Abstract
Background
The aim of this prospective study was to investigate the relationship between lifestyle factors including nutrition intake and the incidence of fall-related fragility fractures in postmenopausal women.
Methods
A total of 1169 female volunteers were recruited from participants at the morning health examinations held at each local public health center in the West Chiayi County of Taiwan at the beginning of the study. Laboratory examinations, anthropometric measurements, and questionnaire interviews inquiring about lifestyle factors, including weekly nutrition intake, were performed. Subsequently, four follow-up telephone interviews at intervals of about 6–12 months were performed to inquire about instances of falls and fractures.
Results
Nine hundred and fifty-three subjects responded at least once to the four telephone interviews, and there were 183 postmenopausal women, with a mean age of 68.8 ± 8.3 (49–87) years, reporting falls. Of the 183 women, 25 had incurred new fractures from low-energy impacts. Statistical analysis revealed that older age and hypertension were associated with increased risks of falling. Intake of other deep-colored (nondark-green) vegetables and light-colored vegetables as well as total vegetable intake were associated with reduced risk of fall-related fragility fracture.
Conclusion
Among postmenopausal women, older age and the presence of hypertension were associated with increased risks of falls. Increased vegetable intake might be helpful to reduce the incidence of fall-related fragility fractures.
Keywords: Fragility fracture, Nutrition, Osteoporosis, Taiwan, Vegetable
At a glance commentary
Scientific background of the subject
Osteoporosis is a major public health issue. To identify modifiable risk factors and to develop corresponding strategies to help prevent osteoporosis is crucial, especially for postmenopausal women. There is increasing evidence suggesting a positive association between intake of vegetables and bone health. The results, however, have not been always consistent.
What this study adds to the field
This prospective study has yielded more evidence that adequate vegetable intake might reduce the risk of fall-related fragility fractures for Taiwanese postmenopausal women. It might be recommended that individuals consume vegetables at least 2 times a day for better bone health.
Osteoporosis is a major public health issue. An estimated 56 million cases of osteoporotic or fragility fractures were reported worldwide in 2000 [1]. Osteoporotic fractures lead to pain, functional limitation, and increased the risk of death. Elderly people, especially postmenopausal women are particularly at increased risk [2], [3]. It was reported that the prevalence of osteoporosis in Taiwanese men and women over 50 years old was 23.9% and 38.3%, respectively [4]. Nearly one-third of Taiwanese women in their lifetime would have at least one fragility or osteoporosis fracture and the mortality rate within the 1st year of hip fracture is as high as 15% [5]. Thus, to identify modifiable risk factors and to develop corresponding strategies to help prevent osteoporosis is crucial.
The association between bone health and lifestyle factors, especially dietary intake has been highly discussed in literature. There is increasing evidence suggesting a positive association between intake of vegetables and bone health. The results, however, have not been always consistent. Some studies revealed a positive association between consuming vegetables and bone mineral density (BMD) [6], [7], [8] and reduced fracture risk [9], [10], but other studies have not shown this same results [11], [12], [13]. A majority of the studies that have produced positive findings were either cross-sectional or had a case-control design. Few prospective or longitudinal studies have shown direct links between vegetable consumption and the incidence of fractures. Besides, most of these studies have been conducted in European or American countries, and the evidence in the Asian populations is still relatively lacking, especially in Taiwan. In addition, dietary patterns and lifestyle may be quite different between Asian and Western populations, and thus the current findings may not be totally applicable to the Asians. For example, the Taiwanese population consumes much less dairy products (0.8–0.9 servings/day) than those in Western countries, but most of them consume an adequate amount of vegetables (around three servings a day) [14]. Therefore, the aim of this prospective study was to investigate the relationship between lifestyle factors, including nutrition intake, and the incidence of fall-related fragility fractures in the Taiwanese postmenopausal women.
Methods
This study was based on community geriatric medical research in the West Chiayi County, Taiwan. Female volunteers were recruited for this prospective observational study from participants in the morning health examinations held at each local public health center. All enrolled subjects underwent fasting blood sampling and anthropometric measurements. Trained research assistants then conducted in-person interviews with all subjects. All subjects were then interviewed by telephone to inquire about the occurrence of any falls or fractures; these interviews were conducted in 12 months, 18 months, 2 years, and 2½ years after the initial interviews. Informed consent was obtained from all participants, and the study was approved by the local Institutional Review Board of Chiayi Chang Gung Memorial Hospital, a regional teaching hospital.
Anthropometric and laboratory examinations
Body weight, height, and waist and hip circumference were measured with participants wearing light clothing and without shoes. Body mass index (BMI, kg/m2) was calculated from body weight and height. Brachial arterial blood pressure levels were obtained with an automatic blood pressure monitor after the subjects were at rest at least for 15 min. Three separate measurements were made. Left side brachial blood pressures were measured first, immediately followed by measurement on the right side, and then on the left once more. Mean systolic and diastolic pressures were established by calculating the mean of the three measurements. Laboratory studies included measurement of the levels of serum blood urea nitrogen, creatinine, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, glucose, and glycohemoglobin (HbA1c).
