Abstract
Despite recent advances unraveling mechanisms of host–pathogen interactions in innate immunity, the participation of purinergic signaling in infection-driven inflammation remains an emerging research field with many unanswered questions. As one of the most-studied oral pathogens, Porphyromonas gingivalis is considered as a keystone pathogen with a central role in development of periodontal disease. This pathogen needs to evade immune-mediated defense mechanisms and tolerate inflammation in order to survive in the host. In this review, we summarize evidence showing that purinergic signaling modulates P. gingivalis survival and cellular immune responses, and discuss the role played by inflammasome activation and cell death during P. gingivalis infection.
Keywords: Purinergic receptors, Innate immunity, Porphyromonas gingivalis, P2X7 receptor, Oral microbes, Inflammasome
Innate immunity and oral microbes
The host organism is always ready to respond to foreign stimuli, such as infection by pathogens. The first response of the host immune system is carried out by innate immunity. Unlike the adaptive response (which includes specific antibodies and lymphocytes), the repertoire of the innate response is common among all normal and healthy individuals. This response involves cellular and humoral activities, as well as chemical (e.g. acidic stomach pH, saliva and tears) and anatomical barriers (e.g. epithelial cells throughout the body).
Cellular responses to pathogens depend on the recognition of evolutionarily conserved structures that are typically present in microbes but not in the host. These molecules, called “Pathogens Associated Molecular Patterns” (PAMPs), are recognized by innate immune cells by “Pattern Recognition Receptors” (PRRs). PRRs include Toll-like receptors (TLRs), nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), retinoic-acid-inducible gene I (RIG-I)-like receptors (RLRs), and the C-type lectin receptors (CLRs), as well as DNA receptors (cytosolic sensors for DNA) [1], [2], [3]. Different PAMPs can be recognized by PRRs, whose ligation leads to activation of transcription factors, such as activator protein 1 (AP-1) and nuclear factor kappa B (NF-ĸB), which in turn, modulate gene transcription of pro-inflammatory cytokines and chemokines [4]. This response is important for the host to control infections and prevent disease.
Knowledge about the human microbiota and its relationship with the host is also crucial for better understanding the mechanisms of immunity, since the microbiota can stimulate and modulate the immune system. The human body contains 10 times more prokaryotic than eukaryotic cells, and humans and microbes have evolved to gradually become dependent on one another [5]. The oral mucosa is associated with hundreds of different viruses and bacterial, archaeal, fungal, and protozoan species, many of which can interact to form biofilms conferring resistance to the microorganisms against mechanical and chemical stress [5], [6]. In this context, bacterial communities found in the oral mucosa are highly complex (comprising around 1000 species) and are one of the most complex in the whole body [6]. Most of the oral microbes live as commensals within the host, but some species can become pathogenic in response to the host genotype, stress, diet or behavior (e.g. smoking) [7].
Not surprisingly, some oral microbes have been related to oral disorders, including periodontal disease (or periodontitis), which is commonly manifested as a chronic and inflammatory condition induced by biofilms and pathogens closely associated with the periodontium (the structures that protect and support teeth, such as gingiva, periodontal ligament and alveolar bone). When the periodontium is damaged, a process of uncontrolled bone resorption is initiated and can lead to tooth loss as a consequence [7], [8]. Socransky and colleagues identified several bacteria present in subgingival biofilms from individuals with periodontitis and grouped these bacteria in complexes according to their association with disease [9]. Among these complexes, the one most strongly associated with periodontitis is the so-called “red complex” bacteria. The “red complex” consists of Tannerela forsythia, Treponema denticola and Porphyromonas gingivalis, which are related to human periodontitis because of their strong association with the diseased sites [9], [10].
P. gingivalis is an anaerobic, asaccharolytic, black-pigmented, non-motile and non-spore forming Gram-negative bacterium, which exists as different strains with variable virulence [11], [12], [13]. P. gingivalis is mainly found during diseased states but it can be also found in healthy individuals, with a prevalence that ranges around 25% in healthy individuals and 79% in individuals with periodontitis [14]. Recent studies have suggested a new paradigm of periodontal pathogenesis, which assigns a larger role for some bacteria from the oral microbiota and host susceptibility in development of disease. Usually, pathogenic bacteria lead to inflammation by direct infection and dysregulation of the commensal microbiota. P. gingivalis was described as a “keystone pathogen” due to its ability to induce dysbiosis and inflammation even in relatively low numbers. Using a murine model of experimental periodontitis, it was shown that, even in low-abundance after inoculation, P. gingivalis can infect the oral mucosa and promote changes in numbers and composition of the oral commensal microbiota, leading to a dysbiotic environment [15]. Dysbiosis is characterized by an imbalance in the relative abundance of species within the microbiota that is related to disease induction [7]. This study also showed that P. gingivalis inoculation in the oral cavity led to bone loss in specific pathogen free (SPF) mice, but P. gingivalis alone could infect but did not induce bone loss in germ-free mice. These data resulted in the “keystone pathogen hypothesis” of periodontal disease [15].
Besides being considered a keystone pathogen, this bacterium is also associated with increased risk of diverse systemic diseases. Oral health is important for overall health since it has been shown that periodontitis increases the patients' risk for atherosclerosis, rheumatoid arthritis, and cancer [16], [17], [18], [19], [20]. In fact, during periodontal disease, bacteria reach blood vessels temporarily and this transient bacteremia is responsible for spreading bacteria to different sites of the organism, such as atherosclerotic plaques (as detected by PCR [17]), where they can accelerate pathogenesis.
