Abstract
Over-prescription of opioid pain medications and increases in heroin use have contributed to the sharp rise in opioid-related hospitalizations and overdose deaths among young adults in the United States, including pregnant women. This has imposed substantial direct and indirect costs to our nation’s health care system. Effective treatment with methadone and buprenorphine is available, but significant barriers to care may restrict access for many. Improved screening tools and expanded access to treatments for substance use disorders are keys to addressing the epidemic of opioid use disorder.
Introduction
Opioid Use Disorder (OUD), a problematic pattern of opioid use that causes clinically significant impairment or distress,1 has become epidemic in the United States, due largely to the over-prescription of opioid pain relievers during the past two decades. Since 1999, the number of opioid prescriptions has quadrupled, as have the number of drug overdose deaths in the U.S., 60% of which involve opioids including heroin.2,3 In 2014, opioids accounted for a record 28,647 overdose deaths in the U.S.4 In that same year, heroin was involved in 338 of Missouri’s 1,067 drug overdose deaths.2 In 2012, U.S. physicians wrote 259 million opioid prescriptions, many for patients, including pregnant women, with chronic, non-cancer related pain.5,6 Each year since 2013 more Americans, including Missourians, have died of drug overdoses than have died in motor vehicle crashes, with young adults aged 18–25 being affected the most.7 The estimated annual cost of substance use disorders in the U.S., including direct health care costs, lost productivity, and costs related to violence and crime, is $442 billion.8 Abuse of prescription drugs has become so prevalent that Federal, State, and local agencies, including the Surgeon General, have called on all physicians and public health agencies to improve prevention efforts, diagnosis and management strategies, and to modify prescribing practices in order to combat the growing problem of OUD in the United States.9
History
In the 1990s, there was renewed attention on the under-treatment of chronic pain as a public health problem in the United States. The Institute of Medicine report, Relieving Pain in America,10 called for more frequent and comprehensive assessment of pain (the “5th vital sign”) by physicians in all specialties, including primary care. Many physicians, inadequately trained in treating chronic pain, found themselves caught between overprescribing opioids and undertreating pain. Over the next decade, liberal prescribing practices and the aggressive marketing of long acting, extended release formulations of opioids (e.g., OxyContinTM) had some unintended consequences. The increase in opioid prescribing coincided with increased rates of opioid addiction, overdose deaths and hospitalizations, and entry into drug treatment programs.11 Practitioners, and the medical community in general, largely overestimated the efficacy of opioids in treating chronic pain and underestimated their associated risks of addiction, overdose, and death. Although very effective for the short term treatment of acute pain with a low risk of addiction, 12 prolonged use of opioid analgesics (i.e., greater than six weeks) for chronic, non-cancer related pain is not efficacious for reducing pain scores or improving functionality or quality of life.13 Further, the risk of addiction for patients on long term opioid therapy for chronic pain is estimated to be 26–35%.14, 15
In response to the epidemic of OUD, State and Federal agencies have tightened regulatory controls on the dispensing and sale of prescription pain medications. In October 2016, the DEA announced new regulations that will reduce the manufacture of some prescription opioids by 25–34% in 2017.16 To assist efforts at the state level, the Centers for Disease Control and Prevention (CDC) has developed the “Prevention for States” program, which provides funding to states with innovative solutions to address prescription drug overdose and abuse. Twenty-nine states (Missouri is not included) have received a share of these funds to institute safer prescribing practices. Prescription Drug Monitoring Programs (PDMPs), electronic databases that track the statewide prescription and dispensing of controlled substances, have been instrumental in limiting opioid prescribing in some states. Laws requiring prescribers in New York and Tennessee to query their state’s PDMP prior to prescribing opioid analgesics have been effective in reducing the number of patients filling multiple prescriptions for the same drugs (“doctor shopping”).17 Similar laws in Florida have stopped high volume pain clinics (“pill mills”) from prescribing and dispensing large quantities of opioid analgesics to patients with no justifiable need for them. Since 2010, overdose deaths in Florida due to oxycodone have decreased by 50%.18 Despite being in the second highest quartile of opioid prescriptions per 100 people, Missouri is the only state without a PDMP.19
Heroin
Although regulatory efforts to curtail opioid prescribing have reduced the supply of prescription opioids, demand by non-medical users remains high because of the experiences or feelings they cause. Increasingly since 2005, heroin has filled this void as an inexpensive, readily available, and easy-to-use alternative to prescription opioids. According to the 2015 U.S. National Survey on Drug Use and Health, over 300,000 American adults reported using heroin in the past year.20 The number of overdose deaths from heroin increased from 1,878 in 2004 to 10,574 in 2014, and have tripled since 2010 when a tamper resistant formulation of OxyContinTM was introduced.21 It is estimated that 80% of new heroin users abused prescription opioids prior to using heroin.22 Over the past 30 years, the cost of a gram of heroin has gone from nearly $3,000 (U.S.) to less than $500.23 One of the authors (FTO) has been told by a patient at the University of Missouri with OUD that a gram of pure heroin can be purchased for as little as $200 in Columbia, Missouri. The gram is then typically divided into 10 “hits” that are sold individually.
