Abstract
Pancreatic cancer is a common gastrointestinal malignancy, and is often associated with a poor prognosis. Challenges to effective screening for pancreatic cancer include low disease prevalence and high cost of screening modalities such as endoscopic ultrasound and cross-sectional imaging. Further, most patients are asymptomatic during the early course of disease, which often leads to delay in diagnosis. Treatment options include surgery, chemotherapy, and palliative care.
Pancreatic cancer remains a devastating diagnosis and the management of patients is facilitated by the coordinated efforts of specialists in gastroenterology, radiology, oncology, and oncologic surgery.
Introduction
Pancreatic cancer is the second most common gastrointestinal malignancy in the United States. Approximately 53,000 people will be diagnosed with pancreatic cancer this year.1
Interestingly, it is more common in African-Americans, slightly more common in men, and is usually a disease of older adults.1 Despite its relative low incidence compared to other more common malignancies (prostate, lung, colorectal, etc), pancreatic cancer represents the fourth leading cause of death in men and women. Cigarette smoking appears to be one of the strongest risk factors, and is likely associated with as many as one quarter of all pancreatic tumors.2 Diabetes mellitus is also suspected to play a role in the development of pancreatic cancer, though the precise mechanism by which this occurs is not clearly established.3 Patients with type 2 diabetes often have years or decades of insulin resistance with compensatory hyperinsulinemia. The mitogenic properties of insulin may explain the association between type 2 diabetes and many types of malignancy. Other known risk factors for pancreatic cancer include alcohol use, obesity, and various genetic syndromes, although these account for less than 10% of all pancreatic cancer. Concomitant risk factors are felt to be synergistic.2
In general, the prognosis for pancreatic cancer is poor. The overall one- and five-year mortality rates are 24% and 6%, respectively.1 Approximately 80% of patients have regional spread or metastatic disease at the time of diagnosis. This highlights the need for improved screening modalities, early detection, accurate pre-operative staging, and improved treatment options.
Pancreatic Adenocarcinoma
The term “pancreatic cancer” is typically used to refer to pancreatic adenocarcinoma. There are other types of pancreatic cancer, but this review will focus on discussion of pancreatic adenocarcinoma. More than 95% of malignant neoplasms of the pancreas arise from the exocrine portions of the gland (ductal and acinar cells), and demonstrate features consistent with adenocarcinoma.4
Patients with pancreatic cancer who have two first-degree relatives with a history of pancreatic cancer meet criteria for familial pancreatic cancer.5 Though hereditary causes account for only approximately one tenth of pancreatic cancers, it is important to identify those patients with higher risk due to genetic mutations early in order to determine appropriate screening and surveillance strategies. Patients with BRCA2 gene mutations account for the highest proportion of known causes of inherited pancreatic cancer.6 Another common etiology of hereditary pancreatitis is an autosomal dominant mutation of the PRSS1 gene on chromosome 7.7 The cumulative risk for development of pancreatic cancer in these patients by age 75 is greater than 50%.7 Other genetic syndromes associated with elevated risk of pancreatic cancer include Peutz-Jeghers syndrome (STK11 gene mutation), familial atypical mole-malignant melanoma (FAMMM) syndrome (germline mutation in the P16 gene), hereditary nonpolyposis colorectal cancer (mutations in MLH1, MSH2, MSH6, PMS2, or the EPCAM gene), and mutations in PALB2.8
Challenges to effective screening for pancreatic cancer include low disease prevalence and cost of screening modalities such as endoscopic ultrasound and cross-sectional imaging. Current medical practice relies on family history as the primary assessment of risk for pancreatic cancer.