P. gingivalis has evolved several virulence factors to evade innate and adaptive immunity and cause disease. There is a large body of evidence describing the ambivalent behavior of this microorganism, which on one hand needs to escape immune-mediated detection and on the other hand tolerates and perpetuates inflammation to survive in the host. It was shown that P. gingivalis fimbriae can highly activate human monocytes and poorly activate epithelial cells with regards to interleukin (IL)-6, IL-8, macrophage colony stimulating factor (M-CSF) and tumor necrosis factor (TNF)-α responses, thus reflecting different strategies used by this bacterium when interacting with distinct host cell types [21]. As another example, P. gingivalis can manipulate host neutrophils through complement C5a receptor and TLR2 pathways in a cooperative crosstalk involving downstream adaptor molecules such as MyD88 [22]. This study showed higher bacterial survival in infected MyD88-deficient mice compared with wild-type mice. In vitro, MyD88-depleted human periodontal fibroblasts showed decreased expression of major inflammatory cytokines such as IL-6 and IL-8 compared to control cells when stimulated with P. gingivalis LPS [23]. Moreover, cysteine proteinases called gingipains from P. gingivalis can influence the composition of polymicrobial biofilms [24]. This work revealed an interdependency between the gingipains of P. gingivalis and T. forsythia or T. denticola, thus suggesting supported survival and virulence of the biofilm community as a whole. The capacity of P. gingivalis to interact with other periodontal pathogens such as Fusobacterium nucleatum was also demonstrated in the case of P. gingivalis' ability to suppress inflammasome activity [25], which will be discussed later in this review. Finally, P. gingivalis expresses hemaglutinins, as well as an atypical and less immunogenic LPS, which can be an antagonist of TLR4 ligation; a serine phosphatase B (SerB) which inhibits IL-8 synthesis by gingival epithelial cells (GECs); fimbriae that are involved in bacterial adhesion; and a nucleoside-diphosphate-kinase (NDK), which hydrolyzes extracellular ATP (eATP) and will be discussed in more detail later in this review.
P. gingivalis can also evade different mechanisms of adaptive immunity. Human neutrophils, peripheral blood mononuclear cells (PBMCs) and GECs infected with P. gingivalis produced IL-1β but not the T-cell chemokine CXCL10 [26]. Thus, P. gingivalis infection inhibited interferon (IFN)-γ-induced and F. nucleatum-induced CXCL10 secretion by epithelial cells. Moreover, P. gingivalis adhesion induced morphological changes, reactive-oxygen-species (ROS) production and increased intracellular Ca+2 levels in T-cells [27]. This periodontal pathogen also inhibited AP-1 and NF-ĸB activity, as well as IL-2 accumulation, by means of its gingipains. Another study demonstrated that gingipains of P. gingivalis cleave immunoglobulin G1 (IgG1) in gingival crevicular fluid of patients and may suppress antibody-dependent antibacterial activity in vivo [28]. Also, it was shown that, upon initial encounter with P. gingivalis in vivo, murine splenic T cells and CD11b+ cells produced IL-10, and this cytokine suppressed IFN-γ T cell responses [29]. Furthermore, it was demonstrated that supernatants of human immune cells infected with two different strains of P. gingivalis-induced T helper cell polarization to a Th17 profile instead of a Th1 profile [30]. Moreover, P. gingivalis favored the generation of Th17-related cytokines such as IL-1β, IL-6 and IL-23 but not the Th1-related cytokine, IL-12. Interestingly, another laboratory showed that subcutaneous vaccination with formalin-killed P. gingivalis protected the mice from alveolar bone resorption and inflammation by downregulation of Th17 cells and IL-17A production, while promoting upregulation of regulatory T (Treg) cells, IL-10 and transforming growth factor-β1 (TGF-β1) [31].
Purinergic signaling in the context of infection and inflammation
ATP is traditionally associated with cellular energy metabolism in all prokaryotic and eukaryotic cell types, but it is also recognized that ATP and other nucleotides are released from cells following stress or injury [32], [33]. It has been shown that controlled and uncontrolled mechanisms of ATP release to the extracellular space takes place during cellular stress, death or tissue injury. It has been demonstrated thus far that ATP is released from necrotic cells via pannexin channels, connexin hemichannels and also via the P2X7 receptor [34], [35]. In the extracellular compartment, nucleotides can be recognized by the host immune system as danger signals and can promote several biological activities in different immune cell types. Examples include: maturation of immature dendritic cells, secretion of pro-inflammatory cytokines by macrophages, chemotaxis and IL-8 production by eosinophils, and costimulation for antigenic stimulation by T and B cells [35], [36]. These molecules bind to purinergic receptors expressed on virtually all immune cell types [33].
Purinergic receptors are divided into two families: P1 and P2 receptors [32]. The G-protein coupled metabotropic P1 receptors recognize exclusively adenosine and can be subdivided into A1, A2A, A2B and A3 receptors, all of which with different binding affinities for adenosine [32], [37]. The receptors A1 and A3 are coupled to protein Gi, and A2A and A2B becoming associated with protein Gs [37], [38]. The P2 receptors can be subdivided into two subtypes: non-selective ion-gated channel P2X receptors (that recognize ATP) and G-coupled P2Y receptors (that recognize ATP, ADP, UTP, UDP and UDP-glucose) [35], [36]. To date, seven P2X receptors (from P2X1 to P2X7) with different affinities for ATP have been described. Among these receptors, the P2X7 receptor has a low affinity for ATP (requiring ≥100 μM to be activated; while others can be activated at lower concentrations) and has been associated with immune responses and inflammation, such as inhibition of infection by intracellular pathogens and activation of the inflammasome, which will be discussed later in this review [4], [39], [40], [41], [41].
ATP ligation of the P2X7 receptor leads to opening of a transmembrane non-selective cationic channel that allows K+ efflux and Na+ and Ca2+ influx and promotes cytoplasmic membrane depolarization [35]. P2X7 receptor activation is associated with pore formation, which depends on the concentration and duration of ATP treatment [41]. Continuous stimulation of the P2X7 receptor with ATP can induce cell death either by necrosis or apoptosis [42], as well as lead to opening of a pore that allows the passage of molecules up to 900 Da [43].
Since recognition of high levels of eATP results in modulation of immune responses, the host has a sophisticated and sensitive mechanism to regulate the composition, duration, intensity and magnitude of purinergic signaling, as reviewed elsewhere [41]. Immune and non-immune cells utilize a group of nucleotide-hydrolyzing enzymes called ecto-nucleotidases to control exacerbated levels of nucleotides and maintain steady-state conditions. The ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases which degrade extracellular tri- and diphosphonucleosides to monophosphonucleosides), ecto-nucleotide pyrophosphatase/phosphodiesterases (E-NPPs that hydrolyze pyrophosphate and phosphodiester bonds in a wide range of substrates), and ecto-5′-nucleotidase (which degrades AMP) are the most relevant ecto-nucleotidases in the context of innate immunity [41]. These enzymes are responsible for maintaining healthy and stable levels of eATP [36] and generating adenosine, a metabolite of ATP breakdown [41].
