ABSTRACT
Spermatogenesis involves the progressive reorganization of heterochromatin. However, the mechanisms that underlie the dynamic remodeling of heterochromatin remain unknown. Here, we identify SCML2, a germline-specific Polycomb protein, as a critical regulator of heterochromatin organization in spermatogenesis. We show that SCML2 accumulates on pericentromeric heterochromatin (PCH) in male germ cells, where it suppresses PRC1-mediated monoubiquitylation of histone H2A at Lysine 119 (H2AK119ub) and promotes deposition of PRC2-mediated H3K27me3 during meiosis. In postmeiotic spermatids, SCML2 is required for heterochromatin organization, and the loss of SCML2 leads to the formation of ectopic patches of facultative heterochromatin. Our data suggest that, in the absence of SCML2, the ectopic expression of somatic lamins drives this process. Furthermore, the centromere protein CENP-V is a specific marker of PCH in postmeiotic spermatids, and SCML2 is required for CENP-V localization on PCH. Given the essential functions of PRC1 and PRC2 for genome-wide gene expression in spermatogenesis, our data suggest that heterochromatin organization and spermatogenesis-specific gene expression are functionally linked. We propose that SCML2 coordinates the organization of heterochromatin and gene expression through the regulation of Polycomb complexes.
KEY WORDS: Pericentromeric heterochromatin, Spermatogenesis, Meiosis, Spermatids, Chromocenter
Summary: Organization of heterochromatin and gene expression is coordinated by SCML2 through the regulation of Polycomb complexes in the male germline of mice.
INTRODUCTION
Spermatogenesis involves the progressive, dynamic reorganization of germ cell nuclei, beginning in mitotic spermatogonia and continuing through to mature sperm (Kimmins and Sassone-Corsi, 2005). A population of self-renewing spermatogonial stem cells supports the continuous production of sperm. Once differentiation commences, spermatogonia undergo mitotic proliferation before entering meiosis, when germ cells go through homologous recombination and reductional divisions. After the completion of meiosis, spermatids go through a nuclear transformation to produce compacted mature sperm. These dynamic nuclear changes are accompanied by the reorganization of heterochromatin in spermatogenesis (Berrios, 2017; Rousseaux et al., 2008) (Fig. 1A).
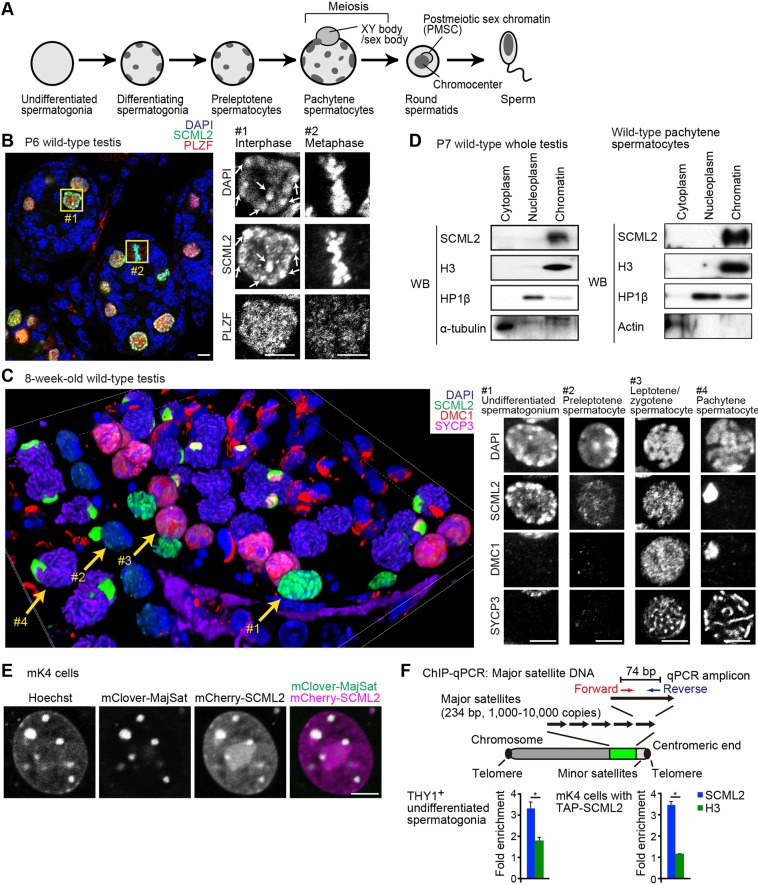
Fig. 1.
SCML2 tightly binds chromatin and localizes on pericentromeric heterochromatin. (A) Schematic of organization of heterochromatin during spermatogenesis. (B) SCML2 localizes on pericentromeric heterochromatin (arrows) in interphase nuclei of PLZF-positive undifferentiated spermatogonia (box 1) and on metaphase chromosomes (box 2) in a testicular section at P6. Boxed regions are magnified in the right panels. Scale bars: 5 µm. (C) Localization of SCML2 during spermatogenesis. Immunostaining of testicular paraffin sections with the indicated antibodies. The image was acquired with a confocal microscope and reconstituted in 3D from 68 z-stacks. Nuclei indicated by yellow arrows are magnified in the right panels as 2D images. Scale bars: 5 µm. (D) Chromatin binding of SCML2 shown through cell fractionation and western blotting. P7 whole testis and isolated pachytene spermatocytes from adult testes are shown. (E) Live imaging of mCherry-SCML2 (magenta) and mClover-MajSat (green), which detects major satellite repeats in mK4 cells; in the far-right panel, the merge of mClover-MajSat and mCherry-SCML2 signals, excluding Hoechst signals, appears white. Scale bar: 5 μm. (F) SCML2 enrichment was detected via ChIP-qPCR using THY1+ undifferentiated spermatogonia and mK4 cells expressing TAP-SCML2. The relative values of ChIP-qPCR data to input samples were normalized with that of rDNA. Mean±s.e.m. (error bars); n=4. *P<0.05, unpaired t-test. The values for rDNA loci are normalized to 1, and relative values are shown.
In somatic cells, pericentromeric heterochromatin (PCH) – a type of chromatin that is formed from the satellite DNA at pericentromeric regions – is consistently enriched with markers such as the silent histone modification H3K9me3 and heterochromatin proteins HP1α, HP1β and HP1γ (Saksouk et al., 2015). Thus, PCH is considered to be ‘constitutive heterochromatin’. In male germ cells, PCH undergoes continuous remodeling as spermatogenesis progresses. As germ cells progress to become differentiating spermatogonia, they become enriched with H3K9me3 and a structural maintenance of chromosomes (SMC) protein, SMC6 (Shirakawa et al., 2013; Verver et al., 2013). During meiosis, the accumulation of H3K9me2, H3K9me3 and HP1γ on autosomal PCH is critical for homologous pairing (Peters et al., 2001; Tachibana et al., 2007; Takada et al., 2011), and the movement of PCH ensures the homology search that occurs during homologous recombination (Scherthan et al., 2014). In contrast to the situation in autosomes, the PCH of the unsynapsed X chromosome is uniquely established in meiosis by the action of the DNA damage response protein BRCA1 (Broering et al., 2014). After the completion of meiosis, PCH is organized into a single chromocenter (Haaf and Ward, 1995), which has been hypothesized to define the nuclear topology that facilitates chromatin condensation in spermiogenesis (Meyer-Ficca et al., 1998). Chromocenter organization is, in part, mediated by BRDT, a testis-specific member of the BET family of double bromodomain-containing proteins (Berkovits and Wolgemuth, 2011). However, the mechanism by which heterochromatin undergoes dynamic remodeling during spermatogenesis remains undetermined.