Patients who carry a known genetic mutation that increases risk for pancreatic cancer and have a family history (as above) that meets criteria for pancreatic cancer screening should be enrolled in a screening program. This includes endoscopic ultrasound and/or magnetic resonance cholangiopancreatography (MRCP), though there is no consensus regarding initiation of screening. Those who meet criteria should be screened every 6 months with MRI or EUS on an alternating schedule. Many practitioners start screening at age 40–50 in accordance with recommendations by the CAPS consortium.5 However, the American Gastroenterological Association (AGA) recommends screening patients with hereditary pancreatitis at age 35, and in other familial pancreatic cancer syndromes, 10 years prior to the age of the index case.9
Autopsy data show that approximately 60–70% of tumors are located in the head of the pancreas, 5–10% in the body, and 10–15% in the tail.10 At diagnosis, the average size of pancreatic cancers located in the head of the pancreas is approximately 3 cm, while those in the body or tail are approximately 6 cm. This is explained by the earlier development of signs and symptoms in proximal tumors related to obstruction of the common bile duct and pancreatic duct. Distal cancers in the body and tail of the pancreas have a higher propensity for extra-pancreatic extension into retroperitoneal tissues, and vasculature such as the portal vein and superior mesenteric artery and vein. Other sites of invasion with extra-pancreatic extension include the spleen, stomach, colon (transverse colon or splenic flexure), and the left adrenal gland. In more advanced disease, common sites of distant metastases include lymph nodes, liver, and peritoneum. Less common sites of metastases are lung and bone.2
Common Presentation
Unfortunately, most patients do not note symptoms during the early course of their disease, which often delays diagnosis. Jaundice is one of the main presenting symptoms, especially with pancreatic head tumors. Some patients present with pancreatic exocrine insufficiency, which can manifest as a wide spectrum of symptoms, including steatorrhea (fatty, frothy, loose, greasy, foulsmelling stools), malabsorption, weight loss, abdominal discomfort, and abdominal bloating. Yet others can present with dull, nonspecific pain, which usually occurs as a result of tumor invasion of celiac or superior mesenteric arterial plexus. Other common clinical manifestations of pancreatic cancer include nausea, anorexia, weight loss, and new-onset diabetes mellitus.
Diagnosis
Routine lab tests are usually non-specific, though elevated liver tests may suggest biliary obstruction. Serum amylase and lipase levels are occasionally elevated.
Serum markers have some utility in the evaluation of pancreatic cancer, though they are not routinely used for screening purposes. Cancer-associated antigen 19-9 (CA 19) is the most well-known and likely the most useful. It serves as an adjunct in diagnosis, and in monitoring response to treatment.11, 12 However, it should be noted that false-positive results (high values of CA 19-9 in the absence of malignancy) can be seen with cholestasis related to non-malignant obstruction (ie choledocholithiasis, cholangitis, and chronic pancreatitis.13
Radiologic Imaging
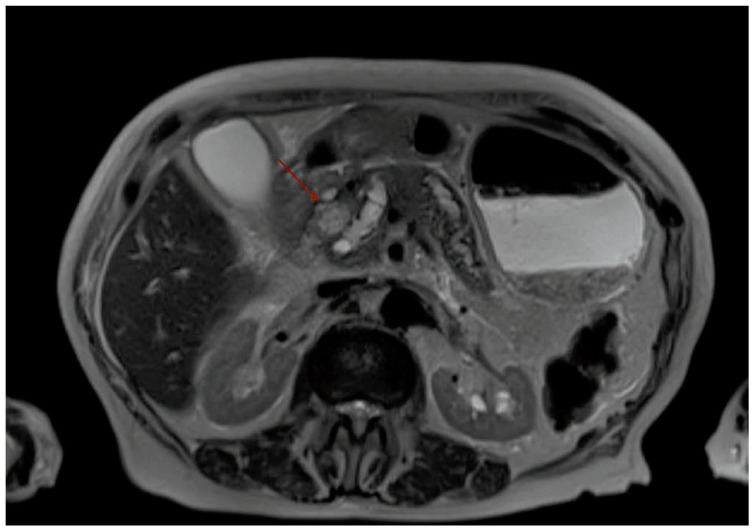
Though many patients presenting with jaundice often undergo trans-abdominal ultrasound as part of the initial evaluation for jaundice, MRI of the pancreas (with MRCP) or CT of the pancreas (pancreatic protocol CT) are the preferred radiologic modalities to identify and help stage pancreatic cancer, with MRI being preferred to CT (Figure 1).
Figure 1.
MRI abdomen showing pancreatic head mass (red arrow). Adjacent to the mass is a dilated pancreatic duct.
CT is associated with a high degree of accuracy in defining unresectable pancreatic cancer, for which the criteria is met with any one of the following: distant metastases (liver, peritoneum, etc), and certain arterial/ vascular involvement (celiac axis or superior mesenteric artery, or occlusion of the portal vein or superior mesenteric vein) which may be institution-specific.14 Advantages of magnetic resonance imaging (with MRCP) include lack of radiation and iodine-free contrast. Cost and limited availability are the main limitations to MRI/ MRCP.15
Positron emission tomography (PET) combined with CT is not useful as a primary diagnostic imaging study for pancreatic cancer due to lack of anatomic detail. However, PET/CT may be useful in assessing tumor recurrence after resection, or after neoadjuvant chemotherapy, and can be helpful in identifying sites of distant metastases.
