Abstract
Sleep disorders are prevalent in Parkinson disease (PD), a disease with well recognized motor dysfunction. Sleep related problems received little attention until the last three decades. Sleep disorders seen in PD patients include insomnia, excessive sleepiness, restless legs syndrome, REM sleep behavior disorder. Some of these can have significant impact and lower the quality of life in these patients. An understanding of sleep issues in PD can help identify them early and result in optimal management.
Introduction
Parkinson disease (PD) is a neurodegenerative disorder first described by James Parkinson in his ‘ Essay on Shaking Palsy’1. It has predominant motor dysfunction. Its prevalence increases with age. In his description, Parkinson briefly mentioned the sleep disturbances seen in the terminal stages of PD. He described severe exhaustion and extreme sleepiness. Though motor symptoms dominate initially, the patients start experiencing non-motor symptoms as the disease advances. These include: dysautonomia (constipation, erectile dysfunction, impaired heat regulation, etc.), mood disorders (anxiety, depression) and sleep problems.
Sleep disturbances in PD are numerous, multifactorial, and result in a significant morbidity even contributing to earlier institutionalization2. Prevalence of sleep problems in PD can vary anywhere from 50%3 to 81%4,5. Although nighttime sleep impairment and excessive daytime sleepiness were reported first, other sleep-related problems in PD received little attention until the last three decades. In this review article, we will discuss the sleep-related issues in Parkinson disease.
Sleep-Wake Architectural Differences and Circadian Variation of Symptoms in Parkinson Disease
With increasing understanding and analysis of sleep in PD, sleep architectural abnormalities are reported in this patient population.
Compared to healthy controls, PD patients have reduced total sleep time6. Sleep fragmentation is a consistent finding and may be seen early during the disease7. Sleep fragmentation is also a common finding in other neurodegenerative disorders8. Sleep efficiency is reduced with increased amount of WASO (Wakefulness After Sleep Onset). Nocturnal arousals are common complaint in PD patients. Up to 80% of PD patients report two to five nocturnal awakenings9. Coexisting problems like benign prostatic hypertrophy, diuretic usage, etc., may further worsen this. Some studies report increased amount of Rapid Eye Movement (REM) sleep with shortened onset to REM especially with coexisting depression10. Increased alpha activity has been noted in drug naive patients with Parkinson disease.
People with PD may show circadian variation of their symptoms11. Some feel better in the morning while some may feel worse. A small proportion may not have this circadian variation9. However, studies analyzing changes in the circadian rhythm in Parkinson disease population did not find any differences between patients and controls. One study showed increased melatonin secretion in patients with significant tremor compared to patients with just akinesia or rigidity. Treatment with levodopa is reported to cause phase advance of the nocturnal melatonin secretion12.
Medications used in PD can also affect sleep architecture. Levodopa can result in REM suppression and increased REM latency6,13. Levodopa can also block REM rebound following REM sleep deprivation14. Dopamine receptors agonists can also suppress REM sleep. Anticholinergic drugs can increase REM latency and suppress REM sleep15. Similar REM sleep suppression is noted with Mono-Amine Oxidase Inhibitors and Catechol-O-Methyl Transferase inhibitors. Deep brain stimulation of subthalamic nucleus was shown to improve total sleep time and sleep efficiency and reduction in WASO16.
Insomnia
Insomnia is persistent difficulty with sleep initiation, duration, consolidation, or quality occurring despite adequate opportunity/circumstances for sleep and resulting in daytime impairment. Daytime symptoms include fatigue, mood issues, irritability, malaise, and cognitive impairment. Some patients with insomnia may also experience physical symptoms like muscle tension, palpitations, and headache. Under the international classification of sleep disorders, insomnia with PD is categorized as “Insomnia due to a Medical condition.” Female sex, disease duration, and presence of depression are associated with higher risk of insomnia in PD patients17.