Here, we identify the germline-specific Polycomb protein SCML2 as a critical regulator of heterochromatin organization in spermatogenesis. Polycomb proteins regulate heritable gene silencing and define cell type-specific gene expression (Aranda et al., 2015; Geisler and Paro, 2015; Laugesen and Helin, 2014). In spermatogenesis, Polycomb repressive complex 2 (PRC2) suppresses somatic genes (Mu et al., 2014), whereas PRC1 suppresses non-lineage-specific genes and directs timely activation of germline genes (Maezawa et al., 2017). SCML2, which was identified as a germline-specific subunit of PRC1, globally suppresses the somatic gene expression program in late spermatogenesis, during meiosis and into postmeiotic stages (Hasegawa et al., 2015). During late spermatogenesis, thousands of genes commonly expressed in both somatic lineages and the progenitors of spermatocytes (e.g. mitotic spermatogonia) – termed somatic/progenitor genes – are largely suppressed; meanwhile, a distinct class of thousands of other genes, termed late spermatogenesis genes, is activated (Hasegawa et al., 2015; Sin et al., 2015).
In this study, we show that SCML2 accumulates on PCH in male germ cells to suppress PRC1-mediated monoubiquitylation of histone H2A at Lysine 119 (H2AK119ub) and we show that SCML2 promotes PRC2-mediated deposition of H3K27me3 on PCH during meiosis. In postmeiotic spermatids, SCML2 is required for heterochromatin organization, as loss of SCML2 leads to ectopic patches of facultative heterochromatin. Furthermore, we demonstrate that the centromere protein CENP-V is a specific marker of PCH in postmeiotic spermatids and that SCML2 is required for CENP-V localization on PCH. Given the essential functions of both PRC1 and PRC2 in spermatogenesis, our data suggest that heterochromatin organization and spermatogenesis-specific gene expression are functionally linked in spermatogenesis.
RESULTS
SCML2 tightly binds chromatin throughout the cell cycle in spermatogenesis
To determine the functions of SCML2, which localizes to the nuclei of germ cells (Hasegawa et al., 2015; Luo et al., 2015), we first performed detailed analyses of SCML2 localization using a confocal microscope during spermatogenesis. Analyses of wild-type testis sections at postnatal day (P)6 revealed that SCML2 accumulates at high levels in the nuclei of germ cells. Indeed, SCML2 accumulated in interphase nuclei of undifferentiated spermatogonia, as detected with PLZF, a nuclear marker that is required for the maintenance of undifferentiated spermatogonia (Buaas et al., 2004; Costoya et al., 2004) and metaphase chromosomes (Fig. 1B). In interphase nuclei, SCML2 localized on DAPI-intense pericentromeric heterochromatin (PCH; arrows in Fig. 1B, box 1). On metaphase chromosomes, SCML2 signals overlapped with DAPI-stained condensed chromatin (Fig. 1B, box 2). PLZF did not show these patterns. These results suggest that SCML2 tightly binds DAPI-stained chromatin throughout the cell cycle.
Next, we examined wild-type 8-week-old testis samples through three-dimensional (3D) reconstruction of two-dimensional (2D) confocal images (Fig. 1C). We observed that SCML2 was strongly enriched at DAPI-intense PCH in undifferentiated spermatogonia (Fig. 1C, arrow 1). Although SCML2 signal intensity decreased in the nuclei of preleptotene spermatocytes (Fig. 1C, arrow 2), SCML2 persisted into the nuclei of leptotene/zygotene spermatocytes (Fig. 1C, arrow 3); leptotene/zygotene spermatocytes were detected using two meiotic markers, DMC1 and SYCP3, which detect, respectively, recombination foci (Terasawa et al., 1995) and meiotic chromosome axes (Dobson et al., 1994). While SCML2 was typically spread throughout the nuclei in the leptotene/zygotene stages, SCML2 disappeared from autosomal regions during the mid pachytene stage of prophase; at the same time, SCML2 became highly enriched on the sex chromosomes during meiosis (Fig. 1C, arrow 4). At this time, meiotic sex chromosome inactivation has taken place, and the sex chromosomes have formed a transcriptionally silent domain termed the XY body (Ichijima et al., 2012; Turner, 2007).
We next examined the chromatin binding of SCML2 during spermatogenesis through cell fractionation followed by western blotting. SCML2 was previously reported as a chromatin binding protein (Luo et al., 2015). In P7 testes, which are enriched for spermatogonia, and in isolated pachytene spermatocytes, SCML2 was tightly bound to chromatin (Fig. 1D). Although a previous study reported that SCML2 is also present in the soluble nuclear extract of P20 testes (Luo et al., 2015), we could not detect SCML2 in the nucleoplasm or cytoplasm fractions of P7 testes and pachytene spermatocytes. Differences in the stages of the materials may account for this. Our experiments verified the quality of the nucleoplasm fraction through the absence of the chromatin marker histone H3, the absence of cytoplasm markers and the presence of HP1β (Fig. 1D), thereby confirming that SCML2 is tightly bound to chromatin.
To confirm the direct binding of SCML2 to PCH, we performed a live-imaging assay that monitors the chromatin binding property of SCML2. In this set-up, we took advantage of somatic cells, where germline-specific SCML2 is not endogenously expressed, to observe full-length SCML2 fused to the fluorescent fusion protein mCherry (mCherry-SCML2). We used the somatic cell line mK4, derived from embryonic kidney mesenchyme (Valerius et al., 2002), to monitor localization of mCherry-SCML2. In mK4 cells, mCherry-SCML2 accumulated on Hoechst-dense PCH (Fig. 1E). The location of major satellites that comprise PCH in mK4 cells was confirmed with mClover, a fluorescent fusion protein that binds major satellites (Miyanari et al., 2013) (Fig. 1E). The binding of SCML2 to PCH and chromatin was further confirmed by live imaging using mK4 cells with ectopically expressed mCherry-SCML2 throughout the cell cycle (Fig. S1). The binding of SCML2 to major satellites was validated with chromatin immunoprecipitation (ChIP) followed by quantitative PCR (qPCR). SCML2 was enriched at major satellites in THY1+ spermatogonia, as well as in mK4 cells with ectopically expressed tandem affinity purification (TAP)-tagged SCML2 (Fig. 1F). Together, we conclude that SCML2 tightly binds PCH and nuclear chromatin throughout the cell cycle, which is in line with the previously identified role of SCML2 as a regulator of heritable epigenetic memories of gene silencing in spermatogenesis (Hasegawa et al., 2015).
SCML2 suppresses PRC1-mediated H2AK119ub but promotes PRC2-mediated H3K27me3 on pericentromeric heterochromatin
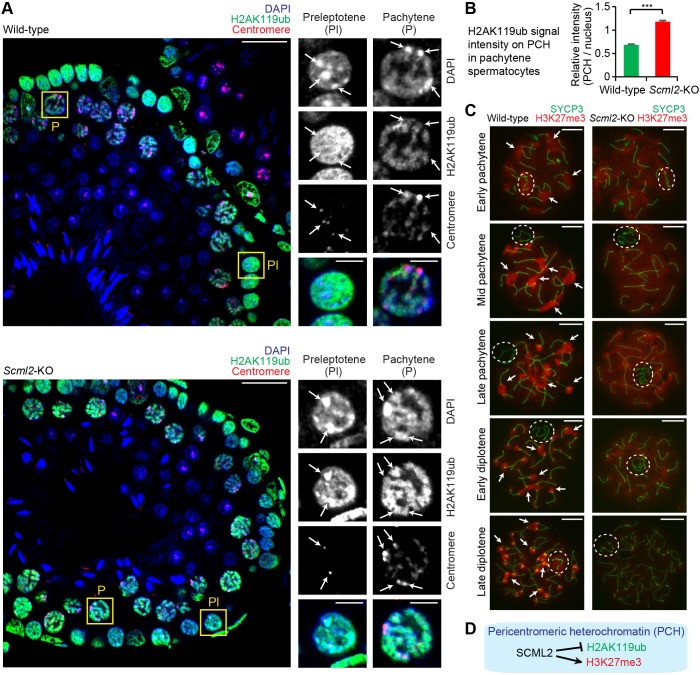
To determine the functions of SCML2 in nuclear organization, we investigated the nuclear distribution of monoubiquitylation of histone H2A at Lysine 119 (H2AK119ub), a PRC1-mediated silencing mark, in germ cells from Scml2-knockout (Scml2-KO) mice. While SCML2 forms a complex with PRC1 to promote H2AK119ub for suppression of somatic/progenitor genes on autosomes (Hasegawa et al., 2015), SCML2 also suppresses H2AK119ub on the sex chromosomes during meiosis (Adams et al., 2018; Hasegawa et al., 2015; Luo et al., 2015). In wild-type spermatogenesis, PCH, detected as DAPI-dense heterochromatin, was largely devoid of H2AK119ub in both preleptotene spermatocytes and pachytene spermatocytes (Fig. 2A, top panels). However, in the Scml2-KO mice, PCH was highly enriched with H2AK119ub in preleptotene spermatocytes and pachytene spermatocytes (Fig. 2A, bottom panels), and signal quantification confirmed that the relative signal intensity of H2AK119ub increased on PCH compared to that of the entire nuclei of Scml2-KO pachytene spermatocytes (Fig. 2B). Together, these data suggest that H2AK119ub is suppressed by SCML2 on PCH before and during meiosis.
