Abstract
With advancements in neonatal intensive care units, the early detection of retinopathy of prematurity in premature infants is extremely important. It is critical for physicians to recognize children at risk and ensure they receive appropriate screening and monitoring. This article will discuss risk factors, screening guidelines, classification and treatment of this disease in premature infants.
Background
Retinopathy of Prematurity (ROP) is a leading cause of vision impairment and blindness worldwide. Normal retinal vasculature begins to develop at the optic nerve around 16 weeks gestational age and continues throughout pregnancy. This process is considered complete when the retinal vessels reach the boundary between the retina and ciliary body, known as the ora serrata. In normal fetal development the retinal vascularization reaches the nasal ora serrata by 36 weeks gestational age and the temporal ora serrata by 40 weeks gestational age.1
ROP develops in infants who are born prematurely with incomplete retinal development at birth. Due to changes in levels of vascular endothelial growth factor (VEGF), insulin-like growth factor I (IGF-I), oxygen and other factors the retinal blood vessels may grow abnormally which can cause permanent damage to the retina. According to the American Association of Pediatric Ophthalmology and Strabismus (AAPOS) there are around 14,000 infants who develop ROP in the United States each year. Of those children, 1,100 to 1,500 develop severe disease which warrants treatment and 400 to 600 become legally blind from ROP.2
Population at Risk
While there are many factors that influence the risk of developing ROP, early gestational age and low birth weight are two of the most important. Other factors may include but are not limited to anemia, blood transfusion, respiratory distress, breathing difficulties and overall poor health of the infant. In the 1940s and 1950s hospitals began using incubators with very high levels of oxygen in the intensive care units to increase infant survival. During this time the incidence of ROP rose substantially and physicians began to realize the increased risk of ROP with high oxygen levels after birth. Over the past several decades the overall incidence of ROP has reduced with improvements in neonatal intensive care medicine and more effective technology to monitor oxygen saturation levels.1
Screening Guidelines
The purpose of the ROP screening guidelines is to twofold: 1) to detect infants who are at risk of developing ROP and closely monitor their retinal development after birth, 2) to identify infants with severe disease who require treatment. It is important that all at-risk infants be screened in a timely manner as ROP can progress to permanent vision loss if it is not identified early.