A variety of reasons are proposed for insomnia in PD. Insomnia is more prevalent in elderly and PD is a disease, typically of elderly. Inadequately controlled motor symptoms can interfere with sleep initiation and maintenance. Associated Restless Legs Syndrome (RLS) can result in sleep onset difficulties. Periodic leg movements in sleep can cause sleep fragmentation with resulting sleep maintenance insomnia. Nocturia is another frequent problem in elderly and can cause repeated awakenings from sleep. This can result from prostatic issues but can also be due to dysautonomia and fluctuations in responses to medication.
Co-existing mood problems contribute to daytime dysfunction and affect night sleep. In fact, depression is common and seen in up to 50% of PD population17. Identification of mood disorders should be part of evaluation of PD patients. Coexisting depression and anxiety can result in sleep fragmentation and early morning awakenings10. Addressing and alleviating depression and anxiety in these patients can improve the day time functioning and help with the night time insomnia.
Pain disorders cause sleep disruption and insomnia and increased prevalence of pain in PD patients may negatively impact sleep. Approximately 46% of patients with PD report chronic pain18. Nocturnal limb dystonia (discussed below) can also result in early morning awakening.
Sleep-Disordered Breathing in PD
Sleep-related breathing disorders include obstructive sleep apnea, central sleep apnea, sleep-related hypoventilation, and sleep-related hypoxemia.
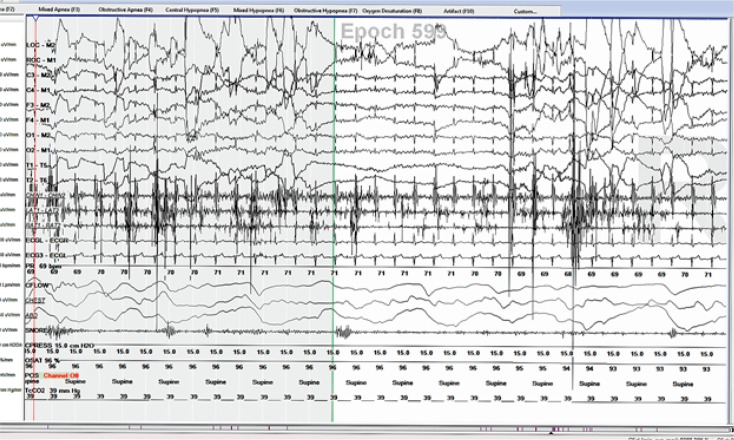
A high incidence of central and obstructive apnea is found in moderate to severe PD19 as compared to early stages19,20. Several factors may be contributory including rigidity, diaphragmatic dyskinesias, abnormal movement of glottis-supraglottic structures, and stridor. These in conjunction with increased tone in upper airways increase the likelihood of obstructive sleep apnea21–23. Sleep disordered breathing can cause chronic sleep deprivation and is a risk factor for cardiovascular and cerebrovascular disease. Untreated sleep apnea can result in sleep deprivation with daytime sleepiness, fatigue, early morning headaches, poor concentration, etc.
Treatment of sleep disordered breathing depends on the type of the disorder. Obstructive sleep apnea is treated with Positive Airway Pressure (PAP) therapies, mandibular advancement devices, positional therapy, and surgery in select cases. Treatment of central sleep apnea involves addressing the causative factors, advanced PAP therapies, and in some cases, respiratory stimulants like acetazolamide. Hypoxemia disorder is treated with supplemental oxygen while hypoventilation is treated with advanced PAP therapies.
Parasomnias and Other Sleep-Related Movement Disorders
Restless Legs Syndrome and Periodic Leg Movements in Sleep
Restless Legs Syndrome (Willis-Ekbom Disease) is associated with urge to move the legs due to unpleasant and uncomfortable sensations. These sensations begin or worsen during periods of rest or inactivity and are partially or totally relieved by movement. The symptoms occur mainly in the evening or nighttime. Sometimes, symptoms may involve arms and very rarely, the whole body. RLS can result in significant distress to the patients. It can interfere with sleep onset and cause insomnia. Daytime fatigue and sleepiness are common associated complaints with restless leg syndrome.
