Abstract
Nonalcoholic steatohepatitis (NASH) is a common cause of chronic liver disease that can progress to cirrhosis and hepatocellular carcinoma. This review provides brief answers to common questions clinicians may have about NASH. The diagnosis of NASH currently requires a liver biopsy, although non-invasive tests are being evaluated and may supplant liver biopsy in the near future. Treatment is focused on lifestyle modifications, with new medications currently in clinical trials.
Nonalcoholic steatohepatitis has emerged as the major form of chronic liver disease of this decade, and NASH cirrhosis is soon to be the leading indication for liver transplantation.
What differentiates nonalcoholic fatty liver (NAFL) from nonalcoholic steatohepatitis (NASH)?
Nonalcoholic fatty liver disease (NAFLD) is characterized by macrovesicular hepatic fat accumulation in ≥ 5% of hepatocytes in those patients who do not have a secondary cause of steatosis (e.g. alcohol consumption, genotype 3 hepatitis C, medications, protein malnutrition, parenteral nutrition). NAFLD is a spectrum ranging from nonalcoholic fatty liver (NAFL) which includes hepatic steatosis without substantial inflammation or hepatocellular injury, to nonalcoholic steatohepatitis (NASH) which is characterized by steatosis, inflammation and evidence of hepatocellular injury in a characteristic pattern. Both can progress to advanced fibrosis and cirrhosis, but the risk of progression is much greater in patients with NASH compared to NAFL.
Why is it important to distinguish between NAFL and NASH?
NASH, unlike NAFL, has the greatest potential to progress to cirrhosis, liver failure, and liver cancer. A multitude of studies have shown that progression of fibrosis is associated with decreased survival.1 Differentiating between the two allows for earlier lifestyle changes, medical interventions, cancer screening, and overall improved outcomes.
What factors help identify the patients with NAFLD who are at greatest risk for progression to cirrhosis?
Identifying factors that place patients with NAFLD at risk for progression to cirrhosis is important for determining potential therapeutic interventions.1 Studies have shown that liver fibrosis, hepatocyte ballooning, and portal inflammation (but surprisingly not the amount of steatosis) correlate with reduced survival.2 A recent meta-analysis not only identified fibrosis as an independent risk factor for liver-related mortality, but also demonstrated an exponential increase in liver related mortality with worsening fibrosis stage.3 These data highlights the importance of targeting fibrosis in the treatment of NAFLD, as well as targeting NASH, the driver of that fibrosis.
What is the prevalence of NAFLD and NASH?
Over the past few decades, the rate of NAFLD has been on the rise. This increase parallels the overall trend of increasing obesity and metabolic syndrome, particularly in developed nations. The prevalence of NAFLD is often underestimated, given that patients are typically asymptomatic until they progress to decompensated cirrhosis. Several studies have examined the epidemiology of NAFLD, and often, a wide range of prevalence rates has been found. This variation is partly related to the criteria used to establish a diagnosis (e.g. elevation in liver enzymes, imaging, histology). Younossi et al. studied the global prevalence and incidence of fatty liver disease spanning 26 years, encompassing 22 countries, and including approximately 8.5 million individuals.4 They estimate that approximately 25% of the adult population worldwide has NAFLD, with the highest prevalence rates in South America (31%) and the Middle East (32%) compared to the lowest rate in Africa (14%).4 The prevalence of NASH in adults has been approximated to be 1.5% – 6.45%.4 Furthermore, NASH is a major cause of chronic liver disease in children, even progressing to cirrhosis in teenagers.5 Comparing years 1999–2002 to 2009–2012, Kabbany et al reported a 2–2.5-fold increase in the prevalence of NASH cirrhosis and NAFLD-associated advanced fibrosis.6 In light of the rising rate of NAFLD, it is not surprising that the percentage of patients with NASH cirrhosis on the liver transplant list has also increased.7
What is the burden of NAFLD on healthcare costs?
Cirrhosis with subsequent decompensation results in frequent hospital admissions and ultimately the need for liver transplantation, although many patients with NASH cirrhosis may not be transplant candidates due to co-existing obesity and cardiovascular complications. The percentage of patients with NASH cirrhosis listed for liver transplantation has been rising and will soon surpass hepatitis C. This reversal is due to a decrease in liver transplants in the latter group which is partly related to new highly efficacious direct acting antiviral therapies for chronic hepatitis C.7 In the coming decade, NAFLD is projected to be the leading indication for liver transplantation.8 This change has resulted in NAFLD patients becoming major contributors to increasing healthcare costs. The 64 million people with NAFLD in the United States have been estimated to contribute $103 billion to direct medical costs annually.9 Similarly, four European nations (Germany, France, Italy, and United Kingdom) are estimated to have 52 million patients with NAFLD with annual combined health care costs of £35 billion.9
What conditions have been associated with NAFLD?
