Introduction
The United States (US) is the largest consumer of medical devices in the world1. Responsible for assuring the “safety and effectiveness” of all medical devices, the Food and Drug Administration (FDA) regulates device manufacturers’ ability to market devices within the US2. How the FDA carries out this task, however, is controversial. Consumer-protection advocacy groups demand tighter regulatory oversight, while businesses, many physicians, and some patient advocacy groups argue that the current bureaucratic regulations stymie innovation and hamper patient access to novel devices3.
It is the authors’ experience that many medical doctors understand FDA oversight of drug development, but most harbor little understanding of the regulatory process for devices. In fact, many erroneously believe the two to be alike. It is important, however, for all physicians to familiarize themselves with device regulation so that they can advocate for their patients and advise them about the devices they may be choosing to have implanted. This review will introduce to general physicians the vast and intricate process of device regulation, as well as highlight several controversies fueling recent debate.
History of FDA Device Regulation
In 1938, President Franklin Roosevelt signed into law the Federal Food, Drug, and Cosmetic Act, which, for the first time, required manufacturers to prove the safety of the drugs they sold. While it also implemented limited oversight of device manufacturing, this pertained only to post-market intervention after damage had already occurred. In 1976, with congressional approval of the Medical Device Amendments Act (MDAA), the FDA finally received its jurisdiction to regulate devices prior to reaching market and authorize their use within the US. An integral forerunner of the MDAA was the Cooper Commission, a government-issued task force that reported over 10,000 documented injuries from medical devices, including 731 deaths. The findings of the Cooper report swayed public opinion and influenced congress to legislate new oversight of manufacturers, resulting in much of the FDA regulatory framework as we know it today4.
To begin managing the vast array of new devices coming to market, the MDAA established a classification system that codified all novel devices into one of three risk classes. Devices already in use, by-and-large, required no further testing and ultimately forwent this system by receiving “pre-amendment” device status. Class I devices present minimal risk (i.e., nonpowered goniometer); Class II devices have moderate risk (i.e., intramedullary nails, screws, and most total joint arthroplasties); Class III devices carry the highest risk, represent a completely novel design, and/or support or sustain human life (i.e., intervertebral body fusion devices that include therapeutic biologics, metal-on-metal hip resurfacing, and cardiac ventricular assist)4,5.
Following passage of the MDAA, most changes to device regulation have revolved around less regulation in response to manufacturers’ concerns about FDA costs and delays, and to promote the timely availability of new products. The most significant change was the passage of the FDA Modernization Act of 1997, which established the “least burdensome provisions.” These mandated the FDA to require only the least burdensome amount of evidence that would result in a reasonable likelihood of device approval. For instance, the Act forbade the FDA from requesting a clinical study for class II devices when a benchtop study would likely result in clearance6. In the most recent effort to further limit regulatory oversight of novel devices, Congress reinforced the “least burdensome” principle in the 21st Century Cures Act, signed into law December 20167.
How Devices Reach Market
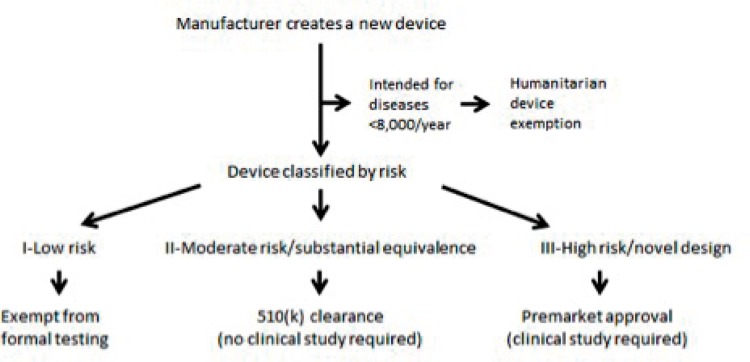
The majority of medical devices subject to FDA regulation progress to market via one of three pathways: Premarket Notification (commonly known as 510(k) Clearance), Premarket Approval (PMA), and Humanitarian Device Exemption (HDE) (See Figure 1). There are other pathways, such as the Breakthrough, Expedited Access, De Novo, and Investigational Device Exemption, however these are less frequently used and beyond the scope of this review1,2,4,5,8.