Prevalence of RLS is estimated to be 5–10% of adult population in North America. It is twice as prevalent in women as in men. Prevalence increases with age. Iron deficiency, certain medications (sedating antihistamines, centrally-acting dopamine blockers, and most antidepressants), pregnancy, chronic renal failure, and prolonged immobility are associated with increased risk of RLS. There is a strong familial association especially with early onset RLS.
Multiple studies show higher RLS prevalence in PD patients than in general population24. RLS profile of PD patients, however, is different from general population. RLS in PD tends to start relatively late, tends to be milder with most patients having no family history of RLS25. The overall impact of RLS on PD patients seems modest in terms of day time sleepiness and quality of life24.
RLS responds to dopaminergic medications especially agonists (pramipexole, ropinirole, rotigotine), already used for treatment of PD. Other medications like gabapentin enacarbil can help especially in patients with coexisting neuropathy. Opioidergic medications are a second line therapy in refractory RLS cases.
Periodic limb movements are repetitive, stereotyped, usually lower extremity movements in sleep, and may cause sleep disruption with daytime symptoms. Symptoms associated with periodic leg movement disorder may result from sleep fragmentation like daytime fatigue, daytime sleepiness, lack of energy etc. Periodic leg movements in sleep can also disrupt the sleep of the bed partner. They are commonly associated with restless legs syndrome.
REM Sleep Behavior Disorder
REM sleep is characterized by rapid eye movements and skeletal muscle atonia. Respiration is diaphragm–based during REM sleep. Dreaming during REM sleep tends to be vivid and detailed, compared to vague content of Non-REM (NREM) sleep. Muscle atonia is mediated by brainstem centers, pedunculopontine nucleus and locus ceruleus, and serves as a protective mechanism preventing dream enactment during REM. However, conditions resulting in disruption of this protective mechanism can lead to physical enactment of dream content during REM26. This phenomenon is called REM Sleep Behavior Disorder (RBD) 27.
Parkinson disease is very strongly associated with RBD. In fact, RBD can predate the initial motor symptoms by years28 and is one of the best known biomarkers for PD. RBD is present in 25–50% of PD patients and is even more frequent in multiple system atrophy and Lewy body dementia29,30. Forty percent of the patients with idiopathic RBD develop a parkinsonian syndrome within a decade, and two-thirds within two decades31–33. Mild cognitive impairment34 and dysautonomia35 were seen in these patients with idiopathic RBD.
As PD advances, higher amount of phasic muscle activity is found in both REM and NREM sleep36. Patients experiencing RBD may have mild, low amplitude limb movements that are often overlooked. Some patients have vocalizations. These movements can be significant and violent with apparent goal directed behaviors that can cause injury with lacerations, ecchymosis and, even death37. Curiously, the motor activity in RBD appears smoother, faster and stronger than the patient’s day time motor activity38. Patients may report falling from their beds during the night. Their bed partners may sometimes be able to understand the dream content based on the patient’s behavior. Some episodes result in arousal from sleep. Usually, RBD episodes do not cause significant sleep disruption.
Management includes careful history from the patient and the spouse. The first step is to make the sleeping environment safe – e.g., putting soft pads around the bed to minimize the impact of falls, using pillows, keeping distance from the bed partner, and removing sharp and potentially injurious objects. Most common medications used in the treatment of RBD are Melatonin and Clonazepam. They should be started at low doses and increased if the RBD behaviors continue. Morning grogginess and day time somnolence are common side effects (especially with clonazepam) and should be part of patient counselling. Refractory cases may require combination of both medications. Of note, presence of medications like Tricyclic antidepressants and Selective Serotonin Reuptake inhibitors can increase the likelihood of causing RBD episodes. Discontinuation of these medications should be considered, especially if there is a temporal correlation between the initiation of medication and the onset of RBD. Unlike most other motor problems of Parkinson disease, RBD does not respond to dopaminergic therapy. See Figure 1.
Figure 1.
Increased EMG activity in the chin and the leg leads suggestive of RBD in a patient with Parkinson disease.