Fig. 2.
SCML2 suppresses PRC1-mediated H2AK119ub but promotes PRC2-mediated H3K27me3 on pericentromeric heterochromatin. (A) Immunostaining of testicular paraffin sections with the indicated antibodies. All images were acquired with a confocal microscope. Boxed regions are magnified in the right panels. Scale bars: 20 µm (left) and 5 µm (right). SCML2 suppresses H2AK119ub on pericentromeric heterochromatin (PCH), shown by arrows. (B) Quantification of relative signal intensity of H2AK119ub on PCH in pachytene spermatocytes. The relative intensity is a ratio of mean signal intensity on PCH to mean signal intensity of a given nucleus after the subtraction of background signal intensity (see Materials and Methods). Wild-type: 123 regions of PCH were quantified in 41 cells from three mice; Scml2-KO: 93 regions of PCH were quantified in 31 cells from three mice. Mean±s.e.m. (error bars); ***P<0.0001, unpaired t-test. (C) Immunostaining of H3K27me3 and SYCP3, a marker of chromosome axes, in chromosome spreads of each substage of pachytene and diplotene spermatocytes. Dashed circles, sex chromosomes; arrows, PCH in wild type. Images were acquired with a wide-field microscope. Scale bars: 10 µm. (D) Summary of the action of SCML2 on PCH in male germ cells.
Since the function of PRC1 is related to another Polycomb complex, PRC2 (Blackledge et al., 2014; Cooper et al., 2014; Kahn et al., 2016), we investigated the function of SCML2 in the nuclear distribution of the PRC2-mediated histone modification trimethylation of histone H3 lysine 27 (H3K27me3). To detail the localization of H3K27me3 in meiotic spermatocytes, we performed immunostaining of H3K27me3 using meiotic chromosome spreads. During meiotic prophase of wild-type mice, H3K27me3 first accumulated on PCH in the early pachytene stage and was notably enriched on PCH from the mid pachytene stage onward (Fig. 2C; Fig. S2). Also, in wild-type mice, H3K27me3 was initially present on the sex chromosome in the early pachytene stage but was largely depleted from the sex chromosomes from the mid pachytene stage onward (Fig. 2C; Fig. S2). Contrastingly, in Scml2-KO mice, the accumulation of H3K27me3 on PCH was markedly diminished from the mid pachytene stage onward, although the localization pattern of H3K27me3 on the sex chromosomes was not affected (Fig. 2C; Fig. S2). Together with the finding that SCML2 binds PCH (Fig. 1), SCML2 is revealed to have two opposing functions on PCH: promotion of PRC2-mediated H3K27me3 and suppression of PRC1-mediated H2AK119ub (Fig. 2D).
The loss of SCML2 leads to abnormal organization of facultative heterochromatin in round spermatids.
Next, by using confocal microscopy, we investigated the consequences of SCML2 deficiency on the nuclear organization of round spermatids (RSs), germ cells that have progressed to a postmeiotic stage. In RSs, PCH is clustered in a central region of the nucleus, forming a nuclear body termed the chromocenter (labeled ‘CC’ in Fig. 3) (Haaf and Ward, 1995). Adjoining the chromocenter, the X or Y chromosome forms a silent heterochromatin domain termed postmeiotic sex chromatin (PMSC), which is associated with the maintenance of gene silencing following meiotic sex chromosome inactivation (Namekawa et al., 2006). In Scml2-KO RSs, in addition to the chromocenter and PMSC, ectopic DAPI-dense, patch-like structures appeared throughout the nucleus (Fig. 3A, arrows). These ectopic patches, which were entirely absent from wild-type RSs (n=30 cells from four independent mice), were consistently present in Scml2-KO RSs: the number of ectopic patches was 6.7±0.17 (n=26 cells, mean±s.e.m., from three independent mice) per nucleus.
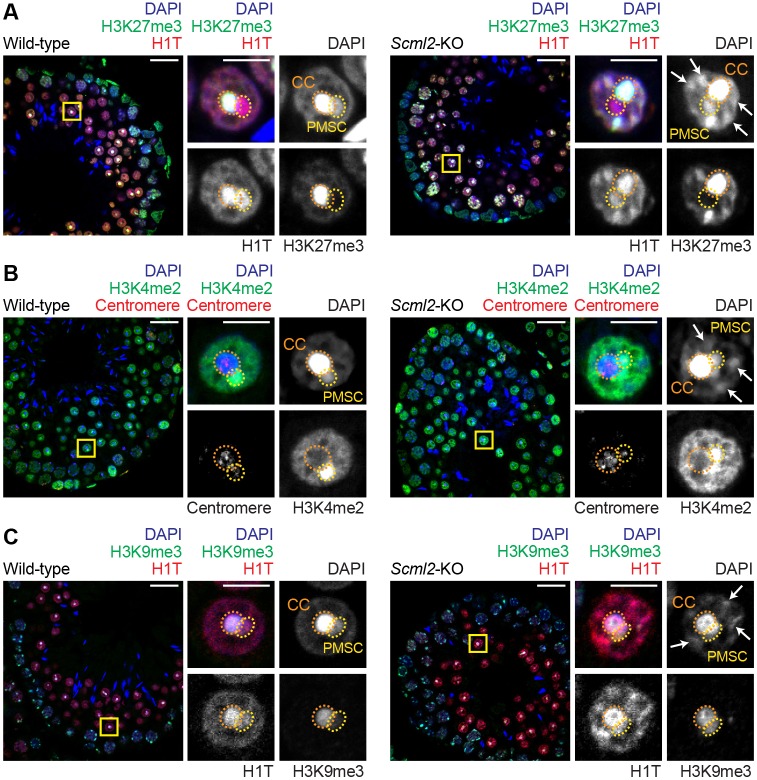
Fig. 3.
Loss of SCML2 leads to ectopic patches of DAPI-stained chromatin in round spermatids. (A-C) Immunostaining of testicular paraffin sections with the indicated antibodies. Images were acquired with a confocal microscope. Boxed regions are magnified in the right panels. CC, chromocenter; PMSC, postmeiotic sex chromatin. Arrows indicate ectopic patches in Scml2-KO RSs. Scale bars: 20 µm (left) and 5 µm (right). Three independent pairs of wild-type and Scml2-KO mice were examined and representative images are shown.
