Abstract
Background
Poststroke depression (PSD) is one of the most frequent and devastating neuropsychiatric consequences of stroke. The purpose of this study was to investigate the incidence and risk factors for PSD in a general hospital in Taiwan.
Methods
One hundred and one patients with ischemic stroke were enrolled initially, and 91 (90.1%) completed the 1-year study. Assessments were performed at baseline, and at the 1st, 3rd, 6th, 9th, and 12th month after enrolment. The definition of PSD was in accordance with the diagnostic criteria of major depressive episode in the Diagnostic and Statistical Manual, fourth edition (DSM-IV).
Results
The accumulated incidence rates of PSD at the 1st, 3rd, 6th, and 9th, month were 4%, 8%, 9%, and 10%, respectively, and the overall incidence at 1 year was 11%. In multivariate regression analysis, female gender, higher depression score, and severity of stroke were significant risk factors. In subgroup analysis, a higher depression score was significantly associated with PSD, regardless of gender; however, stroke severity was a risk factor only in the female group.
Conclusion
The 1-year incidence of PSD was 11%, based on the DSM-IV diagnostic criteria. More attention should be paid to patients with more risk factors to enable earlier detection and intervention.
Keywords: Incidence, Poststroke depression, Risk factors, Taiwan
At a glance commentary
Scientific background on the subject
Poststroke depression (PSD) is a very frequent neuropsychiatric sequel after ischemic stroke. Patients suffering from PSD may be associated with increased disability, poor rehabilitation outcome, and higher mortality. Thus, research into the incidence of PSD and the possible risk factors are important.
What this study adds to the field
Our study recruited ischemic stroke patients and followed them for 1 year to investigate the incidence of poststroke depression (PSD) and its risk factors. We used strict diagnostic criteria for PSD rather than just using the depression screening scale. Our result is valuable for the epidemiological study of PSD in Taiwan and useful also for its clinical implications.
Depression is one of the most frequent neuropsychiatric consequences of stroke and affects about one-third of all stroke patients [1]. Patients who develop depression after acute stroke (so-called poststroke depression [PSD]) have been associated with increased disability, cognitive impairment, and risk of falls [2], [3], [4]. Patients with PSD have also been reported to have a stronger correlation with significant impairment, poor rehabilitation outcome, poor quality of life, and higher mortality than those without significant depressive symptoms [5], [6].
Cultural variations have been reported in the incidence and prevalence of functional depression among the general population [7], and this appears to be true for PSD, with reported prevalence rates ranging from 6% to 79% [8]. In ethnic Chinese populations, the reported prevalence rates of PSD were 16.4% and 17.2% in Hong Kong [9], [10], [11], 34.9% [12] and 62.2% [13] in Taiwan, 43.4% in China [14], and 55% in Singapore [15]. Variations in the peak incidence of PSD have also been reported, including an increased frequency in the initial weeks poststroke, and particularly within the first 3 months [16], [17], [18]; a peak incidence around the 3rd to 6th month after stroke has also been reported [19]. In spite of these inconsistencies in peak incidence, PSD has been reported to occur from the acute phase to at least 2–3 years [20] but with an apparent peak within the first 6 months after stroke [21].
In addition to its high incidence and prevalence, previous studies have reported various risk factors for PSD including gender, age, living alone, lesion location, stroke severity, and functional disability [22], [23], [24], [25]. Previous studies on the incidence and risk factors for PSD in Taiwan have focused on patients in outpatient departments and in the community rather than at the acute stage after stroke. Thus, the current study had 2 aims: (1) To survey the 12-month incidence of PSD among Taiwanese acute ischemic stroke patients, (2) to identify the risk factors associated with PSD.
Methods
Patient enrolment and assessment
This study was conducted from July 2007 to June 2010 in a general teaching hospital with 650 beds in Taiwan. All subjects were screened consecutively when they were admitted to the neurological ward. Patients were included if they had a diagnosis of the first or recurrent ischemic stroke that was image-proven and that had occurred within the past 4 weeks. Patients were excluded from the study if they: (1) Had a transient ischemic attack; (2) had impaired communication or cognitive function (Mini-Mental State Examination [MMSE] score <15); (3) had history of depression, psychosis, or severe substance abuse; (4) had been taking antidepressants within 2 weeks prior to the stroke; or (5) had possible concurrent depression.
