Abstract
Primary care physicians and specialists are frequently involved in the care of surgical patients. Changes in reimbursement have prompted re-examination of preoperative testing and health care expenditures. Physicians have additional incentives to improve health care delivery and reduce costs. The perioperative surgical home concept involves coordinating all aspects of patient care, including behavioral modifications, during the perioperative period. Evidence-based guidelines on preoperative evaluation are available to assist practitioners in managing cardiovascular disease, and communicating surgical risks. Shared decision making in the preoperative period can improve surgical outcomes and patient satisfaction.
Introduction
In the United States, $937 billion was spent on hospital care in 2013 (the most recent year for which data is available), two-thirds of which was related to surgical services.1 Approximately 100 million surgeries are performed each year in the U.S., over half of which are done in ambulatory facilities. As the elderly population increases, surgical volume has also been increasing as more procedures are being performed on older patients with multiple chronic medical conditions. Forty-three percent of all surgical procedures are paid for by Medicare or Medicaid.
Since the Affordable Care Act (ACA) was signed into law in 2010, two of its primary aims have been to improve health care delivery and reduce costs. To this end, there has been a renewed focus on moving from the high costs and fragmented care associated with the traditional volume-based fee for service model to more efficient systems of multidisciplinary coordinated health care delivery. The Patient Centered Medical Home (PCMH) is a well-established concept that designates one physician to coordinate all aspects of the patient’s care and has been shown to improve outcomes and patient satisfaction.2 A similar model of coordinated care for surgical patients is the Perioperative Surgical Home (PSH), a multidisciplinary system designed to shepherd patients through the surgical process from the time that surgery is planned until 30 days after discharge.3 Recent changes in reimbursement have provided incentives for physicians and hospitals to streamline surgical care and meet performance benchmarks such as reducing the rate of readmission for Medicare beneficiaries.4 These changes have provided rich opportunity for primary care physicians and specialists to improve surgical outcomes by participating in the management and optimization of their patients in the days and weeks leading up to surgery.
Preoperative Evaluation
Preoperative evaluation is a process of clinical assessment that precedes the delivery of anesthesia care for surgery and non-surgical procedures. At the very least, it includes a review of medical records and recent test results, a comprehensive medical history, and a physical examination of the cardiovascular system, the pulmonary system, and the airway. As the preoperative evaluation is considered a basic element of anesthetic care, it is often performed in the immediate preoperative period (ie, on the day of surgery), by the anesthesiologist. However, the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation recommends that patients with high disease severity and those undergoing procedures with high surgical invasiveness be evaluated prior to the day of surgery.5
Preoperative Testing
There is no clear consensus on the optimal content and timing of a preoperative evaluation, but it is important to balance the potential benefits of preoperative testing with the known risks and costs. Although preoperative studies and tests are often ordered on a selective basis to guide or optimize perioperative management, routine preoperative testing (ordering tests in the absence of a specific clinical indication or purpose) is generally not recommended, especially prior to low risk surgery.5 Testing of asymptomatic patients can result in a waste of limited health care resources, unnecessary postponement or cancellation of surgery or, at worst, additional diagnostic testing and harmful medical interventions in the name of preoperative clearance for surgery.6 Conversely, substantial cost savings can result from the avoidance of unnecessary preoperative medical testing and office visits prior to low risk procedures such as cataract surgery.
One of the most commonly performed elective procedures in the U.S., cataract surgery accounts for yearly health care expenditures totaling 3.5 billion dollars, 80% of which are paid for by Medicare.7 A safe procedure of short duration, cataract surgery carries less that a 1% risk of a major adverse cardiac event (MACE) or death, even in older patients with multiple chronic medical conditions. Surgery for cataracts is thought to be no more stressful than dental work or even activities of daily living. The majority of these procedures are performed in an ambulatory setting and are done with topical anesthesia and only light sedation. Although it carries low risk, more than half of Medicare patients who underwent cataract surgery in 2012 had at least one preoperative test performed, despite evidence that routine testing before cataract surgery does not reduce adverse events or improve outcomes.8, 9 The extent of preoperative testing has been shown to depend more on individual opthalmologists’ practice patterns rather than adherence to published, evidence-based guidelines on preoperative testing. Therefore, selective preoperative testing should be guided by medical history and physical exam findings rather than a matter of routine practice.
