Hyun J.W., Harreld J.H., Schreiber J.E., Palmer S.L., Li Y., Gajjar A.J., Reddick W.E. Disrupted development and integrity of frontal white matter in patients treated for pediatric medulloblastoma. Neuro-Oncology. 2017;19:1408–1418. doi: 10.1093/neuonc/nox062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.M., Merchant T.E., Billups C.A., Stokes D.C., Broniscer A., Bartels U., Chintagumpala M., Hassall T.E., Gururangan S., McCowage G.B., Heath J.A., Cohn R.J., Fisher M.J., Srinivasan A., Robinson G.W., Gajjar A. Pulmonary Function after Treatment for Embryonal Brain Tumors on SJMB03 that included Craniospinal Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:47–53. doi: 10.1016/j.ijrobp.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill J., Renaux V.K., Bulteau C., Viguier D., Levy-Piebois C., Sainte-Rose C., Dellatolas G., Raquin M.A., Jambaque I., Kalifa C. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- Gudrunardottir T., Morgan A.T., Lux A.L., Walker D.A., Walsh K.S., Wells E.M., Wisoff J.H., Juhler M., Schmahmann J.D., Keating R.F., Catsman-Berrevoets C., Iceland Delphi G. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv. Syst. 2016;32:1195–1203. doi: 10.1007/s00381-016-3093-3. [DOI] [PubMed] [Google Scholar]
- Hoppe-Hirsch E., Brunet L., Laroussinie F., Cinalli G., Pierre-Kahn A., Renier D., Sainte-Rose C., Hirsch J.F. Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv. Syst. 1995;11:340–345. doi: 10.1007/BF00301666. (discussion 345-346) [DOI] [PubMed] [Google Scholar]
- Khong P.L., Kwong D.L., Chan G.C., Sham J.S., Chan F.L., Ooi G.C. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. AJNR Am. J. Neuroradiol. 2003;24:734–740. [PMC free article] [PubMed] [Google Scholar]
- Kun L.E., Mulhern R.K., Jr. Neuropsychologic function in children with brain tumors: II. Serial studies of intellect and time after treatment. Am. J. Clin. Oncol. 1983;6:651–656. [PubMed] [Google Scholar]
- Lassaletta A., Bouffet E., Mabbott D., Kulkarni A.V. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv. Syst. 2015;31:1877–1890. doi: 10.1007/s00381-015-2829-9. [DOI] [PubMed] [Google Scholar]
- Law N., Bouffet E., Laughlin S., Laperriere N., Briere M.E., Strother D., McConnell D., Hukin J., Fryer C., Rockel C., Dickson J., Mabbott D. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: impact on working memory. NeuroImage. 2011;56:2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Mabbott D.J., Noseworthy M.D., Bouffet E., Rockel C., Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro-Oncology. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklewright J.L., King T.Z., Morris R.D., Krawiecki N. Quantifying pediatric neuro-oncology risk factors: development of the neurological predictor scale. J. Child Neurol. 2008;23:455–458. doi: 10.1177/0883073807309241. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I., Bouffet E., Taylor M.D., Laperriere N., Scantlebury N., Law N., Spiegler B.J., Malkin D., Janzen L., Mabbott D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 2014;32:1760–1768. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I., Bouffet E., Taylor M.D., Laperriere N., Sharpe M.B., Laughlin S., Bartels U., Scantlebury N., Law N., Malkin D., Skocic J., Richard L., Mabbott D.J. Vulnerability of white matter to insult during childhood: evidence from patients treated for medulloblastoma. J Neurosurg Pediatr. 2016;18:29–40. doi: 10.3171/2016.1.PEDS15580. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I., Taylor M.D., Bouffet E., Hardy K., Campen C.J., Malkin D., Hawkins C., Laperriere N., Ramaswamy V., Bartels U., Scantlebury N., Janzen L., Law N., Walsh K.S., Mabbott D.J. Intellectual Outcome in Molecular Subgroups of Medulloblastoma. J. Clin. Oncol. 2016;34:4161–4170. doi: 10.1200/JCO.2016.66.9077. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Miller J.H., Shimony J.S., Conturo T.E., Lee B.C., Almli C.R., McKinstry R.C. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Mulhern R.K., Kepner J.L., Thomas P.R., Armstrong F.D., Friedman H.S., Kun L.E. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J. Clin. Oncol. 1998;16:1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- Mulhern R.K., Palmer S.L., Merchant T.E., Wallace D., Kocak M., Brouwers P., Krull K., Chintagumpala M., Stargatt R., Ashley D.M., Tyc V.L., Kun L., Boyett J., Gajjar A. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J. Clin. Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- Nieman B.J., de Guzman A.E., Gazdzinski L.M., Lerch J.P., Chakravarty M.M., Pipitone J., Strother D., Fryer C., Bouffet E., Laughlin S., Laperriere N., Riggs L., Skocic J., Mabbott D.J. White and Gray Matter Abnormalities after Cranial Radiation in Children and mice. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:882–891. doi: 10.1016/j.ijrobp.2015.07.2293. [DOI] [PubMed] [Google Scholar]