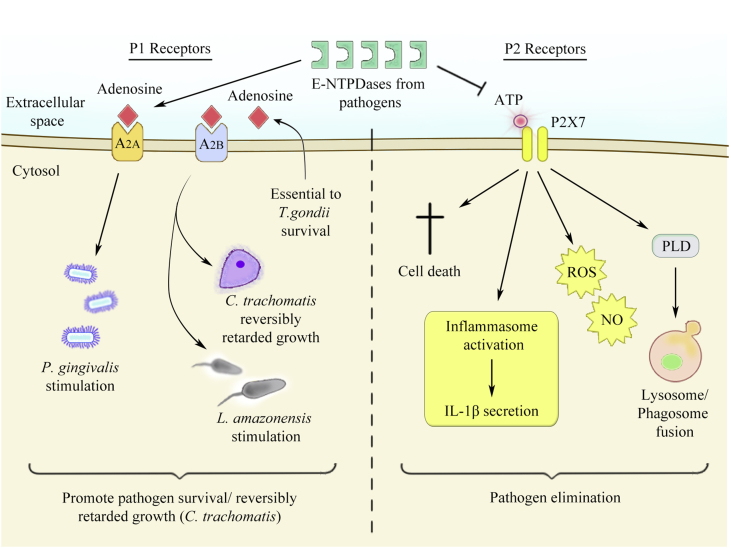
While ATP exhibits pro-inflammatory and stimulatory effects in the immune system and physiology [35], [44], [45], [46], [47], [48], [49], [50], [51], adenosine has primarily anti-inflammatory and inhibitory effects [37], [52], [53], [54], [55], [56]. Therefore, the balance between ATP versus adenosine levels is important in modulating cellular immune responses and pathogen survival [Fig. 1].
Fig. 1.
Schematic figure of the antagonistic effects of P1 and P2 receptors on infected cells. On the left, extracellular adenosine is recognized by P1 receptors (for example, A2A and A2B) and can promote pathogen survival or reversibly retard microbial growth. On the other hand, on the right, eATP released from stressed, dying or infected cells binds to P2 receptors (for example, P2X7) and leads to pathogen elimination through several pathways: (1) host cell death; (2) inflammasome activation and IL-1β secretion; (3) ROS and NO production; (4) or phospholipase D activation, promoting lysosome and phagosome fusion. Importantly, ecto-nucleotidases (E-NTPDases) from several pathogens inhibit pathogen elimination by eATP cleavage and/or favor microbial survival by generating extracellular adenosine.
ATP effects in intracellular pathogen infection
Immune and epithelial cells activate microbicidal pathways and contribute to inflammation after ligation of purinergic P2 receptors by eATP [Fig. 1]. Facultative and obligate intracellular microbes survive inside the host cell, where they acquire nutrients needed for microbial replication and propagation of the infection [53], [57]. For intracellular pathogens, it is advantageous to be able to prevent or delay apoptosis of the host cell in order to promote survival and growth of the pathogen. In this regard, several pathogens evolved different mechanisms to promote their own growth inside the host cell, as for example Mycobacterium tuberculosis, Chlamydia trachomatis, Leishmania species, Toxoplasma gondii and also P. gingivalis.
Unlike activation of surface CD95 (Fas receptor), leading to host cell apoptosis, or complement-mediated host cell lysis, which do not induce mycobacterial death [50], eATP treatment of macrophages enhances their antimicrobial properties in a P2X7 receptor-dependent manner. We and others showed that eATP-related killing of M. tuberculosis and C. trachomatis within human and murine macrophages is mediated by phospholipase D, which is associated with mobilization of intracellular Ca+2 and consequently lysosomal fusion and acidification of the phagosomes containing the pathogen [44], [48], [49]. In another study, it was shown that adenine nucleotides (adenosine, AMP and ATP) can inhibit C. trachomatis growth in epithelial cells [58]. Moreover, millimolar eATP concentrations inhibit chlamydial infection via P2X7 receptor in macrophages [48], while micromolar eATP concentrations reversibly inhibit chlamydial infection via the P2X4 receptor in epithelial cells [58].
Infection with the protozoan parasite Leishmania amazonensis is also controlled by eATP treatment. Murine macrophages infected with L. amazonensis showed enhanced P2X7 receptor expression in vitro and were more responsive to eATP activation in vitro and in vivo, where cells from established cutaneous lesions were more sensitive to eATP than cells from uninfected mice [46]. Additionally, elimination of Leishmania via eATP ligation of the P2X7 receptor is involved with leukotriene B4 production in a 5-lipoxigenase dependent-manner [45], [46]. Another work showed that UTP, but not UDP, inhibits L. amazonensis infection in murine macrophages, inducing morphological damage in the intracellular parasite, promoting apoptosis of macrophages and production of oxygen and nitrogen reactive species, and increasing intracellular Ca+2 concentrations [59]. These effects are believed to occur via P2Y2 and P2Y4 receptors following their upregulation during L. amazonensis infection. Periodate-oxidized ATP also induces morphological changes directly in the parasite, dampening the attachment and entry of the protozoa in murine macrophages [60].
Another intracellular protozoan parasite affected by purinergic signaling is T. gondii. eATP treatment in infected macrophages promotes T. gondii elimination via P2X7 receptor through acidification of the parasitophorous vacuole and ROS production [47], [51]. Additionally, our group recently demonstrated that UTP and UDP treatment in murine macrophages infected with T. gondii promotes 90% elimination of the parasite, without inducing NO, ROS or apoptosis in the host cell [61]. Interestingly, UTP and UDP induced parasite egress from the host cell via P2Y2, P2Y4 and P2Y6, thus compromising infectivity and replication of the egressed parasites.