ACC/AHA Guidelines
In 2014, the American Heart Association and American College of Cardiology updated their guidelines on cardiovascular evaluation and management of patients undergoing non-cardiac surgery.10 The purpose of the guidelines is to
provide patients and their caregivers with evidence-based information about perioperative cardiovascular risk; and
guide management of patients with cardiovascular conditions and comorbidities.
Risk Assessment
Procedures such as cataracts, superficial plastic surgery, and endoscopy are considered low risk procedures because they carry less than a 1% chance of a major adverse cardiac event (MACE) including death or a myocardial infarction in the perioperative period.10 Elevated risk applies to procedures that carry a risk of MACE equal to 1% or greater. The degree of risk depends on procedural and patient factors and can be estimated with the Revised Cardiac Risk Index (RCRI), a validated tool to predict the risk of major cardiac complications.11,12 (See Table 1). A patient who has 0 or 1 of the 6 RCRI risk factors is considered to be at low risk for MACE. Two or more risk factors confers elevated risk of MACE. Other validated risk calculators have also been used to predict procedure-specific risks.13, 14 These tools can be used to determine whether further preoperative evaluation is appropriate.
Table 1.
Revised Cardiac Risk Index
| Revised Cardiac Risk Index |
|---|
| History of ischemic heart disease |
| History of congestive heart failure |
| History of cerebrovascular disease |
| Diabetes mellitus requiring insulin therapy |
| Renal insufficiency (serum creatinine >2 mg/dL) |
| High risk surgery (suprainguinal vascular, intrathoracic, intraperitoneal) |
| Risk of major adverse cardiac event (MACE): 0 risk factors: 0.4%; 1 risk factor 0.9%; 2 risk factors 6.6%; 3 or more 11% |
Management of Coexisting Disease
Coronary Artery Disease
Coronary artery disease (CAD) increases the risk of perioperative morbidity and mortality, especially in the context of a recent myocardial infarction (MI) or coronary revascularization (CABG or PCI). For patients who have had a recent MI (within six months), the risk of both MACE and mortality is highest in the first 30 days following the MI (32.8% and 14.2%, respectively).15 These patients are also at an eight-fold increased risk of stroke.16 It is therefore recommended that elective, non-cardiac surgery be delayed for at least 60 days following an MI without coronary intervention.
Many patients with coronary artery disease who have had percutaneous coronary intervention (PCI) with balloon angioplasty or coronary artery stents present for non-cardiac surgery.17 Up to 26% of patients with either a bare metal stent (BMS) or drug-eluting stent (DES) will require surgery within five years of undergoing PCI.18 Drug eluting stents offer more protection than BMS against re-stenosis from smooth muscle proliferation but are slower to re-endothelialize and thus require a longer period of dual anti-platelet therapy (DAPT) to prevent life threatening stent thrombosis. It is known that premature discontinuation of DAPT increases the risk of stent thrombosis, especially in patients undergoing surgery, which induces a hypercoagulable state. The risk of MACE is highest (10.5%) in those patients with BMS who undergo surgery within 30 days after PCI, and lowest (2.8%) in those who wait at least 90 days.19 Another study found that MACE was highest when major non-cardiac surgery was performed less than 45 days after implantation of any coronary stent.20 Following placement of a DES, the risk of MACE is lowest in those who wait at least 365 days before non-cardiac surgery. In both cases, the risk of MACE is higher when surgery is performed on an emergent basis. For patients undergoing surgery prior to completion of DAPT, the theoretical risk of surgical bleeding while on DAPT must be balanced with the risk of stent thrombosis if dual antiplatelet therapy is held for surgery. Decisions about management of DAPT should be made in concert with the patient’s cardiologist. The updated ACC/AHA guidelines recommend delaying elective non-cardiac surgery for 14 days following balloon angioplasty, at least 30 days following BMS implantation, and 365 days following DES implantation, although in some cases a waiting period of 180 days after DES implantation may be appropriate.