Management of Opioid Use Disorder
The diagnostic criteria for opioid use disorder include the development of tolerance, and a strong desire or urge to use opioids (craving) despite negative social, occupational, and financial consequences.1 Patients who abruptly discontinue opioids, voluntarily or otherwise, after long term regular use are at risk for the physical and psychologic symptoms of acute opioid withdrawal, which include pain, autonomic hyperactivity, sweating, nausea, vomiting, diarrhea, anxiety, and insomnia. Withdrawal symptoms occur when opioids rapidly dissociate from their receptors in the brain and spinal cord. Avoidance of withdrawal symptoms is one reason that patients with opioid use disorder continue to use and misuse opioids, even though they may wish to stop.
The standard of care for treating opioid use disorder is medication-assisted treatment (MAT) that combines daily medically-supervised administration of FDA-approved medications with behavioral therapy and counseling.24 MAT is effective in facilitating recovery from OUD, preventing relapses, improving social and occupational functioning, and in reducing criminal behavior and the spread of infectious diseases.25 The most effective treatment for withdrawal remains opioid replacement therapy with long acting opioids such as methadone and buprenorphine. These medications prevent withdrawal symptoms and opioid cravings by continuous occupation of endogenous opioid receptors. Methadone, a full agonist at the endogenous mu opioid receptor, has long been the mainstay of treatment for opioid addiction. Methadone has a long half-life and is ideally suited for once daily oral dosing, but it carries the risks of respiratory depression and is among the most commonly implicated prescription opioids in overdose deaths.26 Despite its risks, methadone has been shown to reduce mortality in patients with opioid use disorder by half.27 Methadone is available only from federally-regulated specialty clinics approved to prescribe and dispense the drug to patients who meet strict inclusion criteria. Most states provide Medicaid coverage for methadone maintenance therapy, but most of the 17 states that do not are located in areas of the U.S. with high rates of OUD and heroin use (e.g., the South, Midwest, and Ohio River Valley).28
Unlike methadone, buprenorphine is only a partial agonist at the mu receptor. This partial activity prevents withdrawal, but also blocks the action of full opioid agonists taken concomitantly.29 Buprenorphine is thus limited in its potential to cause sedation and respiratory depression, a safety advantage over methadone.30 Buprenorphine is available as a rapidly dissolving sublingual tablet or buccal film, some formulations of which contain small amounts of the opioid antagonist naloxone (NarcanTM) to deter intravenous injection of the drug. Compared to methadone, buprenorphine is not restricted to specialty clinics and is available by prescription from office-based physicians who are specially trained in treating opioid use disorder and have DEA approval to prescribe it. Although outpatient buprenorphine therapy is covered by Medicaid in all 50 states, it may be inaccessible in many areas due to a shortage of physicians licensed by the DEA to prescribe it.
