Abstract
IgG4-related disease (IgG-RD) describes a group of fibroinflammatory diseases that affect a variety of tissues resulting in tumor-like effect and/or organ dysfunction. Clinical presentation varies according to the tissue(s) involved, and diagnosis relies on tissue findings of dense infiltration of IgG4-positive plasma cells and a characteristic storiform fibrosis. Treatment is mainly with glucocorticoids, while multiple immunosuppressive medications can be used as adjuvant agents. Rituximab has showed promising results, but further studies are needed.
Introduction
IgG 4 related disease (IgG4-RD) is a systemic fibroinflammatory disease characterized by dense infiltration of IgG4-positive plasma cells in the affected tissue(s) with or without elevated plasma levels of IgG4.1 The inflammatory infiltration along with a characteristic storiform fibrosis can lead to the development of chronic damage and/or tumefactive lesions1 that may affect any organ. In 2001, Hamano and colleagues published a study that showed the association between elevated plasma IgG4 levels and sclerosing pancreatitis 2, which is now called type 1 autoimmune pancreatitis (AIP). Immunohistochemical examinations of AIP tissues revealed severe infiltration with IgG4-positive plasma cells that further enlightened the existence of an IgG4-RD with a possible systemic process.3 In the years to follow, several previously described syndromes were linked to IgG4 infiltration and now they are labeled as IgG4-RD affecting different tissues or organs. These syndromes include Mikulicz disease4, chronic sclerosing sialadenitis (Kuttner’s tumor)5, Riedels’ thyroiditis6, mediastinal fibrosis, retroperitoneal fibrosis (Ormond’s disease), periaortitis, idiopathic hypocomplementic tubulointerstitial nephritis, multifocal fibrosclerosis, and inflammatory pseudotumor.1
Epidemiology
The incidence of IGG4-RD remains unknown. This is due to challenges in making the diagnosis as the disease is often unrecognized or misdiagnosed. A cohort of 125 biopsy proven IgG4-RD showed a mean age of 55 years old at diagnosis (50 years old at onset of symptoms); 78% were white and 61% were males. Head and neck tissues were the most affected sites.7 Mayo Clinic reported 166 patients with IgG-RD; the median age of onset was 61 years old and the male to female ratio was 3:1; 80 % of patients were white. In the Mayo Clinic report; hepatobiliary IgG4-RD was the most common entity.8 One study from Japan reported 114 of cases with a similar male to female ratio of 3:1. The higher incidence of the disease in males persisted regardless of the tissue affected with the only exception of IgG4-RD in the head and neck (sialadenitis or dacryoadenitis) where the male to female distribution was equal. In this cohort the average age at diagnosis was 64 years old.9 The disease seems to be less common in the pediatrics population. A systematic review found 22 cases of IgG4-RD in children with a median age of 13 years old (age range was 22 months to 17 years); 64% were females in contrast to the male predominance in the adult disease. In the pediatric population ophthalmic IgG4-RD was the most common entity and it was seen in 44% of the cases.10
Pathogenesis
Most studies on the pathogenesis and the genetic associations of IgG4-RD were conducted in Asian populations with AIP. Both human leukocyte antigen (HLA), and non-HLA genes were associated with IgG4-RD. A study from Japan reported that the frequencies of HLA serotypes DRB1_0405 and DQB1_0401 were significantly higher in patients with AIP than in healthy individuals and in patients with chronic calcifying pancreatitis not related to AIP. In addition, single nucleotide polymorphisms (SNPs) in non-HLA genes were linked to AIP. Those genes include Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) and Tumor necrosis factor (TNF)- alpha promoter genes. The frequency of CTLA-4_49A and TNF- alpha promoter_863 haplotypes were higher in Chinese AIP patients than in healthy individuals. A Japanese study found that the CTLA-4_49A haplotype is associated with a higher risk of disease relapse. Another study reported an association between AIP and Fc receptor-like 3 (FCRL3)_110A/A genotype.11
