Abstract
Peptic ulcer disease (PUD) is a common condition that both primary care providers and gastroenterologists encounter. Symptoms of peptic ulcer disease are variable and may include abdominal pain, nausea, vomiting, weight loss and bleeding or perforation with complicated disease. Identifying the risk factors and mechanisms that lead to the development of PUD helps to understand the approach behind diagnostic and treatment strategies.
Peptic ulcers are frequently encountered in the primary care setting and understanding associated risk factors is key to disease prevention and management.
Definition and Epidemiology
Peptic ulcers are acid-induced lesions found in the stomach and duodenum characterized by denuded mucosa with the defect extending into the submucosa or muscularis propria.1 Lesions that do not reach this depth are called erosions (Figure 1). In the United States, the prevalence of self-reported physician-diagnosed peptic ulcer disease was 10% in 1990, and the approximate incidence is about 500,000 new cases per a year.2, 3 Overall, however, the risk of mortality and need for hospitalizations due to PUD has been decreasing worldwide. This is most likely secondary to a decline in Helicobacter pylori (H. pylori) infections due to treatment and improved hygiene.4, 5 Increased use of prescription and over-the-counter acid-suppressing medications and greater caution with non-steroidal anti-inflammatory drugs (NSAIDs) may account partially for this trend as well.5, 6
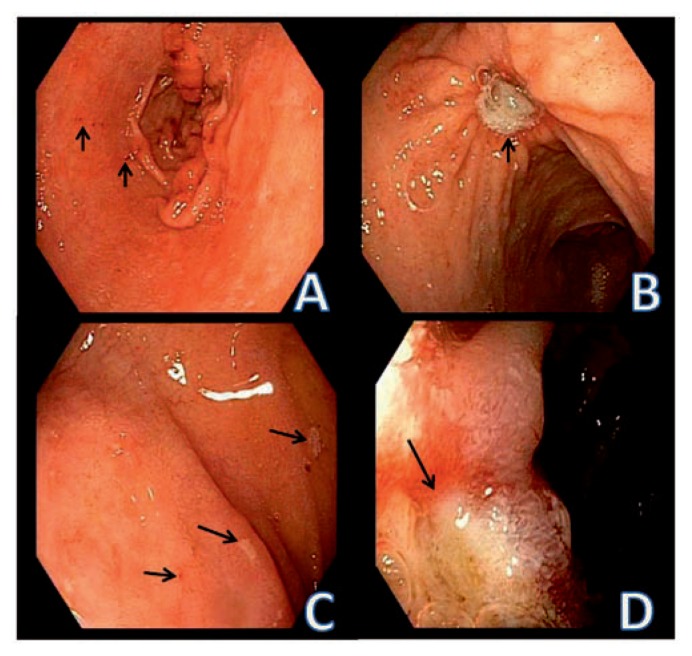
Figure 1.
Peptic erosions and ulcers in the stomach and duodenum. A. small erosions in the gastric antrum. Mucosal breaks with focal hemorrhage are identified by the arrows. B. Benign peptic ulcer in the body of the stomach (arrow). C. Duodenal erosions identified by focal areas of adherent exudate (arrows). D. Duodenal ulcer. The mucosal defect has depth and the margin is identified by the arrow. The surrounding mucosa is edematous.
Etiology and Pathophysiology
The main risk factors for PUD are H. pylori and NSAID use, however not all individuals infected with H. pylori or taking NSAIDs develop PUD.1, 7 Almost half of the world’s population is colonized by H. pylori. 8 The organism is usually acquired in childhood and persists until treated. Risk factors for acquiring the infection include a lower socioeconomic status and unsanitary conditions or crowding. The prevalence of H. pylori is higher in developing countries and more common in certain ethnicities. In the last five years in the United States, there has been a decline in the prevalence of H.pylori in all ages. Yet, there are differences based on ethnicity with rates of infection that are over 60% in Mexican Americans versus 30% in the non-Hispanic white population.9
H. pylori causes an inflammatory response with neutrophils, lymphocytes, plasma cells, and macrophages within the mucosal layer and causes epithelial cell degeneration and injury. Gastritis is usually more severe in the antrum, with little or no inflammation in the corpus. All patients found to have peptic ulcers should be tested for H. pylori. There are both invasive and noninvasive methods for testing that are summarized in Table 1. Of all the noninvasive methods, the urea breath test and stool antigen tests are the most feasible and are more accurate than serologic testing.10 Although invasive, endoscopy allows for biopsy and includes a variety of methods for testing such as histology, culture, or rapid urease test. All methods other than serology are affected by use of acid-suppressing medications such as proton pump inhibitors and may produce false negatives.