The onset of stroke was defined as the occurrence of abnormal neurological symptoms based on the patient's statements. The follow-up period was 12 months after enrolment. The first visit (baseline) was the patient's admission due to ischemic stroke. The initial assessments included recording demographic data, and an initial clinical diagnostic interview to exclude history of depression, concurrent depression, substance abuse, or psychosis. A self-report assessment scale for depression, the Taiwanese depression questionnaire (TDQ), was used to assess the severity of depressive symptoms. The TDQ is a 4-point scale with 18 items that has been shown to be a culturally specific depression screening instrument effective in screening depressive symptoms in Taiwanese with satisfactory reliability and validity [26]. It has also been reported to be superior to the Beck Depression Inventory in screening depression in a medical setting in Taiwan [27]. In addition, the National Institute of Health Stroke Scale (NIHSS) [28], instrumental activities of daily living scale (IADL) [29] and the MMSE [30] were used at the first visit.
Study protocol and diagnosis of poststroke depression
After being enrolled, the patients were followed up at the 1st, 3rd, 6th, 9th, and 12th month, and a clinical diagnostic interview was performed at each visit. The diagnosis of PSD in the study was based on the diagnostic criteria of major depressive episode in the Diagnostic and Statistical Manual, fourth edition (DSM-IV), and was made by a certificated psychiatrist (JA Su). If a patient dropped out, the reason was clarified and recorded.
Ethical review and informed consent
This study and the informed consent form were approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital and were performed in accordance with the World Medical Association Declaration of Helsinki and the Good Clinical Practice guidelines. All the participants had sufficient mental capacity and written informed consents were obtained from the participants following a complete explanation and consideration.
Statistical analysis
The t-test and Chi-square test were used to compare differences between those with and without PSD. The cumulative incidence curve of PSD was plotted using Kaplan–Meier Method and the effects of risk factors were compared with the log-rank test. Univariate and multivariate Cox proportional hazard analyses were further used to determine the risk factors for PSD. A p < 0.05 was used to indicate statistical significance. All analyses were performed using SPSS for Windows, Version 18 (SPSS Inc., Chicago, IL, USA).
Results
Socio-demographic and clinical profiles
A total of 519 patients admitted to our neurological wards due to ischemia during the study period were screened; 101 fulfilled the enrolment criteria and agreed to participate in the trial. Of these 101 patients, 69 (68.3%) were male, and 84 (82.2%) were married. The TDQ, NIHSS, IADL, and MMSE scores at baseline are shown in Table 1.
Table 1.
Baseline demographic data and clinical assessment scores.
| Characteristics | n (%) or mean ± SD (n = 101) |
|---|---|
| Gender | |
| Female | 32 (31.7) |
| Male | 69 (68.3) |
| Age (years) | 64.6 ± 11.0 |
| Marital status | |
| Single | 3 (3.0) |
| Married | 84 (82.2) |
| Widowed/divorced | 14 (14.8) |
| Occupation | |
| None | 46 (45.5) |
| Farmer and fisher | 27 (26.7) |
| Others | 28 (27.7) |
| Educational level | |
| None | 29 (28.7) |
| Primary school | 45 (44.6) |
| Higher than secondary school | 27 (26.7) |
| Location of stroke | |
| Left hemisphere | 47 (47.5) |
| Right hemisphere | 52 (52.5) |
| TDQ | 6.1 ± 5.9 |
| NIHSS | 2.6 ± 2.8 |
| IADL | 13.7 ± 7.5 |
| MMSE | 25.0 ± 4.0 |
| Completion rate | 91 (90.1) |
Abbreviations: SD: Standard deviation; TDQ: Taiwanese Depression Screening Questionnaire; NIHSS: National Institutes of Health Stroke Scale; IADL: Instrumental activities of daily living scale; MMSE: Mini-mental status examination; PSD: Poststroke depression.
Ninety-one patients (90.1%) completed the 12 months of the study. There were no significant differences in baseline socio-demographic data or assessment scores between the patients who did and did not complete the study. The reasons for withdrawal from the study were unwillingness to participate (40%) and inability to cooperate with the study protocol (20%).