Lifestyle questionnaire
All participants were interviewed with a comprehensive questionnaire. Dietary habits and lifestyle factors including lacto-ovo vegetarianism, vegetarianism, smoking, milk, coffee, tea and alcohol consumption, exercise, and employment as a manual laborer were recorded. Further information was collected about the amounts, frequencies, and duration of the individual's lifestyle habits if the subject exhibited certain dietary habits. Use of supplemental calcium and additional medicine for osteoporosis were also recorded with showing the samples or pictures of common anti-osteoporotic drugs including Fosamax, Evista, salmon calcitonin nasal spray, and Forteo to the subjects. Habitual tea or coffee drinkers were defined as those who had drunk tea or coffee for 4 or more days/week for 5 years or longer. Subjects with habitual milk consumption were defined as those who had drunk seven or more glasses per week for 5 years or longer. Habitual exercisers were defined as those who had exercised 3.5 or more hours/week for 5 years or longer.
Each subject's past medical history (including hypertension, diabetes mellitus, hyperlipidemia, liver diseases, renal diseases, blurred vision, stroke, previous fracture, and menstrual status) was recorded. If the subject reported having liver or renal disease, the liver or renal disease was defined further. A supplemental questionnaire (also face to face) involving semi-quantitative nutrition intake was administered 6 months to 1 year later because these information not included in the initial questionnaire were found to be possibly helpful after initial analysis. In this questionnaire, subjects were asked about the frequency (the frequency per day and week were used to calculate the total weekly frequency) and duration of several kinds of food consumed. These foods included fruits (including fresh fruit juice); dark-green vegetables (including green peppers, broccoli, and dark-green leafy vegetables such as spinach, chrysanthemum, and bok choy); other deep-colored vegetables (including deep yellow vegetables such as carrots, pumpkin, sweet potatoes, tomatoes, and yellow pepper; dark-red vegetables such as red peppers, red phoenix vegetables, and red amaranth; and others such as eggplant); light-colored vegetables (such as kale, cabbage, bamboo shoots, radish, cauliflower, white gourd, cabbage, Chinese cabbage, and celery); algae (such as a variety of mushrooms, shiitake, fungus, kelp, wakame, and seaweed); soy products (such as bean bag, bean curd, and tofu); eggs (including chicken, duck, and quail eggs); fish; meats (including pork, chicken, and duck); and organ meats (such as liver, heart, kidney, and large and small intestine from a variety of animals).
Telephone interview on falls and fractures and the definition of fragility fracture
Participants were asked about any falls or fractures that had occurred since the interview. A fall was defined as “falling with landing on the ground or lower level.” When a fracture was reported, the site and cause of fracture were also recorded. Fractures from accidental falls that occurred during standing, walking, running, jumping, or riding a bicycle at slow speed were defined as fragility fractures and were thought to be closely related to reduced bone strength. Meanwhile, fractures caused by falling from a height (falling more than one floor) and due to traffic accidents involving a car, motorcycle, or by riding a bicycle at high speed were defined as high-energy-impact fractures, these fractures are not attributed to bone strength but an irresistible trauma.
Data analysis
The variables between subjects with and without fall accident were analyzed. Among the subjects with at least once of fall accident, the variables between subjects with or without fragility fracture were also analyzed. The independent sample t-test or Mann–Whitney U-test (for variables not normally distributed) were used for continuous variables analysis while Pearson's Chi-square test or Fisher's exact test were used for categorical variables. Binary logistic regression analyses were used for multivariate analysis, and odds ratios were calculated with a 95% confidence interval (CI). A p < 0.05 was considered significant for all tests. Statistical analysis was carried out using SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA).
Results
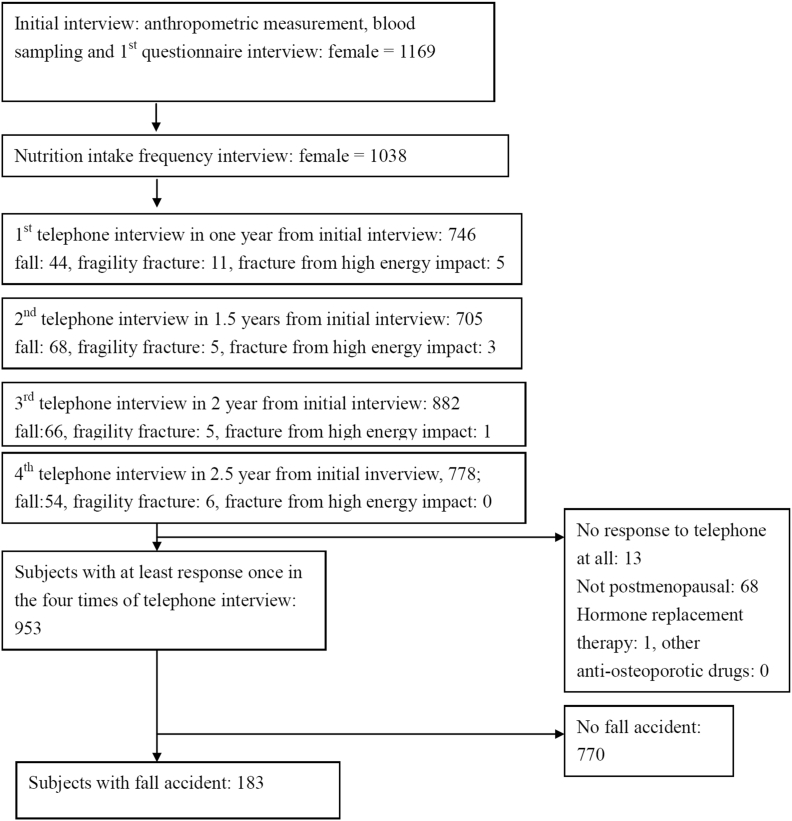
During the 3 years, from September 2008 to August 2011, a total of 1169 subjects were included in the beginning of the study and there were 1038 female completed the two comprehensive questionnaires. Among 1038 subjects, the number of participants who responded to the first to fourth telephone interviews was around 700–900 with a responsive rate of 71.9%, 67.9%, 85.0%, and 75.0%, respectively [Fig. 1]. After excluding participants who were not menopausal or those who had received anti-osteoporotic treatments, such as hormone replacement therapy, 953 subjects had responded at least once during the four telephone interviews. Among these, 183 women, with a mean age of 68.8 ± 8.3 (49–87) years, had experienced at least one fall [Fig. 1].