While impaired function or a decreased number of regulatory T cells (Treg cells) is believed to be an important step in the development of autoimmune disorders11, Treg cells seem to play an important role in the pathogenesis of IgG4-RD. The expression of Th2 cytokines (IL-4, IL-5, and IL-13) and regulatory cytokines (IL-10 and TGF-β) was up-regulated in the affected tissues of type 1 AIP12, and in IgG4-related tubulointerstitial nephritis.11 Similar findings were reported in Mickulicz disease.13 The overproduction of IL-4, IL-5, and IL-13 can explain the higher frequency of peripheral eosinophilia and IgE levels in patients with IgG4-RD. IL-10 causes increased production of IgG4 and can lead to fibrosis by increasing the expression of Transforming Growth Factor- beta (TGF-β), which is a fibrogenic cytokine.12 A recent study showed that CD4+ effector/memory T cells with a cytolytic phenotype were expanded in IgG4-RD patients. These CD4+ cytotoxic lymphocytes (CD4+ CTLs) might play a major role in the pathogenesis of the disease. Plasmablasts, which are increased in IGG4-RD, are thought to be responsible for the reactivation of the CD4+CTLs and induce them to make fibrosing-inducing cytokines including IL-1β and TGF-β1.14
Clinical Presentation
The symptoms of IgG4-RD vary considerably depending on the organ(s) or tissues involved. The most common presentation is a mass lesion or organ enlargement. The most commonly affected organs are salivary and lacrimal glands, pancreas and biliary tract, and the kidneys; but any organ could be involved and multiorgan disease can be also seen.15 The inflammatory infiltration and fibrosis can result in tissue or organ dysfunction in addition to tumor-like effects that can cause obstruction or compression complications.16 Among the clinical features observed are exophthalmos including orbital pseudotumor, salivary gland enlargement, pancreatic failure, lymph node enlargement, retroperitoneal fibrosis, kidney disease with proteinuria and subsequent renal failure, and aortitis-related aortic aneurysm.17 A number of cases will present as an incidental finding on imaging. Presentation is usually subacute, and some patients might have more than one organ affected at the same time or years after the initial diagnosis. Many patients have an existing allergic condition. Peripheral eosinophilia is not uncommon while fever is rare.1
Diagnosis
The diagnosis of IgG4-RD can be challenging and the approach to diagnosis will depend on the site affected. A thorough process should always be followed to rule out the different diseases that can mimic IgG4-RD. These diseases include a broad spectrum of conditions including malignancy, lymphoproliferative disorders, Antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, sarcoidosis, Sjogren’s syndrome, Castleman’s disease and others.16, 17
It is important to remember that elevated plasma IgG4 levels are neither specific nor sensitive for IgG4-RD. Normal IgG4 can be seen in nearly one-fourth of the cases9, and using a cutoff level for IgG4 of 135 mg/dl has a positive predictive value for IGG4-RD of only 34%.18 While tissue biopsy is very helpful in ruling out cancer and other possible disease processes, IgG4+ cellular staining on biopsy alone is not sufficient to make a definite diagnosis of IgG4-RD as the presence of IGG4+ plasma cells in the tissue can be seen in other diseases including malignancy. In addition, certain sites affected by IgG4-RD, like the retroperitoneum, orbital cavity and pancreas, are very difficult to biopsy.1,16,17 Elevated numbers of circulating plasmablasts can be seen in a variety of inflammatory conditions but a significant elevation (>2,000 cells/ml) was observed in IgG4- RD and may serve as a marker for both diagnosis and measurement of disease activity. Plasmablasts elevation in IgG4-RD was seen even in patients who had normal IgG4 levels.19 However, the testing of plasmablasts might not be readily available in all clinical settings and more studies are needed to advise a universal use of this test.17 Comprehensive diagnostic criteria for IgG4-RD and organ-specific IgG4-RD (like AIP and IgG4-related kidney disease) exist and they take in consideration the clinical, serological, radiographic and histopathologic al manifestations.16 However, some of the proposed criteria had low sensitivity (as low as 70%).16 Also, a major caveat was the ability to make IgG4-RD diagnosis without obtaining a biopsy, which can be problematic.17 The use of these criteria should not replace the need for obtaining a biopsy from the suspected lesion.