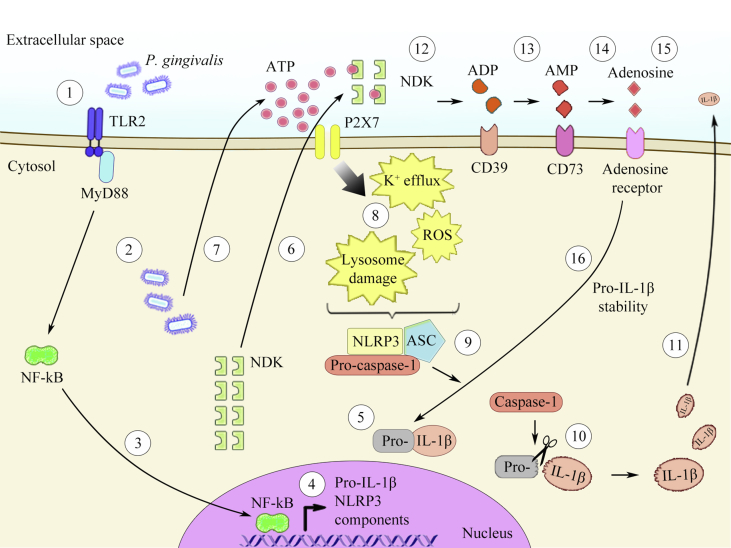
The expression of P2X2, P2X4, P2X5, P2X6 and P2X7 receptors was also reported in GECs, and eATP, unlike other extracellular nucleotides such as UTP and ADP, induced apoptosis of these cells [62]. These studies also demonstrated that P. gingivalis infection inhibits eATP-induced apoptosis in GECs through a mechanism that depends on a homolog of nucleoside-diphosphate-kinase (NDK), which is secreted by P. gingivalis [62], [63]. NDK is an ubiquitous enzyme that is highly conserved in prokaryotes and eukaryotes, including plants [64], [65]. The main role of NDK is to catalyze the transfer of terminal phosphate groups from 5′-triphosphate- to 5′-diphosphate-nucleotides [64], [65], [66]. Thus NDK has the ability to hydrolyze (and also synthesize) any NTP/dNTP [65], [67]. In this context, the NDK from P. gingivalis can hydrolyze eATP after infection in human GECs. The enzyme can thus (1) diminish eATP-induced apoptosis [62]; (2) inhibit eATP-induced ROS via P2X7/NADPH oxidase signaling [63]; and (3) attenuate eATP-induced inflammasome activation, therefore impairing IL-1β release [68]. In contrast, the presence of NDK from Pseudomonas aeruginosa [69] and P. gingivalis during infection in murine macrophages contributes to pro-IL-1β production/stability and induces IL-1β secretion [unpublished data – Fig. 2], thus demonstrating differences in the role of NDK from P. gingivalis after infection in different cell lines.
Fig. 2.
Schematic figure of the effects of P1 receptors, P2 receptors, and ecto-nucleotidases on P. gingivalis-induced inflammasome activation. (1) As the first signal required for inflammasome activation, P. gingivalis is recognized by the host cell through TLR2, and (2) reaches the cytosolic compartment. (3) Recognition of the bacterium via TLR2 promotes the translocation of the transcriptional factor NF-ĸB to the nucleus. (4) Once in the nucleus, NF-ĸB induces pro-inflammatory cytokines and transcription of inflammasome components (5) and promotes pro-IL-1β synthesis. (6) Concomitantly, P. gingivalis secretes NDK, which may be released to the extracellular compartment. (7) In addition, after infection, this oral bacterium induces ATP release from the host cell to the extracellular space. As a second signal for inflammasome activation, (8) ATP ligation to the P2X7 receptor can promote K+ efflux, ROS generation and/or lysosome damage, (9) which can activate the NLRP3 inflammasome. The NLRP3 inflammasome activates procaspase-1 to the mature caspase-1, and (10) this enzyme, in turn, proteolytically processes pro-IL-1β into IL-1β, (11) which is released from the cell. (12) NDK from P. gingivalis hydrolyzes eATP, generating its own metabolites, such as ADP, (13) which are recognized and cleaved via ecto-nucleotidases from the host, such as CD39, generating AMP. (14) AMP binds to CD73 on the host cell, and this enzyme can generate adenosine. (15) Adenosine interaction via adenosine receptor (16) can promote pro-IL-1β stability and thus, supports IL-1β secretion.
Adenosine effects on P. gingivalis infection
Adenosine is also considered as a danger signal, which can be released from stressed, necrotic or dying cells, or can be generated via dephosphorylation of eATP by the ecto-nucleotidases CD39 and CD73, as reviewed elsewhere [41], [70]. In favor of the role of adenosine in downregulating immune responses and inflammation [37], [52], [53], [55], [56] [Fig. 1], a recent study has found that agonist of adenosine receptors, when added exogenously in vitro, stimulates the growth of P. gingivalis in GECs [54]. This work demonstrated that GECs express all the adenosine receptors, and stimulation of the A2A receptor via CGS21680 specific-agonist could enhance proliferation of P. gingivalis. On the other hand, a high-affinity adenosine receptor agonist NECA, or adenosine by itself, could reversibly inhibit growth of the intracellular bacterium C. trachomatis via A2B receptors [71]. Adenosine is also important in survival of protozoan parasites, such as the obligate intracellular pathogen T. gondii. CD73-generated extracellular adenosine is important for T. gondii survival because this pathogen can not to make its own [53]. Thus, CD73-deficient mice infected with T. gondii have lower parasite levels and are protected from chronic infection when compared with wild-type mice. Since mice lacking adenosine receptors have no effect in cyst formation, CD73 expression is thought to promote T. gondii differentiation and cyst formation by a mechanism dependent on adenosine generation, but independent of adenosine receptor signaling [53], suggesting that the parasite utilizes adenosine as a substrate for its own metabolism. Thus, adenosine effects on cells infected with intracellular pathogens depends on the specific type of pathogen and may depend on which adenosine receptor is stimulated [Fig. 1].