Fig. 1.
Subjects flow diagram showing the steps of the case enrollments.
Older age and a past medical history of diabetes, hypertension, and lower weekly intake frequency of fruit were associated with a fall, but HbA1c level, mean systolic or diastolic blood pressure, and the other food items weekly intake frequencies were not related to an increased risk of falling. Subjects who had a previous history of fracture or shorter stature tended to have a greater risk of falling, although this did not reach statistical significance. Binary logistic regression included all of the variables that had an impact on accidental falls noted in univariate analysis and revealed that older age and the presence of hypertension were independently associated with falling. Subjects with diabetes or a previous history of fracture tended to have a higher risk of falling, although this did not reach statistical significance [Table 1, other variables not associated with fall were not shown].
Table 1.
Characteristics of subjects with fall or not.
| Variables | Subjects with fall |
Subjects without fall |
Univariate analysis |
Binary logistic regression analysis |
|||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD, percentage | n | Mean ± SD, percentage | p | ORe (95% CI) | pe | |
| Age (year-old) | 183 | 68.8 ± 8.3 | 770 | 66.4 ± 8.4 | <0.001a | 1.02 (1.00–1.05) | 0.027 |
| Body height (cm) | 182 | 151.7 ± 5.1 | 765 | 152.5 ± 5.6 | 0.064a | 0.98 (0.95–1.02) | 0.308 |
| Body weight (kg) | 182 | 58.1 ± 9.1 | 765 | 57.9 ± 9.3 | 0.760a | ||
| BMI (kg/m2) | 182 | 25.3 ± 3.7 | 765 | 24.9 ± 3.6 | 0.196a | ||
| Waist circumflex (cm) | 182 | 83.2 ± 9.6 | 762 | 82.3 ± 9.4 | 0.283a | ||
| Hip circumflex (cm) | 175 | 96.6 ± 8.5 | 747 | 96.2 ± 7.9 | 0.552a | ||
| Systolic blood pressure (mmHg) | 182 | 140.7 ± 20.0 | 761 | 139.1 ± 19.6 | 0.331a | ||
| Diastolic blood pressure (mmHg) | 182 | 83.5 ± 11.4 | 761 | 82.6 ± 10.8 | 0.349a | ||
| Hypertension | 183 | 43.2 (79/183) | 770 | 29.9 (230/770) | 0.001b | 1.54 (1.08–2.18) | 0.016 |
| Diabetes | 183 | 17.5 (32/183) | 770 | 10.9 (84/770) | 0.014b | 1.58 (1.00–2.50) | 0.051 |
| Fasting glucose | 178 | 108.9 ± 38.4 | 759 | 105.3 ± 29.6 | 0.230a | ||
| HbA1c | 180 | 6.1 ± 1.2 | 760 | 6.0 ± 1.1 | 0.192a | ||
| Fruit intake (frequency) | 183 | 6.0 ± 4.1 | 770 | 6.5 ± 3.9 | 0.023c | 0.97 (0.93–1.02) | 0.192 |
| Previous fracture history | 183 | 23.0 (42/183) | 770 | 17.1 (132/770) | 0.068b | 1.44 (0.97–2.15) | 0.072 |
| Blurred vision | 183 | 2.2 (4/183) | 770 | 2.1 (16/770) | >0.99d | ||
| Stroke | 183 | 0.5 (1/183) | 770 | 0.5 (4/770) | >0.99d | ||
| Exercise | 183 | 22.0 (39/183) | 770 | 25.9 (195/770) | 0.283b | ||
| Labor worker | 183 | 15.4 (20/183) | 770 | 17.9 (98/770) | 0.499b | ||
Abbreviations: n: Case number; BMI: Body mass index; CI: Confidence interval; SD: Standard deviation; HbA1c: Glycohemoglobin.
Determined by independent sample t-test.
Determined by Pearson's Chi-square test.
Determined by Mann–Whitney U-test.
Determined by Fisher's exact test.
Determined by binary logistic regression (dependent variable: Fall, covariates: age, body height, hypertension, diabetes, previous fracture history).