Incidence of poststroke depression
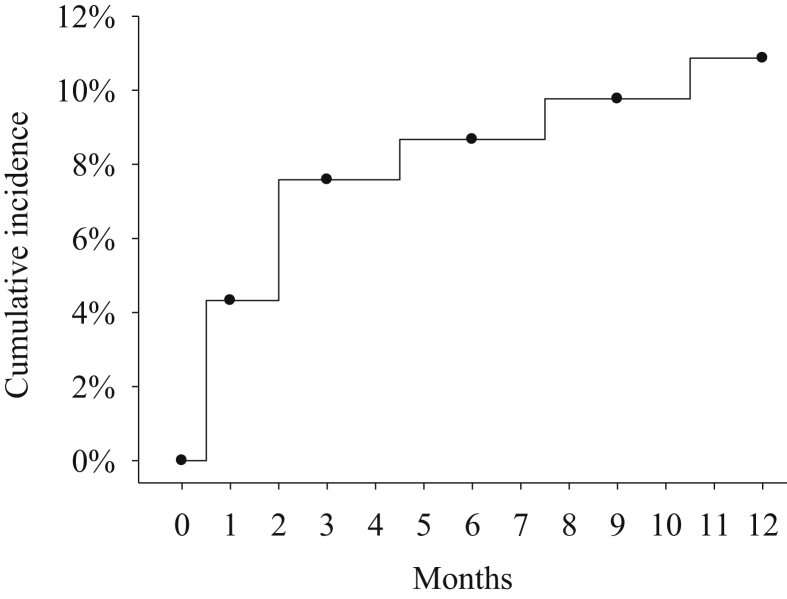
During the 12-month study period, 10 patients developed PSD; 4 were found at the 1st month visit, 3 at the 3rd month visit, and 1 each at the 6th, 9th, and 12th month visit after being enrolled. The incidence rates of PSD at the 1st, 3rd, 6th, and 9th month were 4%, 8%, 9%, and 10%, respectively, and the overall incidence at 1 year was 11% [Fig. 1].
Fig. 1.
Incidences of poststroke depression.
Risk factors for poststroke depression
The effects quartile classification of age, TDQ, NIHSS, IADL, and MMSE and other factors on PSD incidence were compared using log-rank test. Gender, TDQ, and NIHSS showed significant effects in Table 2. Further univariate Cox analysis revealed that PSD was significantly associated with female gender, higher TDQ and NIHSS scores, and a lower IADL score [Table 3]. After multivariate Cox regression analysis, female gender and higher TDQ and NIHSS scores still had a significant correlation [Table 3]. When the patients were divided into two groups based on gender, a higher TDQ score was significantly associated with PSD, regardless of gender. However, higher NIHSS scores, which were significantly associated with PSD, were observed only in the female group. The MMSE score may have been a protective factor for PSD in the male group; however, the significance disappeared after adjusting for the TDQ [Table 4].
Table 2.
The related incidences of PSD by different demographic data and clinical assessment scores.
| Characteristics | PSD incidence n (%) | pa |
|---|---|---|
| 1-year incidence of PSD | 10/91 (11.0) | |
| Gender | ||
| Male | 4/63 (6.3) | 0.034 |
| Female | 6/28 (21.4) | |
| Age (years) | 0.493b | |
| Marital status | ||
| Married | 10/76 (13.2) | 0.146 |
| Single/widowed/divorced | 0/15 (0.0) | |
| Occupation | ||
| None | 5/39 (12.8) | 0.625 |
| Others | 5/52 (9.6) | |
| Educational level | ||
| None | 5/26 (19.2) | 0.110 |
| Others | 5/65 (7.7) | |
| Location of stroke | ||
| Left hemisphere | 5/42 (11.9) | 0.873 |
| Right hemisphere | 5/47 (10.6) | |
| TDQ | 0.011b | |
| NIHSS | 0.045b | |
| IADL | 0.067b | |
| MMSE | 0.154b | |
Abbreviations: PSD: Poststroke depression; TDQ: Taiwanese Depression Screening Questionnaire; NIHSS: National Institutes of Health Stroke Scale; IADL: Instrumental activities of daily living scale; MMSE: Mini-mental status examination.
p values for the log-rank test.
Tested among quartile groups.
Table 3.