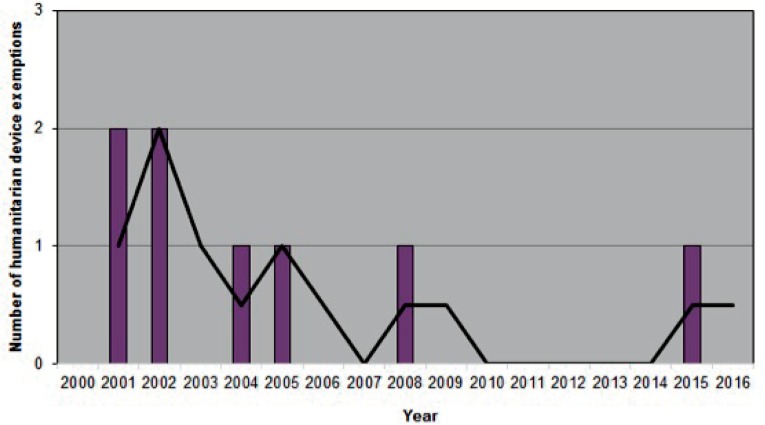
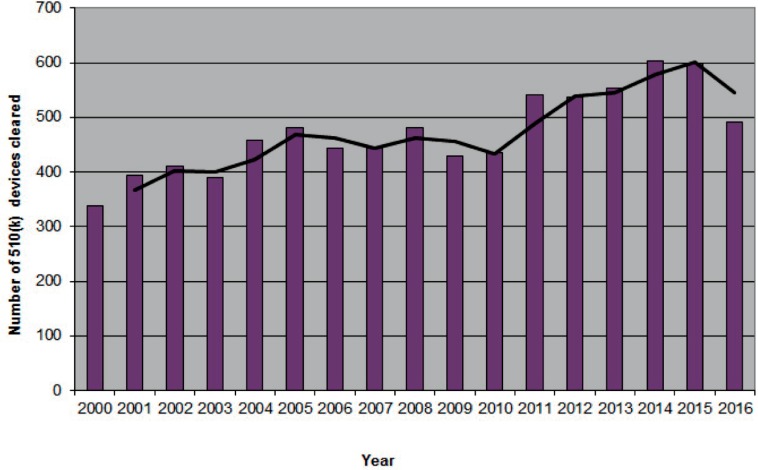
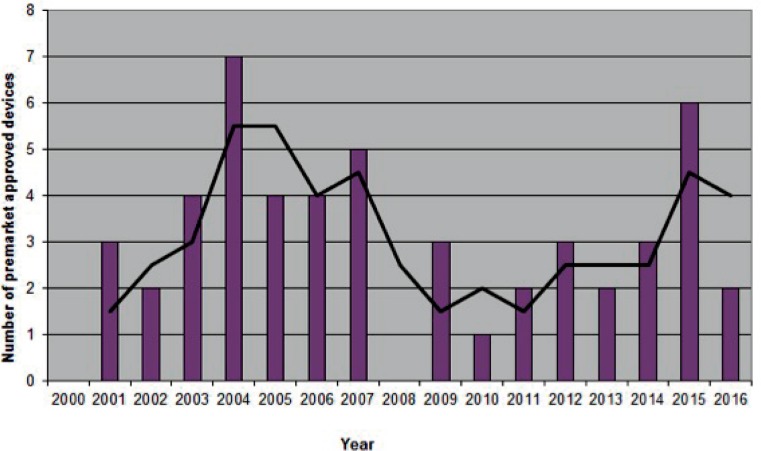
Figures 1a/1b/1c.
Number of orthopaedic devices advanced to market between 2000–2016
1a. Humanitarian device exemptions Data acquired from the Humanitarian Device Exemption Database found at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfHDE/hde.cfm. Advisory Committee: Orthopedic. Accessed February 27, 2017. Each device was counted only once.