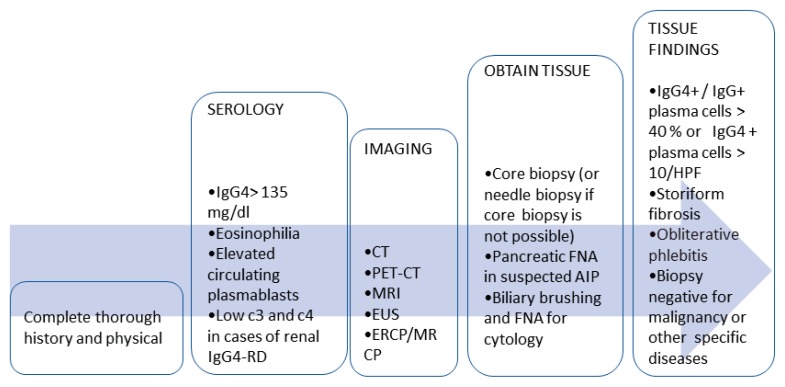
The assessment should start with complete history and physical examination. Then when the disease is suspected lab testing and appropriate radiology evaluation will depend on the site involved. Plasma IgG4 levels should be obtained in all patients. Most cases of renal IgG4-RD will have low complements17, which often correlates with disease activity17, thus complement levels should be obtained when renal IgG4-RD is suspected. Circulating plasmablasts measurement, if available, can be helpful especially if the serum IgG4 level is normal. Selection of the imaging modality in the assessment of IgG4-RD will depend on the organ under evaluation, local radiology expertise, and availability. Computed tomography (CT), CT with positron emission tomography (CT-PET), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), Endoscopic retrograde cholangiopancreatography (ERCP), and endoscopic ultrasound (EUS) are modalities that are commonly used to evaluate IgG4-RD.17 Biopsy remains the cornerstone step in evaluation. As noted, the presence of high level of IgG4+ plasma cells (> 10/HPF or IgG4+/IgG4>40%) is typical but not diagnostic.9 Findings of storiform fibrosis and obliterative phlebitis increase the specificity of the diagnosis.16,17 Diagnosing biliary or pancreatic IgG4- RD is even more challenging due to difficulties in obtaining adequate tissue samples. The characteristic imaging findings and serologic testing become the main diagnostic criteria. Fine needle aspiration of the pancreas and brush cytology of the biliary system, however, remain important to rule out malignancy.16, 17
The diagnosis is made by clinical correlation of the laboratory, imaging and histopathologic findings in absence of malignancy or other disease processes that could explain the presentation (Figure 1).
Figure (1).
Suggested flow chart for IgG4-RD diagnostic work up. HPF: high-power field. FNA: Fine needle aspiration.
Treatment
Some patients with asymptomatic IgG4-RD can be watched without treatment, e.g. asymptomatic lymphadenopathy or mild submandibular gland enlargement.17 However, most patients will require initiation of therapy. Special attention should be made to active IgG4-RD in the pancreas, biliary tree, aorta, mediastinum, kidneys, retroperitoneum and mesentery as in those cases starting therapy in an urgent fashion must be considered.17 Pachymeningitis is one of the manifestations of IgG4-RD that also requires urgent treatment. 17
Glucocorticoids remain the main initial therapy agents with expected good and quick response. A meta-analysis that included 62 different studies with total number of 3034 patients showed that glucocorticoids are the most commonly used medication to initiate therapy (74% of reported cases) with a mean starting dose of 0.6 mg/kg. The reported response rate for glucocorticoids monotherapy was 97% but a complete response rate was 65 %.15 Also, relapse can occur after stopping glucocorticoids. The actual rate for relapse is unknown but one study reported a relapse rate of 54 % after cessation of steroids therapy in AIP.20 The efficacy of corticosteroids was less in American studies compared to studies from Asia.15
Initial glucocorticoids dose should be maintained for 2–4 weeks then tapered slowly over 3–6 months depending on response.17 Adding a steroid-sparing agent is reasonable especially if prednisone can’t be tapered because of persistently active disease. Options include Methotrexate, Azathioprine, Mycophenolate, 6-Mercaptopurine and cyclophosphamide.17 To date there is no randomized controlled studies that compared prednisone monotherapy to combination of steroids with any immunosuppressive agent in IgG4-RD. The use of Rituximab (RTX) monotherapy to induce remission has demonstrated promising results. Rituximab was associated with significant reductions in both IgG4 levels21, 22, and circulating plasmablasts.22 A prospective study of Rituximab showed that 77% patients met the primary outcome of response with no relapses for six months.21
Maintenance therapy for IgG4-RD after remission induction is recommended for patients with higher risk of organ dysfunction or high risk of relapse. There is no consensus on the optimal regimen or duration for maintenance therapy, however. Maintenance with small dose of glucocorticoids is the most practiced approach in Japan for AIP.17 A steroid sparing agent and Rituximab for maintenance can also be used. Disease relapse or flares should be managed with glucocorticoids and if the patient is not already on another immunosuppressive then starting an agent should be strongly considered.17
Biography
Orwah M. Al-Khalili, MD, (above), is a Rheumatology Fellow, University of Nebraska Medical Center, Omaha, Neb. Alan R. Erickson, MD, is an Associate Professor of Internal Medicine and Rheumatology, University of Nebraska Medical Center, Omaha, Neb.