Although both are effective, the choice between methadone and buprenorphine depends on individual patient factors such as proximity to treatment programs. Whenever long term opioid therapy is elected for, patients are encouraged to participate in educational programs as well as cognitive and behavioral therapy such as individual or group counseling and participation in self-help groups like Narcotics Anonymous. Although maintenance therapy with methadone or buprenorphine can be tapered down over time and in some cases discontinued altogether, most individuals require lifelong treatment as relapse rates are high.31
In addition to its proven efficacy, medication-assisted treatment of OUD is cost saving. Every dollar spent on treatment of substance use disorders saves $4 in health care costs and $7 in criminal justice costs.32
Pregnancy
Since 2002, the incidence of first time heroin use has doubled among women and among young adults aged 18–25 years, a demographic that includes many pregnant women.33 According to the American College of Obstetrics and Gynecology, 1% of pregnant women report nonmedical use of opioid pain medications.34 Pregnant women with opioid use disorder are at increased risk for adverse pregnancy outcomes including preterm labor, fetal death, growth restriction, and neonatal abstinence syndrome, which increased by 300% from 2000 to 2009.35,36
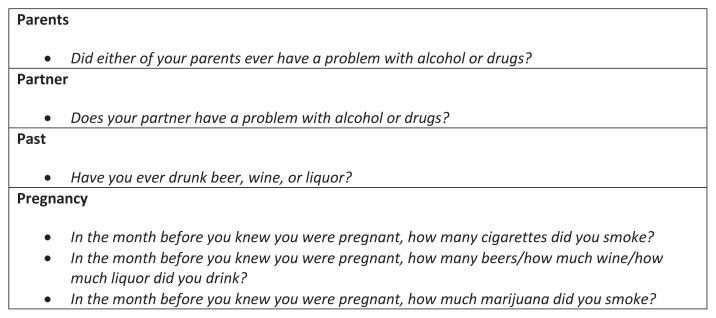
Universal screening for substance use disorders, including opioid use disorder, is recommended in pregnancy, and should occur at the initial prenatal visit and be performed again once per trimester for those who screen positive for past use. This should be done using a validated screening tool such as the 4Ps Plus questionnaire (see Figure 1),37 an instrument to quickly identify obstetric patients at risk for misusing alcohol, tobacco, and illicit drugs, or the three question set of “In the past year how many times have you had more than four alcoholic drinks per day, used tobacco, or taken illegal drugs or used prescription drugs for non-medical purposes?”.38 Screening by means of a questionnaire is recommended over the use of universal urine drug screens, which have poor sensitivity and specificity for chronic use, and it is essential that the topic of substance use disorder be broached in a non-judgmental fashion. Care for women with opioid use disorder should be approached in a manner similar to that for women with other chronic conditions such as diabetes, chronic hypertension, obesity, or any of the myriad of maternal comorbidities that complicate pregnancy.
Figure 1.
The 4Ps Plus©
Reprinted with permission, NTI Upstream
The goal of treating maternal OUD is to use the lowest possible dose of methadone or buprenorphine in order to eliminate cravings and withdrawal. While it has traditionally been taught that maternal withdrawal during opioid detoxification is harmful to the fetus, a recent publication by Bell, et al. challenges this notion.39 Patients will frequently state a desire to wean off of MAT during pregnancy in order to minimize the risk of neonatal abstinence syndrome (NAS). We generally recommend against this approach due to the unacceptably high risk of relapse, which ranges from 35 to 75% depending on the intensity of outpatient therapy.
In regards to the fetus, maternal opioid use disorder carries significant risks. Maternal overdose and the associated risk of coma, aspiration, hypothermia, and cardiovascular collapse can be life threatening for the developing fetus. Neonates exposed to opioids in utero have a risk of neonatal abstinence syndrome (NAS) that is as high as 90%.34 NAS is characterized by neonatal irritability, temperature dysregulation, poor feeding, failure to thrive, and in some cases seizures. This condition may last for up to 10 weeks after delivery and may necessitate a prolonged NICU admission.40 Some data suggests a lower rate of NAS with buprenorphine therapy as opposed to methadone, but this data is far from conclusive.41 Although buprenorphine alone (SubutexTM) has been traditionally preferred over buprenorphine-naloxone (SuboxoneTM) for pregnant women due to concerns about the fetal effects of naloxone, oral naloxone is biologically inert and a growing body of data supports the safety of buprenorphine-naloxone use in pregnancy.
The peripartum period is a particularly challenging time to provide care for women with OUD. Due to chronic opioid use and the development of tolerance, these individuals may require larger doses of pain medications than providers are typically accustomed to giving. This can lead to the patient being accused of ‘drug seeking behavior’ and her pain going untreated.