Endoscopic Imaging
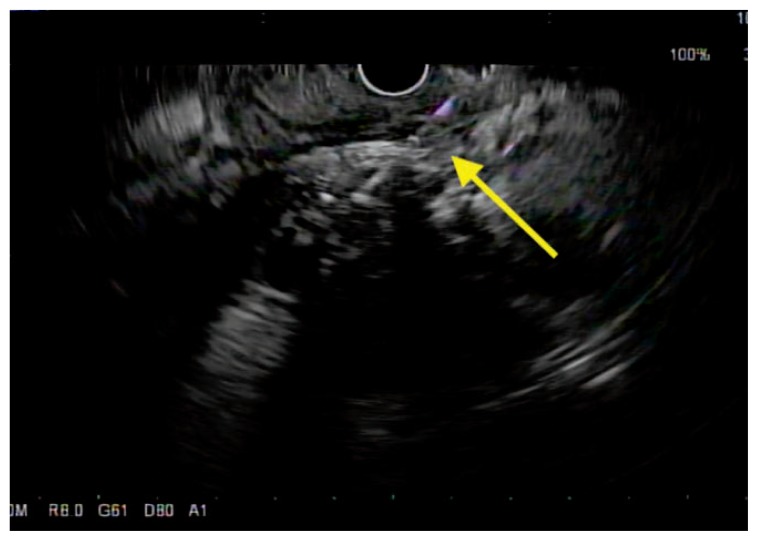
Endoscopic ultrasound (EUS) is felt to be the most accurate test for the diagnosis of pancreatic cancer.16 It has been shown to have a higher sensitivity and specificity for detecting pancreatic masses than CT, although there have been no prospective head-to-head studies comparing the two modalities.16 The ability to add fine needle aspiration (FNA) cytology to EUS increases the specificity of detecting pancreatic cancer, compared to imaging studies (Figure 2).
Figure 2.
EUS-guided fine-needle aspiration with a 22-gauge needle (yellow arrow points to the needle) of the same pancreatic head tumor via transduodenal approach. The tip of the needle is seen inside the mass.
The caveat, however, is that EUS has a high level of operator dependence, and requires significant experience. Therefore, this study is best when performed at a high volume center.17
Endoscopic retrograde cholangiopancreatography (ERCP) allows for radiographic visualization of the biliary and pancreatic ducts via X-ray. However, ERCP should be reserved for therapeutic treatment of ductal obstruction and not used as the sole tool to evaluate for pancreatic cancer. ERCP does allow for tissue sampling as well as interventions such as biliary or pancreatic stenting, if necessary.15, 18 A “double duct” sign, which represents simultaneous dilatation of the common bile duct and pancreatic duct due to obstruction, is a worrisome finding seen on ERCP (and on imaging studies such as CT or MRI) as it can represent obstruction due to pancreatic cancer. When this “double duct” sign is seen, it is of paramount importance to evaluate for pancreatic cancer before being complacent with other diagnostic possibilities.
Staging of Pancreatic Cancer
The American Joint Committee on Cancer (AJCC) staging system was recently revised in July 2017, and is shown in Table 1.19
Table 1.
TNM Pancreatic Cancer Staging (American Joint Committee on Cancer.)
| Stage | Definition |
|---|---|
| Primary Tumor (T) | |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to pancreas, ≤ 2cm |
| T2 | Tumor limited to pancreas, ≥ 2cm |
| T3 | Extension into peri-pancreatic tissues (excluding arteries) |
| T4 | Tumor involves celiac axis or superior mesenteric artery |
| Regional lymph nodes (N) | |
| Nx | Regional lymph nodes not assessed |
| N0 | No metastatic regional lymph nodes |
| N1 | Metastatic regional lymph nodes |
| Distant metastases (M) | |
| M0 | No distant metastatic disease |
| M1 | Distant metastatic disease |
American Joint Committee on Cancer (AJCC) TNM staging system, September 6, 2013. American Cancer Society. Available at http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-staging. April 5, 2016; Accessed November 28, 2017.
Once the diagnosis of pancreatic cancer has been made, staging can include CT or MRI of the chest, abdomen, and pelvis with IV contrast. EUS is also very important not only for tissue acquisition for diagnosis, but for staging as well.