1b. 510(k) clearances
Data acquired from the 510(k) Premarket Notification Database found at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm. Panel: Orthopedic. Accessed February 27, 2017.
1c. Premarket approvals
Data acquired from the Premarket Approval Database found at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm. Advisory Committee: Orthopedic. Supplement Type: Originals Only. Accessed February 27, 2017.
510(k) Clearance, the most frequently utilized pathway, stipulates that a new device must demonstrate “substantial equivalence” to a device already approved or cleared by the FDA, or to a pre-amendment device that was in use prior to May 28, 1976. Manufacturers accomplish this usually without producing supporting clinical data, and instead rely on simpler bench-top biomechanical studies2,5. Premarket Approval, the most rigorous process, requires supporting empirical clinical data demonstrating safety and effectiveness, though the methodologic rigor of these studies has been called into question by multiple authors5,9. Humanitarian Device Exemption is a path exempt from clinical evidence of effectiveness, and is intended for devices that will be utilized for diseases found in no more than 8,000 patients per year (changed from 4,000 prior to the 21st Century Cures Act)10.
The decision of which pathway a device must enter typically follows the aforementioned classification system related to perceived risk. Forty-seven percent of all medical devices are Class I, 95% of which are exempt from undergoing any formal clearance or approval, while other Class I devices, like Class II devices, require clearance through the 510(k)11. Class III devices undergo the more demanding PMA2,5. (See Figure 2) After initial evaluation, a device may be selected to undergo additional independent evaluation by the Medical Devices Advisory Committee, which consists of multiple medical specialty panels, each made up of FDA-designated experts in the respective specialty, though the decision to approve a device ultimately rests in the hands of the FDA12.
Figure 2.
Common FDA pathways to market
Controversies in Device Approval
510(k) Overutilization
The 510(k) is by far the most commonly utilized FDA regulatory pathway. (See Figure 1) These devices never receive FDA approval, but rather clearance. Though subtle, the difference is significant as approval by the FDA implies certain protections, such as immunity from litigation in instances of harm directly related to the device. This statute was confirmed in the Riegel vs Medtronic court decision3.
Furthermore, devices cleared by 510(k) most often do not require supporting empirical clinical research. While this simplifies, and cheapens, the process for a manufacturer, it diminishes “real-world” testing prior to wide spread dissemination and implantation of the product. The metal-on-metal DePuy ASR XL total hip replacement exemplifies the worst-case scenario of this policy. By claiming “substantial equivalence” to 95 different previously marketed devices, including pre-amendment devices in use prior to 1976, it cleared without any clinical evaluation in 2008. The ASR XL was recalled just two years later, at which time 100,000 devices had already been implanted and there was a reported 49% failure rate at 6-year follow-up5,13,14.
As a result of this and other public controversies, the criteria for submission to and clearance by the 510(k) pathway have been called into question5,15. In 2011, the Institute of Medicine conducted a thorough investigation of the 510(k) process 35 years after its implementation. In their conclusion, they reported “the committee believes that a move away from the 510(k) clearance process should occur as soon as reasonably possible but recognizes that it will take time to obtain the information needed to design the new framework”15. To the authors’ knowledge, the investigation has not amounted to any significant FDA policy change.
Comparison to European Device Regulation
Critics of the FDA often compare the US system of device regulation to that of Europe. (See Table 1). In their 2010 survey of over 200 medical device manufacturers, Makower, et. al. proffered a scathing analysis of the FDA1. They found that 76% of the responding companies believed the European process good or excellent, compared to only 16% expressing that sentiment toward the US system. In addition, they found the FDA to be much less efficient, which often led to US patients receiving innovative technologies nearly two years later than their European counterparts1.
Table 1.