Nocturnal Limb Dystonia
Limb dystonia may occur in the affected limb during the night as off phenomenon and can be painful. Painful extension of the great toe called ‘Striatal toe’ is an ‘off phenomenon’ and happens as the concentration of Levodopa declines during the night. It can cause early morning awakenings. Addition of long-acting Levodopa/Carbidopa formulation or a dopamine agonist (which typically tends to have a longer effect than Levodopa formulations) can help.
Excessive Daytime Sleepiness
Excessive daytime sleepiness (EDS) is a common problem in PD patients. Up to one-third of the patients can have disabling sleepiness39. The incidence of excessive sleepiness increases as the disease advances40 and can have a significant impact on the quality of life measures in PD patients. Early recognition and appropriate management is very important. See Figure 2.
Figure 2.
It is important to establish the temporal relationship between increased sleepiness and the initiation of medication before the medication changes are planned.
There are multiple potential reasons for increased sleepiness in Parkinson disease. Based on the analysis of the PSG data, Arnulf et al. suggested sleepiness as an integral part of neurodegeneration in PD41. Neurodegenerative disorders in general tend to have EDS as a common feature. This is thought to be due to involvement of alerting mechanisms and reduction in the levels of neurotransmitters (like acetyl choline, dopamine, serotonin, and norepinephrine) that increase alertness. Reduction in some of the same neurotransmitters is also implicated in mood disorders seen in PD. Recently, it has been found that PD is associated with loss of orexin producing neurons42,43 – same neurons that are lost in narcolepsy. These neurons play a critical role in alertness and wakefulness.
In addition, medications to treat PD may also contribute to the EDS. Dopamine agonists (DA) have a greater tendency to cause sleepiness than Levodopa formulations. This effect is dose dependent but it is not uncommon to see intolerable sleepiness in some patients even with low doses. Though these medications are used for addressing the cardinal symptoms of PD, they may also be started to treat RLS in this patient population. DAs may cause sleep attacks, which are episodes of sudden onset of irresistible sleep out of a relative alert state. These can be dangerous and potentially result in accidents and injuries, and should always be inquired in PD patients on dopaminergic therapy. They were first reported in eight patients taking non-ergot dopamine agonists at high doses44. Sleepiness in PD has been found to increase with the duration of dopaminergic therapy40.
Though sleep attacks are less likely with Levodopa formulations45, they may happen. Amantadine, another medication used in managing Parkinson disease, may be associated with sleepiness. Amantadine has both anti-NMDA and anti-cholinergic properties along with dopaminergic activity. Antagonism of acetyl-choline appears to be the main reason for increased sleepiness caused by amantadine. Tolerance to amantadine gradually decreases as people get older.
Another reason for daytime sleepiness is nocturnal sleep fragmentation causing chronic sleep deprivation. Insomnia may also contribute to daytime sleepiness and fatigue. Some patients may also have irregular sleep-wake routine causing sleepiness during the day and insomnia at night.
Clinicians should have a high index of suspicion for EDS. Addressing the EDS can sometimes be challenging. The dopaminergic medications that are helping the patient in alleviating the motor symptoms may be causing sleepiness. It is important to establish the temporal relationship between increased sleepiness and the initiation of medication before the medication changes are planned. Younger patients tend to tolerate DAs better than older patients. When DAs are intolerable, levodopa formulations should be introduced for symptomatic treatment of PD symptoms. Sometimes, tremors may not respond to dopaminergic therapy and require anti-cholinergic agents. Sleepiness from anti-cholinergics may limit their use and in fact, centrally-acting anti-cholinergics are usually intolerable when used in adult patients making their use very rare (the exception is amantadine which is commonly used). At times, patients can’t tolerate any of the symptomatic treatments due to disabling sleepiness, and DBS can be explored as an option in this select group. Appropriate patient selection for DBS is beyond the scope of this paper.
Other Miscellaneous Sleep-Related Issues
Sweating in Parkinson Disease
Thermoregulary and sweating disturbances are well recognized in PD patients. The disturbances in sweating can be both hypo- and hyperhidrosis, and are related to the spectrum of dysautonomia seen in this disease. Up to two-thirds of the patients with PD report a problem with sweating and this can have a considerable impact on the quality of life in PD patients. This may trouble the patients at night causing discomfort and arousals from sleep. Both central and peripheral mechanisms are thought to be involved46.