Medical Decision-Making
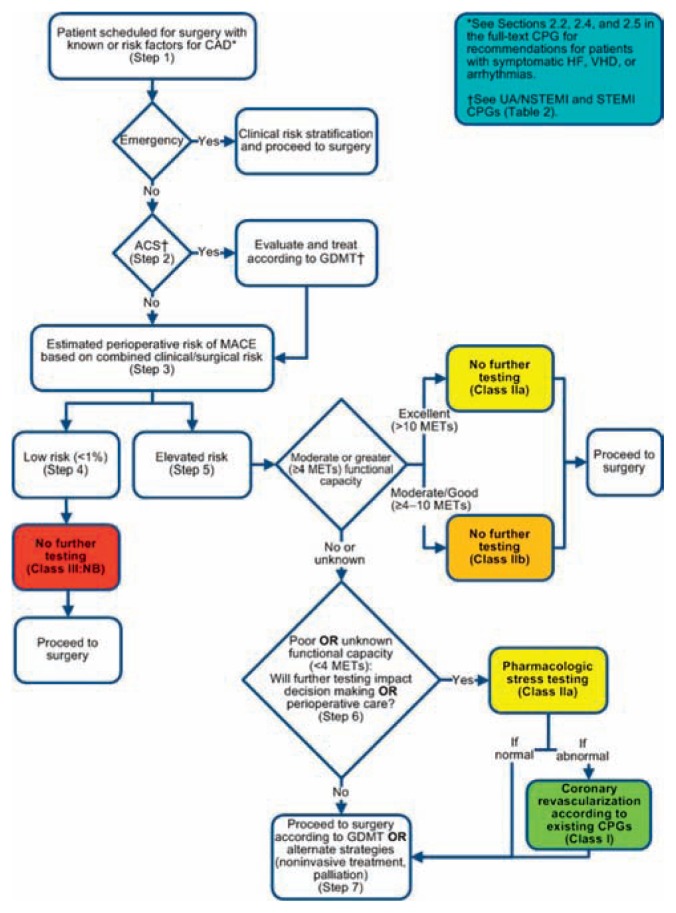
For practitioners involved in the evaluation of patients with coronary artery disease preparing for surgery, a stepwise approach is available to assist in patient evaluation and estimation of risk. (See Figure 1). Patients who are planning surgery with low risk of MACE and those at elevated risk of MACE with at least moderate functional capacity (>4 METS as defined by the ability to climb a flight of stairs without stopping, walking uphill with ease, or gardening) can proceed to surgery without further evaluation. Patients with poor or unknown functional capacity may benefit from pharmacologic stress testing if it will influence their decision to have surgery or change perioperative management. Patients who elect to undergo stress testing may be candidates for coronary angiography or revascularization prior to surgery. Similar recommendations are available for managing patients with heart failure, arrhythmias, and heart valve disease.10
Figure 1.
Stepwise Approach to Perioperative Cardiac Assessment: Treatment Algorithm.
Reprinted with permission Circulation.
2014;130:2215–2245 ©2014 American Heart Association, Inc.
As part of any assessment of cardiovascular health, it is important to differentiate between active cardiac conditions and stable clinical risk factors. Patients with active cardiac conditions (acute coronary syndromes, decompensated heart failure, significant arrhythmias, or severe valvulopathy) are at high risk for perioperative MACE and should be evaluated and treated according to guideline-directed medical therapy.21 For those with clinical risk factors (history of CVA, history of ischemic heart disease, history of congestive heart failure, diabetes, or kidney failure), the revised cardiac risk index or a similar risk calculator should be used along with procedural factors to estimate the risk perioperative MACE prior to surgery.
Additional tests and studies such as electrocardiograms (ECGs), chest x-rays, and blood tests are often ordered as a matter of routine—actions that are often based on medicolegal concerns or perceived expectations rather than on evidence based guidelines.22 Twelve lead ECGs may be informative in patients with known cardiovascular disease, but are not indicated for asymptomatic patients undergoing low risk procedures.10 As many as 45% of asymptomatic patients have abnormal ECG findings.5 There is also no agreed upon minimum age requirement for ECG testing among asymptomatic patients. Similarly, chest x-rays were once included as part of any preoperative evaluation, but abnormal findings may be present in up to 60% of asymptomatic patients and can lead to costly and unnecessary postponement or cancellation of surgery, changes in medical management that otherwise would not have occurred, and excess radiation exposure. The same is true of laboratory tests such as hemoglobin and hematocrit measures, serum chemistries, and coagulation profiles. It is now recommended that these tests should only be ordered when clearly indicated (i.e., to answer a specific question and only if it will lead to a change in management).