We next sought to determine specific modifications that comprise the ectopic patches in Scml2-KO RS. One such modification was H3K27me3, which was present on ectopic patches, in addition to the chromocenter, in Scml2-KO RS; in contrast, in wild-type RSs, H3K27me3 was confined to the chromocenter (Fig. 3A). We observed the intense accumulation of another modification, the testis-specific histone variant H1T, at ectopic patches in Scml2-KO RSs; however, in wild-type RSs, the intense accumulation of H1T was observed solely at the chromocenter and PMSC (Fig. 3A). 3D reconstruction of 2D confocal images confirmed that the ectopic patches observed in Scml2-KO RSs were present throughout nuclei and were absent from wild-type nuclei (Figs S3 and S4). The ectopic patches did not contain centromeres (Fig. 3B) and were devoid of H3K9me3, a marker of constitutive heterochromatin (Fig. 3C). Thus, these results suggest that the ectopic patches are not constitutive heterochromatin surrounding centromeres.
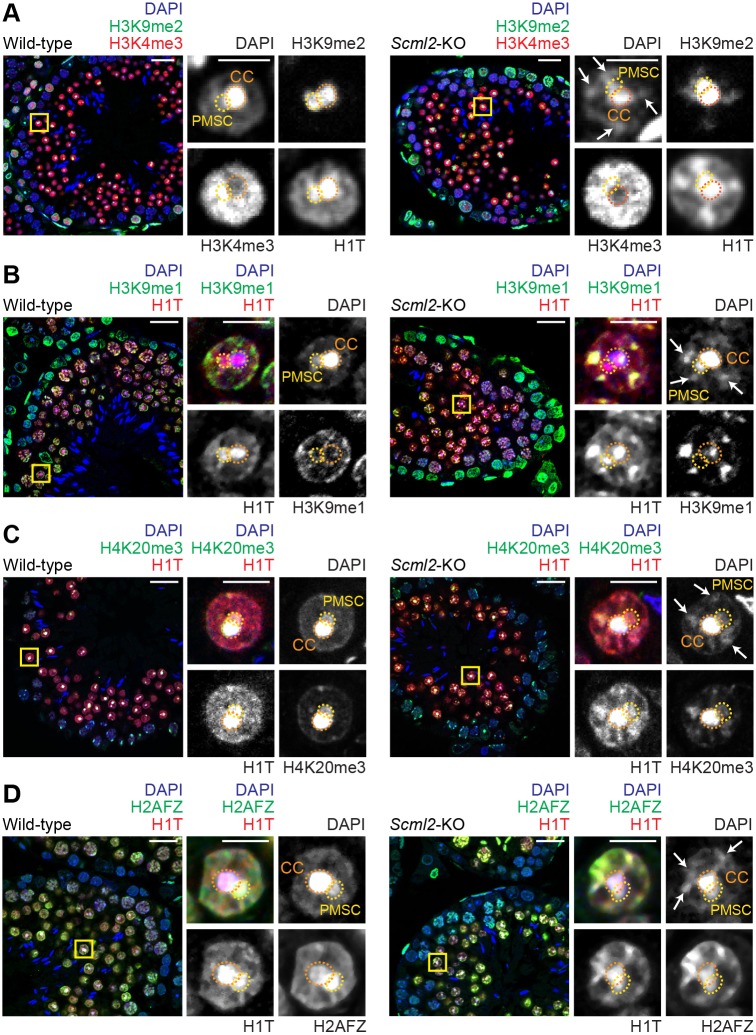
Our data revealed that the ectopic patches in Scml2-KO RSs were enriched with H3K27me3, which is typically associated with facultative heterochromatin, and did not comprise the constitutive heterochromatin typically associated with centromeres. Thus, we sought to examine the localization of H3K9me2, a modification that is also associated with facultative heterochromatin (Trojer and Reinberg, 2007). We observed the accumulation of H3K9me2 in ectopic patches in Scml2-KO RSs; this contrasted with wild-type RSs, in which H3K9me2 was highly enriched on PMSC and, to a lesser extent, the chromocenter (Fig. 4A). Another form of H3K9 methylation, H3K9me1, was intensely enriched in the ectopic patches of Scml2-KO RSs; however, in wild-type RSs, H3K9me1 accumulated at the nuclear periphery and was absent from both the chromocenter and PMSC (Fig. 4B). We further identified that H4K20me3, a histone modification associated with the repression of transcription (Wang et al., 2008), accumulated in the ectopic patches of Scml2-KO RSs; this accumulation pattern deviated from that in wild-type RSs, where H4K20me3 was concentrated on the chromocenter with slight deposition on PMSC too (Fig. 4C). The combination of histone modifications H3K9me1/2 on the ectopic patches of Scml2-KO RSs led us to conclude that they represent a form of facultative heterochromatin. In support of this conclusion, the ectopic patches in the Scml2-KO RSs were not associated with apoptosis (Fig. S5A), suggesting that they are independent of the fragmented DNA associated with apoptosis. And although we observed polynucleated spermatogenic cells in the Scml2-KO testes, these cells were infrequent (Hasegawa et al., 2015), indicating that the ectopic patches in Scml2-KO RSs are independent of polynucleation.
Fig. 4.
Ectopic patches in Scml2-KO round spermatids are detected with H3K9me2, H3K9me1, H4K20me3 and H2AFZ. (A-D) Immunostaining of testicular paraffin sections with the indicated antibodies. Images were acquired with a confocal microscope. Boxed regions are magnified in the right panels. CC, chromocenter; PMSC, postmeiotic sex chromatin. Arrows indicate ectopic patches in the Scml2-KO RSs. Scale bars: 20 µm (left) and 5 µm (right). Three independent pairs of wild-type and Scml2-KO mice were examined and representative images are shown.
To further investigate the features of ectopic patches in Scml2-KO RSs as facultative heterochromatin, we examined the localization of active modifications that are usually excluded from heterochromatin. H3K4me2 is an active histone mark, but it is counterintuitively associated with PMSC, a silent compartment (Fig. 3B); this is because, on PMSC, H3K4me2 is associated not with postmeiotic silencing but instead with the activation of sex chromosome-linked genes that escape postmeiotic silencing (Sin et al., 2012). In Scml2-KO RSs, the ectopic patches were largely devoid of H3K4me2, while H3K4me2 remained on PMSC. Another active modification, H3K4me3, did not highly accumulate on the ectopic patches of the Scml2-KO RSs (Fig. 4A). These results support the heterochromatic nature of the ectopic patches. However, H2AFZ, a histone variant associated with both active and inactive genes (Raisner et al., 2005), accumulated at the ectopic patches of Scml2-KO RSs (Fig. 4D). Similar to H3K4me2, H2AFZ accumulation on PMSC is associated not with postmeiotic silencing but instead with the activation of the sex chromosome-linked genes that escape postmeiotic silencing (Sin et al., 2012). H2AFZ was also associated with the chromocenter in Scml2-KO RSs, similar to the wild-type RSs (Fig. 4D). Thus, H2AFZ is associated with both active and repressive states as previously reported (Raisner et al., 2005).
Next, we sought to evaluate the persistence of the ectopic patches into the later stages of spermatogenesis in the Scml2-KO. Although the ectopic patches were evident in late RSs (Fig. S5B), the detection limit of our confocal microscope made the ectopic patches increasingly difficult to observe, as germ cells condensed into elongating spermatids and testicular spermatozoa (Fig S5C,D). Yet notably, the Scml2-KO has abnormal spermatozoa (Hasegawa et al., 2015), raising the possibility that chromatin abnormalities persist into spermatozoa. Although histone-to-protamine exchange occurred in Scml2-KO spermatozoa (Fig. S6A), the size of testicular tubules in Scml2-KO was smaller than that of the wild-type (Fig. S6A), presumably due to progressive spermatogenic defects that occur over multiple stages of spermatogenesis.