With staging, patients are often placed into one of four distinct groups, which determines treatment plan. Patients in the first group are those with stage 1 (clearly resectable) disease, and should be referred as soon as possible to a surgeon, to determine if they are candidates for resection based on other medical comorbidities. The second group includes those with “borderline resectable” disease; these patients are more likely to derive benefit from neoadjuvant chemoradiation. Third, we include those with locally advanced disease, without metastases, but are still unresectable. These patients may be candidates for neoadjuvant chemoradiation, and potentially surgery after successful downstaging. The fourth and final group is comprised of patients with metastatic disease, who do not benefit from surgical resection. Treatment for this group of patients includes chemotherapy and palliative care.
Approximately 1 in 4 patients with CT-demonstrated localized pancreatic cancer are found to have metastatic disease at laparoscopy.20, 21 As a result, staging diagnostic laparoscopy is recommended (to identify small lesions with visual inspection that may be missed on imaging studies) in most patients prior to surgical exploration.6, 22
Treatment Options
Surgery
Surgery is the only treatment modality for pancreatic cancer that is potentially curative.23 Unfortunately, as mentioned above, only 15–20% of patients are candidates for pancreatectomy, due to the high proportion of advanced disease at presentation. An absolute contraindication to surgery is extra-pancreatic disease. Vascular involvement is a relative contraindication – in some cases, vascular resection and reconstruction are performed with good success.
Routine pre-operative ERCP and biliary duct stenting to relieve jaundice has not been shown to improve outcomes, and is not currently recommended in patients with resectable tumors.24 Patients for whom pre-operative ERCP may be appropriate include those with an expected delay in surgery, and those receiving neoadjuvant therapy. In patients with comorbidities that preclude surgery, ERCP with biliary stenting (with expandable metal stents) is an excellent palliative option.
The most common surgical procedure for pancreatic cancer localized to the head or uncinate region or both is pancreaticoduodenectomy, commonly referred to as the Whipple procedure. This involves en bloc removal of the head of the pancreas and duodenum, distal common bile duct, and proximal jejunum. A pancreaticojejunal anastomosis is also established. The mortality rate with the Whipple procedure has improved significantly and is now less than 3% when performed at a center of excellence for pancreatic surgery.25 Patients with tumors localized to the body and or tail who are surgical candidates typically undergo distal pancreatectomy and splenectomy.
Predictors of better outcomes after surgery include tumor size < 3 cm, lack of lymph node metastases, negative resection margins, well-differentiated tumors, and intra-operative blood loss of < 750 mL.26
Still, prognosis for pancreatic cancer remains poor, even after potentially curative surgery. The five-year survival rate after resection remains approximately 25%, similar to that of 30 years ago.1
Adjuvant and Neoadjuvant Therapy
Neoadjuvant therapy is defined as treatment (chemotherapy and/or radiation) administered prior to surgery. Potential benefits include early therapy to decrease the likelihood of distant disease and tumor downstaging to optimize surgical resection. One concern with neoadjuvant therapy is the possibility that during therapy, the cancer may progress from potentially resectable to unresectable terminal cancer. It should also be noted that neoadjuvant therapy protocols tend to be institution-specific.
Adjuvant therapy is defined as treatment administered after surgery, with the goal of preventing recurrence. Recurrence of pancreatic cancer typically manifests as distant metastatic disease, and isolated local tumor bed recurrence only occurs 10–15% of the time. Though current guidelines from the National Cancer Center Network recommend adjuvant therapy, its value has been questioned.12
Chemotherapy for metastatic disease is not curative. Further, the potential for toxic side effects is significant. Current recommendations for treatment of patients with metastatic disease include combination therapy with FOLFIRINOX (folinic acid, fluorouracil, irinotecan, oxaliplatin) or gemcitabine plus nab-paclitaxel or gemcitabine alone (based on the patient’s performance status).12 Response to treatment is assessed with serial imaging studies, serum markers (such as CA19-9), and changes in tumor-related symptoms.
Locally advanced pancreatic cancer may be treated with stereotactic body radiation therapy (SBRT), a form of radiation therapy that involves high-dose targeted radiation over a short time course – usually three to five days.12 This usually requires EUS-guided placement of fiducial markers at the site of the tumor. Common treatment-related toxicities include fatigue, nausea, and less common risks are gastrointestinal bleeding and perforation.
A new ablation therapy for locally advanced pancreatic cancer is irreversible electroporation (IRE). It achieves soft tissue ablation with short (70 to 90 microseconds), high voltage (3,000 volts) pulses causing cell membrane perforation, electrolyte instability with disruption of cellular homeostasis, and apoptosis.27 It is unique in that it does not cause coagulative necrosis, such as in radiation therapy, but immune-mediated cell death. A major advantage is that surrounding structures and vasculature are not injured.27 Though not a standard-of-care practice at this time, with further research, IRE may serve as a useful adjunct in the optimal treatment of pancreatic cancer.