Among 183 women who had falls, 25 had fragility fractures. The case number of each fragility fracture site is shown in Table 2. Table 3 provides a comparison of anthropometry, laboratory test results, lifestyle, and nutrition intake variables between subjects with and without fragility fractured. The weekly intake of other deep-colored vegetables, light-colored vegetables, and total amount of vegetables (including dark-green, other deep-colored, and light-colored vegetables) was significantly lower among women in the fragility fracture group than among those in the nonfragility fracture group. For some variables, the case numbers were so small that these variables were not considered to be valuable for statistical analysis and thus are not included. Some of these variables are smoking (n = 6), habitual coffee drinking (n = 12), alcohol consumption (n = 17), rheumatoid arthritis (n = 5) in the analysis for comparison of subjects with or without falling; and blurred vision (n = 4), stroke (n = 1), lacto-ovo vegetarianism (n = 4) and vegetarianism (n = 1) in the analysis for comparison of subjects with or without fragility fracture. None of the subjects reported having liver cirrhosis or severe renal disease. For variables reaching statistical significance, such as other deep-colored vegetables, light-colored vegetables, and total vegetable consumption, binary logistic regression was used to investigate the odds ratio, adjusted by age and BMI. Subjects were divided into two groups by different intake frequency for comparison. The results of all of the possible groupings by each intake frequency were analyzed. The highest statistical significant cut point of weekly intake frequency for other deep-colored vegetable and light-colored vegetable were both 6 times/week, and that for total vegetable was 15 times/week. For example, for light-colored vegetable, the p value of binary logistic regression in comparison of weekly intake frequency with six versus fewer than six, seven versus fewer than seven, eight versus fewer than eight were 0.029, 0.036, and 0.163, respectively. Therefore, 6 times was the cut point frequency of such kind of food. Each corresponding value of odds ratio are shown in Table 3.
Table 2.
Case number of each fragility fracture sites.
| Fracture sites | n |
|---|---|
| Hand and wrist | 5 |
| Humerus | 2 |
| Rib | 3 |
| Spine | 4 |
| Femur (including hip) | 5 |
| Lower limbs other than femur | 6 |
| Total | 25 |
Table 3.
Comparison between subjects with and without fragility fracture.
| Variables | Subjects with fragility fracture |
Subjects without fragility fracture |
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD, percentage | n | Mean ± SD, percentage | p | ORe (95% CI) | pe | |
| Age (year-old) | 25 | 69.0 ± 8.8 | 158 | 68.7 ± 8.2 | 0.870a | ||
| Body height (cm) | 25 | 151.2 ± 6.7 | 157 | 151.7 ± 4.8 | 0.634a | ||
| Body weight (kg) | 25 | 56.7 ± 9.6 | 157 | 58.4 ± 9.0 | 0.384a | ||
| BMI (kg/m2) | 25 | 24.7 ± 3.6 | 157 | 25.3 ± 3.7 | 0.440a | ||
| Waist circumflex (cm) | 25 | 83.1 ± 7.0 | 157 | 83.2 ± 10.0 | 0.990a | ||
| Hip circumflex (cm) | 25 | 97.5 ± 6.7 | 157 | 96.4 ± 8.8 | 0.570a | ||
| Bun | 24 | 17.5 ± 5.2 | 156 | 15.9 ± 4.5 | 0.108a | ||
| Creatinine | 24 | 0.8 ± 0.2 | 156 | 0.8 ± 0.2 | 0.419a | ||
| Cholesterol | 24 | 216.1 ± 41.1 | 157 | 208.0 ± 38.2 | 0.338a | ||
| HDL | 25 | 59.0 ± 11.9 | 158 | 57.0 ± 12.7 | 0.445a | ||
| LDL | 25 | 137.5 ± 32.9 | 157 | 131.9 ± 34.8 | 0.453a | ||
| Triglyceride | 24 | 131.5 ± 63.6 | 157 | 125.6 ± 67.6 | 0.687a | ||
| Fasting glucose | 24 | 114.3 ± 39.9 | 154 | 108.1 ± 38.2 | 0.468a | ||
| HbA1c | 24 | 6.2 ± 1.4 | 156 | 6.1 ± 1.2 | 0.618a | ||
| Uric acid | 24 | 5.5 ± 1.9 | 157 | 5.5 ± 1.3 | 0.983a | ||
| Hypertension | 25 | 36.0 (9/25) | 158 | 44.3 (70/158) | 0.436b | ||
| Diabetes mellitus | 25 | 20.0 (5/25) | 158 | 17.1 (27/158) | 0.777c | ||
| Previous fracture history | 25 | 20.0 (5/25) | 158 | 23.4 (37/158) | 0.706b | ||
| Exercise habit | 25 | 32.0 (8/25) | 158 | 29.5 (47/158) | 0.819b | ||
| Labor worker | 25 | 12.0 (3/25) | 158 | 10.8 (17/158) | >0.99c | ||
| Calcium tablet | 25 | 32 (8/25) | 158 | 23.4 (37/158) | 0.354b | ||
| Habitual milk intake | 25 | 16.0 (4/25) | 158 | 20.9 (33/158) | 0.572b | ||
| Habitual tea intake | 25 | 8.0 (2/25) | 158 | 10.8 (17/158) | >0.99c | ||
| Various food intake frequency | |||||||
| Egg | 25 | 1.7 ± 1.3 | 158 | 2.0 ± 1.8 | 0.919d | ||
| Fish | 25 | 7.2 ± 4.3 | 158 | 7.0 ± 4.6 | 0.739d | ||
| Meat | 25 | 3.7 ± 2.8 | 158 | 4.5 ± 3.7 | 0.475d | ||
| Offal food | 25 | 0.1 ± 0.3 | 158 | 0.3 ± 0.8 | 0.230d | ||
| Algae | 25 | 1.2 ± 1.2 | 158 | 1.9 ± 2.2 | 0.120d | ||
| Soy product | 25 | 1.4 ± 1.7 | 158 | 1.7 ± 2.1 | 0.471d | ||
| Fruit | 25 | 6.1 ± 3.7 | 158 | 6.0 ± 4.2 | 0.570d | ||
| Dark-green vegetable | 25 | 7.9 ± 4.4 | 158 | 8.6 ± 4.4 | 0.431d | ||
| Other deep-colored vegetable | 25 | 5.1 ± 3.8 | 158 | 6.6 ± 4.2 | 0.024d | 3.00f (1.06–8.53) | 0.039 |