Our approach to caring for women on MAT at the time of delivery is to maximize the use of multimodal analgesia and to use regional anesthesia whenever possible. Neuraxial anesthesia (i.e., spinal or epidural) is the preferred method for both vaginal and cesarean delivery. Of note, there is no contraindication to intrathecal morphine in the setting of MAT use. In women with OUD undergoing vaginal delivery, we also treat pain with acetaminophen and NSAIDS and if necessary increase the dose of methadone or buprenorphine. In women who continue to have refractory pain, we will use adjunctive opioid therapy in the form of oral medication or patient controlled analgesia (PCA) as appropriate (intravenous or transdermal fentanyl in the setting of buprenorphine use) with early outpatient follow up with the patient’s MAT provider.
Pregnant women on MAT with buprenorphine and methadone require careful attention in regards to medication interactions. The use of opioid agonist/antagonists such as butorphanol (Stadol™) or nalbuphine (Nubain™) is absolutely contraindicated in patients on methadone or buprenorphine due to the risk of precipitated withdrawal. Additionally, our practice is to avoid the concurrent use of full opioid agonists with buprenorphine. Because of buprenorphine’s higher binding affinity for the mu receptor compared with full agonists (except fentanyl),42 individuals undergoing induction of buprenorphine therapy must be free of opioids and in withdrawal before starting buprenorphine. In an individual with opioid dependence who has a full opioid agonist in her system and is therefore not in withdrawal, giving a dose of buprenorphine will result in the partial agonist buprenorphine displacing the full agonist from the mu receptors, which would in turn precipitate withdrawal due to the lower mu agonist activity of buprenorphine. Furthermore, an individual on long term buprenorphine therapy with refractory pain is unlikely to achieve adequate analgesia with typical mu receptor agonists (oxycodone, hydrocodone, morphine, etc) because large doses of these medications will be necessary to overcome the higher binding affinity of the buprenorphine.
As part of the treatment plan, it is essential to assure patients that the care team will do everything possible to manage the pain associated with labor and delivery and that the treatment of surgical pain will not increase the risk of relapse. If a patient enters prenatal care on MAT, it is recommended that the same provider continue to manage her MAT during pregnancy. In cases where MAT is initiated by an obstetric provider, arrangements should be made for continued treatment with MAT post-partum, which may entail cooperation with a methadone clinic, primary care provider, or addiction psychiatrist as appropriate. It is also important to arrange for consultation between the patient and the pediatricians or neonatologists who will be caring for the baby after delivery. The care of the child affected by maternal opioid use does not end at delivery and the patient should have an opportunity to ask questions regarding the post-partum care plan.
Although MAT with either methadone or buprenorphine is recognized as the standard of care for treating OUD during pregnancy, significant barriers to care exist and prevent more than half of pregnant women with OUD from receiving the recommended treatments.43 Many women with OUD live in states where methadone is not covered under Medicaid, or live in areas where there are no physicians who are licensed to prescribe buprenorphine. Women in the criminal justice system are even less likely to receive standardized care for OUD. In addition to issues relating to accessibility and funding, women are significantly less likely to receive MAT (or even to seek prenatal care at all) in states that permit child abuse charges for illicit drug use during pregnancy. Although Tennessee is currently the only state in which a woman can face criminal charges for substance abuse during pregnancy, 18 states permit civil child abuse charges that may result in termination of parental rights. Missouri has no such laws and does not require drug testing or physician reporting of suspected substance use disorders during pregnancy.28 Missouri is also one of only 12 states that offers pregnant women priority access to treatment programs for substance use disorders and does not impose limits on the dose or duration of buprenorphine therapy.
Future Directions
The opioid epidemic continues to shape Federal, State, and local health care policies on substance use disorders. The Comprehensive Addiction and Recovery Act, signed into law in July 2016, provides new funding for measures to prevent and treat opioid use disorder, and such strategies must continue to evolve.
Prevention
Primary prevention of substance use disorders depends on effective prevention education. School and community-based prevention programs (e.g., Communities that Care) that teach skills to resist negative social influences have proven to reduce rates of initiation and escalation of alcohol, tobacco, and drug use among adolescents and young adults.21 Many such programs, though effective, are underutilized and poorly implemented. It is estimated that only 8–10% of U.S. high schools utilize evidence-based programs to prevent substance misuse.44 An emerging field within public health is improving the implementation and dissemination of evidence-based prevention programs at the community level, where they are most effective.