Risk factors associated with PSD in Cox regression.
| Factors | Univariate model |
Multivariate model 1 |
Multivariate model 2 |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | Adjusted HRa (95% CI) | p | Adjusted HRb (95% CI) | p | |
| Age | 1.00 (0.95–1.06) | 0.886 | ||||
| Female versus male | 3.52 (0.99–12.49) | 0.051 | 15.51 (2.50–96.01) | 0.003 | 15.95 (2.64–96.31) | 0.003 |
| TDQ | 1.15 (1.08–1.24) | <0.001 | 1.26 (1.12–1.41) | <0.001 | 1.26 (1.12–1.41) | <0.001 |
| NIHSS | 1.22 (1.02–1.46) | 0.030 | 1.46 (1.02–2.11) | 0.041 | 1.52 (1.15–2.01) | 0.003 |
| IADL | 0.91 (0.84–1.00) | 0.045 | 0.98 (0.86–1.11) | 0.742 | ||
| MMSE | 0.87 (0.75–1.01) | 0.060 | ||||
Abbreviations: HR: Hazard ratio; CI: Confidence interval; TDQ: Taiwanese Depression Screening Questionnaire; NIHSS: National Institutes of Health Stroke Scale; IADL: Instrumental activities of daily living scale; MMSE: Mini-Mental Status Examination; PSD: Poststroke depression.
Multivariate model 1 included gender, TDQ, NIHSS, and IADL.
Multivariate model 2 included gender, TDQ, and NIHSS.
Table 4.
Risk factors associated with PSD by gender.
| Factors | Univariate model |
Multivariate model 1 |
Multivariate model 2 |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | Adjusted HRa (95% CI) | p | Adjusted HRb (95% CI) | p | |
| Female | ||||||
| Age | 1.00 (0.93–1.07) | 0.990 | ||||
| TDQ | 1.21 (1.03–1.42) | 0.021 | 1.30 (1.09–1.56) | 0.004 | ||
| NIHSS | 1.33 (1.02–1.74) | 0.037 | 1.70 (1.16–2.50) | 0.007 | ||
| IADL | 0.89 (0.79–1.01) | 0.080 | ||||
| MMSE | 0.98 (0.82–1.17) | 0.836 | ||||
| Male | ||||||
| Age | 1.02 (0.93–1.13) | 0.657 | ||||
| TDQ | 1.20 (1.07–1.34) | 0.002 | 1.18 (1.04–1.33) | 0.009 | 1.20 (1.07–1.34) | 0.002 |
| NIHSS | 1.24 (0.95–1.63) | 0.111 | ||||
| IADL | 0.94 (0.82–1.07) | 0.352 | ||||
| MMSE | 0.73 (0.54–0.98) | 0.035 | 0.82 (0.64–1.05) | 0.111 | ||
Abbreviations: HR: Hazard ratio; CI: Confidence interval; TDQ: Taiwanese Depression Screening Questionnaire; NIHSS: National Institutes of Health Stroke Scale; IADL: Instrumental activities of daily living scale; MMSE: Mini-mental status examination; PSD: Poststroke depression.
Female multivariate model 1 included TDQ and NIHSS, and male model 1 included TDQ and MMSE.
Male multivariate model 2 included TDQ only.
Discussion
In this study, the incidence rates of PSD in the 1st, 3rd, 6th, and 9th month were 4%, 8%, 9%, and 10%, respectively, and the overall incidence at 1 year was 11%. Female gender and higher TDQ and NIHSS scores were risk factors associated with PSD.
Previous studies have reported that about 20%–40% of poststroke patients experience depressive symptoms [18], [31], [32], [33]. These discrepancies may be due to methodological heterogeneity, such as differences in the definition of stroke, the time of assessment after stroke, the diagnostic tools used to assess depression, the inclusion criteria, and the characteristics of the sample. In the present study, we used the DSM-IV diagnostic criteria of the major depressive episode to identify PSD, and this strict definition was also used in the studies from Hong Kong. Moreover, we excluded the patients with impairments in communication or cognitive function (MMSE score <15), out of concern for potential difficulties in conducting interviews, and history of depression, which has been reported to be a risk factor for PSD [22], [34], [35]. However, our study indicated the 1-year incidence of PSD rather than the prevalence, and thus it was difficult to compare our study with others.
We found that the severity of stroke was not as great among patients with lower NIHSS scores. Our results indicate that stroke severity is highly correlated with the incidence of PSD, and this may be another reason why the incidence in the current study was lower than that in other studies. In addition, cultural differences may be another issue. Ethnic Chinese are not considered to be psychologically-minded – they tend to deny depression due to stigmatization and express depression in other ways, such as neurasthenia or somatization [36]. It has also been reported that many verbal expressions of feeling in the Chinese language do not clearly discriminate between physical complaints and emotional distress [37]. Together, these factors may interfere with the detection and identification of PSD; the incidence of PSD might have been higher if depression rating scales with more somatic items were used to identify PSD, instead of the DSM-IV diagnostic criteria.