Because of the role of adenosine in facilitating survival of some microorganisms, some pathogens have evolved mechanisms to stimulate extracellular adenosine generation independently of the host. Staphylococcus aureus produces adenosine synthase A (AdsA) as a virulence factor, a cell wall-anchored enzyme which allows the bacteria to escape from phagocytic clearance and favoring the formation of organ abscesses [52]. Moreover, the same study showed that other prominent bacteria from the oral cavity including Enterococcus faecalis and Streptococcus mutans possess uncharacterized homologs of adenosine synthase.
Furthermore, some pathogens have evolved extracellular nucleotide-hydrolyzing enzymes that mimic the ecto-nucleotidases expressed in the host [Fig. 1]. For example, the surface of Trypanosoma cruzi expresses a Mg2+-dependent ecto-ATPase activity, whose function is 20 times greater in trypomastigotes than in epimastigotes, suggesting a role for this enzyme in promoting infection in the vertebrate host [72]. A comparison of the Mg2+-ecto-ATPase activities of the three forms of T. cruzi showed that the noninfective epimastigotes are less efficient at hydrolyzing eATP than the infective trypomastigote and amastigote stages [73]. Another parasite with Mg2+-dependent ecto-ATPase is L. amazonensis, where the avirulent promastigotes are less efficient than the virulent promastigotes in hydrolyzing eATP, suggesting that virulent strains acquire adenosine and utilize it in its favor [74].
Interestingly, some bacteria secrete ATP during their growth [75], which may play a role in bacterial physiology. In the oral cavity, it was found that Aggregatibacter actinomycetemcomitans but not P. gingivalis, Prevotella intermedia, or F. nucleatum secrete ATP into the culture supernatant during its growth [76]. Infection in general can induce release of ATP from the host cell [77], and P. gingivalis infection stimulates ATP liberation after infection in GECs [63], THP-1 macrophages [78], and murine macrophages (unpublished data). It is tempting to speculate that eATP may be secreted during infection by some pathogens during their growth, such as the common bacteria Escherichia coli, Staphylococcus, Acinetobacter, and others; while supporting the survival of other pathogens during infection – for example during infection by L. amazonensis, T. gondii and T. cruzi, due to their ability to hydrolyze eATP generating adenosine, which is necessary for their survival.
Inflammasomes and purinergic signaling associated with P. gingivalis infection
Inflammasomes, discovered in 2002, are multi-protein complexes assembled in the host cell in response to infection or cellular stress, leading to a type of cell death called pyroptosis and/or maturation and secretion of pro-inflammatory cytokines, such as IL-1β and IL-18 [79], [80], [81]. Pyroptosis is a non-homeostatic and lytic cell death dependent on caspase-1 and/or caspase-11 activation [80], [82], [82]. P2X7 receptor was shown to activate NLRP3 inflammasomes [83]; and recently, caspase-11-induced pyroptosis was shown to require pannexin-1 channels and the P2X7 receptor [84]. This kind of cell death presents similarities to necrosis such as an increase in the cytoplasmic volume and rupture of the cellular plasma membrane. In the context of inflammasome activation, pro-inflammatory cytokines are important for eliminating pathogens [4], [82]. Pyroptosis is important because of the cytokines, chemokines and DAMPs which are released to the extracellular compartment, and also because this type of cell death exposes intracellular bacteria to extracellular immune surveillance, thus allowing their destruction by antimicrobial peptides, immunoglobulins, and the complement system, and their uptake by immune cells [79]. IL-1β affects virtually all cells and organs of the body and is one of the most important cytokines that mediate autoimmunity, infections and degenerative diseases [85]. This cytokine has a role in the central nervous system as an endogenous pyrogenic agent, and it can also induce inflammation, leukocyte recruitment, and Th17 profile immune responses [85], [86]. Inflammasomes are usually studied in immune cells, but they can also be activated in several types of epithelial cells, including GECs [87].
Canonical inflammasomes convert procaspase-1 into the catalytically active enzyme, caspase-1, whereas a still completely undefined non-canonical inflammasome promotes activation of procaspase-11 [82], [88]. Several canonical inflammasome complexes have been identified depending on the receptor that recognizes the PAMPs (for example, NLRP1, NLRP3, AIM2, NLRC4), while the non-canonical inflammasome can be activated by cytosolic LPS derived from Gram-negative bacteria. One of the best characterized inflammasomes contains the NLR member, NLRP3, the adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC), and the protease, caspase-1. The NLRP3 inflammasome can be activated by different stimuli, such as bacterial, viral, and fungal pathogens, pore-forming toxins, crystals, silica and DAMPs (for example, eATP) [82], [89]. The activation of the canonical NLRP3 inflammasome typically requires two signals: (1) a PAMP, such as LPS, leading to transcription of NF-κB, upregulating genes encoding pro-inflammatory cytokines, chemokines and proteins involved in the inflammasome platform; and (2) a DAMP, such as eATP, which induces inflammasome activation after ligation to the P2X7 receptor [39]. Once activated, these complexes promote activation of the protease caspase-1, which cleaves pro-IL-1β and pro-IL-18 into their active forms: IL-1β and IL-18.
As reviewed elsewhere [79], inflammasome activation occurs in response to microbial invasion and is important for controlling infections. The murine NLRP1 inflammasome recognizes the cytosolic Bacillus anthracis lethal toxin, and mutations in the Nlrp1b gene confer susceptibility to anthrax lethal toxin-induced macrophage death. A defective NLPR3 inflammasome leads to mice susceptibility to Candida albicans and Aspergillus fumigatus infections [79]. Besides stimulating secretion of IL-1β and IL-18, the induction of pyroptosis is a critical in vivo mechanism by which the NLRC4 inflammasome clears flagellin-expressing bacteria, such as Legionella pneumophila and Burkholderia thailandensis. Moreover, Francisella tularensis activates the AIM2 inflammasome, as shown by the increased susceptibility of caspase-1-deficient mice to infection with this pathogen [79].