Palliative Care
Patients with unresectable disease often have significant jaundice and duodenal obstruction. These patients benefit greatly from either endoscopic biliary or duodenal stenting. Endoscopy is associated with a high success rate of palliation, as well as low procedurerelated morbidity and mortality.
Pain in patients with unresectable pancreatic cancer can be difficult to manage. These patients should be referred for EUS-guided celiac plexus neurolysis given the high-success rate of 70–80% reduction in pain post-procedure.28 Patients who are deemed nontreatment candidates or who do not wish to pursue treatment should be referred to hospice. Finally, several nonprofit organizations are useful resources for patients with pancreatic cancer as well as for their families. One such organization is PANCAN, the Pancreatic Cancer Action Network.
Summary
Pancreatic cancer is the second most common gastrointestinal malignancy in the United States, and carries a poor prognosis, with overall one and five-year mortality rates of 24% and 6%, respectively.
Risk factors include older age, cigarette smoking, type 2 diabetes mellitus, and various genetic syndromes.
Endoscopic ultrasound (EUS) is felt to be the most accurate test for the diagnosis of pancreatic cancer. It has been shown to have a higher sensitivity and specificity for detecting pancreatic masses than CT.
Patients with stage 1 disease should be referred promptly for evaluation for surgical resection, as surgery is the only treatment modality for pancreatic cancer that is potentially curative
Surgery, chemotherapy, radiation therapy, and other modalities of treatment are best decided after discussions with the patient, at a high volume referral center with a multi-disciplinary approach.
Patients with advanced disease should be referred for palliative chemotherapy and/or hospice.
Biography
Ashley A. Vareedayah, MD, (top left), is a Fellow, and Samer Alkaade, MD, (top right), and Jason R. Taylor, MD, (bottom), are Associate Professors of Internal Medicine in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, St. Louis, Mo.
Contact: Jason.Taylor@health.slu.edu



Footnotes
Disclosure
None reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson SC, Permert J, Håkansson N, Näslund I, Bergkvist L, Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93:1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40:612–623. doi: 10.1016/j.humpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 7.Rebours V, Lévy P, Ruszniewski P. An overview of hereditary pancreatitis. Dig Liver Dis. 2012;44:8–15. doi: 10.1016/j.dld.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:404–412. doi: 10.1158/1055-9965.EPI-16-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association Gastroenterology. 1999;117:1464–1484. doi: 10.1016/s0016-5085(99)70298-2. [DOI] [PubMed] [Google Scholar]
- 10.Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/Management. Tenth edition ed. Philadelphia, PA: Saunders/Elsevier; 2016. [Google Scholar]
- 11.Berger AC, Garcia M, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 13.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20:10740–10751. doi: 10.3748/wjg.v20.i31.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 16.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki R, Lee JH, Krishna SG, Ramireddy S, Qiao W, Weston B, et al. Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointestin Liver Dis. 2013;22:183–187. [PubMed] [Google Scholar]
- 18.Gemmel C, Eickhoff A, Helmstädter L, Riemann JF. Pancreatic cancer screening: state of the art. Expert Rev Gastroenterol Hepatol. 2009;3:89–96. doi: 10.1586/17474124.3.1.89. [DOI] [PubMed] [Google Scholar]
- 19.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2017 doi: 10.1245/s10434-017-6025-x. [DOI] [PubMed] [Google Scholar]
- 20.Garcea G, Cairns V, Berry DP, Neal CP, Metcalfe MS, Dennison AR. Improving the diagnostic yield from staging laparoscopy for periampullary malignancies: the value of preoperative inflammatory markers and radiological tumor size. Pancreas. 2012;41:233–237. doi: 10.1097/MPA.0b013e31822432ee. [DOI] [PubMed] [Google Scholar]
- 21.Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 22.Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era? J Am Coll Surg. 2009;208:87–95. doi: 10.1016/j.jamcollsurg.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. Am J Gastroenterol. 2007;102:1377–1382. doi: 10.1111/j.1572-0241.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 24.van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, Wargo JA, Ferrone CR, Thayer SP, et al. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery. 2012;152:S56–63. doi: 10.1016/j.surg.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 27.Martin RC, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20(Suppl 3):S443–449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda I, Wang HP. Endoscopic ultrasound-guided celiac plexus block and neurolysis. Dig Endosc. 2017;29:455–462. doi: 10.1111/den.12824. [DOI] [PubMed] [Google Scholar]