Medications
Many patients with cardiovascular disease presenting for preoperative evaluation are on chronic antihypertensive therapy. The use of beta adrenergic antagonists (beta blockers) has been the subject of several studies in recent years.23, 24 It is now recommended that patients who are on chronic beta blocker therapy remain so throughout the perioperative period. For beta blocker naive patients, beta blockers may reduce perioperative cardiac risk, but are associated with adverse effects such as bradycardia, hypotension, and stroke. Therefore, initiating beta blocker therapy on the day of surgery is not recommended. Patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs) on the day of surgery may experience transient hypotension, but are at no increased risk of adverse cardiac events, and continuing these medications in the perioperative period is reasonable.10
Early Patient Engagement and Prehabilitation
One strategy to reduce length of stay and prevent postoperative complications is the concept of early patient engagement in the preoperative period or “prehabilitation” (enhancing functional capacity in preparation for a stressful event such as surgery).25 This is especially relevant to elderly patients with chronic respiratory disease who are at increased risk for postoperative pulmonary complications, which they tolerate poorly. Ergina et al. reported that identifying patients with COPD who smoke and have poor functionality and instituting perioperative regimens that include smoking cessation, bronchodilators, chest physiotherapy, postural drainage, and deep breathing exercises reduced the incidence of postoperative pulmonary complications.26 Other investigators have reported on the benefits of preoperative aerobic exercise training on postoperative recovery from colorectal surgery.27, 28 Others have shown that prehabilitation programs have a positive impact on length of stay and health-related quality of life measures.29
Smoking Cessation
Despite the long-known health risks of tobacco use, 19.2% of American adults report smoking every day or some days.30 Tobacco-related diseases are the leading cause of preventable deaths worldwide, contributing to 443,000 deaths per year in the U.S. and nearly $200 billion annually in medical expenses and lost productivity.31 One half of people who continue to smoke will die of a tobacco-related illness. Lung cancer is the most prevalent type of cancer in the world and the leading cause of cancer death in the United States, accounting for 27% of the expected 589,000 U.S. cancer deaths in 2015. The majority of lung cancer deaths are caused by smoking.
The benefits of smoking cessation prior to surgery are well known. Within hours of stopping, blood levels of carbon monoxide and nicotine decline, leading to improved blood flow and oxygen delivery to tissues.32, 33 After several weeks, some aspects of airway inflammation and hyperreactivity improve, including mucociliary clearance, symptoms of coughing and wheezing, and the decline of pulmonary function as measured by lung spirometry. Conversely, patients who continue to smoke in the perioperative period are at increased risk for infection and impaired wound healing, as well as perioperative pulmonary complications such as respiratory failure requiring unplanned ICU admission, pneumonia, and airway complications related to anesthesia. The precise amount of time required to fully realize the benefits of smoking cessation are unknown, with some requiring up to 6 months, and even brief periods of cessation are helpful, but longer is assumed to be better.33 A recent review of the literature found that compared with current smokers, those who abstain from smoking for at least four weeks (and preferably for at least eight weeks) prior to surgery experience lower rates of respiratory complications and fewer instances of impaired wound healing.34
Surgery presents a unique opportunity for health professionals to encourage smoking cessation. A teachable moment occurs when a patient is faced with the recent diagnosis of a serious illness or the prospect of surgery.35 Quit rates are higher following major surgery, especially surgery related to conditions associated with smoking. Heightened awareness of risks and potential negative consequences may provide additional motivation to reduce or quit smoking and patients may be more receptive to anti-smoking discussions, especially when initiated by a physician or other health care professional.32
For surgical patients (and non-surgical patients) who are willing to make a quit attempt and be smoke-free for surgery, and for the physicians who care for them, there are effective smoking cessation resources available in the form of tobacco dependence counseling and medication treatments.36
The Brief Intervention is a practical tool that busy clinicians can use during a routine preoperative clinic visit.37 It is effective in reducing smoking rates in surgical patients and is based on the “5 A” model for reducing tobacco use and dependence: Ask—“Do you smoke?” and “Do you want to quit?” Advise— Strongly urge all tobacco users to quit. Assess—Determine willingness to make a quit attempt. Assist—Provide counseling and medication. Arrange—Ensure follow-up contact. Although intensive behavioral counselling that involves problem solving training and social support is most effective, national telephone tobacco quitlines (1-800-QUIT-NOW) and web-based resources (http://www.smokefree.gov) are also useful.38 Smoking cessation counseling is effective even for those not yet willing to make a quit attempt.39
All patients attempting to quit smoking should be encouraged to use effective first line medications unless medically contraindicated (e.g., Bupropion SR, varenicline, and nicotine replacement therapy, which includes gum, lozenges, and patches). When used together, tobacco dependence counseling and medications are most effective in increasing quitting success and reducing withdrawal symptoms.40
Evidence-based smoking cessation strategies are not only efficacious, but are also cost effective and consistent with the Healthy People 2020 objective of reducing the prevalence of cigarette smoking among U.S. adults to less than 12%. To this end, the 2010 Patient Protection and Affordable Care Act provides expanded coverage for evidence-based smoking cessation treatments.