IL-1β regulates innate immune responses and is critical for host defense against bacterial infection. However, excessive IL-1β production or inflammasome components [78], as well as increased P2X7 receptor and NLRP3 mRNA levels [90], [91], are linked to periodontal disease in human gingival tissues. Recently, our group demonstrated that in murine macrophages, eATP-induced IL-1β secretion is impaired by P. gingivalis fimbriae in a P2X7-dependent manner [92]. In human macrophages, P. gingivalis induces IL-1β secretion and inflammatory cell death via caspase-1 activation (pyroptosis). Moreover, IL-1β secretion and pyroptotic cell death requires both NLRP3 and AIM2 inflammasome activation by this oral pathogen. P. gingivalis infection induces ATP release from macrophages, which is mediated by NLRP3 inflammasome activation via P2X7 receptor stimulation and lysosomal damage [78]. In addition, GECs express the inflammasome components, NLRP3, NLRC4 and NLRP1 [93]. P. gingivalis stimulated expression of IL-1β mRNA and intracellular accumulation of pro-IL-1β, although IL-1β secretion required on the addition of eATP in vitro. In GECs, eATP, but not P. gingivalis alone, induced caspase-1 activation [93]. Thus, P. gingivalis infection can provide the signals necessary for synthesis of pro-IL-1β, but an exogenous danger signal, such as eATP, must activate the inflammasome, allowing the infected cell to secrete mature IL-1β.
eATP induces ROS production through a complex consisting of the P2X4, P2X7 receptor and pannexin-1, and the P2X7-mediated ROS production can activate the NLRP3 inflammasome and caspase-1 [94]. Interestingly, P. gingivalis infection in GECs induces partially reduction of NLRP3 mRNA levels compared with uninfected GECs [93], suggesting that P. gingivalis can inhibit inflammasome components to promote its own survival. Consistent with the idea that P. gingivalis suppresses immune responses, P. gingivalis also suppresses inflammasome activation due to infection by another oral bacterium, F. nucleatum. This repression affects IL-1β and IL-18 processing and cell death, in both human and murine macrophages. F. nucleatum activates IL-1β secretion via the NLRP3 inflammasome, but when macrophages are co-infected with F. nucleatum and P. gingivalis, activation of the inflammasome and caspase-1, as well as IL-1β secretion, are inhibited by P. gingivalis [25]. Since inflammasome activation is important for controlling infection, and P. gingivalis-induced inflammasome activation is linked to induction of periodontitis, it is still unclear if deficiencies in inflammasome activation could favor P. gingivalis infection or, instead, impair periodontitis and alveolar bone loss induced by this periodontal pathogen.
Concluding remarks
Purinergic signaling can up- or down-modulate immune responses depending on the danger signals present, the purinergic receptor that is activated, and the cell type involved in the process [Fig. 1]. A large body of evidence demonstrates that purinergic signaling affects clearance or persistence of infection by P. gingivalis and others pathogens. P1 as well as P2 receptors modulate P. gingivalis infection as a function of the danger signal involved. Moreover, ecto-enzymes from the host cell or from the pathogens can modulate the course of infection by influencing the availability of nucleotides in the microenvironment. Finally, P2X7 receptor is involved in the activation of inflammasomes and its activation can control different infections. Because of the purinergic signaling can modulate different intracellular infections including those with the oral pathogen P. gingivalis, this field represents an important focus for future research regarding survival and elimination of different pathogens.
Conflicts of interest
All authors have declared that are no conflicts of interest.
Acknowledgments
This work was supported by funds from Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq - 311362/2014-1; 448152/2014-2), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Science Without Borders (CAPES - 038/2012), Programa de Núcleos de Excelência (PRONEX), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ - E-26/010.002985/2014). This study was approved and partially funded by Science Without Borders Federal Government Program to RCS and DMO and with a post-doctoral scholarship to ACM (2013/16113-9 and 2015/20954-4).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K., Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:14. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- 4.Said-Sadier N., Ojcius D.M. Alarmins, inflammasomes and immunity. Biomed J. 2012;35:437–449. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila M., Ojcius D.M., Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade W.G. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 10.Socransky S.S., Haffajee A.D. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 11.Zambon J.J., Reynolds H.S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981;32:198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffen A.L., Lyons S.R., Becker M.R., Moeschberger M.L., Leys E.J. Porphyromonas gingivalis strain variability and periodontitis. J Clin Microbiol. 1999;37:4028–4033. doi: 10.1128/jcm.37.12.4028-4033.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igboin C.O., Griffen A.L., Leys E.J. Porphyromonas gingivalis strain diversity. J Clin Microbiol. 2009;47:3073–3081. doi: 10.1128/JCM.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffen A.L., Becker M.R., Lyons S.R., Moeschberger M.L., Leys E.J. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 1998;36:3239–3242. doi: 10.1128/jcm.36.11.3239-3242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G., Liang S., Payne M.A., Hashim A., Jotwani R., Eskan M.A. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kholy K.E., Genco R.J., Van Dyke T.E. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26:315–321. doi: 10.1016/j.tem.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Haraszthy V.I., Zambon J.J., Trevisan M., Zeid M., Genco R.J. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg K., Wegner N., Yucel-Lindberg T., Venables P.J. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol. 2010;6:727–730. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- 19.de Aquino S.G., Abdollahi-Roodsaz S., Koenders M.I., van de Loo F.A., Pruijn G.J., Marijnissen R.J. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol. 2014;192:4103–4111. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- 20.Whitmore S.E., Lamont R.J. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskan M.A., Hajishengallis G., Kinane D.F. Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infect Immun. 2007;75:892–898. doi: 10.1128/IAI.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maekawa T., Krauss J.L., Abe T., Jotwani R., Triantafilou M., Triantafilou K. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morandini A.C., Chaves Souza P.P., Ramos-Junior E.S., Souza Costa C.A., Santos C.F. MyD88 or TRAM knockdown regulates interleukin (IL)-6, IL-8, and CXCL12 mRNA expression in human gingival and periodontal ligament fibroblasts. J Periodontol. 2013;84:1353–1360. doi: 10.1902/jop.2012.120496. [DOI] [PubMed] [Google Scholar]