Table 1.
Diagnostic Tests for H. pylori
| Test | Sensitivity | Specificity | Advantages | Disadvantages |
|---|---|---|---|---|
| Serology | 85–92% | 79–83% | Only test not influenced by PPI or antibiotic use | Cannot confirm cure |
| Urea Breath Test | 95% | 96% | Confirms cure | Accuracy affected by PPI and antibiotic use |
| Fecal antigen testing | 95% | 94% | Confirms cure | Accuracy affected by PPI and antibiotic use |
| Rapid urease test | 98% | 99% | Inexpensive, confirms cure | Requires endoscopy, less accurate after treatment or after PPI use |
| Histology | >95% | >95% | Permits visualization, confirms cure | Requires endoscopy, affected by PPI and antibiotic use |
| Culture | 70–90% | 100% | Allows determination of antimicrobial sensitivity, confirms cure | Requires endoscopy, result takes several days, affected by PPI and antibiotic use |
PPI, proton pump inhibitor
NSAIDs are widely used for a variety of conditions to help reduce pain and inflammation; however, many users develop gastrointestinal side effects. NSAIDs account for over 90% of all ulcers and approximately 25% of NSAID users will develop peptic ulcer disease.11 Aspirin users are also twice as likely to develop peptic ulcers as the general population.12, 13 Others develop a milder degree of topical injury, which is seen as mucosal hemorrhages and erosions and are referred to as NSAID gastropathy. These multiple small erosions are usually located in the antrum but may also be seen in the body.
NSAIDs induce mucosal injury by several mechanisms. The majority of NSAIDs are weak acids and become protonated and cross lipid membranes to enter epithelial cells when exposed to acidic gastric juice (pH 2). In the epithelial cell (pH 7.4), the NSAID ionizes and releases its H+ and cannot cross the lipid membrane and thus becomes trapped. This leads to uncoupling of oxidative phosphorylation, leading to decreased mitochondrial energy production, reduced cellular integrity, and increased cellular permeability. This can result in a topical injury and rapid epithelial cell death, superficial hemorrhage, and erosions.14
The other major mechanism by which NSAIDs cause mucosal injury is by inhibition of cyclooxygenase-1 (COX-1), which is responsible for prostaglandin synthesis. Prostaglandins increase secretion of bicarbonate and mucous, increase mucosal blood flow, and inhibit cell proliferation to maintain the mucosal barrier.5 Aspirin acetylates cyclooxygenase and irreversibly inhibits the enzyme, whereas NSAIDs inhibit the enzyme reversibly in a concentration-dependent manner. Among these pathophysiologic responses, reduction in blood flow is thought to be the primary mechanism responsible for NSAIDs-induced injury.14
Two isoforms of COX exist: COX-1 is primarily responsible for prostaglandin synthesis in the GI tract, whereas COX-2 is responsible for prostaglandin synthesis at sites of inflammation. NSAIDs such as ibuprofen, naproxen, aspirin, and indomethacin inhibit both COX-1 and COX-2 and are classified as non-selective. COX-2 specific NSAIDs such as celocoxib or rofecoxib inhibit COX-2 without inhibiting COX-1, making them potentially safer in the GI tract. Endoscopic studies of patients taking COX-2 inhibitors have demonstrated lower incidences of ulceration of approximately 3–5% when compared to traditional NSAIDs which have a 20–40% incidence. However, COX-2 selective NSAIDs have been shown to increase risk of cardiac disease and many have been taken off the market.