Because of the adverse effects of PSD, it is important to identify risk factors to allow for an early diagnosis and management, thereby decreasing the negative effects in stroke patients. We identified several risk factors associated with PSD, including female gender, stroke severity, and disability in daily living activities, which were consistent with previous studies [22], [24], [25], [38], [39], [40]; however, lesion laterality and educational level had no association with PSD, consistent with former studies [23], [39], [41]. Furthermore, being single, widowed, or divorced was not a risk factor for PSD in our study, in contrast to previous studies [39], [42]. This may be due to the small proportion of these patients and thus the inability to show a difference. As for depressive severity at baseline, we used the TDQ as an indicator of subclinical depressive symptoms. Higher depressive scores might lead to PSD afterward. In terms of clinical implications, clinicians should use the depression scale to screen ischemic stroke patients and then pay more attention to those with higher scores, since they might develop PSD. In addition, we also found some variations between male and female patients in the current study. The TDQ score was associated with PSD in both genders, so a higher depressive score at baseline predicted increased PSD,. However stroke severity in the NIHSS was significantly associated with PSD in the female group only. This suggests that the nature of PSD may differ in males and females. Gender differences in PSD were addressed in a systematic review study [43], which found that the prevalence of PSD was higher in females in most relevant studies. The postulated reasons for this included genetic factors, psychosocial issues, and the course-related to recovery [44]. Paradiso and Robinson reported that females are twice as likely to suffer from PSD as males and that this is due to more left hemisphere lesions occurring in females [45]. Further studies are needed to elucidate the exact relationship between gender and PSD.
In spite of the lack of consistency regarding the peak incidence of PSD, most patients in our study developed PSD within the first 3 months, which is similar to previous reports [16], [17], [18]. Thus, stroke patients should be carefully monitored, especially in the first 3 months after stroke in order to detect PSD earlier and allow for interventions.
There are several limitations to this study. First, enrolment did not include all stroke patients in the acute phase, and the most had mild severity. Therefore, the findings may not be applicable to all stroke patients. Second, this study was carried out in only one general hospital, so the results may not be generalized to other settings. Third, on the basis of exploratory analysis, our findings in present study need to be confirmed in further larger studies. Even so, we believe our findings are valuable. We addressed differences in risk factors between genders, which have not frequently been reported, and we found that the TDQ score and stroke severity may be predictors of PSD in stroke patients in Taiwan.
Conclusion
Our reported incidence of PSD was around 11% based on the criteria for major depressive episode in the DSM-IV. Given the negative impact of PSD, early detection and intervention are important. The clinical implication of our findings is that close observation of stroke patients and screening for depression are needed, especially during the first 3 months after stroke and in those with risk factors for PSD.
Source of support
The authors are very grateful to the research team for their dedication to this study and for grants from the Chang-Gung Medical Research Program (CMRPG 690493).
Conflicts of interest
None declared.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Hackett M.L., Yapa C., Parag V., Anderson C.S. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 2.Austin M.P., Mitchell P., Goodwin G.M. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 3.Lenze E.J., Rogers J.C., Martire L.M., Mulsant B.H., Rollman B.L., Dew M.A. The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry. 2001;9:113–135. [PubMed] [Google Scholar]