Comparison of device regulation in the United States and European Union
| United States | European Union | |
|---|---|---|
| Title Granted | FDA Approval/Clearance | Conformité Européenne |
| Burden of Proof | Devices must demonstrate effectiveness | Devices must “function as intended” |
| Oversight | Manufacturer interact with single federal entity throughout process | Numerous (>70) Private groups (Notified Bodies) compete in open market for opportunity to approve devices |
| Marketability | Available within US | Approval by one NB allows marketability in each member country |
| Time of Approval | 3–11 months for 510(k), 9–54 months for PMA | For 510(k) and PMA equivalents, 4 and 11 months, respectively |
It is true that devices take longer to obtain approval in the US than Europe, but the actual discrepancy varies dramatically depending on the source. Manufacturers claim it takes 11 months for FDA 510(k) clearance and 54 months for PMA approval, whereas for the European equivalents, it takes only four months and 11 months, respectively. The FDA publishes three months for 510(k) clearance and nine months for PMA approval1.
Yet, these comparisons may be flawed from the inception. The FDA emerged out of public health concern for consumer and patient protection with the intent to mitigate harm from untested devices4. The Conformité Européenne (CE) mark for medical devices, the European equivalent of FDA approval, stemmed from the New Approach Directives in the 1990s, which attempted to streamline innovation and create uniformity across the nations of the European Union (EU). Public health concerns in Europe largely fall within the purview of the Competent Authority of each individual country. This difference in founding principles allows for a burden of proof substantially weaker for European approval than that in the US. While both systems require some assurance of safety, within the EU a device manufacturer must prove only a device “functions as intended,” whereas in the US they must demonstrate effectiveness8.
To carry this out, Notified Bodies (NB) formed as private, for-profit enterprises to evaluate novel devices and approve them for marketing within the European Union. With little central oversight, a manufacturer may seek approval from any NB within the EU and still be granted rights to market throughout the trading bloc. Multiple studies have pointedly shown enormous variation among the more than 70 NB across Europe. In essence, while a manufacturer may apply to only a single NB at a time, they can peruse the market for the most favorable NB, i.e., one that is faster, cheaper, and most likely to approve the device. Published in the British Medical Journal, Cohen described the process for obtaining approval for a made-up metal-on-metal hip replacement from a fictitious company, Changi. The dossier for the device included comparisons to two recalled devices, as well as serum metal ion levels known to be toxic. They presented their data to 14 different NBs across Europe, including one called Alberk, located “in an office above a garage in the back streets of Istanbul,” and discovered devices rarely fail to obtain approval16. Each NB competes openly to obtain business from manufacturers, and tighter scrutiny drives away business8.
Balancing Innovation and Regulation
An appropriate balance must exist between innovation and regulation to ensure patient safety. Multiple authors have called for more robust FDA checkpoints and supporting clinical data in order for devices to reach market17. Schemitsch, et. al. proposed a four-tiered system, similar to the pathway required in the more rigorous drug industry, involving multiple research trials17. Although such a change would potentially come with a substantial cost. The current cost for a single drug to reach market in the US is reported to be $350 million, and estimated up to $5 billion when taking into account multiple failed attempts that accompany the development of a safe and effective medication18. To bring a device to market in the US, on the other hand, currently costs roughly $31 million for 510(k) and $94 million for PMA devices1.
Unlike the European market in, despite earlier approval, payors (notably, the government) control aggressive spread of costly and unproven technologies, in the US devices can integrate quickly to usher in staggering profits. A bone morphogenetic protein (BMP) product, Infuse Bone Graft, was approved in 2002 for single level LT-Cage lumbar fusion and in 2004 for use in open acute tibial shaft fractures treated with intramedullary nail fixation. Between 2002–2011, Infuse yielded over $4 billion in sales19. Despite multiple studies in peer-reviewed journals, including a dedicated exposé issue in The Spine Journal in 2011, outlining ineffectiveness, higher rates of complications, inappropriate disclosure of financial arrangements with the lead authors of the pivotal studies, as well as a Senate investigation into Medtronic’s promotion of off-label use of BMP, the device still profited $471 million in the 2013–2014 fiscal year, an 11% decrease from the year before20.