Tremor and Other Motor Symptoms of Parkinson Disease in Sleep
The resting tremor of PD usually ceases with the onset of stage I sleep. Even when it persists, the amplitude of the tremor is typically reduced in sleep by up to 50%, compared to waking level47. However, it may result in sleep onset insomnia in some people. Unlike essential tremor which is predominantly an action tremor, PD tremor is predominantly at rest. If the tremor is bothering the patient causing sleep onset insomnia, medications may have to be used to address the symptoms during sleep wake transition. Sometimes, patients may also experience bothersome tremor when they get up in the middle of the night. A long acting dopaminergic agent may help to address this issue. In some patients, the tremor improves during the morning hours suggesting a beneficial effect of sleep on tremor.
Similar to tremor, other features of PD like rigidity and bradykinesia can cause discomfort to the patients and sleep initiation problems. Other motor abnormalities described in PD patients include increased muscle tone, simple and complex movements, repeated blinking, blepharospasm at the onset of REM sleep, and prolonged tonic contractions of limb muscles during NREM sleep48.
Dreaming in Parkinson Disease
Parkinson disease patients on dopaminergic therapy report higher prevalence of dreams that are intense, unpleasant, frightening, and recurrent as compared to controls. Nightmares and nocturnal vocalizations are reported and increased with treatment. Patients who experience altered dreams frequently hallucinate and vice versa9,49. It has been suggested that a kindling mechanism consisting of progressive mesolimbic dopamine receptor hypersensitivity might be the reason for this strong association50.
Summary
Patients with PD can have a range of sleep disorders. They may be a feature of the disease process, result of other motor and non-motor symptoms, related to medications, or represent a primary sleep disorder. They can affect not just the quality of sleep but also the quality of life in patients with PD. Their recognition and appropriate treatment can play a vital role in the overall management and functioning of patients with PD.
Biography
Pradeep C. Bollu, MD, (top), and Pradeep Sahota, MD, FAAN, (below), MSMA member since 2003, are both in the Department of Neurology, University of Missouri School of Medicine.
Contact: BolluP@health.missouri.edu


Footnotes
Disclosure
None reported.
References
- 1.Parkinson J. An Essay on the Shaking Palsy (Sherwood, Neely, and Jones, London, 1817); MH Polymeropoulos, et al. Science. 1997;276:2045–2047. [Google Scholar]
- 2.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri K. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Movement Disorders. 2011;26(3):399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 3.Riedel O, Klotsche J, Spottke A, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. Journal of neurology. 2010;257(7):1073–1082. doi: 10.1007/s00415-010-5465-z. [DOI] [PubMed] [Google Scholar]
- 4.Nausieda PA, Weiner WJ, Kaplan LR, Weber S, Klawans HL. Sleep disruption in the course of chronic levodopa therapy: an early feature of the levodopa psychosis. Clinical neuropharmacology. 1982;5(2):183–194. doi: 10.1097/00002826-198205020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Van Hilten J, Weggeman M, Van der Velde E, Kerkhof G, Van Dijk J, Roos R. Sleep, excessive daytime sleepiness and fatigue in Parkinson’s disease. Journal of Neural Transmission-Parkinson’s Disease and Dementia Section. 1993;5(3):235–244. doi: 10.1007/BF02257678. [DOI] [PubMed] [Google Scholar]
- 6.Wetter TC, Collado-Seidel V, Pollmächer T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep: Journal of Sleep Research & Sleep Medicine. 2000. [PubMed]
- 7.Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson’s disease. Movement Disorders. 1998;13(6):895–899. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 8.Petit D, Gagnon J-F, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. Journal of psychosomatic research. 2004;56(5):487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Factor SA, McAlarney T, Sanchez Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Movement disorders. 1990;5(4):280–285. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- 10.Kostic VS, Susic V, Przedborski S, Sternic N. Sleep EEG in depressed and nondepressed patients with Parkinson’s disease. The Journal of neuropsychiatry and clinical neurosciences. 1991. [DOI] [PubMed]