Patients with nonalcoholic fatty liver disease are often obese. They typically have some, if not all, of the other components of metabolic syndrome including insulin resistance, type 2 diabetes mellitus, hypertension, and dyslipidemia. This places patients with NAFLD at an increased risk of cardiovascular disease and its associated complications. Even without metabolic syndrome, NAFLD is an independent risk factor for cardiovascular disease.10 In fact, cardiovascular disease is the leading cause of death in people with NAFLD. Established conditions associated with NAFLD include hepatocellular carcinoma (including in non-cirrhotic patients), extrahepatic tumors (e.g. colorectal cancer) and osteoporosis.10, 11 There have also been associations between NAFLD and sleep apnea, psoriasis, endocrinopathies, and polycystic ovary syndrome independent of obesity.5 The importance of recognizing these associations is that it provides clinicians the opportunity to screen patients with NAFLD for these conditions and provide early medical management to reduce morbidity and mortality in this population.
Which patients with NAFLD are at an increased risk for hepatocellular carcinoma?
It has been well established that cirrhosis is a significant risk factor for the development of hepatocellular carcinoma (HCC). A recent study of US veterans has also shown that NAFLD, in the absence of cirrhosis, also confers an increased risk of HCC.12 Mittal et al concluded that non-cirrhotic NAFLD patients developed HCC more frequently compared to non-cirrhotic patients with other etiologies for their chronic liver disease.12 Although this data begs the question of whether the criteria for HCC screening should be expanded to include NAFLD patient without cirrhosis, current guidelines recommend screening only those patients with cirrhosis.5
How are NAFLD and NASH diagnosed?
Patients with NAFLD are often asymptomatic until they develop signs or symptoms of decompensated cirrhosis. Often it is incidental elevation in serum aminotransferases (ALT and AST) or incidental steatosis on imaging that clue clinicians to the possibility of NAFLD. Persistently elevated aminotransferases can be associated with an increased risk of disease progression. However, even with normal aminotransferases, those patients with metabolic syndrome who are found to have steatosis on imaging are at increased risk for nonalcoholic steatohepatitis.5 Up to 30–60% of biopsy-proven NASH patients can have a normal ALT, and it is only through histology that one can distinguish steatohepatitis from steatosis.13 Although biopsy is the gold standard to delineate among the spectrum of NAFLDs, it is associated with higher costs, increased risk of complications, and sampling error. These hurdles have inspired researchers to seek non-invasive means of assessing hepatic steatosis and hepatic fibrosis.
Ultrasound (US), computerized tomography (CT) and magnetic resonance imaging (MRI) have been used to assess patients for steatosis. US has 93% sensitivity in diagnosing steatosis when hepatic fat accumulation is greater than 33%, but the sensitivity decreases when hepatic fat accumulation is less than 30%.14, 15 In contrast, MRI is able to detect steatosis greater than 5.56% nearly 100% of the time.16 CT is also sensitive for detecting liver fat in which the liver appears lower in density than the spleen or paraspinal muscles, but it is not used routinely for this purpose because of the radiation exposure. A significant limitation of imaging studies is their inability to distinguish steatosis from steatohepatitis or detect early stages of liver fibrosis.
Noninvasive assessments of advanced fibrosis currently available include clinical scoring systems (e.g. NAFLD fibrosis score [NFS], Fibrosis-4 index [FIB-4]), imaging techniques (vibration controlled transient elastography [VCTE], MR elastography [MRE]), and biomarkers (e.g. enhanced liver fibrosis [ELF] panel, FibroTest). Of all the risk scoring systems available, NFS (calculated based on age, BMI, hyperglycemia, platelet count, albumin, and AST/ALT ratio) and FIB-4 (an algorithm based on age, platelet count, AST, and ALT) have been shown to be as reasonable at predicting advanced fibrosis as MR is elastography when compared to biopsy-proven NAFLD.17 VCTE (FibroScan) is a noninvasive measure of liver stiffness used for assessing liver fibrosis. When comparing the two modalities, MRE was better than VCTE at identifying fibrosis stage 2 and above, however, both were equally as effective at detecting fibrosis stage 3 or above.17 One emerging option is to combine the results of Fibroscan and the FIB-4 index to identify patients needing further evaluation. The ELF panel (comprised of hyaluronic acid, tissue inhibitor of metalloproteinase 1, and N-terminal procollagen III-peptide) has also been shown to have 80% sensitivity and 90% specificity for detecting advanced fibrosis, but it is not available in the United States.18
When is a liver biopsy the next step in the evaluation of patients with NAFLD?