Dilated fundus examinations should be performed on all infants born at 30 weeks gestational age or younger and infants with birth weight less than 1500g. If an infant does not fall into either of these categories, but has had a very unstable clinical course the pediatrician can identify the child as high risk for developing ROP and request ophthalmology screening. The first examination should occur at 4 weeks post-natal age or a corrected gestational age of 30 to 31 weeks, whichever occurs later. All dilated fundus examinations should be performed by an ophthalmologist with sufficient knowledge and training in ROP.3 While there are many ophthalmic dilating drops on the market, Cyclomydril is preferred for small infants due to its dilute concentration of phenylephrine and cyclopentolate. Some infants may be sensitive to the neurologic and cardiopulmonary side effects of systemic absorption of these medications, so they should be monitored closely after drop instillation. A nurse should also be present during all dilated fundus examinations in the neonatal intensive care unit as infants can experience apnea and bradycardia during the examination. For more information and up to date screening recommendations please visit the American Academy of Pediatrics website.3
Classification
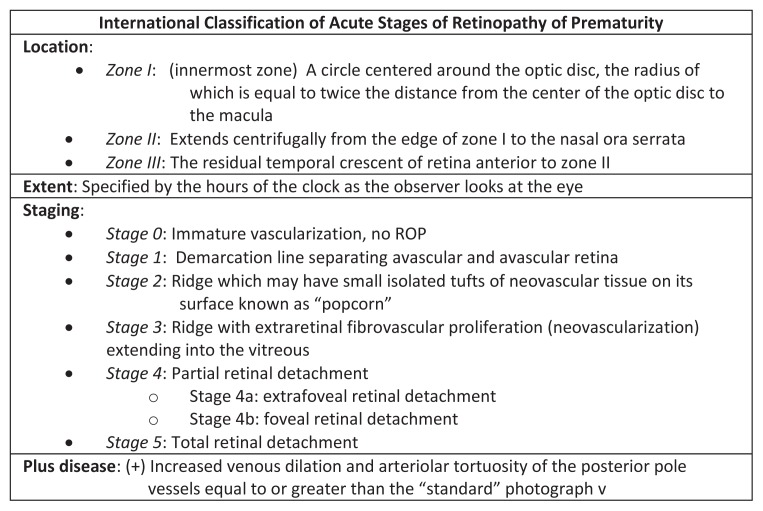
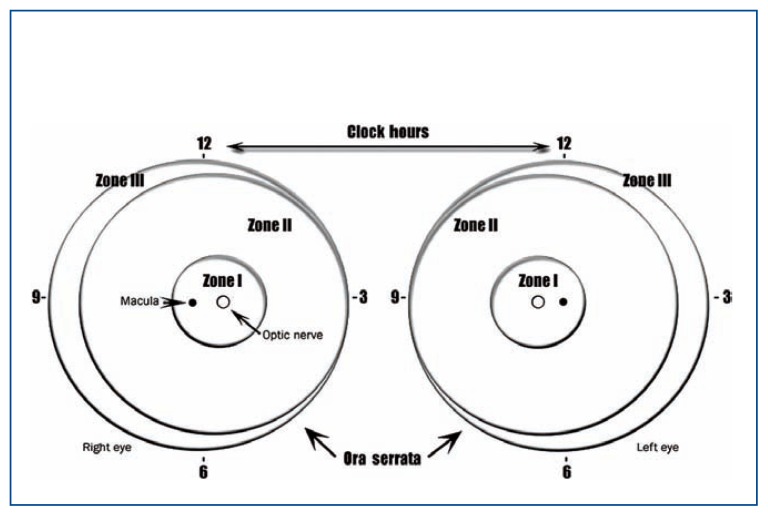
Retinopathy of prematurity is classically described in terms of the zone and stage of the disease which is summarized by the International Classification of Retinopathy of Prematurity (ICROP) chart in Figure 1. The zone indicates the location of the leading edge of retinal vascularization, and the retina is divided into three separate zones (See Figure 2). Zone I refers to a circle around the optic disc with a diameter twice the distance between the center of the optic nerve and the center of the macula. Zone II is a concentric circle centered on the optic disc with its radius extending to the nasal ora serrata. Zone III includes the remaining temporal crescent of retina.4
Figure 1.
The International Classification of Retinopathy of Prematurity (ICROP) describes the extent and severity of ROP disease.4
Figure 2.
Retinal drawing describing the location of the zones for staging ROP according to the ICROP system.3
The stage refers to the presence and severity of ROP. Each stage defines a specific retinal and vascular pattern at the interface between the vascular and avascular retina. Stage 0 represents immature vascularization with no visible abnormal vessel growth present. Stage 1 describes a line of tissue and stage 2 an elevated ridge of tissue at the junction of vascular and avascular retina. In stage 3 the ridge has developed fibrovascular proliferation which has the potential to cause traction on the underlying retina. Stage 4 indicates a partial retinal detachment and stage 5 a total retinal detachment in which visual prognosis is very poor.4
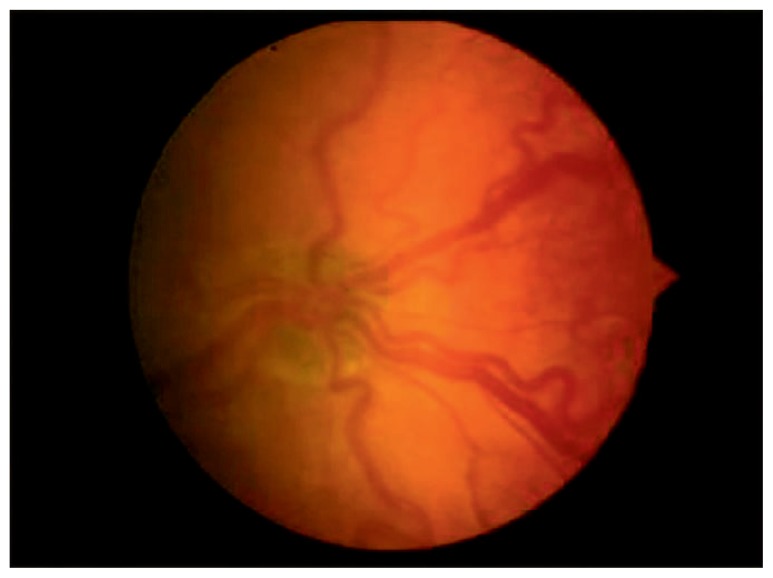
In addition to zones and stages, ROP is also described in terms of the presence or absence of Plus disease. Plus disease refers to arteriolar tortuosity and venous engorgement of the posterior pole vessels. It is diagnosed by comparing the patient’s vessel pattern with that of a standard photograph (See Figure 3). When present, plus disease indicates vascular shunting and severe ROP.4
Figure 3.