Similarly, hyaluronic acid (HA) injections (considered a device by the FDA) have yielded substantial profits that dwarf the investment costs for FDA approval. Eight HA injection products indicated for painful knee arthritis were premarket approved between 2001–2015. Among these, Synvisc-One alone accounted for $274 million in US sales in 201421. Despite this staggering value, quality evidence supporting HA use is lacking. The original blinded randomized-controlled trial for Orthovisc, another HA injection approved in 2004, failed to show any significant difference between the investigational cohort and saline placebo. It instead relied upon a post-hoc analysis with altered endpoints22. Monovisc, a similar HA device, likewise initially failed to demonstrate significant improvement over saline placebo, but ultimately was approved on the basis of non-inferiority to Orthovisc23. This, among other evidence, has led the American Academy of Orthopaedic Surgeons to publish a strong recommendation against the use of HA injections for knee osteoarthritis24. Despite this expert recommendation, it remains unlikely that sales will subside in the near future.
The assumption that all novel devices are truly innovative is false according to the Institute of Medicine’s definition of innovation: “Something that improves the quality of, efficiency of, or access to healthcare”15. Currently, there is no FDA requirement that a device must meet this standard to achieve approval or clearance. The current standard for high-risk devices seeking PMA, for example, often mandates a non-inferiority research design, using an existing treatment as the comparator, such as proving that a novel lumbar or cervical disc replacement is not inferior to spinal fusion. There need not be an empirical demonstration of improvement, efficiency, or better access over the comparator.
Conclusion
Tens of thousands of devices reach market annually in the United States, with each offering the opportunity to improve the quality of life for millions of patients. This benefit, however, must be weighed against the potential for harm from devices lacking sufficient clinical testing prior to their widespread use. While we recommend strengthening the pre-market clinical analysis of devices by the FDA, there are pragmatic limitations such as cost, as well as concerns from industry of excessive regulation limiting innovation. As it stands now, the FDA and European governing bodies are trending towards following post-market surveillance techniques such as MedWatch and EudaMed, respectively8,25. While device manufacturers are required to report adverse events to the FDA, MedWatch is a voluntary reporting system that allows both consumers and providers to report device issues to the FDA8,12,25. The greatest contributors are nurses (25%), while physicians only contribute 8% of reports8,12. As we progress into an era that places more emphasis on post-market surveillance, it becomes ever more important for physicians to understand and participate in the process of device regulation.
Biography
Madelyn Lauer, MD, and Jordan P. Barker, MD, are orthopaedic surgery residents at the University of Missouri-Kansas City. Mitchell Solano, BA, MSMA member since 2012, is a medical student at the UMKC. Jonathan Dubin, MD, (above) is the Chief of Orthopaedic Trauma at Truman Medical Center and an Assistant Professor in the Department of Orthopaedic Surgery at UMKC.
Contact: jonathan.dubin@tmcmed.org

Footnotes
Disclosure
None reported.
References
- 1.Makower J, Meer A, Denend L. FDA Impact on U.S. Medical Technology Innovation: A Survey of Over 200 Medical Technology Companies. November 2010. 2010 http://advamed.org/res.download/30. Accessed May 28, 2016. [Google Scholar]
- 2.U.S. Food and Drug Administration Overview of Device Regulation. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/default.htm Accessed March 4, 2017.
- 3. Public Citizen. Substantially Unsafe: Medical Devices Pose Great Threat to Patients; Safeguards Must be Strengthened, Not Weakened. https://www.citizen.org/documents/substantially-unsafe-medical-device-report.pdf2012.
- 4.Monsein LH. Primer on medical device regulation. Part III. Regulatory mechanisms and import/export regulation. Radiology. 1997;205(1):19–25. doi: 10.1148/radiology.205.1.9314954. [DOI] [PubMed] [Google Scholar]
- 5.Barker JP, Dubin J. Recent Trends in Orthopedic Device Regulation. Orthopedics. 2015;38(7):410–412. doi: 10.3928/01477447-20150701-02. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration The Least Burdensome Provisions of the FDA Modernization Act of 1997: Concept and Principles; Final Guidance for FDA and Industry. . https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm085994.htm#2. Accessed March 4, 2017. [Google Scholar]
- 7.U.S. Food and Drug Administration Drug Administration 21st Century Cures Act: Making Progress on Shared Goals for Patients. https://blogs.fda.gov/fdavoice/index.php/2016/12/21st-century-cures-act-making-progress-on-shared-goals-for-patients/. Accessed March 4, 2017.