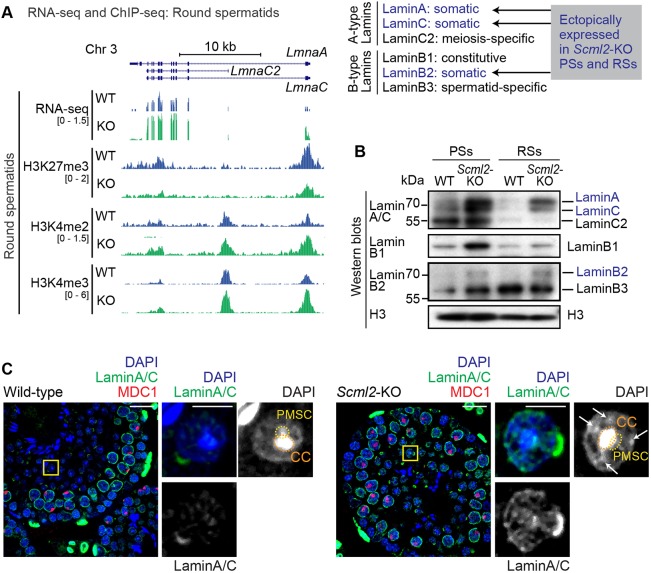
Loss of SCML2 leads to ectopic expression of somatic lamins in round spermatids
The ectopic patches of facultative heterochromatin in the Scml2-KO RSs suggest abnormal nuclear organization. To test this possibility, we investigated the expression of lamins in Scml2-KO RSs. Lamins are major factors in the arrangement of chromatin and the structural organization of the nucleus (Dechat et al., 2008). During late spermatogenesis, the expression of somatic lamins is shut off, instead replaced with the expression of spermatogenesis-specific lamins (Schütz et al., 2005). The mRNA expression of somatic lamin genes LmnaA/C are suppressed in late spermatogenesis in wild-type mice (Fig. 5A). However, in Scml2-KO, LmnaA/C were derepressed (Fig. 5A). This is in accord with our recent finding wherein SCML2 facilitates H3K27me3 at the sites of bivalent genomic domains (as defined by the presence of H3K27me3 and H3K4me2/3 on transcription start sites) (Maezawa et al., 2018a). During spermatogenesis, bivalent domains are established on developmental regulator genes and somatic/progenitor genes that are suppressed in pachytene spermatocytes and RSs (Lesch et al., 2013, 2016; Lesch and Page, 2014; Sin et al., 2015). In the Scml2-KO, disruption of bivalent domains (that is, the depletion of H3K27me3 and concomitant increase in H3K4me2/3) is associated with transcriptional derepression of somatic/progenitor genes in late spermatogenesis, during meiosis and into postmeiotic stages (Maezawa et al., 2018a). Thus, in Scml2-KO, somatic lamins were derepressed (Fig. 5A).
Fig. 5.
Loss of SCML2 leads to ectopic expression of somatic lamins in round spermatids. (A) Distribution of RNA-seq and ChIP-seq reads at the Lamin gene locus. Somatic lamins (LmnaA and LmnaC) were derepressed at the transcriptional level in round spermatids of Scml2-KO. A summary of A- and B-type lamins is shown on the right. (B) Western blotting with anti-Lamin antibodies using purified pachytene spermatocytes (PSs) and round spermatids (RSs) from wild-type and Scml2-KO mice. (C) Immunostaining of testicular paraffin sections with the indicated antibodies. Images were acquired with a confocal microscope. Boxed regions are magnified in the right panels. CC, chromocenter; PMSC, postmeiotic sex chromatin. Arrows indicate ectopic patches in the Scml2-KO RSs. Scale bars: 20 µm (left) and 5 µm (right). Three independent pairs of wild-type and Scml2-KO mice were examined and representative images are shown.
In agreement with this change in transcription, western blots revealed that somatic lamins, such as LaminA/C and LaminB2, evince ectopic protein expression in the Scml2-KO (Fig. 5B). Furthermore, confocal microscopy revealed that LaminA/C remain throughout the nuclei in Scml2-KO RSs, in sharp contrast to their disappearance in wild-type RSs (Fig. 5C). Therefore, the disruption of germline-specific bivalent domains leads to the ectopic presence of somatic lamins in Scml2-KO RSs. And since lamins regulate nuclear organization (Dechat et al., 2008), it is conceivable that the ectopic persistence of somatic lamins catalyzes the abnormal heterochromatin formation observed in Scml2-KO RSs. Together, these results suggest that SCML2 is required for global heterochromatin organization in RSs.
SCML2 is required for CENP-V localization on pericentromeric heterochromatin in round spermatids
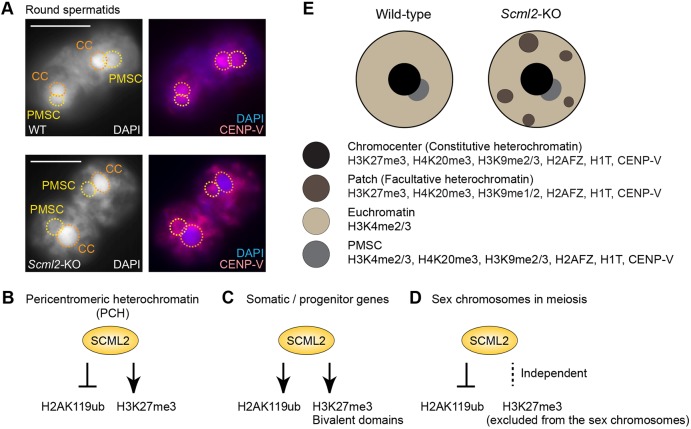
Although the persistence of somatic lamins is a potential cause of heterochromatin disorganization in Scml2-KO RSs (Fig. 5), SCML2 accumulates at high levels on PCH in spermatogonia (Fig. 1) and regulates Polycomb-mediated marks on PCH (Fig. 2). This led us to postulate that SCML2 performed two actions for heterochromatin regulation: direct action on PCH and indirect regulation through the suppression of somatic lamins. To determine whether SCML2 directly regulates properties of the chromocenter – the cluster of PCH – in RSs, we tested the localization of the centromere protein CENP-V (Tadeu et al., 2008). We initially identified CENP-V through a proteomics screening to identify proteins associated with γH2AX-containing nucleosomes purified from testes (Broering et al., 2015; Hasegawa et al., 2015) and we found that CENP-V localized on the chromocenter and PMSC in wild-type RSs (Fig. 6A, top panels). However, CENP-V did not localize on the γH2AX-enriched XY body, although it did localize on centromeres in meiotic spermatocytes (data not shown). Surprisingly, in Scml2-KO RSs, the accumulation of CENP-V on the chromocenter was diminished while enriched in the ectopic patches (Fig. 6A, bottom panels). In the Scml2-KO RSs, CENP-V accumulation on PMSC appeared unchanged compared with that in wild-type RSs. These results suggest that the chromocenter of the RSs, in comparison to previous stages, has a distinct feature for the localization of CENP-V and that SCML2 directly regulates the chromocenter.
Fig. 6.
SCML2 is required for CENP-V localization on pericentromeric heterochromatin in round spermatids. (A) Immunostaining of CENP-V in slides that preserve the relative nuclear structure of spermatogenic cells. Pairs of round spermatids are shown. CC, chromocenter; PMSC, postmeiotic sex chromatin. Images were acquired with a wide-field microscope. Scale bars: 10 µm. Three independent pairs of wild-type and Scml2-KO mice were examined and representative images are shown. (B-D) Summary of action of SCML2 in spermatogenesis. (E) Schematic of round spermatid nuclei in wild-type and Scml2-KO mice.
In addition to the direct regulation of PCH by SCML2, our results indicate that the regulation of multiple layers of epigenetic modifications and nuclear organization are required for normal spermatogenesis. We infer that the absence of these key mechanisms is detrimental to reproductive fitness and, collectively, underlies the infertility phenotype of Scml2-KO mice (Hasegawa et al., 2015).