Despite the advances in noninvasive methods of assessing steatosis and fibrosis, there are instances when a biopsy is necessary. Liver biopsy is considered in those patients in whom competing etiologies of chronic liver disease (e.g. autoimmune hepatitis, Wilsons, hemochromatosis) cannot be excluded, when noninvasive imaging studies are equivocal, and in those patients who are at increased risk of having steatosis and/or advanced fibrosis (e.g. patients with metabolic syndrome, high NFS or FIB-4 score, or increased liver stiffness measured by MRE or VCTE).5
Are there any genetic associations with NAFLD?
There have been genome-wide association studies to identify genetic links found in NAFLD. These studies have elucidated associations between genetic polymorphisms, notably PNPLA-3, and NAFLD.19 An allele in PNPLA3 has been strongly associated with increased hepatic fat content and inflammation; this allele is most common in Hispanics, the ethnic group with the highest prevalence of NAFLD.19 However, it will be some time before these genetic associations will be applicable in routine clinical settings. Nonetheless, patients with first degree relatives with advanced NASH should be followed with a higher index of suspicion.
What is the treatment for NAFLD?
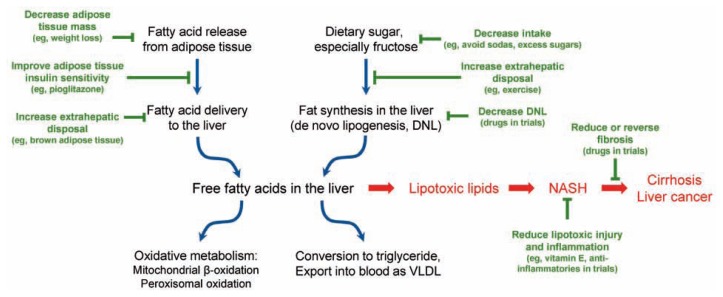
The role of treatment approaches currently available and those being studied in clinical trials is shown in Figure 1. The cornerstone of NAFLD management consists of healthy eating habits and regular exercise.5 Weight loss alone has been shown to decrease histologic features of NASH and induce regression of fibrosis with greatest improvement occurring after 10% weight reduction has been attained.20 Decreasing daily caloric intake by 500–1000 kcal is an effective method for achieving weight loss, but it is often challenging to maintain this lifestyle modification.20 Various diets have also been researched, and studies show that the content of the diet may play a role in the treatment of NAFLD independent of weight loss. A recent randomized controlled trial found that a Mediterranean diet aids in the reduction of fat accumulation and liver stiffness in addition to improving insulin sensitivity.21 Exercise has also been shown to reduce fat accumulation in the liver as well as reduce aminotransferase levels; however, its effect on liver histology remains to be fully established.22 Nonetheless, exercise provides multiple other benefits including improved cardiovascular health and reduced peripheral, adipose, and hepatic insulin resistance independent of weight loss.13 Studies suggest that specifically aerobic exercise reduces more intrahepatic fat content compared to resistance exercise, however, further investigation is required to determine the specifics regarding exercise and its impact in the treatment of NAFLD.22 Although there are no randomized controlled trials evaluating bariatric surgery in patients with NAFLD, cohort studies that have shown improvement in steatohepatitis following bariatric surgery, which suggests that surgery may be a reasonable option in a subset of NAFLD patients.23
Figure 1.
Figure. NASH develops in the setting of excess fat being transported to the liver or being produced in the liver, in conjunction with limitations on the capacity of the liver to oxidize fat or return it to the circulation as very low density lipoprotein (VLDL). Dietary factors such as fructose and glucose consumption contribute to this process. The role of genetics, epigenetic changes, and other modifying factors, such as the gut microbiome, are currently being explored in research studies. Multiple treatment options that target differents steps of these pathways are undergoing evaluation in clinical trials. Reducing caloric intake, especially from carbohydrates, and increasing disposal through regular aerobic exercise remain the primary treatment recommendations.
Are there any pharmacologic agents recommended for the treatment of NAFLD?
In addition to lifestyle modification, pharmacologic agents for the management of NAFLD have been explored. Currently, there are no medications approved for the treatment of NAFLD, but studies have identified subgroups of NASH patients who may benefit from vitamin E or pioglitazone. In a randomized controlled trial, both vitamin E and pioglitazone were shown to significantly improve nonalcoholic steatohepatitis in non-diabetic patients.24 A recent trial also demonstrated improvement in steatohepatitis and fibrosis in diabetic patients who took pioglitazone.25 These findings have influenced current AASLD guidelines, which recommend considering treatment with vitamin E in non-diabetics and pioglitazone in patients with biopsy confirmed NASH.5 Additionally, obeticholic acid (an FXR ligand), elafibranor (a PPARγ/PPARδ ligand), selonsertib (an ASK-1 inhibitor) and cenicriviroc (a chemokine receptor 2 and 5 inhibitor) are currently in phase 3 trials as potential therapies for NASH.