| Light-colored vegetable | 25 | 5.6 ± 3.3 | 158 | 7.7 ± 4.3 | 0.021d | 2.70f (1.07–6.85) | 0.029 |
| Sum of vegetable | 25 | 18.6 ± 9.9 | 158 | 22.8 ± 11.1 | 0.037d | 2.88g (1.22–6.84) | 0.016 |
Abbreviations: n: Case number; BMI: Body mass index; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; HbA1c: Glycohemoglobin; CI: Confidence interval; OR: Odds ratio.
Determined by independent sample t-test.
Determined by Pearson's Chi-square test.
Determined by Fisher's exact test.
Determined by Mann–Whitney U-test.
Determined by binary logistic regression. Dependent variable: Fragility fracture or not, covariates: Food item divided into two groups, age and body mass index. Each food item (including dark-green, other deep-colored, light-colored vegetable and sum of vegetable) was analyzed by each binary logistic regression model to avoid multicollinearity.
OR for weekly intake frequency <6 versus ≥6.
OR for weekly intake frequency <15 versus ≥15.
Discussion
The results of this study revealed that older age is associated with increased risk of accidental falls, a fact that is generally known [15], [16]. Falls are a major threat to the elderly, and the risk for such falls is greater among women [17]. Women are also more likely to develop osteoporosis, which results in more fall-related osteoporotic fractures [18]. In some studies, diabetes and previous falls with a fracture history were also reported to be associated with increased risk of falling [19], [20]. In the present study, women with self-reported diabetes and a history of fracture tended to have greater risk of falling, although this did not reach statistical significance.
A self-reported history of hypertension disregarding the value of blood pressure is an independent risk factor for falling. Several studies have addressed the relationship between blood pressure and the risk of falling in the elderly. One has pointed out that aging could result in insufficient blood pressure homeostasis, such as orthostatic hypotension, which could be exacerbated by hypertension [21]. Gangavati et al. reported that orthostatic hypotension occurred mostly in the elderly with uncontrolled hypertension rather than among individuals with controlled hypertension or without hypertension. The elderly who have uncontrolled hypertension with systolic orthostatic hypotension are at greater risk of falling [22]. However, on the other hand, many other studies have reported that strict antihypertensive pharmacotherapy could also increase the risk of falling in the elderly [23], [24], which might partially explain why the value of blood pressure did not show a significant association with the risk of fall in this study. Since preventing falls among the elderly is a nationally important health promotion issue [25], efforts should be made to pay even more attention to older people with chronic diseases such as hypertension and diabetes.
Subjects with fall-related fragility fractures were compared with those who had fallen but had not incurred fractures, and the results indicate that total vegetable intake is inversely associated with fall-related fragility fracture incidence in postmenopausal women. In contrast, other variables such as milk consumption, BMI, and exercise habit had no obvious association with fracture risk. Since the subjects without any fall accident in the observation period was already excluded in the analysis, the results here might be considered as that increased vegetable intake was associated with better bone strength rather than decreased the risk of falling. Although our study results show that other deep-colored vegetables and light-colored vegetables (but not dark-green vegetables) are associated with reduced risk of fragility fractures, one cannot interpret this as that other deep-colored vegetables and light-colored vegetables are superior to dark-green vegetables for bone health. In fact, our subjects consumed dark-green vegetables more frequently than the other two kinds of vegetables [Table 3], and the results should be interpreted in light of the overall relatively low intake of the two other kinds of vegetables. There is currently no specific evidence addressing which kinds of vegetables are more valuable for bone health. Besides, dark-green vegetables, especially green leafy vegetables are reported to be a very important source of dietary calcium and usually contribute more calcium than other vegetables [8], [14].
Although there was increasing evidence of the benefits of vegetable intake on bone health, the exact mechanisms remain unclear. Vegetables contain many nutrients that might be beneficial to bone health including many minerals, flavonoids, carotenoids, omega-3-fatty acids, and Vitamins A, C, E, and K [26], [27]. Vegetables are the second most important dietary source of calcium for the Taiwan's elder population (149.0 mg/day), just after dairy products (257.5 mg/day), which comprises 24% of total daily calcium intake [14].