Those who are at highest risk for NSAID-induced ulcers are patients with a history of peptic ulcers or hemorrhage, those concomitantly using steroids or anticoagulants, anyone over the age of 65 and those taking high doses or combinations of more than one NSAID (including low dose aspirin). If these patients need multiple agents, they should be started on treatment to prevent ulcers. Furthermore, using medications such as selective serotonin-reuptake inhibitors, corticosteroids, aldosterone antagonists, or anticoagulants increases the risk of bleeding.15 Older age and a greater number of comorbidities also affect the clinical course of patients with H. pylori and NSAIDs.16, 17 Interaction between H. pylori and NSAIDs is controversial but current American College of Gastroenterology guidelines recommend testing and treating for H. pylori if an individual is to start long-term NSAIDs and testing could be considered in those taking long term low dose aspirin as well.5, 18
About a fifth of PUD cases are not related to H. pylori, NSAIDs or aspirin, but the accuracy of this value has been challenged due to false negative H. pylori testing or accidental (or underreported) NSAID ingestion.19,20 This idiopathic PUD may be due to an imbalance between factors that contribute to mucosal integrity and aggressive insults, including a hypersecretory status. Other etiologies for PUD include ischemia causing stress ulcers, medications (steroids, alendronate, potassium chloride, and chemotherapeutic agents), viral infections (CMV, HSV), gastric bypass surgery, metabolic disturbances, radiotherapy, histamine, eosinophilic infiltration, and basophilia.5, 21
Diagnosis
Diagnosis begins with clinical suspicion when patients present with symptoms such as epigastric abdominal pain, burning, post-prandial fullness, or early satiety.1 Classically, patients with duodenal ulcers complain of worsening abdominal pain on an empty stomach and describe hunger or abdominal pain two to three hours after meals or at night. In contrast, patients with gastric ulcers report nausea, vomiting, weight loss and post-prandial abdominal pain. Elderly patients are often minimally symptomatic and some patients with untreated PUD may have intermittent symptoms due to spontaneous healing and then relapse due to persistence of risk factors, such as continued NSAIDs use or H. pylori infection.5
If clinical symptoms suggest possible peptic ulcer disease and no alarm symptoms are noted, empiric treatment with anti-secretory therapy can be started. Furthermore, since H. pylori is a common cause of PUD, a test and treat strategy with a non-invasive test for H. pylori (stool antigen or urea breath test) is recommended in patients less than 55 years of age without alarm features, in geographic regions were gastric cancer is uncommon and the prevalence of H. pylori is greater than 20%.22 In older patients and those with alarm symptoms, endoscopy is recommended to establish a diagnosis. Alarm symptoms include GI bleeding, weight loss, early satiety, dysphagia or odynophagia, family history of upper GI malignancy, iron deficiency anemia or new upper GI symptoms in patients older than 55.23 Esophagogastroduodenoscopy (EGD) or upper endoscopy is the gold standard for the diagnosis of PUD. It is can be used to detect H. pylori with gastric biopsies and can also rule out malignancy.