DISCUSSION
Functions of SCML2 on pericentromeric heterochromatin in male germ cells
Here, we demonstrate that SCML2 regulates heterochromatin organization in different stages of spermatogenesis. In spermatogonia, SCML2 binds to PCH and suppresses PRC1-mediated H2AK119ub, while SCML2 promotes PRC2-mediated H3K27me3 on PCH in meiotic prophase (Fig. 6B). Therefore, on PCH of male germ cells, the activities of PRC1 and PRC2 are distinctly regulated by SCML2. Previously, we demonstrated two distinct functions of SCML2: (1) On the gene promoters of somatic/progenitor genes, SCML2 works with PRC1 to promote H2AK119ub to suppress somatic/progenitor genes (Hasegawa et al., 2015) and facilitate H3K27me3 to establish bivalent genomic domains (Maezawa et al., 2018a) (Fig. 6C); (2) on the other hand, SCML2 has an independent function on the sex chromosomes during meiosis, where SCML2 uniformly binds sex chromosome chromatin during the process of meiotic sex chromosome inactivation. In this context, SCML2 binds to the deubiquitylase USP7 to remove H2AK119ub from the sex chromosomes (Adams et al., 2018; Hasegawa et al., 2015; Luo et al., 2015). However, H3K27me3 is excluded from the sex chromosomes in an SCML2-independent manner (Hasegawa et al., 2015). Therefore, although SCML2 suppresses H2AK119ub, it does not regulate H3K27me3 on the sex chromosomes (Fig. 6D).
Together with these results, the function of SCML2 on PCH seems to be distinct from its functions on gene promoters and the sex chromosomes (Fig. 6B-D). One possible reason for this difference is that the heterochromatin environment of PCH is affected by the presence of H3K9me3. In early zygotes, the absence of H3K9me3 on paternal PCH induces PRC1 recruitment to PCH (Puschendorf et al., 2008). Notably, PRC1 recruitment in early zygotes is independent of PRC2 (Puschendorf et al., 2008), in contrast to a classic model that postulates that the recruitment of PRC1 is mediated by PRC2 (Cao et al., 2002). Therefore, it is conceivable that, on the PCH of male germ cells, the presence of H3K9me3 prevents the recruitment of PRC1-mediated H2AK119ub, whereas SCML2 promotes recruitments of PRC2 to mediate H3K27me3.
Curiously, this relationship between PRC1, PRC2 and PCH is different from that in embryonic stem (ES) cells, where PRC1-mediated H2AK119ub is required for the recruitment of PRC2 to chromatin in response to the loss of DNA methylation (Cooper et al., 2014). Furthermore, when DNA methylation was ablated, SCML2 was identified as the most abundantly recruited protein to the PCH of ES cells (Saksouk et al., 2014), raising the possibility that SCML2 acts on PCH in ES cells to facilitate PRC2-mediated H3K27me3. In terms of DNA methylation and H3K9 methylation, further characterization of PCH is warranted to determine the mechanistic differences among forms of PCH regulation in different cell contexts.
Despite the abnormal regulation of H3K27me3 and H2AK119ub, Scml2-KO mice can undergo normal chromosome pairing and synapsis (Hasegawa et al., 2015). This phenotype is milder than that of loss-of-function mutants of PRC2 (Mu et al., 2014) and YY1, another Polycomb protein (Wu et al., 2009). Since H3K9me3 and its binding protein HP1γ are required for chromosome pairing (Peters et al., 2001; Takada et al., 2011), H3K9me3-HP1 accumulation is likely to be critical for chromosome dynamics in meiosis. Indeed, abrogated H3K9me3 signals were reported in YY1-mutant spermatocytes (Wu et al., 2009). We suggest that SCML2's function is independent of the H3K9me3-HP1 accumulation, thereby enabling the meiotic progression of Scml2-KO spermatocytes.
Importantly, SCML2 and Polycomb complexes have a pivotal role in the regulation of genome-wide gene expression in spermatogenesis (Hasegawa et al., 2015; Maezawa et al., 2017; Mu et al., 2014). Particularly, SCML2 is required for global suppression of a somatic/progenitor genes in late spermatogenesis, during meiosis and into postmeiotic stages (Hasegawa et al., 2015; Sin et al., 2015). Therefore, it is possible that the SCML2-dependent regulation of PCH is related to global gene expression programs in late spermatogenesis. We propose that SCML2 coordinates heterochromatin organization and spermatogenesis-specific gene expression through the regulation of Polycomb complexes.
Heterochromatin organization in spermatids
After the completion of meiosis, PCH is organized into a single chromocenter. At this transition, SCML2 has an important role in organization of heterochromatin. One potential role of SCML2 is to suppress the transcription of somatic lamins, which are major factors in the structural organization of the nucleus and chromatin (Dechat et al., 2008). The nuclear lamina is associated with large chromatin domains, called lamina-associated domains, which are associated with gene repression and heterochromatin (van Steensel and Belmont, 2017). This raises a possible interpretation of the ectopic patches of facultative heterochromatin in Scml2-KO RSs: the ectopic expression of somatic lamins leads to the ectopic retention of heterochromatin at the nuclear lamina of RSs. Thus, one intriguing possibility, in wild-type RSs, is that heterochromatin may be internalized to form a single chromocenter in the absence of this lamin-mediated retention mechanism. In support of this possibility, we found another notable feature of lamin localization in spermatogenesis: a constitutive lamin, LaminB1, localizes on heterochromatin and nuclear envelopes in diplotene spermatocytes and accumulates at high concentrations on chromocenters in round spermatids (Fig. S6B). This is another possible reason why the chromocenter is internalized in round spermatids. This LaminB1 localization did not change in the Scml2-KO RSs (data not shown). Together, we propose that, in normal spermatogenesis, the coordinated disappearance of somatic lamins, coupled with the localization change of LaminB1, enables the organization of heterochromatin in a single chromocenter.
The appearance of ectopic patches in the Scml2-KO RSs provides information on the structural organization of the chromocenter. This is because, in normal spermatogenesis, in addition to markers of constitutive heterochromatin such as H3K9me3 and HP1 (Namekawa et al., 2006, 2007), the chromocenter contains various markers of facultative heterochromatin such as H3K27me3 and H3K9me2 (Fig. 6E). Intriguingly, in the Scml2-KO, these markers of facultative heterochromatin are present on the ectopic patches (Fig. 6E). This result raises an interesting possibility that the chromocenter consists of two types of heterochromatin: constitutive and facultative heterochromatin. Since the loss of SCML2 leads to ectopic patches of facultative heterochromatin, SCML2 may be required for the organization of facultative heterochromatin on the chromocenter. In support of this notion, CENP-V is detected on the ectopic patches but not on the chromocenter in Scml2-KO RSs (Fig. 6A). Thus, CENP-V could be a specific marker of the facultative heterochromatin constituting the chromocenter.
Another interesting aspect of this study is that many of the markers detected at the ectopic patches, such as H3K9me2 and H3K9me3, are present on PMSC, the heterochromatic structure of the sex chromosomes in RSs (Namekawa et al., 2006, 2007). Although PMSC is not associated with H3K27me3 (Namekawa et al., 2006), it is likely that PMSC share features with the facultative heterochromatin that comprises, in part, the chromocenter in spermatids.
Finally, it is worth mentioning that, in normal condensing spermatids, PCH undergoes specific reprogramming (Govin et al., 2007). While a spermatid-specific histone variant, H2A.L.2, ensures a genome-wide histone-to-protamine exchange (Barral et al., 2017), H2A.L.2 remains on PCH along with other nucleosomal histones and participates in the formation of new nucleoprotein structures (Carone et al., 2014; Govin et al., 2007). Therefore, it is possible that, in the Scml2-KO, defects on PCH in an earlier stage lead to abnormal reprogramming of PCH in later stages, impacting male fertility.