Despite some successful efforts to curtail the prescription and consumption of opioids, opioid pain relievers are still the most prescribed class of drugs in the United States.45 Prescription drug monitoring programs have shown promise, but in many states they are underutilized because their use is voluntary.46 A national PDMP, or interconnecting those of individual states could potentially prevent patients from crossing state lines to obtain and fill multiple prescriptions, but would require further investments in health information technologies and infrastructure. More widespread adoption of programs such as the CDC Guideline for Prescribing Opioids for Chronic Pain can help promote responsible and safe prescribing practices.
Another key aspect of preventing OUD is stemming the flow of heroin and illegally produced fentanyl, as well as counterfeit prescription opioids, into the United States. Most of the heroin sold in the U.S. comes from Mexico and South America, where it enters the Southwestern U.S. via established drug trafficking routes.47 Heroin seizures at the border have tripled over the past five years. In response to this, the Office of National Drug Control Policy’s 2015 National Drug Control Strategy includes a Southwest Border Counternarcotics Strategy to combat drug trafficking and organized criminal activity. Similar law enforcement strategies are in place at the U.S.-Canada and U.S.-Caribbean borders.
Treatment
Given the increasing burden of opioid use disorder in the U.S., we face the formidable public health challenge of expanding access to proven treatment modalities for people who need them most. It is estimated that only 1 in 10 persons with a substance use disorder receives medication-assisted treatment (MAT). It is therefore imperative to develop health care policies that make MAT more widely available and integrate it into existing general health care settings, thereby improving access to care and reducing the stigma of substance use disorders. The Office of National Drug Control Policy has advocated for therapeutic, rather than punitive, approaches to addressing opioid use disorder and treating it as a chronic medical condition rather than a moral failing or lack of willpower.
The 2010 Affordable Care Act (ACA) requires health care plans to provide coverage for mental health and substance use disorder services, one of the 10 designated categories of “essential health benefits”.21 However, a relative shortage of physicians who are certified to treat patients with OUD is a significant barrier to delivering care. The 2000 Drug Addiction Treatment Act increased the number of physicians permitted to prescribe buprenorphine for treating OUD by waiving the requirement to obtain a separate DEA registration for that purpose. In 2016, the Federal Substance Abuse and Mental Health Services Administration (SAMHSA) increased the buprenorphine-naloxone prescriber limit from 100 patients to 275 patients. However, fewer than 4% of office-based physicians in the U.S. are authorized to prescribe buprenorphine as part of MAT, and even fewer obstetricians can provide such care. According to SAMHSA, 215 physicians in Missouri are licensed to prescribe buprenorphine, including one of the authors (DLJ). Increasing prescribing capacity will be necessary to meet the increasing demand for OUD treatment. Information on obtaining a DEA physician waiver for prescribing buprenorphine is available at http://www.samhsa.gov/medication-assisted-treatment.
Another provision of the ACA allows states to expand Medicaid coverage to include individuals who qualify based on income, many of whom are affected by, or at risk for developing, substance use disorders including OUD. This expanded coverage includes mental health services and substance use disorder treatments in addition to general medical care. Missouri is one of 19 states not currently participating in the voluntary expansion of Medicaid. An estimated 3 million people who would qualify for expanded Medicaid coverage live in states that don’t participate in Medicaid expansion.48
Advances in health information technology have given providers increased leverage in confronting the burden of substance use disorders. Telehealth, computerized Prescription Drug Monitoring Programs, and electronic medical records allow greater efficiency in treating patients with OUD and have much potential to expand OUD treatment services into areas where they are currently unavailable. Another barrier to providing treatment for OUD is a lack of reimbursement for screening and counseling services. To this end, the Office of National Drug Control Policy is pursuing initiatives to update coding and increase reimbursement for Screening, Brief Intervention, and Referral to Treatment (SBIRT), an evidence-based practice to identify and treat OUD.
Conclusions
Though significant progress has been made on many fronts in the fight against the opioid epidemic, more research is needed in order to gain a better understanding of the determinants of addiction, effective prevention strategies, and ways to more effectively deliver treatment to those struggling with the many facets of opioid use disorder.