Treatment
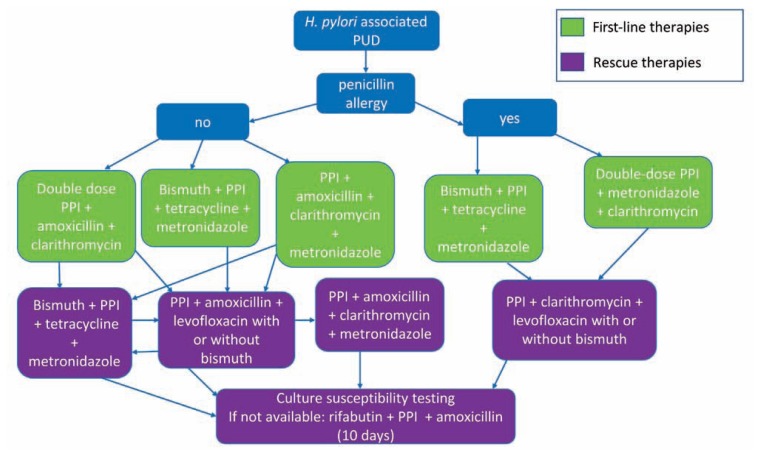
Treatment is usually directed at identifying the factors that lead to PUD. For H. pylori-associated PUD, eradication alone will lead to ulcer healing and prevent further mucosal injury. However, due to rising antibiotic resistance in H. pylori, treatment has become more difficult (Figure 2). First line therapy for H. pylori eradication includes a proton pump inhibitor (PPI), clarithromycin and amoxicillin or metronidazole (for penicillin-allergic patients) for seven to 14 days.24 PPIs work synergistically with antibiotics to eradicate H. pylori.25 Due to increasing antibiotic resistance, the efficacy of triple therapy has fallen below 70% in many countries. 24 As susceptibility testing is often not available in clinical practice, clarithromycin-based regimens should be avoided when local clarithromycin resistance rates are greater than 15%.24 Clarithromycin resistance rates are high (>20%) across the United States.26 When using clarithromycin-based triple therapy, eradication rates can be increased with use of high dose PPI and by extending the duration of treatment from seven to 14 days.5 For areas with high clarithromycin resistance, bismuth-containing quadruple therapy with a PPI, bismuth, tetracycline and a nitroimidazole (metronidazole or tinidazole) for 14 days or PPI, clarithromycin, amoxicillin, and a nitroimidazole for 14 days is the preferred as first line treatment.18 There have been issues with the cost and availability of tetracycline and the data have been mixed on whether doxycycline can be substituted. The regimens discussed above yield eradication rates greater than 90%.5
Figure 2.
Multiple treatment regimens for H. pylori can be considered and the standard treatment duration is 14 days. The doses of the drugs used are: proton pump inhibitor (PPI, standard or double dose), clarithromycin 500 mg BID, amoxicillin 1 gm BID, bismuth subsalicylate 300 mg QID, metronidazole 500 mg TID, tetracycline 500 mg QID, levofloxacin 500 mg QD, rifabutin 300 mg QD.
All patients treated for H. pylori, should be tested to confirm eradication at least four weeks after completing therapy. Second line therapy should be prescribed if a first line regimen fails (Figure 2) and should not include repeating metronidazole or clarithromycin.18 Furthermore, susceptibility testing should be considered after two treatment failures or after one treatment failure when endoscopy is performed (for other reasons such as follow up of gastric ulcer). If culture for H. pylori is not available to evaluate for resistance or after three recommended treatments have failed, rifabutin-based triple therapy (PPI, rifabutin, and amoxicillin) for 10 days can be considered. If symptoms do not improve after H. pylori eradication, endoscopy should be pursued if not already performed.
In NSAID- or aspirin-associated PUD, ulcers heal more than 85% of the time with 6–8 weeks of PPI therapy if the offending agent is discontinued. Ulcer healing is still attainable but delayed with continued NSAIDs use. Anti-secretory therapy can be started for prevention of PUD in patients on aspirin. Although PPIs, H2 blockers, sucralfate, and misoprostol can all be considered to treat NSAID-associated PUD, PPIs are far more effective than other agents.25 Sucralfate is effective for treating NSAID-associated duodenal ulcers but not for the treatment or prevention of NSAID-associated gastric ulcers. In addition to its poor efficacy, misoprostol is often limited by its side effect profile, which includes gastrointestinal upset and abortifacient reactions. In cases of refractory ulcers, both drug compliance with PPI use and inadvertent use of NSAIDs should be explored.27 All gastric ulcers require repeat endoscopy in six to eight weeks to evaluate for healing. If a gastric ulcer is not healed, biopsies must be taken at time of repeat endoscopy to rule out gastric cancer. For refractory ulcers, doubling the PPI dose can be recommended for six to eight weeks, although the evidence supporting this is weak. Furthermore, evaluating for false-negative H. pylori testing (via serology), malignancies, infections, Crohn’s disease, vasculitis, upper abdominal radiotherapy, cocaine use, and Zollinger-Ellison syndrome should be considered for ulcers that have been treated appropriately and have not healed.5
Complications