Another hypothesis centers on the effect of acid-base balance on bone. Adequate protein and energy intake are thought to be essential for bone health [28]; however, the dietary source of major energy and protein are all acid-forming, such as meat, fish, soybean, dairy products, and cereal. Thus, bone mineral dissolution to buffer these acid loads can develop if a diet lacks enough alkaline-forming foods such as fruits and vegetables [29]. This is shown in research examining the relationship between urinary calcium excretion, markers of bone turnover, and dietary intake [30], [31]. The mean daily protein intake for Taiwanese women is 59.5 g/day (16.8% of daily energy intake), which is higher than recommended levels (10–14% of daily energy intake) [14]. In this case, adequate vegetable intake might be crucial.
Some studies have reported that intake of calcium or dairy products is associated with increased BMD or reduced fracture risk; however, the results have not always been conclusive [32]. Dairy products are the most important dietary sources of calcium and Vitamin D and are thought to be important in osteoporosis prevention [14], [26]. However, in several recent long-term prospective studies with fragility fracture as the observation end-point, no obvious association was found between the intake of dairy products and the occurrence of fracture [33], [34], [35]. It is worth mentioning that in Benetou et al.'s study, intake of dairy products showed no obvious association with hip fracture incidence. However, there is a weak and statistically nonsignificant evidence to suggest that vegetables are helpful in reducing fracture risk [33]. This puzzling result might be due to the fact that milk or dairy products contain some nutrients that are also detrimental to bone health and that may offset its benefit, for example, protein acid load or Vitamin A [36]. In addition, behavioral changes due to health problems may also confound the investigation of the causal relationship in longitudinal observational studies. Subjects who knew they were likely to have greater risk of osteoporosis would have higher motivation to increase their intake of milk or calcium supplements [34] which would interfere with analysis of the effect of these two variables.
BMI is well-known to be positively associated with BMD; however, its impact on fracture risk can be complicated and site-dependent. It has been reported that obesity is protective for hip fracture but associated with increased humerus, ankle, and leg fractures [37], [38]. In this study, various fracture sites were included, and therefore, it was not expected that there would be a significant correlation between BMI and fragility fracture.
Similarly, exercise is known to be positively associated with BMD in pre- and post-menopausal women [39], but its impact on fall-related fragility fractures is still obscure. In Kemmler et al.'s systematic review and meta-analysis, the authors stated that although there is evidence to suggest exercise can reduce the overall incidence of fractures and to a lesser degree, vertebral fractures in the elderly, however, publication biases were obviously noted in the analysis [40]. This is a reflection of the fact that trials showing a positive connection between exercise and reduced fracture risk are published much more often than those showing negative or null findings. Furthermore, one recent Japanese study addressing lifestyle variables and osteoporosis in middle-aged and elderly women revealed that exercise is associated with increased incidence of fractures [41]. This might be partially explained by the fact that some types of exercise are associated with the increased risk of falls such as bicycling. It indicates that exercise should be carefully performed, especially for the elderly.
There were several limitations in the present study. First, the sample size and observation time were limited, which could cause insufficient statistical power for analysis of some variables and limited reproducibility of the results. Second, the subjects were not randomly enrolled but were volunteers drawn from people attending the morning health examinations. These people might pay more attention to health than those in the general population and were not nationally representative. Therefore, such selection bias should be considered while interpreting the results of this study. For example, they might consume more dark-green vegetables than the general population and this might reduce the significance of the relationship between dark-green vegetables intake and fall-related fractures in the analysis. Third, possible recall bias and information bias should also be considered since the information about lifestyle variables and the occurrence of fracture were obtained through questionnaire and telephone interview, respectively. For example, use of medicine such as anti-osteoporotic drugs might be under-reported as some subjects could not recognize or remember the drugs they used. On the other hand, lack of awareness of vertebral compression fractures in some subjects might result in underestimated fracture incidence. However, since most fractures, especially nonvertebral fracture would cause severe symptoms including intractable pain, swelling, and functional disability, leading individuals to seek medical assistance; we assume most participants could respond adequately about the occurrence of fractures during the telephone interviews within the 6–12-month interval. Nevertheless, on the other hand, the response rate of each telephone call interview to collect the information about falls and fracture was only 67.9–85.0%. Thus, among 183 subjects with accidental falls, those who responded more often or at the later times would have more chances to report a fracture. All of these factors would result in nondifferential misclassification and could thus dilute the association between variables. The fourth limitation was that the measurement of various kinds of food intake was done semi-quantitatively by total weekly frequency. Detailed information about the amount of intake of each nutrient component is not available. Therefore, the results were analyzed based merely on intake frequency rather than exact amount of food. Besides, BMD report of the subjects was not available in this study. Thus, lifestyle related BMD change could not be known. However, the results of the present study might be more directly related to fracture risk itself by using the incidence of fall-related fragility fracture as the outcome measure, since the bone strength is determined not only by BMD but also other factors such as bony micro-architecture.