Biography
Frederick T. O’Donnell, MD, (left), Assistant Professor of clinical Anesthesiology, Department of Anesthesiology and Perioperative Medicine; Daniel L. Jackson, MD, (right), Assistant Professor of Clinical Obstetrics and Gynecology, Department of Obstetrics, Gynecology and Women’s Health, University of Missouri Women’s and Children’s Hospital, Columbia, Missouri.
Contact: odonnellf@health.missouri.edu


Footnotes
Disclosure
None reported.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Injury Prevention & Control. Opioid Overdose. CDC; [Accessed 09/24/16.]. https://www.cdc.gov/drugoverdose/ [Google Scholar]
- 3.Betses M, Brennan T. Abusive prescribing of controlled substances -- a pharmacy view. N Engl J Med. 2013;369:989–991. doi: 10.1056/NEJMp1308222. [DOI] [PubMed] [Google Scholar]
- 4.Rudd RA, Aleshire N, Zibbel JE, Gladden RM. Increases in drug and opioid overdose deaths — United States, 2000–2014. MMWR. 2016;64(50):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 5.CDC vital signs. Opioid painkiller prescribing. Jul, 2014. [Accessed 11/04/16]. Available at: http://www.cdc.gov/vitalsigns/opioid-prescribing/
- 6.Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, US, 2007–2012. American Journal of Preventive Medicine. 2015;49(3):409–413. doi: 10.1016/j.amepre.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: 2016. [PubMed] [Google Scholar]
- 8.National Drug Intelligence Center. National drug threat assessment. Washington, DC: U.S. Department of Justice; 2011. [Google Scholar]
- 9.U.S. Department of Health and Human Services (HHS), Office of the Surgeon General, Facing Addiction in America. The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: HHS; Nov, 2016. [PubMed] [Google Scholar]
- 10.Pizzo P, Clark N, Carter-Pokras OD, et al. Relieving pain in America: A blueprint for transforming prevention, care Education, and Research. OIM (Institute of Medicine); 2011. [Google Scholar]
- 11.Drug overdose deaths in the United States continue to increase in 2015. CDC; [Accessed 10/01/16]. https://www.cdc.gov/drugoverdose/epidemic/index.html. [Google Scholar]
- 12.Kunins HV, Farley TA, Dowell D. Guidelines for opioid prescription: why emergency physicians need support. Ann Intern Med. 2013;158:841–842. doi: 10.7326/0003-4819-158-11-201306040-00631. [DOI] [PubMed] [Google Scholar]
- 13.Manchikanti L, Ailinani H, Koyyalagunta D, et al. A systematic review of randomized trials of long-term opioid management for chronic noncancer pain. Pain Physician. 2011;14:91–121. [PubMed] [Google Scholar]
- 14.Frieden TR, Houry D. Reducing the risks of relief—the CDC opioid-prescribing guideline. N Engl J Med. 2013;374:501–1504. doi: 10.1056/NEJMp1515917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boscarino JA, Rukstalis MR, Hoffman SN, et al. Prevalence of prescription opioid-use disorder among chronic pain patients: comparison of the DSM-5 vs. DSM-4 diagnostic criteria. J Addict Dis. 2011;30(3):185–94. doi: 10.1080/10550887.2011.581961. [DOI] [PubMed] [Google Scholar]
- 16.DEA puts quota on production of opioid painkillers. [Accessed 11/04/16]. https://medlineplus.gov/news/fullstory_161326.html.