The complications of PUD include unabated symptoms, bleeding, perforation, penetration, gastric outlet obstruction, and gastric malignancy (adenocarcinoma and MALT lymphoma). Bleeding is the most common complication and occurs in about 15–20% of patients. PUD accounts for a large proportion (about 40–60%) of acute upper GI bleeding.28 Upper GI bleeding is an emergency which requires patients to be evaluated and triaged immediately. Alerting a GI consultant early in the evaluation of a bleeding patient is helpful to coordinate care for critically ill patients. The Glasgow-Blatchford score and Rockall scores have been used for risk stratification.29 Appropriate resuscitation with IV fluids and blood products to maintain a goal hemoglobin above 7 is vital in management.30
Intravenous PPI therapy should be started on all patients considered to have an upper GI bleed immediately on presentation, as IV PPIs reduce the risk of finding high-risk stigmata during endoscopy and in accordance, re-bleeding risk and need for surgery as well.25 They work by increasing intra-gastric pH, encouraging platelet aggregation and providing clot stability. Prokinetic agents such as erythromycin or metoclopramide can also be considered to improve endoscopic visualization and diagnostic yield.31 Early endoscopy which is ideally performed within 24 hours provides both prognostic and therapeutic results. After endoscopy, it is recommended to either start or continue PPI (IV if high-risk stigmata of bleeding are found). Studies have shown that IV PPI given twice daily is as efficacious as a continuous PPI drip32, 33 with significant cost savings. Recurrent bleeding is associated with high mortality and may require repeat endoscopy, angiographic embolization by interventional radiology, or surgery.
If patients have coagulopathy due to warfarin, reversal agents such as vitamin K, fresh frozen plasma (FFP), prothrombin complex concentrates (PCC) or recombinant factor VIIa should be considered as a part of management. These agents need to be used judiciously however, as they can have adverse effects. For instance, high doses of vitamin K can cause prolongation of the time to achieve therapeutic warfarin levels after bleeding stops and therapeutic anticoagulation needs to be restarted (e.g., in the setting of mechanical heart valves). FFP has a risk of causing volume overload and recombinant factor VIIa has an increased risk of thrombosis and is expensive. Novel oral anticoagulants (NOACs) are being used more commonly and these agents cannot be reversed with vitamin K. Compared to warfarin, the NOACs rivaroxaban and dabigatran increase the risk of GI bleeding although apixaban does not seem to have an increased risk of GI bleeding.34 Activated charcoal can be given within four hours of ingestion to treat a NOAC overdose. Hemodialysis and idarucizumab can be used for life-threatening bleeding associated with dabigatran.5 Anticoagulant and antiplatelet agents can be restarted once hemostasis has been achieved with the timing dependent on the urgency to restore anticoagulation. Patients on dual antiplatelet therapy after placement of drug-eluting stents should avoid stopping both agents, as they are at high risk for in-stent thrombosis. For patients on aspirin for cardiovascular disease, aspirin should be resumed soon (between one to three days, within seven days) after hemostasis is achieved.31 For patients with a high risk of thrombosis (such as mechanical mitral valve) bridging with low molecular weight heparin is recommended while warfarin levels are sub-therapeutic.
Perforation is the next most common complication of PUD, occurring in 2–10% of peptic ulcers, and can present as sudden severe abdominal pain with hemodynamic instability or shock.35 Physical exam findings may include initially hyperactive bowel sounds that can diminish and progress to a rigid abdomen with rebound, suggesting peritonitis. The presence of free air on imaging is supportive of this diagnosis and endoscopy should be avoided in this setting. Surgery is usually the treatment of choice for a perforated peptic ulcer.3 In patients who are poor surgical candidates and with perforation for more than 24 hours that is contained (based on water soluble contrast studies), medical treatment with nasogastric (NG) suction, IV fluids, antibiotics and acid suppression is an option. Penetrating ulcers can also erode into nearby organs such as pancreas, liver, bile duct, or colon.