Are statins safe for the treatment of dyslipidemia in patients with NAFLD?
Cardiovascular disease is common in patients with NAFLD, and it accounts for twice as many deaths as those that occur by liver-related causes.13 It is vital for health care providers to remain vigilant regarding the recognition and early management of cardiovascular risk factors that place NAFLD patients at highest risk of morbidity and mortality. Despite the overwhelming benefit of statins in cardiovascular disease, physicians have been hesitant to utilize these medications in their NAFLD patients given concerns for potential hepatotoxicity. Studies have confirmed the safety of statin use in NAFLD patients; furthermore, there is no proof that pre-existing liver disease is a reason to avoid statins to reduce cardiovascular disease risks.26 There is no clear evidence that statins result in improvement in the underlying liver disease itself but statins should certainly be considered for cardiovascular risk reduction.
What are the concerns regarding tamoxifen use in the development and progression of NAFLD?
Multiple medications have been identified as steatogenic with the potential to cause or worsen nonalcoholic fatty liver disease. Tamoxifen is an anti-estrogen medication frequently used as adjuvant therapy in patients with estrogen receptor positive breast cancer. Studies have demonstrated that NAFLD develops in 30–50% of patients treated with tamoxifen.27 A small proportion of these patients, especially those with metabolic syndrome, have the potential to progress to NASH.28 Tamoxifen is thought to cause NAFLD by inhibiting mitochondrial beta-oxidation of fatty acids and suppressing estrogen synthesis.27 It is important for health care providers to understand the association between tamoxifen and NAFLD when considering treatment options for estrogen receptor positive breast cancer, especially in patients who also have some or all of the components of metabolic syndrome.
Summary
NASH has emerged as a common cause of chronic liver disease, cirrhosis and hepatocellular carcinoma. Identifying patients with NASH has historically required a liver biopsy, although non-invasive markers may soon be validated as diagnostic tools which may be used in the management of these patients. Current treatment is focused on healthy eating habits, exercise, and weight loss. Vitamin E and pioglitazone may play a role in the treatment of selected patients. Multiple new drugs being evaluated in ongoing clinical trials may be available in the next 2–5 years. NASH is a complex disease that likely results from a combination of environmental and genetic factors that may vary among different patients. Thus, treatment in the future will likely be personalized to each patient based on their risk factors.
Biography
Meron Tesfay, MD, (top left), and W. Joseph Goldkamp, MD, (top right), are Fellows in Gastroenterology and Brent A. Neuschwander-Tetri, MD, (bottom), is a Professor of Internal Medicine at Saint Louis University School of Medicine, St. Louis, Mo.
Contact:brent.tetri@health.slu.edu



Footnotes
Disclosure
None reported.
References
- 1.Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. doi: 10.1186/s12916-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease--meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017 doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 6.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: An analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112:581–587. doi: 10.1038/ajg.2017.5. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of hepatitis c virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099. e1091. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 11.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinar y team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131. e121. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinella ME. Nonalcoholic fatty liver disease: A systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 14.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 15.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34 doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637. e627. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 19.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPL A3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. e365. doi: 10.1053/j.gastro.2015.04.005. quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 21.Abenavoli L, Greco M, Milic N, Accattato F, Foti D, Gulletta E, et al. Effect of mediterranean diet and antioxidant formulation in nonalcoholic fatty liver disease: A randomized study. Nutrients. 2017;9 doi: 10.3390/nu9080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based inter ventions for nonalcoholic fatty liver disease: A meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Corey KE, Rinella ME. Medical and surgical treatment options for nonalcoholic steatohepatitis. Dig Dis Sci. 2016;61:1387–1397. doi: 10.1007/s10620-016-4083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 26.Bays H, Cohen DE, Chalasani N, Harrison SA The National Lipid Association’s Statin Safety Task F. An assessment by the statin liver safety task force: 2014 update. J Clin Lipidol. 2014;8:S47–57. doi: 10.1016/j.jacl.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Yang YJ, Kim KM, An JH, Lee DB, Shim JH, Lim YS, et al. Clinical significance of fatty liver disease induced by tamoxifen and toremifene in breast cancer patients. Breast. 2016;28:67–72. doi: 10.1016/j.breast.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, et al. Incidence and risk factors for non-alcoholic steatohepatitis: Prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]