Standard photograph showing the minimum venous dilation and arteriolar tortuosity needed for the diagnosis of Plus disease.4
ROP Treatment
Once an infant has been identified as having ROP it is important to recognize when the disease becomes severe enough to warrant treatment. There have been several large, multicenter ROP trials that have helped to shape our current treatment guidelines. In 1988 the results of the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study were published which enhanced our understanding of the clinical course and treatment outcomes of ROP. In this study infants were randomized to receive peripheral retinal ablation with cryotherapy versus serial monitoring without treatment once they reached threshold ROP. Threshold disease was defined by at least 5 contiguous or 8 noncontiguous clock-hours of stage 3 ROP in zones I or II with plus disease. Short-term results showed a 50% decrease in the retinal detachment rate of patients who were treated with cryotherapy. Long-term follow up of these patients showed that there is a gradual increase in unfavorable structural outcomes in both the treated and untreated groups with time and visual acuity outcomes were unsatisfactory.5–7 After publication of the CRYO-ROP data, further studies revealed that peripheral retinal ablation with diode laser had better structural and visual outcomes than cryotherapy. For this reason laser therapy has largely replaced cryotherapy in current practice.8–9
In 1999 the National Eye Institute funded the Early Treatment for Retinopathy of Prematurity (ET-ROP) study in hopes identifying earlier treatment options for patients at high risk of ROP progression. The ET-ROP study investigated the risks and benefits of earlier treatment with cryotherapy or laser therapy for infants with prethreshold ROP. Results showed a statistically significant benefit of earlier treatment which was greatest in eyes with more posterior disease. A clinical algorithm was created to indicate when peripheral retinal ablation was most beneficial in prethreshold ROP. Authors recommended treatment for patients with Type I ROP which includes any stage zone I ROP with plus disease, zone I stage 3 with or without plus disease or zone II stage 2 or 3 ROP with plus disease. Observation was recommended for patients with Type II ROP which includes zone I stage 1 or 2 without plus disease or zone II stage 3 without plus disease. This algorithm serves as a guideline to identify patients with prethreshold ROP who are at highest risk for retinal detachment and blindness while also minimizing unnecessary treatment in patients who’s ROP would spontaneously regress.10
In more recent years we have developed a greater understanding of the important role that VEGF plays in the pathogenesis of ROP. In 2004 the FDA approved the use of IV bevacizumab, an anti-VEGF medication, for treatment of metastatic colon cancer. Since then, it has been used off-label in ophthalmology for the treatment of many retinoproliferative disorders including ROP. The study Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) evaluated the use of bevacizumab versus laser therapy for the treatment of zone I or II, stage 3 ROP with plus disease. Results of this study showed ROP recurrence rates were higher in the laser treatment group for patients with zone I, but not zone II disease; however recurrence occurred significantly later in the bevacizumab treatment group. Unlike laser therapy, intravitreal bevacizumab does not cause permanent destruction of peripheral retinal tissue. While the BEAT-ROP study shows promise for the treatment of aggressive posterior disease with bevacizumab, there are no studies to evaluate the systemic absorption of the medication and its long term effects in infants.11
The landmark studies listed above have contributed to the following guidelines in the treatment for ROP. Laser therapy should be initiated for patients with prethreshold Type 1 disease (any stage zone I with plus disease, zone I stage 3 with or without plus disease or zone II stage 2 or 3 with plus disease). Intravitreal bevacizumab therapy can be considered for patients with aggressive zone I disease or who are too unstable to tolerate laser therapy.