Gastric outlet obstruction (GOO) is another complication of PUD and can present with early satiety, bloating, weight loss, indigestion, nausea and vomiting. On physical exam, a succussion splash may be heard due to trapped air and fluid in the stomach. Ulcers that manifest with GOO are often located in the pyloric channel or duodenal bulb. Medical therapy usually involves NG suction and anti-secretory therapy. Endoscopic balloon dilation of pylorus or surgery are options to relieve chronic obstruction.35
Conclusion
PUD is a disease with decreasing clinical burden due to the decline in H. pylori infections, as well as increased accessibility to anti-secretory therapy and more judicious use of NSAIDs. However, due to its continued high lifetime prevalence and varied clinical presentation, recognition and appropriate management of PUD are key to avoid and minimize significant complications. Testing and treating H. pylori as well as limiting mucosal injury caused by NSAIDs (via concurrent PPI prophylaxis or choosing COX-2 selective NSAIDs if available) are the strategies to consider when evaluating PUD. Resuscitation, anti-secretory therapy, endoscopy and management of antithrombotic agents are the key steps in treatment of PUD bleeding, which is the most common complication.
Biography
Mechu Narayanan, MD, (top left), and Kavya M. Reddy, MD, (top right), are Fellows, and Elizabeth Marsicano, MD, (bottom), is an Assistant Professor of Internal Medicine in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, St. Louis, Mo.
Contact: Elizabeth.Marsicano@health.slu.edu



Footnotes
Disclosure
None reported.
References
- 1.Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s gastrointestinal and liver disease : pathophysiology/diagnosis/management. Tenth edition ed. 2 volumes. Philadelphia, PA: Saunders/Elsevier; 2016. p. xxxi.p. 2369.p. 2389. [Google Scholar]
- 2.Sonnenberg A, Everhart JE. The prevalence of self-reported peptic ulcer in the United States. Am J Public Health. 1996;86:200–205. doi: 10.2105/ajph.86.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007;76:1005–1012. [PubMed] [Google Scholar]
- 4.Sonnenberg A. Review article: historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther. 2013;38:329–342. doi: 10.1111/apt.12380. [DOI] [PubMed] [Google Scholar]
- 5.Lanas A, Chan FKL. Peptic ulcer disease. The Lancet. 2017;390:613–624. doi: 10.1016/S0140-6736(16)32404-7. [DOI] [PubMed] [Google Scholar]
- 6.Lanas-Gimeno A, Lanas A. Risk of gastrointestinal bleeding during anticoagulant treatment. Expert Opinion on Drug Safety. 2017;16:673–685. doi: 10.1080/14740338.2017.1325870. [DOI] [PubMed] [Google Scholar]
- 7.Zhang BB, Li Y, Liu XQ, Wang PJ, Yang B, Bian DL. Association between vacA genotypes and the risk of duodenal ulcer: a meta-analysis. Mol Biol Rep. 2014;41:7241–7254. doi: 10.1007/s11033-014-3610-y. [DOI] [PubMed] [Google Scholar]
- 8.Papatheodoridis GV, Archimandritis AJ. Role of Helicobacter pylori eradication in aspirin or non-steroidal anti-inflammatory drug users. World J Gastroenterol. 2005;11:3811–3816. doi: 10.3748/wjg.v11.i25.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chey WD, Wong BC Practice Parameters Committee of the American College of G. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 11.Lanza FL, Chan FK, Quigley EM Practice Parameters Committee of the American College of G. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 12.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 13.Lanas A, Carrera-Lasfuentes P, Arguedas Y, Garcia S, Bujanda L, Calvet X, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13:906–912. e902. doi: 10.1016/j.cgh.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Somasundaram S, Sigthorsson G, Simpson RJ, Watts J, Jacob M, Tavares IA, et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther. 2000;14:639–650. doi: 10.1046/j.1365-2036.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 15.Masclee GM, Valkhoff VE, Coloma PM, de Ridder M, Romio S, Schuemie MJ, et al. Risk of upper gastrointestinal bleeding from different drug combinations. Gastroenterology. 2014;147:784–792. e789. doi: 10.1053/j.gastro.2014.06.007. quiz e713–784. [DOI] [PubMed] [Google Scholar]