- 24.Bao K., Belibasakis G.N., Thurnheer T., Aduse-Opoku J., Curtis M.A., Bostanci N. Role of Porphyromonas gingivalis gingipains in multi-species biofilm formation. BMC Microbiol. 2014;14:258. doi: 10.1186/s12866-014-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taxman D.J., Swanson K.V., Broglie P.M., Wen H., Holley-Guthrie E., Huang M.T. Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J Biol Chem. 2012;287:32791–32799. doi: 10.1074/jbc.M112.401737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jauregui C.E., Wang Q., Wright C.J., Takeuchi H., Uriarte S.M., Lamont R.J. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun. 2013;81:2288–2295. doi: 10.1128/IAI.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalaf H., Bengtsson T. Altered T-cell responses by the periodontal pathogen Porphyromonas gingivalis. PLoS One. 2012;7:e45192. doi: 10.1371/journal.pone.0045192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guentsch A., Hirsch C., Pfister W., Vincents B., Abrahamson M., Sroka A. Cleavage of IgG1 in gingival crevicular fluid is associated with the presence of Porphyromonas gingivalis. J Periodontal Res. 2013;48:458–465. doi: 10.1111/jre.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaddis D.E., Maynard C.L., Weaver C.T., Michalek S.M., Katz J. Role of TLR2-dependent IL-10 production in the inhibition of the initial IFN-gamma T cell response to Porphyromonas gingivalis. J Leukoc Biol. 2013;93:21–31. doi: 10.1189/jlb.0512220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moutsopoulos N.M., Kling H.M., Angelov N., Jin W., Palmer R.J., Nares S. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39:294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Guan N., Jin Y., Lin X., Gao H. Subcutaneous vaccination with Porphyromonas gingivalis ameliorates periodontitis by modulating Th17/Treg imbalance in a murine model. Int Immunopharmacol. 2015;25:65–73. doi: 10.1016/j.intimp.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnstock G., Boeynaems J.M. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegatti P., Falzoni S., Pinton P., Rizzuto R., Di V.F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Idzko M., Ferrari D., Eltzschig H.K. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 37.Hasko G., Linden J., Cronstein B., Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobson K.A., Gao Z.G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutinho-Silva R., Ojcius D.M. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 2012;14:1271–1277. doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coutinho-Silva R., Monteiro da C.C., Persechini P.M., Ojcius D.M. The role of P2 receptors in controlling infections by intracellular pathogens. Purinergic Signal. 2007;3:83–90. doi: 10.1007/s11302-006-9039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morandini A.C., Savio L.E., Coutinho-Silva R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed J. 2014;37:169–177. doi: 10.4103/2319-4170.127803. [DOI] [PubMed] [Google Scholar]
- 42.Solle M., Labasi J., Perregaux D.G., Stam E., Petrushova N., Koller B.H. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 43.Coutinho-Silva R., Persechini P.M. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- 44.Kusner D.J., Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–388. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 45.Chaves M.M., Marques-da-Silva C., Monteiro A.P., Canetti C., Coutinho-Silva R. Leukotriene B4 modulates P2X7 receptor-mediated Leishmania amazonensis elimination in murine macrophages. J Immunol. 2014;192:4765–4773. doi: 10.4049/jimmunol.1301058. [DOI] [PubMed] [Google Scholar]
- 46.Chaves S.P., Torres-Santos E.C., Marques C., Figliuolo V.R., Persechini P.M., Coutinho-Silva R. Modulation of P2X(7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009;11:842–849. doi: 10.1016/j.micinf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Correa G., Marques da S.C., de Abreu Moreira-Souza A.C., Vommaro R.C., Coutinho-Silva R. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Coutinho-Silva R., Stahl L., Raymond M.N., Jungas T., Verbeke P., Burnstock G. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–422. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 49.Kusner D.J., Barton J.A. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J Immunol. 2001;167:3308–3315. doi: 10.4049/jimmunol.167.6.3308. [DOI] [PubMed] [Google Scholar]
- 50.Lammas D.A., Stober C., Harvey C.J., Kendrick N., Panchalingam S., Kumararatne D.S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 51.Lees M.P., Fuller S.J., McLeod R., Boulter N.R., Miller C.M., Zakrzewski A.M. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thammavongsa V., Kern J.W., Missiakas D.M., Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahamed D.A., Mills J.H., Egan C.E., Denkers E.Y., Bynoe M.S. CD73-generated adenosine facilitates Toxoplasma gondii differentiation to long-lived tissue cysts in the central nervous system. Proc Natl Acad Sci USA. 2012;109:16312–16317. doi: 10.1073/pnas.1205589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spooner R., DeGuzman J., Lee K.L., Yilmaz O. Danger signal adenosine via adenosine 2a receptor stimulates growth of Porphyromonas gingivalis in primary gingival epithelial cells. Mol Oral Microbiol. 2014;29:67–78. doi: 10.1111/omi.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitkovsky M.V., Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Thiel M., Caldwell C.C., Sitkovsky M.V. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003;5:515–526. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 57.Coutinho-Silva R., Correa G., Sater A.A., Ojcius D.M. The P2X(7) receptor and intracellular pathogens: a continuing struggle. Purinergic Signal. 2009;5:197–204. doi: 10.1007/s11302-009-9130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pettengill M.A., Marques-da-Silva C., Avila M.L., d'Arc dos Santos O.S., Lam V.W., Ollawa I. Reversible inhibition of Chlamydia trachomatis infection in epithelial cells due to stimulation of P2X(4) receptors. Infect Immun. 2012;80:4232–4238. doi: 10.1128/IAI.00441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marques-da-Silva C., Chaves M.M., Chaves S.P., Figliuolo V.R., Meyer-Fernandes J.R., Corte-Real S. Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host-cell susceptibility to UTP-mediated apoptosis. Cell Microbiol. 2011;13:1410–1428. doi: 10.1111/j.1462-5822.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 60.Figliuolo V.R., Chaves S.P., Santoro G.F., Coutinho C.M., Meyer-Fernandes J.R., Rossi-Bergmann B. Periodate-oxidized ATP modulates macrophage functions during infection with Leishmania amazonensis. Cytometry A. 2014;85:588–600. doi: 10.1002/cyto.a.22449. [DOI] [PubMed] [Google Scholar]