A previous study showed that a layer of pachytene spermatocytes was missing in a portion of Scml2-KO tubules (Luo et al., 2015), suggesting a possible cause of abnormal cell-to-cell communication between germ cells and Sertoli cells. However, this phenotype was not penetrant. So, although it is still possible that an indirect effect such as abnormal cell-to-cell communication between germ cells and Sertoli cells may disturb spermatogenesis in Scml2-KO mice, the penetrant heterochromatin defects observed in this study suggest that cell-intrinsic defects are the major cause of the Scml2-KO phenotype. We rarely observed Scml2-KO sperm in epididymides, and the Scml2-KO sperm we did observe showed an abnormal morphology wherein they were abnormally surrounded by their midpieces (Maezawa et al., 2018a). This abnormal phenotype could be attributed to a cell-intrinsic defect in Scml2-KO mice.
MATERIALS AND METHODS
Animals
Scml2-KO mice on the C57BL/6 background were previously reported (Hasegawa et al., 2015). All experimental work was approved by the Institutional Animal Care and Use Committee protocol no. IACUC2015-0032. At least three independent mice or, for comparisons between wild-type and Scml2-KO samples, three independent littermate pairs of mice were used for each analysis.
Cell culture
Mouse embryonic kidney (mK4) cells (Teneng et al., 2007) were recently authenticated. mK4 cells were cultured in DMEM with GlutaMAX (Thermo Fisher Scientific), 10% fetal bovine serum and antibiotics. Transient transfections were performed in OptiMEM (Thermo Fisher Scientific) with Lipofectamine 3000 reagent (Thermo Fisher Scientific) according to the manufacturer's instructions. For ChIP-qPCR experiments, transfected cells were selectively collected via the MACSelect 4 System (Miltenyi Biotec) according to the manufacturer's instructions. Briefly, cells were co-transfected with pMACS 4.1 vector, which encodes a truncated CD4 marker. Ectopically expressed tandem affinity purification (TAP)-tagged-SCML2 was driven by the CMV promoter, and the TAP tag includes the following tags: 3×FLAG-tag, Strep-Tag II and 6×-His tag. Transfected cells expressing truncated CD4 at the cell surface were labeled with MACS4 microbeads and collected via AutoMACS.
Antibodies
Full details of all antibodies used in this study are available in Table S1.
Immunohistochemistry
For preparation of testicular paraffin blocks, testes were fixed with 4% paraformaldehyde (PFA) at 4°C overnight. Testes were dehydrated and embedded in paraffin. For histological analyses, paraffin sections at 7 µm thickness were deparaffinized and autoclaved in Target Retrieval Solution (DAKO) at 121°C for 10 min. The sections were blocked with Blocking One Histo (Nacalai USA) for 1 h at room temperature, and then incubated with primary antibodies at 4°C overnight. The resulting signals were detected by incubation with secondary antibodies conjugated to fluorophores (Thermo Fisher Scientific, Biotium or Jackson ImmunoResearch). For detection of apoptotic cells, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed using an In Situ Cell Death Detection Kit (Roche) according to the manufacturer's instructions. Sections were counterstained with DAPI. To precisely compare wild-type and Scml2-KO samples, all of these steps were performed at the same time, in parallel with the wild-type and Scml2-KO samples. Images were obtained with a confocal laser-scanning microscope (A1R, Nikon). Between the wild-type and Scml2-KO samples, confocal microscope images were taken under the same settings for scan speed, laser power and detector gain. Images were processed with NIS-Elements (Nikon), Imaris (Bitplane) and ImageJ (National Institutes of Health). Signal intensity on PCH was quantified using ImageJ. Relative signal intensity was calculated as a ratio between the signal intensity on PCH (after subtracting the background intensity) and the signal intensity on the entire nucleus (having subtracted the background intensity). Illustrator (Adobe) was used for composing figures. The following RGB colors were used throughout the figures: magenta=255, 0, 255; green=0, 255, 0; white=255, 255, 255; red=255, 0, 0; yellow=255, 255, 0.
Preparation and immunofluorescence of surface spreads of meiotic chromosomes
Meiotic chromosome spreads (Fig. 2C; Fig. S2) were prepared as previously described (Alavattam et al., 2016). Slides preserving the relative structure of spermatogenic cells (Fig. 6A) were prepared as previously described (Namekawa, 2014; Namekawa and Lee, 2011). For immunostaining experiments, surface spreads were washed in PBST (PBS with 0.1% Tween 20) for 30 min and blocked with antibody dilution buffer (PBST with 0.15% BSA) for 60 min. Primary antibodies (see Table S1 for full details) were added to surface spreads and incubated overnight in humid chambers at room temperature or 4°C. The slides were incubated with secondary antibodies conjugated to fluorophores (Thermo Fisher Scientific, Biotium, or Jackson ImmunoResearch) for 30 min at room temperature in humid chambers in darkness. Slides were mounted with #1.5 thickness coverslips (Thermo Fisher Scientific) under one of two conditions: (1) using Vectashield (Vector Laboratories) containing DAPI (1.5 µg/ml) or (2) using Prolong Gold (Thermo Fisher Scientific) after incubation in PBS containing DAPI (1 µg/ml) for 30 min in darkness, after which slides were cured overnight before imaging. Images were obtained with an ECLIPSE Ti-E microscope (Nikon) equipped with a Zyla 5.5 sCMOS camera (Andor Technology) and 100× CFI Apochromat TIRF oil immersion objective NA 1.4 (Nikon), and processed with NIS-Elements (Nikon) and Photoshop (Adobe).
Time-lapse microscopy
Cells were grown in Phenol Red-free MEMα (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum, 20 mM HEPES and antibiotics on CELLview glass-bottom cell culture dishes (Greiner Bio-One). Where indicated, cells were transfected with plasmids encoding mCherry-SCML2 and pTALYM3B15 (Addgene), which expresses TALE-mClover against mouse major satellite sequences (Miyanari et al., 2013); Clover is the brightest green fluorescent protein (GFP) characterized to date (Lam et al., 2012), and mClover is the monomeric version of Clover. One hour before imaging, DNA was stained in live cells with 0.2 μg/ml Hoechst 33342. Cells were maintained at 37°C in the humidity-saturated atmosphere of an incubation chamber (Tokai Hit) during imaging. Images were obtained with a Ti-E SpectraX wide-field microscope (Nikon) equipped with a Zyla 4.2 sCMOS camera (Andor Technology) and 40× Plan Apochromat λ lens NA 0.95 (Nikon) for time-lapse imaging, or a confocal laser-scanning microscope (A1R, Nikon) equipped with a 60× (NA 1.2) water immersion objective for obtaining single confocal sections or three-dimensional stacks. Images were processed with NIS-Elements (Nikon) and ImageJ (National Institutes of Health).
Germ cell isolation
Pachytene spermatocytes and round spermatids were isolated from adult testes through BSA gravity sedimentation as described (Bellve, 1993). Purity was confirmed by nuclear staining with 0.2 μg/ml Hoechst 33342 via fluorescence wide-field microscopy (ECLIPSE Ti-E microscope (Nikon) as described above); ≥90% purity was confirmed as previously described (Maezawa et al., 2018b). THY1+ spermatogonia were isolated as described previously (Maezawa et al., 2018b). ≥95% purity was confirmed for all THY1+ spermatogonia as previously described (Maezawa et al., 2018b).