Despite these limitations, to our knowledge, this is the first prospective study addressing the direct link between incidents of fall-related fractures and nutrition intake in Taiwan. The results revealed that subjects who consumed other deep-colored vegetables and light-colored vegetables fewer than 6 times a week or who consumed all types of vegetables fewer than 15 times/week were at greater risk for developing fragility fracture (odds ratio: 2.88). In light of these findings, it might be recommended that individuals consume vegetables at least 2 times a day for better bone health, and it is better also to include other deep-colored vegetable and light-colored vegetable in their regular diet. Further prospective cohort studies including larger sample sizes with longer observation periods are warranted to provide more evidence of modifiable risk factors for fragility fracture prevention.
In summary, among postmenopausal women, older age and the presence of hypertension were associated with increased risks of falls. Increased vegetable intake might be helpful to reduce the incidence of fall-related fragility fractures.
Financial support and sponsorship
This study was supported by grants CMRPG670091, CMRPG670092, CMRPG670093 and CMRPG670094 provided by the Chang Gung Medical Research Fund.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are honored to acknowledge the assistance of the Chiayi County Health Bureau and local public health centers.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook P., Cooper C. Osteoporos Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh M.K., Chen L.H., Chen W.J. Current concepts of percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures: evidence-based review. Biomed J. 2013;36:154–161. doi: 10.4103/2319-4170.112544. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y.C., Pan W.H. Bone mineral density in adults in Taiwan: results of the nutrition and health survey in Taiwan 2005–2008 (NAHSIT 2005–2008) Asia Pac J Clin Nutr. 2011;20:283–291. [PubMed] [Google Scholar]
- 5.Ho C.A., Li C.Y., Hsieh K.S., Chen H.F. Factors determining the 1-year survival after operated hip fracture: a hospital-based analysis. J Orthop Sci. 2010;15:30–37. doi: 10.1007/s00776-009-1425-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.M., Ho S.C., Woo J.L. Greater fruit and vegetable intake is associated with increased bone mass among postmenopausal Chinese women. Br J Nutr. 2006;96:745–751. [PubMed] [Google Scholar]
- 7.Prynne C.J., Mishra G.D., O'Connell M.A., Muniz G., Laskey M.A., Yan L. Fruit and vegetable intakes and bone mineral status: a cross-sectional study in 5 age and sex cohorts. Am J Clin Nutr. 2006;83:1420–1428. doi: 10.1093/ajcn/83.6.1420. [DOI] [PubMed] [Google Scholar]
- 8.Fujii H., Noda T., Sairenchi T., Muto T. Daily intake of green and yellow vegetables is effective for maintaining bone mass in young women. Tohoku J Exp Med. 2009;218:149–154. doi: 10.1620/tjem.218.149. [DOI] [PubMed] [Google Scholar]
- 9.Zeng F.F., Wu B.H., Fan F., Xie H.L., Xue W.Q., Zhu H.L. Dietary patterns and the risk of hip fractures in elderly Chinese: a matched case-control study. J Clin Endocrinol Metab. 2013;98:2347–2355. doi: 10.1210/jc.2013-1190. [DOI] [PubMed] [Google Scholar]
- 10.Xu L., Dibley M., D'Este C., Phillips M., Porteous J., Attia J. Food groups and risk of forearm fractures in postmenopausal women in Chengdu, China. Climacteric. 2009;12:222–229. doi: 10.1080/13697130802626958. [DOI] [PubMed] [Google Scholar]
- 11.Langsetmo L., Poliquin S., Hanley D.A., Prior J.C., Barr S., Anastassiades T. Dietary patterns in Canadian men and women ages 25 and older: relationship to demographics, body mass index, and bone mineral density. BMC Musculoskelet Disord. 2010;11:20. doi: 10.1186/1471-2474-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McTiernan A., Wactawski-Wende J., Wu L., Rodabough R.J., Watts N.B., Tylavsky F. Low-fat, increased fruit, vegetable, and grain dietary pattern, fractures, and bone mineral density: the Women's Health Initiative Dietary Modification Trial. Am J Clin Nutr. 2009;89:1864–1876. doi: 10.3945/ajcn.2008.26956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monma Y., Niu K., Iwasaki K., Tomita N., Nakaya N., Hozawa A. Dietary patterns associated with fall-related fracture in elderly Japanese: a population based prospective study. BMC Geriatr. 2010;10:31. doi: 10.1186/1471-2318-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S.J., Chang Y.H., Wei I.L., Kao M.D., Lin Y.C., Pan W.H. Intake levels and major food sources of energy and nutrients in the Taiwanese elderly. Asia Pac J Clin Nutr. 2005;14:211–220. [PubMed] [Google Scholar]
- 15.O'Loughlin J.L., Robitaille Y., Boivin J.F., Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 16.Chu L.W., Chi I., Chiu A.Y. Incidence and predictors of falls in the Chinese elderly. Ann Acad Med Singap. 2005;34:60–72. [PubMed] [Google Scholar]