- 17.Prescription Drug Monitoring Programs (PDMPs) CDC; [Accessed 11/04/16]. https://www.cdc.gov/drugoverdose/pdmp/index.html. [Google Scholar]
- 18.CDC. Decline in drug overdose deaths after state policy changes—Florida, 2010–2012. MMWR. 2014;63:569–74. [PMC free article] [PubMed] [Google Scholar]
- 19.Patrick SW, Fry CE, Jones TF, et al. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Affairs. 2016;35:1324–32. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services (HHS), Office of the Surgeon General, Facing Addiction in America. The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: HHS; Nov, 2016. [PubMed] [Google Scholar]
- 22.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 23.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–163. doi: 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- 24.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375:357–368. doi: 10.1056/NEJMra1604339. [DOI] [PubMed] [Google Scholar]
- 25.McLellan, et al. The Effects of Psychosocial Services in Substance Abuse Treatment. JAMA. 1993 Apr 21;269(15):1953–9. [PubMed] [Google Scholar]
- 26.Ossiander EM. Using textual cause-of-death data to study drug poisoning. Am J Epidemiol. 2014 Apr 1;179(7):884–94. doi: 10.1093/aje/kwt333. [DOI] [PubMed] [Google Scholar]
- 27.Cousins G, Boland F, Courtney B, Barry J, Lyons S, Fahey T. Risk of mortality on and off methadone substitution treatment in primary care: a national cohort study. Addiction. 2016;111:73–82. doi: 10.1111/add.13087. [DOI] [PubMed] [Google Scholar]
- 28.Rinaldo SG, Rinaldo DW. Availability without accessibility? State Medicaid coverage and authorization requirements for opioid dependence medications. American Society of Addiction Medicine; 2013. [Accessed October 31, 2016]. Available: www.asam.org/docs/defaultsource/advocacy/aaam_implications-for-opioid-addictiontreatment_final. [Google Scholar]
- 29.Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1–14. doi: 10.2147/SAR.S45585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahan A, Yassan A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96:627. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 31.Hser YI, Hoffman V, Grella CE, et al. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- 32.Ettner SL, Huang D, Evans E, et al. Benefit-cost in the California treatment outcome project: Does substance abuse treatment “pay for itself ”? Health Services Research. 2006;41(1):192–213. doi: 10.1111/j.1475-6773.2005.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. Vital Signs: Demographic and substance use trends among heroin users— United States, 2002–2013. MMWR. 2015;64:719–25. [PMC free article] [PubMed] [Google Scholar]
- 34.Women, ACoG cfU and M. American Society of Addiction, ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 35.Patrick SW, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 36.Saia KA, Schiff D, Wachman EM, et al. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Curr Obstet Gynecol Rep. 2016;5:257–263. doi: 10.1007/s13669-016-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chasnoff IJ, Hung WC. The 4 P’s Plus© Screen for Substance Use in Pregnancy. Chicago, IL: NTI Upstream; 2002. For permission please contact http://ntiupstream.com/contactnti. [Google Scholar]
- 38.McNeely J, Cleland CM, Strauss SM, Palamar JJ, Rotrosen J, Saitz R. Validation of self-administered single-item screening questions (SISQs) for unhealthy alcohol and drug use in primary care patients. Journal of General Internal Medicine. 2015;30(12):1757–1764. doi: 10.1007/s11606-015-3391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell J, et al. Detoxification from opiate drugs during pregnancy. Am J Obstet Gynecol. 2016;215(3):374.e1–6. doi: 10.1016/j.ajog.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Brady KL. Katie, Addiction and Substance Abuse. Clinical Updates in Women’s Health Care. 2012;11(1) [Google Scholar]
- 41.Noormohammadi A, et al. Buprenorphine Versus Methadone for Opioid Dependence in Pregnancy. Ann Pharmacother. 2016;50(8):666–72. doi: 10.1177/1060028016648367. [DOI] [PubMed] [Google Scholar]
- 42.Buprenorphine: an alternative to methadone. Med Lett Drugs Ther. 2003;45(1150):13–5. [PubMed] [Google Scholar]
- 43.Angelotta C, Weiss CJ, Angelotta JW, et al. A moral or medical problem? The relationship between legal penalties and treatment practices for opioid use disorders in pregnant women. Womens Health Issues. 2016;26(6):595–601. doi: 10.1016/j.whi.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Ringwalt C, Hanley S, Vincus AA, Ennett ST, Rohrbach LA, Bowling JM. The prevalence of effective substance use prevention curricula in the Nation’s high schools. The Journal of Primary Prevention. 2008;29(6):479–488. doi: 10.1007/s10935-008-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, US, 2007–2012. American Journal of Preventive Medicine. 2015;49(3):409–413. doi: 10.1016/j.amepre.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA. 2015;313(9):891–892. doi: 10.1001/jama.2014.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.2015 National Drug Threat Assessment Summary DEA DEA-DCT-DIR-008-16. Drug Enforcement Administration Office of Intelligence; Department of Homeland Security Office of Intelligence and Analysis; Oct, 2015. [Google Scholar]
- 48.Garfield R, Damico A, Cox C. New estimates of eligibility for ACA coverage among the uninsured. Washington, DC: Kaiser Family Foundation; 2015. [Google Scholar]