Conclusion
The preoperative evaluation offers physicians and other health care professional a unique opportunity to help patients optimize their health prior to surgery. Updated, evidence-based guidelines can assist providers in selecting the most appropriate methods of patient evaluation while making the most efficient use of limited health care resources. This includes encouraging healthy behavioral modifications. It is important to bear in mind that the patient always has the final say in any decision to undergo surgery. Therefore, these guidelines are best used to aid shared decision-making, taking into consideration the patient’s perspective on the risks and benefits of surgery.
Biography
Frederick T. O’Donnell, MD, is in the University of Missouri-Columbia Health Care Department of Anesthesiology and Perioperative Medicine.
Contact: odonnellf@health.missouri.edu

Footnotes
Disclosure
None reported.
References
- 1.National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville, MD: 2015. [PubMed] [Google Scholar]
- 2.Vetter TR, Goeddel LA, Boudreaux AM, et al. The Perioperative Surgical Home: how can it make the case so everyone wins? BMC Anesthesiology. 2013;13(1):6. doi: 10.1186/1471-2253-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee A, Kerridge RK, Chui PT, et al. Perioperative systems as a quality model of perioperative medicine and surgical care. Health Policy. 2011;102(2):214–222. doi: 10.1016/j.healthpol.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal D, Abrams M, Nuzum R. The affordable care act at 5 years. N Engl J Med. 2015;372(25):2451–2458. doi: 10.1056/NEJMhpr1503614. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation. Anesthesiology. 2012;116(3):1–17. doi: 10.1097/ALN.0b013e31823c1067. [DOI] [PubMed] [Google Scholar]
- 6.Welch HG. Less medicine, more health. Boston, MA: Beacon Press; 2015. [Google Scholar]
- 7.Sweitzer BJ. Preoperative evaluation for ambulatory procedures. ASA Newsletter: ambulatory anesthesia. 2015;79(8):28–9. [Google Scholar]
- 8.Chen CL, Lin GA, Bardach NS. Preoperative testing in Medicare patients undergoing cataract surgery. N Engl J Med. 2015;372(16):1530–8. doi: 10.1056/NEJMsa1410846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery: study of medical testing for cataract surgery. N Engl J Med. 2000;342(3):168–75. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 10.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e278–e333. doi: 10.1161/CIR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 11.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 12.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336–46.e1. doi: 10.1016/j.jamcollsurg.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–7. doi: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 15.Livhits M, Ko CY, Leonardi MJ, et al. Risk of surgery following recent myocardial infarction. Ann Surg. 2011;253:857–64. doi: 10.1097/SLA.0b013e3182125196. [DOI] [PubMed] [Google Scholar]
- 16.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114:1289–96. doi: 10.1097/ALN.0b013e318216e7f4. [DOI] [PubMed] [Google Scholar]
- 17.Vetter TR, Short RT, Hawn MT, et al. Perioperative management of the patient with a coronary artery stent. Anesthesiology. 2014;121(5):1093–98. doi: 10.1097/ALN.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 18.Hawn MT, Graham LA, Richman JR, Itani KM, Plomondon ME, Altom LK, Henderson WG, Bryson CL, Maddox TM. The incidence and timing of noncardiac surgery after cardiac stent implantation. J Am Coll Surg. 2012;214:658–66. doi: 10.1016/j.jamcollsurg.2011.12.011. discussion 666–7. [DOI] [PubMed] [Google Scholar]
- 19.Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology. 2008;109:588–95. doi: 10.1097/ALN.0b013e318186ddf8. [DOI] [PubMed] [Google Scholar]
- 20.Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126:1355–62. doi: 10.1161/CIRCULATIONAHA.112.102715. [DOI] [PubMed] [Google Scholar]