- 4.Jørgensen L., Engstad T., Jacobsen B.K. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33:542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 5.Paolucci S., Antonucci G., Grasso M.G., Morelli D., Troisi E., Coiro P. Post-stroke depression, antidepressant treatment and rehabilitation results. A case-control study. Cerebrovasc Dis. 2001;12:264–271. doi: 10.1159/000047714. [DOI] [PubMed] [Google Scholar]
- 6.House A., Knapp P., Bamford J., Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32:696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 7.Chi I., Chou K.L. Social support and depression among elderly Chinese people in Hong Kong. Int J Aging Hum Dev. 2001;52:231–252. doi: 10.2190/V5K8-CNMG-G2UP-37QV. [DOI] [PubMed] [Google Scholar]
- 8.Lökk J., Delbari A. Management of depression in elderly stroke patients. Neuropsychiatr Dis Treat. 2010;6:539–549. doi: 10.2147/NDT.S7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W.K., Ungvari G.S., Chiu H.F., Sze K.H., Woo J., Kay R. Psychiatric morbidity in first time stroke patients in Hong Kong: a pilot study in a rehabilitation unit. Aust N Z J Psychiatry. 2002;36:544–549. doi: 10.1046/j.1440-1614.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 10.Tang W.K., Chan S.S., Chiu H.F., Wong K.S., Kwok T.C., Mok V. Can the geriatric depression scale detect poststroke depression in Chinese elderly? J Affect Disord. 2004;81:153–156. doi: 10.1016/S0165-0327(03)00163-0. [DOI] [PubMed] [Google Scholar]
- 11.Tang W.K., Ungvari G.S., Chiu H.F., Sze K.H., Yu A.C., Leung T.L. Screening post-stroke depression in Chinese older adults using the hospital anxiety and depression scale. Aging Ment Health. 2004;8:397–399. doi: 10.1080/13607860410001725027. [DOI] [PubMed] [Google Scholar]
- 12.Li S.C., Wang K.Y., Lin J.C. Depression and related factors in elderly patients with occlusion stroke. J Nurs Res. 2003;11:9–18. doi: 10.1097/01.jnr.0000347614.44660.b5. [DOI] [PubMed] [Google Scholar]
- 13.Fuh J.L., Liu H.C., Wang S.J., Liu C.Y., Wang P.N. Poststroke depression among the Chinese elderly in a rural community. Stroke. 1997;28:1126–1129. doi: 10.1161/01.str.28.6.1126. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q. A correlative study on post-stroke depression and CT, physical, psychological and social parameters. Zhonghua Shen Jing Jing Shen Ke Za Zhi. 1992;25:203–207. 252. [PubMed] [Google Scholar]
- 15.Loong C.K., Kenneth N.K., Paulin S.T. Post-stroke depression: outcome following rehabilitation. Aust N Z J Psychiatry. 1995;29:609–614. doi: 10.3109/00048679509064975. [DOI] [PubMed] [Google Scholar]
- 16.Paolucci S., Gandolfo C., Provinciali L., Torta R., Toso V., DESTRO Study Group The Italian multicenter observational study on post-stroke depression (DESTRO) J Neurol. 2006;253:556–562. doi: 10.1007/s00415-006-0058-6. [DOI] [PubMed] [Google Scholar]
- 17.Aben I., Verhey F., Strik J., Lousberg R., Lodder J., Honig A. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581–585. doi: 10.1136/jnnp.74.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aström M., Adolfsson R., Asplund K. Major depression in stroke patients. A 3-year longitudinal study. Stroke. 1993;24:976–982. doi: 10.1161/01.str.24.7.976. [DOI] [PubMed] [Google Scholar]
- 19.Whyte E.M., Mulsant B.H. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52:253–264. doi: 10.1016/s0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- 20.Berg A., Palomäki H., Lehtihalmes M., Lönnqvist J., Kaste M. Poststroke depression: an 18-month follow-up. Stroke. 2003;34:138–143. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- 21.Dafer R.M., Rao M., Shareef A., Sharma A. Poststroke depression. Top Stroke Rehabil. 2008;15:13–21. doi: 10.1310/tsr1501-13. [DOI] [PubMed] [Google Scholar]
- 22.Andersen G., Vestergaard K., Ingemann-Nielsen M., Lauritzen L. Risk factors for post-stroke depression. Acta Psychiatr Scand. 1995;92:193–198. doi: 10.1111/j.1600-0447.1995.tb09567.x. [DOI] [PubMed] [Google Scholar]
- 23.Carson A.J., MacHale S., Allen K., Lawrie S.M., Dennis M., House A. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356:122–126. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann N., Black S.E., Lawrence J., Szekely C., Szalai J.P. The Sunnybrook stroke study: a prospective study of depressive symptoms and functional outcome. Stroke. 1998;29:618–624. doi: 10.1161/01.str.29.3.618. [DOI] [PubMed] [Google Scholar]