- 61.Moreira-Souza A.C., Marinho Y., Correa G., Santoro G.F., Coutinho C.M., Vommaro R.C. Pyrimidinergic receptor activation controls Toxoplasma gondii infection in macrophages. PLoS One. 2015;10:e0133502. doi: 10.1371/journal.pone.0133502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yilmaz O., Yao L., Maeda K., Rose T.M., Lewis E.L., Duman M. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi C.H., Spooner R., DeGuzman J., Koutouzis T., Ojcius D.M., Yilmaz O. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell Microbiol. 2013;15:961–976. doi: 10.1111/cmi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attwood P.V., Wieland T. Nucleoside diphosphate kinase as protein histidine kinase. Naunyn Schmiedeb Arch Pharmacol. 2015;388:153–160. doi: 10.1007/s00210-014-1003-3. [DOI] [PubMed] [Google Scholar]
- 65.Dar H.H., Chakraborti P.K. Intermolecular phosphotransfer is crucial for efficient catalytic activity of nucleoside diphosphate kinase. Biochem J. 2010;430:539–549. doi: 10.1042/BJ20100026. [DOI] [PubMed] [Google Scholar]
- 66.Spooner R., Yilmaz O. Nucleoside-diphosphate-kinase: a pleiotropic effector in microbial colonization under interdisciplinary characterization. Microbes Infect. 2012;14:228–237. doi: 10.1016/j.micinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sundin G.W., Shankar S., Chugani S.A., Chopade B.A., Kavanaugh-Black A., Chakrabarty A.M. Nucleoside diphosphate kinase from Pseudomonas aeruginosa: characterization of the gene and its role in cellular growth and exopolysaccharide alginate synthesis. Mol Microbiol. 1996;20:965–979. doi: 10.1111/j.1365-2958.1996.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 68.Johnson L., Atanasova K.R., Bui P.Q., Lee J., Hung S.C., Yilmaz O. Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes Infect. 2015;17:369–377. doi: 10.1016/j.micinf.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y.J., Lee J.H., Lee Y., Jia J., Paek S.H., Kim H.B. Nucleoside diphosphate kinase and flagellin from Pseudomonas aeruginosa induce interleukin 1 expression via the Akt/NF-kappaB signaling pathways. Infect Immun. 2014;82:3252–3260. doi: 10.1128/IAI.02007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alam M.S., Costales M.G., Cavanaugh C., Williams K. Extracellular adenosine generation in the regulation of pro-inflammatory responses and pathogen colonization. Biomolecules. 2015;5:775–792. doi: 10.3390/biom5020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettengill M.A., Lam V.W., Ojcius D.M. The danger signal adenosine induces persistence of chlamydial infection through stimulation of A2b receptors. PLoS One. 2009;4:e8299. doi: 10.1371/journal.pone.0008299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bisaggio D.F., Peres-Sampaio C.E., Meyer-Fernandes J.R., Souto-Padron T. Ecto-ATPase activity on the surface of Trypanosoma cruzi and its possible role in the parasite-host cell interaction. Parasitol Res. 2003;91:273–282. doi: 10.1007/s00436-003-0965-8. [DOI] [PubMed] [Google Scholar]
- 73.Meyer-Fernandes J.R., Saad-Nehme J., Peres-Sampaio C.E., Belmont-Firpo R., Bisaggio D.F., Do Couto L.C. A Mg-dependent ecto-ATPase is increased in the infective stages of Trypanosoma cruzi. Parasitol Res. 2004;93:41–50. doi: 10.1007/s00436-003-1066-4. [DOI] [PubMed] [Google Scholar]
- 74.Berredo-Pinho M., Peres-Sampaio C.E., Chrispim P.P., Belmont-Firpo R., Lemos A.P., Martiny A. A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys. 2001;391:16–24. doi: 10.1006/abbi.2001.2384. [DOI] [PubMed] [Google Scholar]
- 75.Mempin R., Tran H., Chen C., Gong H., Kim H.K., Lu S. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013;13:301. doi: 10.1186/1471-2180-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding Q., Quah S.Y., Tan K.S. Secreted ATP from A. actinomycetemcomitans triggers chemokine response. Mol Oral Microbiol. 2015:1–12. doi: 10.1111/omi.12143. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Sikora A., Liu J., Brosnan C., Buell G., Chessel I., Bloom B.R. Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- 78.Park E., Na H.S., Song Y.R., Shin S.Y., Kim Y.M., Chung J. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun. 2014;82:112–123. doi: 10.1128/IAI.00862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamkanfi M., Dixit V.M. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- 80.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 82.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Mariathasan S., Weiss D.S., Newton K., McBride J., O'Rourke K., Roose-Girma M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 84.Yang D., He Y., Munoz-Planillo R., Liu Q., Nunez G. Caspase-11 requires the Pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garlanda C., Dinarello C.A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dinarello C.A. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santana P.T., Martel J., Lai H.C., Perfettini J.L., Kanellopoulos J.M., Young J.D. Is the inflammasome relevant for epithelial cell function? Microbes Infect. 2015;18(2):93–101. doi: 10.1016/j.micinf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Kayagaki N., Warming S., Lamkanfi M., Vande W.L., Louie S., Dong J. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 89.Luna-Gomes T., Santana P.T., Coutinho-Silva R. Silica-induced inflammasome activation in macrophages: role of ATP and P2X7 receptor. Immunobiology. 2015;220:1101–1106. doi: 10.1016/j.imbio.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Bostanci N., Emingil G., Saygan B., Turkoglu O., Atilla G., Curtis M.A. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 2009;157:415–422. doi: 10.1111/j.1365-2249.2009.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramos-Junior E.S., Morandini A.C., Almeida-da-Silva C.L., Franco E.J., Potempa J., Nguyen K.A. A dual role for P2X7 receptor during Porphyromonas gingivalis infection. J Dent Res. 2015;94:1233–1242. doi: 10.1177/0022034515593465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morandini A.C., Ramos-Junior E.S., Potempa J., Nguyen K.A., Oliveira A.C., Bellio M. Porphyromonas gingivalis fimbriae dampen P2X7-dependent interleukin-1beta secretion. J Innate Immun. 2014;6:831–845. doi: 10.1159/000363338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yilmaz O., Sater A.A., Yao L., Koutouzis T., Pettengill M., Ojcius D.M. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung S.C., Choi C.H., Said-Sadier N., Johnson L., Atanasova K.R., Sellami H. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]