- 17.Campbell A.J., Spears G.F., Borrie M.J. Examination by logistic regression modelling of the variables which increase the relative risk of elderly women falling compared to elderly men. J Clin Epidemiol. 1990;43:1415–1420. doi: 10.1016/0895-4356(90)90110-b. [DOI] [PubMed] [Google Scholar]
- 18.Ribot C., Pouillès J.M. Postmenopausal osteoporosis: clinical characteristics in patients first vertebral crush fracture. Results of the GRIO National Multicenter Survey. Groupe de Recherche et d'Information sur les Osteoporoses. Rev Rhum Ed Fr. 1993;60:427–434. [PubMed] [Google Scholar]
- 19.Schwartz A.V., Hillier T.A., Sellmeyer D.E., Resnick H.E., Gregg E., Ensrud K.E. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 20.Nevitt M.C., Cummings S.R., Hudes E.S. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46:M164–M170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 21.Lipsitz L.A. Abnormalities in blood pressure homeostasis that contribute to falls in the elderly. Clin Geriatr Med. 1985;1:637–648. [PubMed] [Google Scholar]
- 22.Gangavati A., Hajjar I., Quach L., Jones R.N., Kiely D.K., Gagnon P. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gribbin J., Hubbard R., Gladman J.R., Smith C., Lewis S. Risk of falls associated with antihypertensive medication: population-based case-control study. Age Ageing. 2010;39:592–597. doi: 10.1093/ageing/afq092. [DOI] [PubMed] [Google Scholar]
- 24.Callisaya M.L., Sharman J.E., Close J., Lord S.R., Srikanth V.K. Greater daily defined dose of antihypertensive medication increases the risk of falls in older people – a population-based study. J Am Geriatr Soc. 2014;62:1527–1533. doi: 10.1111/jgs.12925. [DOI] [PubMed] [Google Scholar]
- 25.Wang H.H., Tsay S.F. Elderly and long-term care trends and policy in Taiwan: challenges and opportunities for health care professionals. Kaohsiung J Med Sci. 2012;28:465–469. doi: 10.1016/j.kjms.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diet, nutrition, and the prevention of chronic diseases. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1990;797:1–204. [PubMed] [Google Scholar]
- 27.Nieves J.W. Skeletal effects of nutrients and nutraceuticals, beyond calcium and Vitamin D. Osteoporos Int. 2013;24:771–786. doi: 10.1007/s00198-012-2214-4. [DOI] [PubMed] [Google Scholar]
- 28.Jesudason D., Clifton P. The interaction between dietary protein and bone health. J Bone Min Metab. 2011;29:1–14. doi: 10.1007/s00774-010-0225-9. [DOI] [PubMed] [Google Scholar]
- 29.New S.A. Nutrition Society Medal lecture. The role of the skeleton in acid-base homeostasis. Proc Nutr Soc. 2002;61:151–164. doi: 10.1079/PNS2002159. [DOI] [PubMed] [Google Scholar]
- 30.Buclin T., Cosma M., Appenzeller M., Jacquet A.F., Décosterd L.A., Biollaz J. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. 2001;12:493–499. doi: 10.1007/s001980170095. [DOI] [PubMed] [Google Scholar]
- 31.Lin P.H., Ginty F., Appel L.J., Aickin M., Bohannon A., Garnero P. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133:3130–3136. doi: 10.1093/jn/133.10.3130. [DOI] [PubMed] [Google Scholar]
- 32.Weinsier R.L., Krumdieck C.L. Dairy foods and bone health: examination of the evidence. Am J Clin Nutr. 2000;72:681–689. doi: 10.1093/ajcn/72.3.681. [DOI] [PubMed] [Google Scholar]
- 33.Benetou V., Orfanos P., Zylis D., Sieri S., Contiero P., Tumino R. Diet and hip fractures among elderly Europeans in the EPIC cohort. Eur J Clin Nutr. 2011;65:132–139. doi: 10.1038/ejcn.2010.226. [DOI] [PubMed] [Google Scholar]
- 34.Feskanich D., Willett W.C., Colditz G.A. Calcium, Vitamin D, milk consumption, and hip fractures: a prospective study among postmenopausal women. Am J Clin Nutr. 2003;77:504–511. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 35.Feskanich D., Willett W.C., Stampfer M.J., Colditz G.A. Milk, dietary calcium, and bone fractures in women: a 12-year prospective study. Am J Public Health. 1997;87:992–997. doi: 10.2105/ajph.87.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feskanich D., Singh V., Willett W.C., Colditz G.A. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287:47–54. doi: 10.1001/jama.287.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Prieto-Alhambra D., Premaor M.O., Fina Avilés F., Hermosilla E., Martinez-Laguna D., Carbonell-Abella C. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Min Res. 2012;27:294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 38.Compston J.E., Watts N.B., Chapurlat R., Cooper C., Boonen S., Greenspan S. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124:1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace B.A., Cumming R.G. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;67:10–18. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- 40.Kemmler W., Häberle L., von Stengel S. Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporos Int. 2013;24:1937–1950. doi: 10.1007/s00198-012-2248-7. [DOI] [PubMed] [Google Scholar]
- 41.Tatsuno I., Terano T., Nakamura M., Suzuki K., Kubota K., Yamaguchi J. Lifestyle and osteoporosis in middle-aged and elderly women: Chiba bone survey. Endocr J. 2013;60:643–650. doi: 10.1507/endocrj.ej12-0368. [DOI] [PubMed] [Google Scholar]