- 8.Maak TG, Wylie JD. Medical Device Regulation: A Comparison of the United States and the European Union. The Journal of the American Academy of Orthopaedic Surgeons. 2016;24(8):537–543. doi: 10.5435/JAAOS-D-15-00403. [DOI] [PubMed] [Google Scholar]
- 9.Dhruva SS, Bero LA, Redberg RF. Strength of study evidence examined by the FDA in premarket approval of cardiovascular devices. JAMA : the journal of the American Medical Association. 2009;302(24):2679–2685. doi: 10.1001/jama.2009.1899. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration Drug Administration. Humanitarian Use Devices and Humanitarian Device Exemption. https://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm249423.htm. Accessed March 4, 2017.
- 11.U.S. Food and Drug Administration Learn if a Medical Device Has Been Cleared by FDA for Marketing. https://www.fda.gov/medicaldevices/resourcesforyou/consumers/ucm142523.htm. Accessed March 4, 2017.
- 12.Maisel WH. Medical device regulation: an introduction for the practicing physician. Ann Intern Med. 2004;140(4):296–302. doi: 10.7326/0003-4819-140-4-200402170-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a metal-on-metal hip implant. The New England journal of medicine. 2013;368(2):97–100. doi: 10.1056/NEJMp1211581. [DOI] [PubMed] [Google Scholar]
- 14.Curfman GD, Redberg RF. Medical devices--balancing regulation and innovation. The New England journal of medicine. 2011;365(11):975–977. doi: 10.1056/NEJMp1109094. [DOI] [PubMed] [Google Scholar]
- 15.IOM (Institute of Medicine) Medical Devices and the Public’s Health: The FDA 510(k) Clearance Process at 35 Years. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 16.Cohen D. How a fake hip showed up failings in European device regulation. BMJ. 2012;345:e7090. doi: 10.1136/bmj.e7090. [DOI] [PubMed] [Google Scholar]
- 17.Schemitsch EH, Bhandari M, Boden SD, et al. The evidence-based approach in bringing new orthopaedic devices to market. The Journal of bone and joint surgery American. 2010;92(4):1030–1037. doi: 10.2106/JBJS.H.01532. [DOI] [PubMed] [Google Scholar]
- 18.Herper M. The Cost Of Creating A New Drug Now $5 Billion, Pushing Big Pharma To Change. Forbes. 2013 [Google Scholar]
- 19.Carlson J. Star Tribune. 2015. New federal data shine a light on Medtronic payments to doctors. http://www.startribune.com/new-federal-data-shine-a-light-on-medtronic-payments-to-doctors/313962621/ Accessed March 6, 2017.
- 20.Carlson J. Star Tribune. 2014. New research says Medtronic’s InFuse safe for most patients. http://www.startribune.com/new-research-says-medtronic-s-infuse-safe-for-most-patients/282782331/. Accessed March 6, 2017.
- 21.Sanofi. 2015. FORM 20-F 2015. http://en.sanofi.com/Images/45889_Sanofi_20-F_2015_V2.pdf. . 54, Rue La Boétie, 75008 Paris, France.
- 22. SUMMARY OF SAFETY AND EFFECTIVENESS DATA, ORTHOVISC® High Molecular Weight Hyaluronan, Anika Therapeutics, Inc. http://www.accessdata.fda.gov/cdrh_docs/pdf3/P030019b.pdf. Accessed March 5, 2017.
- 23. SUMMARY OF SAFETY AND EFFECTIVENESS DATA, MONOVISC™, Anika Therapeutics, Inc. http://www.accessdata.fda.gov/cdrh_docs/pdf9/P090031b.pdf. Accessed March 5, 2017.
- 24.Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. The Journal of the American Academy of Orthopaedic Surgeons. 2013;21(9):577–579. doi: 10.5435/JAAOS-21-09-577. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration Reporting Serious Problems to FDA. https://www.fda.gov/Safety/MedWatch/HowToReport/default.htm. . Accessed March 5, 2017.