- 11.Askenasy J. Sleep in Parkinson’s disease. Acta neurologica scandinavica. 1993;87(3):167–170. doi: 10.1111/j.1600-0404.1993.tb04095.x. [DOI] [PubMed] [Google Scholar]
- 12.Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of l-dopa. Journal of Neural Transmission: Parkinson’s Disease and Dementia Section. 1993;5(3):227–234. doi: 10.1007/BF02257677. [DOI] [PubMed] [Google Scholar]
- 13.Gillin JC, Post R, Wyatt RJ, Goodwin FK, Snyder F, Bunney WE. REM inhibitory effect of L-DOPA infusion during human sleep. Electroencephalography and clinical neurophysiology. 1973;35(2):181–186. doi: 10.1016/0013-4694(73)90174-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa Y, Tachibana H, Kotorii M, Ogata M. Effects of L DOPA on Natural Night Sleep and on Rebound of REM Sleep. Psychiatry and Clinical Neurosciences. 1973;27(3):223–230. doi: 10.1111/j.1440-1819.1973.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 15.Pal P, Calne S, Samii A, Fleming J. A review of normal sleep and its disturbances in Parkinson disease. Parkinsonism & related disorders. 1999;5(1):1–17. doi: 10.1016/s1353-8020(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 16.Arnulf I, Bejjani B, Garma L, et al. Improvement of sleep architecture in PD with subthalamic nucleus stimulation. Neurology. 2000;55(11):1732–1735. doi: 10.1212/wnl.55.11.1732. [DOI] [PubMed] [Google Scholar]
- 17.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson disease: a community-based study. Archives of neurology. 1996;53(2):175–179. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- 18.Goetz CG, Wilson RS, Tanner CM, Garron DC. Relationships among pain, depression, and sleep alterations in Parkinson’s disease. Advances in neurology. 1987;45:345. [PubMed] [Google Scholar]
- 19.Hardie R, Efthimiou J, Stern G. Respiration and sleep in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1986;49(11):1326. doi: 10.1136/jnnp.49.11.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferini-Strambi L, Franceschi M, Pinto P, Zucconi M, Smirne S. Respiration and heart rate variability during sleep in untreated Parkinson patients. Gerontology. 1992;38(1–2):92–98. doi: 10.1159/000213312. [DOI] [PubMed] [Google Scholar]
- 21.Hovestadt A, Bogaard J, Meerwaldt J, van Der Meche F, Stigt J. Pulmonary function in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1989;52(3):329–333. doi: 10.1136/jnnp.52.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincken WG, Gauthier SG, Dollfuss RE, Hanson RE, Darauay CM, Cosio MG. Involvement of upper-airway muscles in extrapyramidal disorders: a cause of airflow limitation. New England Journal of Medicine. 1984;311(7):438–442. doi: 10.1056/NEJM198408163110704. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick A. Reply-Upper airway obstruction in Parkinson’s disease. Anaesthesia and Intensive Care. 1996;24(1):122–122. [PubMed] [Google Scholar]
- 24.Ondo WG. Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease. Springer; 2015. Restless Legs Syndrome and Periodic Limb Movements in Parkinson’s Disease; pp. 159–171. [Google Scholar]
- 25.Fereshtehnejad SM, Shafieesabet M, Shahidi G, Delbari A, Lökk J. Restless legs syndrome in patients with Parkinson’s disease: a comparative study on prevalence, clinical characteristics, quality of life and nutritional status. Acta Neurologica Scandinavica. 2015;131(4):211–218. doi: 10.1111/ane.12307. [DOI] [PubMed] [Google Scholar]
- 26.Boeve BF, Silber M, Saper C, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(11):2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 27.Schenck CH, Bundlie SR, Ettinger MG, Mohowald M. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. SLEEP-NEW YORK-. 2002;25(2):119–119. [PubMed] [Google Scholar]
- 28.Postuma R, Gagnon J, Vendette M, Fantini M, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comella CL, Nardine TM, Diederich NJ, Stebbins GT. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson’s disease. Neurology. 1998;51(2):526–529. doi: 10.1212/wnl.51.2.526. [DOI] [PubMed] [Google Scholar]