- 16.Crooks CJ, West J, Card TR. Comorbidities affect risk of nonvariceal upper gastrointestinal bleeding. Gastroenterology. 2013;144:1384–1393. e1381–1382. doi: 10.1053/j.gastro.2013.02.040. 1393. quiz e1318–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Perez A, Saez ME, Johansson S, Nagy P, Garcia Rodriguez LA. Risk factors associated with uncomplicated peptic ulcer and changes in medication use after diagnosis. PLoS One. 2014;9:e101768. doi: 10.1371/journal.pone.0101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 19.Kanno T, Iijima K, Koike T, Abe Y, Shimada N, Hoshi T, et al. Accommodation in a refugee shelter as a risk factor for peptic ulcer bleeding after the Great East Japan Earthquake: a case-control study of 329 patients. J Gastroenterol. 2015;50:31–40. doi: 10.1007/s00535-014-0940-4. [DOI] [PubMed] [Google Scholar]
- 20.Kiss S, Zsikla V, Frank A, Willi N, Cathomas G. Helicobacter-negative gastritis: polymerase chain reaction for Helicobacter DNA is a valuable tool to elucidate the diagnosis. Aliment Pharmacol Ther. 2016;43:924–932. doi: 10.1111/apt.13564. [DOI] [PubMed] [Google Scholar]
- 21.McColl KE. Helicobacter pylori-negative nonsteroidal anti-inflammatory drug-negative ulcer. Gastroenterol Clin North Am. 2009;38:353–361. doi: 10.1016/j.gtc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Agreus L, Talley NJ, Jones M. Value of the “Test & Treat” Strategy for Uninvestigated Dyspepsia at Low Prevalence Rates of Helicobacter pylori in the Population. Helicobacter. 2016;21:186–191. doi: 10.1111/hel.12267. [DOI] [PubMed] [Google Scholar]
- 23.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–1780. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 25.Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JY, Dunbar KB, Mitui M, Arnold CA, Lam-Himlin DM, Valasek MA, et al. Helicobacter pylori Clarithromycin Resistance and Treatment Failure Are Common in the USA. Dig Dis Sci. 2016;61:2373–2380. doi: 10.1007/s10620-016-4091-8. [DOI] [PubMed] [Google Scholar]
- 27.Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:1124–1133. doi: 10.1016/0016-5085(95)90570-7. [DOI] [PubMed] [Google Scholar]
- 28.Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, Ponce M, Quintero E, Perez-Aisa MA, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33:585–591. doi: 10.1111/j.1365-2036.2010.04563.x. [DOI] [PubMed] [Google Scholar]
- 29.Stanley AJ, Dalton HR, Blatchford O, Ashley D, Mowat C, Cahill A, et al. Multicentre comparison of the Glasgow Blatchford and Rockall Scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011;34:470–475. doi: 10.1111/j.1365-2036.2011.04747.x. [DOI] [PubMed] [Google Scholar]
- 30.Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 31.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–360. doi: 10.1038/ajg.2011.480. quiz 361. [DOI] [PubMed] [Google Scholar]
- 32.Sachar H, Vaidya K, Laine L. Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:1755–1762. doi: 10.1001/jamainternmed.2014.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CH, Ma MH, Chou HC, Yen ZS, Yang CW, Fang CC, et al. High-dose vs non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170:751–758. doi: 10.1001/archinternmed.2010.100. [DOI] [PubMed] [Google Scholar]
- 34.Lanas-Gimeno A, Lanas A. Risk of gastrointestinal bleeding during anticoagulant treatment. Expert Opin Drug Saf. 2017;16:673–685. doi: 10.1080/14740338.2017.1325870. [DOI] [PubMed] [Google Scholar]
- 35.Behrman SW. Management of complicated peptic ulcer disease. Arch Surg. 2005;140:201–208. doi: 10.1001/archsurg.140.2.201. [DOI] [PubMed] [Google Scholar]