- Palmer S.L., Goloubeva O., Reddick W.E., Glass J.O., Gajjar A., Kun L., Merchant T.E., Mulhern R.K. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J. Clin. Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Palmer S.L., Reddick W.E., Glass J.O., Gajjar A., Goloubeva O., Mulhern R.K. Decline in corpus callosum volume among pediatric patients with medulloblastoma: longitudinal MR imaging study. AJNR Am. J. Neuroradiol. 2002;23:1088–1094. [PMC free article] [PubMed] [Google Scholar]
- Perreault S., Lober R.M., Cheshier S., Partap S., Edwards M.S., Yeom K.W. Time-dependent structural changes of the dentatothalamic pathway in children treated for posterior fossa tumor. AJNR Am. J. Neuroradiol. 2014;35:803–807. doi: 10.3174/ajnr.A3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack I.F. Brain tumors in children. N. Engl. J. Med. 1994;331:1500–1507. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- Radcliffe J., Bunin G.R., Sutton L.N., Goldwein J.W., Phillips P.C. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: age-dependent effects of whole brain radiation. Int. J. Dev. Neurosci. 1994;12:327–334. doi: 10.1016/0736-5748(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Reddick W.E., Mulhern R.K., Elkin T.D., Glass J.O., Merchant T.E., Langston J.W. A hybrid neural network analysis of subtle brain volume differences in children surviving brain tumors. Magn. Reson. Imaging. 1998;16:413–421. doi: 10.1016/s0730-725x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Reddick W.E., Russell J.M., Glass J.O., Xiong X., Mulhern R.K., Langston J.W., Merchant T.E., Kun L.E., Gajjar A. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn. Reson. Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- Reddick W.E., Glass J.O., Palmer S.L., Wu S., Gajjar A., Langston J.W., Kun L.E., Xiong X., Mulhern R.K. Atypical white matter volume development in children following craniospinal irradiation. Neuro-Oncology. 2005;7:12–19. doi: 10.1215/S1152851704000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs L., Bouffet E., Laughlin S., Laperriere N., Liu F., Skocic J., Scantlebury N., Wang F., Schoenhoff N.J., Strother D., Hukin J., Fryer C., McConnell D., Mabbott D.J. Changes to memory structures in children treated for posterior fossa tumors. J. Int. Neuropsychol. Soc. 2014;20:168–180. doi: 10.1017/S135561771300129X. [DOI] [PubMed] [Google Scholar]
- Ris M.D., Packer R., Goldwein J., Jones-Wallace D., Boyett J.M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J. Clin. Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- Ris M.D., Walsh K., Wallace D., Armstrong F.D., Holmes E., Gajjar A., Zhou T., Packer R.J. Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr. Blood Cancer. 2013;60:1350–1357. doi: 10.1002/pbc.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckriegel S.M., Driever P.H., Blankenburg F., Ludemann L., Henze G., Bruhn H. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:859–866. doi: 10.1016/j.ijrobp.2009.02.054. [DOI] [PubMed] [Google Scholar]
- Scantlebury N., Bouffet E., Laughlin S., Strother D., McConnell D., Hukin J., Fryer C., Laperriere N., Montour-Proulx I., Keene D., Fleming A., Jabado N., Liu F., Riggs L., Law N., Mabbott D.J. White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology. 2016;30:425–438. doi: 10.1037/neu0000258. [DOI] [PubMed] [Google Scholar]
- Silber J.H., Radcliffe J., Peckham V., Perilongo G., Kishnani P., Fridman M., Goldwein J.W., Meadows A.T. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J. Clin. Oncol. 1992;10:1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., MacKay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Walker D., Thomas S.A., Talbot E.J., Bennett E.J., Starza-Smith A., Da Silva S.L. Cerebellar mutism: the rehabilitation challenge in pediatric neuro-oncology: case studies. J. Pediatr. Rehabil. Med. 2014;7:333–340. doi: 10.3233/PRM-140309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.