Complications of ROP
While retinal detachment and blindness can result from untreated ROP, there are also many complications that can occur in patients who have undergone treatment. These risks can persist throughout the patient’s lifetime. One of the most common sequelae of ROP is myopia which can continue to increase throughout childhood. Dragging of the macula may also occur which can give rise to pseudostrabismus and decreased vision. Patients with ROP are at an increased risk of developing conditions such as glaucoma, cataract, amblyopia, strabismus and retinal detachment despite early treatment. These children must be closely monitored throughout childhood to detect any late complications.1,5
Conclusion
Retinopathy of prematurity is a potentially blinding disorder affecting children worldwide. It is extremely important for all premature infants born at 30 weeks gestational age or younger and infants with birth weight less than 1500g to be screened by a trained ophthalmologist. There are many factors that place an infant at higher risk of developing ROP such as low birth rate and early gestational age. The ICROP classification system is the global standard to describe the severity and extent of ROP. Many large scale studies such as the CRYO-ROP, ET-ROP and BEAT-ROP have formed guidelines for treating infants with severe disease. Current recommendations include laser peripheral retinal ablation for patients with prethreshold Type 1 ROP. The recent development of anti-VEGF inhibitors such as bevacizumab has shown promising results for treatment of aggressive zone I ROP, however there is a need for further research into the systemic and long term effects of these medications in premature infants.
Biography
Emily C. Broxterman, MD, (left) is Pediatric Ophthalmology Fellow at Children’s Mercy Hospitals and Clinics in Kansas City. Denise A. Hug, MD, (right), MSMA member since 2015, is a Pediatric Ophthalmologist at Children’s Mercy Hospitals and Clinics in Kansas City and Associate Professor of Pediatric Ophthalmology at University of Missouri-Kansas City school of Medicine.
Contact: ecbroxterman@cmh.edu


Footnotes
Disclosures
None reported.
References
- 1.Wright KW, Strube YN. Pediatric Ophthalmology and Strabismus. New York, NY: Oxford University Press; 2012. Retinopathy of Prematurity; pp. 957–992. [Google Scholar]
- 2.Retinopathy of Prematurity. American Association for Pediatric Ophthalmology and Strabismus; Jul, 2013. Web. http://aapos.org/terms/conditions/94. [Google Scholar]
- 3.American Academy of Pediatrics. Screening Examination of Premature Infants for Retinopathy of Prematurity. PEDIATRICS. 2014;131(1):189–195. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 4.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity Revisited. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 5.Cryotherapy for Retinopathy of Prematurity Cooperative Group. 15-Year Outcomes Following Threshold Retinopathy of Prematurity. Arch Ophthalmology. 2005;123:311–318. doi: 10.1001/archopht.123.3.311. [DOI] [PubMed] [Google Scholar]
- 6.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: Ophthalmological Outcomes at 10 Years. Arch Ophthalmol. 2001;119:1110–1118. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- 7.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: Preliminary Results. Arch Ophthalmol. 1988;106:471–479. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 8.Ng EY, Connolly BP, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years. Ophthalmology. 2002;109(5):928–934. doi: 10.1016/s0161-6420(01)01017-x. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DG, Repka MX. Diode Laser Photocoagulation for Threshold Retionpathy of Prematurity: A Randomized Study. Ophthalmology. 1993;100(2):238–244. doi: 10.1016/s0161-6420(93)31664-7. [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment for Retinopathy of Prematurity Cooperative Group. Final Results of the Early Treatment for Retinopathy of Prematurity (ETROP) Randomized Trial. Trans Am Ophthalmol Soc. 2004;102:233–250. [PMC free article] [PubMed] [Google Scholar]
- 11.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. N Engl J Med. 2011;364:603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]