- 25.Singh A., Black S.E., Herrmann N., Leibovitch F.S., Ebert P.L., Lawrence J. Functional and neuroanatomic correlations in poststroke depression: the Sunnybrook stroke study. Stroke. 2000;31:637–644. doi: 10.1161/01.str.31.3.637. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y., Yang M.J., Lai T.J., Chiu N.M., Chau T.T. Development of the Taiwanese depression questionnaire. Chang Gung Med J. 2000;23:688–694. [PubMed] [Google Scholar]
- 27.Lee Y., Lin P.Y., Hsu S.T., Cing-Chi Y., Yang L.C., Wen J.K. Comparing the use of the Taiwanese Depression Questionnaire and Beck Depression Inventory for screening depression in patients with chronic pain. Chang Gung Med J. 2008;31:369–377. [PubMed] [Google Scholar]
- 28.Kunitz S.C., Gross C.R., Heyman A., Kase C.S., Mohr J.P., Price T.R. The pilot stroke data bank: definition, design, and data. Stroke. 1984;15:740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 29.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 30.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Burvill P.W., Johnson G.A., Jamrozik K.D., Anderson C.S., Stewart-Wynne E.G., Chakera T.M. Prevalence of depression after stroke: the Perth community stroke study. Br J Psychiatry. 1995;166:320–327. doi: 10.1192/bjp.166.3.320. [DOI] [PubMed] [Google Scholar]
- 32.Berg A., Palomäki H., Lehtihalmes M., Lönnqvist J., Kaste M. Poststroke depression in acute phase after stroke. Cerebrovasc Dis. 2001;12:14–20. doi: 10.1159/000047675. [DOI] [PubMed] [Google Scholar]
- 33.Di Michele V., Bolino F. Post-stroke depression. Br J Psychiatry. 2000;176:94–95. doi: 10.1192/bjp.176.1.94. [DOI] [PubMed] [Google Scholar]
- 34.Kauhanen M., Korpelainen J.T., Hiltunen P., Brusin E., Mononen H., Määttä R. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30:1875–1880. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 35.Paolucci S., Gandolfo C., Provinciali L., Torta R., Sommacal S., Toso V., DESTRO Study Group Quantification of the risk of post stroke depression: the Italian multicenter observational study DESTRO. Acta Psychiatr Scand. 2005;112:272–278. doi: 10.1111/j.1600-0447.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 36.Parker G., Gladstone G., Chee K.T. Depression in the planet's largest ethnic group: the Chinese. Am J Psychiatry. 2001;158:857–864. doi: 10.1176/appi.ajp.158.6.857. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y.P., Xu L.Y., Shen Q.J. Styles of verbal expression of emotional and physical experiences: a study of depressed patients and normal controls in China. Cult Med Psychiatry. 1986;10:231–243. doi: 10.1007/BF00114698. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh L.P., Kao H.J. Depressive symptoms following ischemic stroke: a study of 207 patients. Acta Neurol Taiwan. 2005;14:187–190. [PubMed] [Google Scholar]
- 39.Tang W.K., Chan S.S., Chiu H.F., Ungvari G.S., Wong K.S., Kwok T.C. Poststroke depression in Chinese patients: frequency, psychosocial, clinical, and radiological determinants. J Geriatr Psychiatry Neurol. 2005;18:45–51. doi: 10.1177/0891988704271764. [DOI] [PubMed] [Google Scholar]
- 40.Sit J.W., Wong T.K., Clinton M., Li L.S. Associated factors of post-stroke depression among Hong Kong Chinese: a longitudinal study. Psychol Health Med. 2007;12:117–125. doi: 10.1080/14622200500358978. [DOI] [PubMed] [Google Scholar]
- 41.Desmond D.W., Remien R.H., Moroney J.T., Stern Y., Sano M., Williams J.B. Ischemic stroke and depression. J Int Neuropsychol Soc. 2003;9:429–439. doi: 10.1017/S1355617703930086. [DOI] [PubMed] [Google Scholar]
- 42.Hayee M.A., Akhtar N., Haque A., Rabbani M.G. Depression after stroke-analysis of 297 stroke patients. Bangladesh Med Res Counc Bull. 2001;27:96–102. [PubMed] [Google Scholar]
- 43.Poynter B., Shuman M., Diaz-Granados N., Kapral M., Grace S.L., Stewart D.E. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics. 2009;50:563–569. doi: 10.1176/appi.psy.50.6.563. [DOI] [PubMed] [Google Scholar]
- 44.Grace S.L., Abbey S.E., Pinto R., Shnek Z.M., Irvine J., Stewart D.E. Longitudinal course of depressive symptomatology after a cardiac event: effects of gender and cardiac rehabilitation. Psychosom Med. 2005;67:52–58. doi: 10.1097/01.psy.0000151486.28349.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paradiso S., Robinson R.G. Gender differences in poststroke depression. J Neuropsychiatry Clin Neurosci. 1998;10:41–47. doi: 10.1176/jnp.10.1.41. [DOI] [PubMed] [Google Scholar]