Contact: orwah.alkhalili@unmc.edu

Footnotes
Disclosure
None reported.
References
- 1.Stone J, Zen Y, Deshpande V. IgG4-Related Disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Hammano H, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 4.Yammamoto M, et al. Clinical and pathological differences between Mikulicz’s disease and Sjogren’s syndrome. Rheumatology. 2005;44:227–234. doi: 10.1093/rheumatology/keh447. [DOI] [PubMed] [Google Scholar]
- 5.Geyer J, et al. AM J Surj Path 2010. Chronic sclerosing sialadenitis (Kuttner Tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34:202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 6.Stan M, et al. Riedel’s thyroiditis association with IgG4-related disease. Clinical Endocrinology. 2017;86:425–430. doi: 10.1111/cen.13238. [DOI] [PubMed] [Google Scholar]
- 7.Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol. 2010;34:1812–1819. doi: 10.1097/PAS.0b013e3181f7266b. [DOI] [PubMed] [Google Scholar]
- 8.Wallace Z, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheum. 2015;67:2466–2475. doi: 10.1002/art.39205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekiguchi H, et al. IgG4-related disease: retrospective analysis of one hundred sixty-six patients. Arthritis Rheum. 2016;68:2290–2299. doi: 10.1002/art.39686. [DOI] [PubMed] [Google Scholar]
- 10.Karim F, et al. IgG4-related disease: a systematic review of this unrecognized disease in pediatrics. Pediatric Rheumatology. 2016;14:18. doi: 10.1186/s12969-016-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zen y, Nakanuma Y. Pathogenesis of IgG4-related disease. Current Opinion in Rheumatology. 2011;23:114–118. doi: 10.1097/BOR.0b013e3283412f4a. [DOI] [PubMed] [Google Scholar]
- 12.Zen Y, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. HEPATOLOGY. 2007;45:1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 13.Miyake K, et al. Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz’s disease associated with lymphadenopathy and pleural effusion. Mod rheumatology. 2008;18(1):86–90. doi: 10.1007/s10165-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 14.Mattoo H, et al. Clonal expansion of CD4+ Cytotoxic T Lymphocytes in IgG4-related disease. Allergy Clin Immunol. 2016;138(3):825–838. doi: 10.1016/j.jaci.2015.12.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brito-Zerón P, et al. Therapeutic approach to IgG4-related disease: A systematic review. Medicine. 2016;95:26. doi: 10.1097/MD.0000000000004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umehara H, et al. Current approach to the diagnosis of IgG4-related disease – Combination of comprehensive diagnostic and organ-specific criteria. Mod Rheum. 2017;27(3):381–391. doi: 10.1080/14397595.2017.1290911. [DOI] [PubMed] [Google Scholar]
- 17.Khosroshahi A, et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheum. 2015;67:1688–1699. doi: 10.1002/art.39132. [DOI] [PubMed] [Google Scholar]
- 18.Carruthers MN, et al. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015;74:14–18. doi: 10.1136/annrheumdis-2013-204907. [DOI] [PubMed] [Google Scholar]
- 19.Wallace Z, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190–195. doi: 10.1136/annrheumdis-2014-205233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raina A, et al. Evaluation and management of autoimmune pancreatitis: experience at a large us center. Am J Gastroenterol. 2009;104:2295–2306. doi: 10.1038/ajg.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carruthers MN, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–1177. doi: 10.1136/annrheumdis-2014-206605. [DOI] [PubMed] [Google Scholar]
- 22.Khosroshahi A, Carruthers MN, et al. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine. 2012;91:57–66. doi: 10.1097/MD.0b013e3182431ef6. [DOI] [PubMed] [Google Scholar]