- 30.Gagnon J-F, Bédard M-A, Fantini M, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59(4):585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 31.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46(2):388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 32.Iranzo A, Molinuevo JL, Santamaría J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. The Lancet Neurology. 2006;5(7):572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 33.Gagnon J-F, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. The Lancet Neurology. 2006;5(5):424–432. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 34.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Annals of neurology. 2009;66(1):39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 35.Lanfranchi PA, Fradette L, Gagnon J, Colombo R, Montplaisir J. Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder. SLEEP-NEW YORK THEN WESTCHESTER-. 2007;30(8):1019. doi: 10.1093/sleep/30.8.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bliwise DL, Trotti LM, Greer SA, Juncos JJ, Rye DB. Phasic muscle activity in sleep and clinical features of Parkinson disease. Annals of neurology. 2010;68(3):353–359. doi: 10.1002/ana.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenck CH, Lee SA, Bornemann MAC, Mahowald MW. Potentially lethal behaviors associated with rapid eye movement sleep behavior disorder: review of the literature and forensic implications. Journal of forensic sciences. 2009;54(6):1475–1484. doi: 10.1111/j.1556-4029.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 38.De Cock VC, Vidailhet M, Leu S, et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain. 2007;130(2):450–456. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 39.Arnulf I, Leu-Semenescu S. Sleepiness in Parkinson’s disease. Parkinsonism & related disorders. 2009;15:S101–S104. doi: 10.1016/S1353-8020(09)70792-8. [DOI] [PubMed] [Google Scholar]
- 40.Valko P, Waldvogel D, Weller M, Bassetti C, Held U, Baumann C. Fatigue and excessive daytime sleepiness in idiopathic Parkinson’s disease differently correlate with motor symptoms, depression and dopaminergic treatment. European journal of neurology. 2010;17(12):1428–1436. doi: 10.1111/j.1468-1331.2010.03063.x. [DOI] [PubMed] [Google Scholar]
- 41.Arnulf I, Konofal E, Merino–Andreu M, et al. Parkinson’s disease and sleepiness an integral part of PD. Neurology. 2002;58(7):1019–1024. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- 42.Thannickal TC, Lai Y-Y, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130(6):1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson’s disease. Brain. 2007;130(6):1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 44.Frucht S, Rogers J, Greene P, Gordon M, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999;52(9):1908–1908. doi: 10.1212/wnl.52.9.1908. [DOI] [PubMed] [Google Scholar]
- 45.Paus S, Brecht HM, Köster J, Seeger G, Klockgether T, Wüllner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Movement Disorders. 2003;18(6):659–667. doi: 10.1002/mds.10417. [DOI] [PubMed] [Google Scholar]
- 46.Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N. Sweating dysfunction in Parkinson’s disease. Movement disorders. 2003;18(12):1459–1463. doi: 10.1002/mds.10586. [DOI] [PubMed] [Google Scholar]
- 47.Stern M, Roffwarg H, Duvoisin R. The parkinsonian tremor in sleep. The Journal of nervous and mental disease. 1968;147(2):202–210. doi: 10.1097/00005053-196808000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Mouret J. Differences in sleep in patients with Parkinson’s disease. Electroencephalography and clinical neurophysiology. 1975;38(6):653–657. doi: 10.1016/0013-4694(75)90168-6. [DOI] [PubMed] [Google Scholar]
- 49.Sharf B, Moskovitz C, Lupton M, Klawans H. Dream phenomena induced by chronic levodopa therapy. Journal of neural transmission. 1978;43(2):143–151. doi: 10.1007/BF01579073. [DOI] [PubMed] [Google Scholar]
- 50.Klawans H, Moskovitz C, Nausieda P, Weiner W. Levodopa-induced dopaminergic hypersensitivity in the pathogenesis of psychiatric and neurologic disorders. International journal of neurology. 1979;13(1–4):225. [PubMed] [Google Scholar]