- 21.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Circulation. 2007;116:e418–e499. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
- 22.Brown SR, Brown J. Why do physicians order unnecessary preoperative tests? A qualitative study. Fam Med. 2011;43:338–43. [PubMed] [Google Scholar]
- 23.Devereaux PJ, Yang H, Guyatt GH, et al. Rationale, design, and organization of the PeriOperative ISchemic Evaluation (POISE) trial: a randomised controlled trial of metoprolol versus placebo in patients undergoing noncardiac surgery. Am Heart J. 2006;152:223–30. doi: 10.1016/j.ahj.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 24.London MJ, Hur K, Schwartz GG, et al. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309:1704–13. doi: 10.1001/jama.2013.4135. [DOI] [PubMed] [Google Scholar]
- 25.Kash B, Cline K, Menser T, et al. The perioperative surgical home: a comprehensive literature review for the American society of anesthesiologists. Texas A&M University Center for Health Organization Transformation; 2014. [Google Scholar]
- 26.Ergina PL, Gold SL, Meakins JL. Perioperative care of the elderly patient. World J Surg. 1993;17(2):192–198. doi: 10.1007/BF01658926. [DOI] [PubMed] [Google Scholar]
- 27.Mayo Nancy E, Feldman Liane, Scott Susan, Zavorsky Gerald, Kim Do Jun, Charlebois Patrick, Carli Francesco. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 28.Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–97. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 29.O’Doherty AF, West M, Jack S, Grocott MPW. Preoperative aerobic exercise training in elective intra-cavity surgery: a systematic review. British journal of anaesthesia. 2013;110(5):679–689. doi: 10.1093/bja/aes514. [DOI] [PubMed] [Google Scholar]
- 30.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults—United States, 2005–2012. MMWR. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 31.King BA, Dube SR, Tynan MA. Current tobacco use among adults in the United States: Findings from the national adult tobacco survery. Am J Public Health. 2012;102(11):e93–100. doi: 10.2105/AJPH.2012.301002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bottorff JL, Seaton CL, Viney N. The stop smoking before surgery program: impact on awareness of smoking-related perioperative complications and smoking behavior in northern Canadian communities. J Prim Care Community Health. 2015 Sep 28; doi: 10.1177/2150131915604827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner DO. Perioperative abstinence from cigarettes: Physiologic and clinical consequences. Anesthesiology. 2006;104:356–67. doi: 10.1097/00000542-200602000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Wong J, Lam DP, Abrishami A, et al. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anesth. 2012;59:268–279. doi: 10.1007/s12630-011-9652-x. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Warner DO. Surgery as a teachable moment for smoking cessation. Anesthesiology. 2010;112(4):102–107. doi: 10.1097/ALN.0b013e3181c61cf9. [DOI] [PubMed] [Google Scholar]
- 36.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May, 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 37.Sachs R, Wild TC, Thomas L. Smoking cessation interventions in the pre-admission clinic: assessing two approaches. Can J Anesth. 2012;59:662–69. doi: 10.1007/s12630-012-9716-6. [DOI] [PubMed] [Google Scholar]
- 38.Stead L, Perera R, Lancaster T. Telephone counseling for smoking cessation. Cochrane Database Syst Rev. 2006;3:CD002850. doi: 10.1002/14651858.CD002850.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Rennard SI, Daughton DM. Smoking cessation. Chest. 2000;117:360S–4S. doi: 10.1378/chest.117.5_suppl_2.360s. [DOI] [PubMed] [Google Scholar]
- 40.Lindstrom D, Sadr Azodi O, Wladis A, Tonnesen H, Linder S, Nasell H, Ponzer S, Adami J. Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Ann Surg. 2008;248:739–45. doi: 10.1097/SLA.0b013e3181889d0d. [DOI] [PubMed] [Google Scholar]