Cell fractionation
Cell fractionation was performed as described (Mendez and Stillman, 2000). Briefly, cells were resuspended in buffer A [10 mM HEPES-KOH pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, protease inhibitors (Roche)], Triton X-100 (0.1%) was added and the cells were incubated for 5 min on ice. Nuclei were collected in a pellet by low-speed centrifugation at 1300 g for 6 min at 4°C. The supernatant was further clarified by high-speed centrifugation at 20,000 g for 15 min at 4°C to remove cell debris and insoluble aggregates; the clarified supernatant was used as the cytoplasmic fraction. Nuclei were washed once in buffer A, lysed with buffer B (3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, protease inhibitors), incubated for 30 min at 4°C and centrifuged at 1700 g for 4 min at 4°C. The supernatant was transferred to a new tube and used as the nucleoplasmic fraction. Pellets were washed once in buffer B, centrifuged again under the same conditions, and then used as the chromatin fraction.
Western blotting
For western blotting, fractionated proteins as described above (Fig. 1D) or total proteins from pachytene spermatocytes (PSs) and RSs (Fig. 5B) were dissolved in SDS-PAGE sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol and 0.02% Bromophenol Blue) with Benzonase (NEB), separated by SDS-PAGE and blotted on an Immobilon-P PVDF Membrane (Millipore) via the Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked in StartingBlock T20 (TBS) Blocking Buffer (Thermo Fisher Scientific) for 30 min, probed with primary antibody in 1:20 diluted blocking buffer at 4°C overnight, and then incubated with an HRP-conjugated secondary antibody for 1 h at room temperature. Blots were developed using Immobilon Western Chemiluminescent HRP Substrate (Millipore) and exposed onto Super RX-N X-ray film (Fujifilm). For the re-probing of blots, membranes were incubated in Restore Western Blot Stripping Buffer (Thermo Fisher Scientific).
ChIP-qPCR
Cells were suspended in buffer A (described above). Triton X-100 (0.1%) was added, and the cells were incubated for 5 min on ice. Nuclei were collected in a pellet by low-speed centrifugation at 1300 g for 6 min at 4°C. Nuclei were washed once in buffer A, resuspended with buffer B (described above), disrupted by sonication, and incubated for 30 min on ice. EGTA (final 10 mM), KCl (final 300 mM) and NP-40 (final 0.2%) were added to the lysates. The lysates were incubated for 1 h at 4°C with rotation at 20 rpm and then centrifuged at 8000 g for 10 min at 4°C. Using the supernatant, chromatin immunoprecipitation was carried out with an SX-8X IP-STAR Compact Automated System (Diagenode). Briefly, Dynabeads Protein G were pre-incubated with 0.1% BSA for 2 h. Then, the chromatin fractions were incubated with the beads conjugated to antibodies against SCML2, histone H3 or control IgG at 4°C for 4 h, and then washed 4 times with wash buffer (20 mM HEPES-KOH pH 7.9, 300 mM KCl, 10% glycerol, 0.05% NP-40, and 0.5 mM DTT). The beads were incubated with elution buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA, 250 mM NaCl, 0.3% SDS, 0.1 μg/μl proteinase K) at 42°C for 30 min. ChIPped DNA was purified by Agencourt AMPure XP (Beckman Coulter). qPCR was performed with FAST SYBR Green Master Mix (Thermo Fisher Scientific) on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). Primer sequences are as follows: MajorSat-ChIP-Fw-1, GAC GAC TTG AAA AAT GAC GAA ATC; MajorSat-ChIP-Rv-1, CAT ATT CCA GGT CCT TCA GTG TGC; rDNA-ChIP-Fw, CCT GTG AAT TCT CTG AAC TC; rDNA-ChIP-Rv, CCT AAA CTG CTG ACA GGG TG.
RNA-sequencing and ChIP-sequencing data analysis
The RNA-seq data analyzed in this paper was previously deposited in GEO GSE55060 (Hasegawa et al., 2015); ChIP-seq data was previously deposited in GEO GSE89502 (Maezawa et al., 2018a). Data analyses for RNA-seq and ChIP-seq were performed in the BioWardrobe Experiment Management System (https://github.com/Barski-lab/biowardrobe; Kartashov and Barski, 2015).
Supplementary Material
Acknowledgements
We thank Ho-Su Sin and Tyler Broering for technical assistance; Yueh-Chiang Hu, Jérôme Déjardin, Eda Yildrim, and members of the Namekawa lab for discussion and helpful comments regarding the manuscript; Katie Gerhardt for editing the manuscript; Mary Ann Handel for providing the H1T antibody; and Bill Earnshaw for providing the CENP-V antibody.
Footnotes
Competing interests
A.B. is a cofounder of Datirium, LLC. The remaining authors declare no competing financial interests.
Author contributions
Conceptualization: S.M., K.H., S.H.N.; Methodology: S.M.; Software: A.B.; Validation: S.M.; Formal analysis: S.M., M.F., T.S., S.H.N.; Investigation: S.M., K.H., K.G.A., S.H.N.; Resources: A.B., S.H.N.; Data curation: S.M., S.H.N.; Writing - original draft: S.M., S.H.N.; Writing - review & editing: K.G.A.; Visualization: S.M.; Supervision: S.H.N.; Project administration: S.H.N.; Funding acquisition: A.B., S.H.N.
Funding
This work was supported by the March of Dimes Foundation (FY13-510 to S.H.N.) and the National Institutes of Health (GM119134 and HL098691 to A.B.; GM098605 and GM122776 to S.H.N.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.217125.supplemental
References
- Adams S. R., Maezawa S., Alavattam K. G., Abe H., Sakashita A., Shroder M., Broering T. J., Sroga Rios J., Thomas M. A., Lin X. et al. (2018). RNF8 and SCML2 cooperate to regulate ubiquitination and H3K27 acetylation for escape gene activation on the sex chromosomes. PLoS Genet. 14, e1007233 10.1371/journal.pgen.1007233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavattam K. G., Kato Y., Sin H.-S., Maezawa S., Kowalski I. J., Zhang F., Pang Q., Andreassen P. R. and Namekawa S. H. (2016). Elucidation of the fanconi anemia protein network in meoisis and its function in the regulation of histone modifications. Cell Rep. 17, 1141-1157. 10.1016/j.celrep.2016.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda S., Mas G. and Di Croce L. (2015). Regulation of gene transcription by Polycomb proteins. Sci. Adv. 1, e1500737 10.1126/sciadv.1500737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral S., Morozumi Y., Tanaka H., Montellier E., Govin J., de Dieuleveult M., Charbonnier G., Coute Y., Puthier D., Buchou T. et al. (2017). Histone variant H2A.L.2 guides transition protein-dependent protamine assembly in male germ cells. Mol. Cell 66, 89-101.e8. 10.1016/j.molcel.2017.02.025 [DOI] [PubMed] [Google Scholar]
- Bellve A. R. (1993). Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 225, 84-113. 10.1016/0076-6879(93)25009-Q [DOI] [PubMed] [Google Scholar]
- Berkovits B. D. and Wolgemuth D. J. (2011). The first bromodomain of the testis-specific double bromodomain protein Brdt is required for chromocenter organization that is modulated by genetic background. Dev. Biol. 360, 358-368. 10.1016/j.ydbio.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios S. (2017). Nuclear architecture of mouse spermatocytes: chromosome topology, heterochromatin, and nucleolus. Cytogenet Genome Res. 151, 61-71. 10.1159/000460811 [DOI] [PubMed] [Google Scholar]
- Blackledge N. P., Farcas A. M., Kondo T., King H. W., McGouran J. F., Hanssen L. L. P., Ito S., Cooper S., Kondo K., Koseki Y. et al. (2014). Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445-1459. 10.1016/j.cell.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering T. J., Alavattam K. G., Sadreyev R. I., Ichijima Y., Kato Y., Hasegawa K., Camerini-Otero R. D., Lee J. T., Andreassen P. R. and Namekawa S. H. (2014). BRCA1 establishes DNA damage signaling and pericentric heterochromatin of the X chromosome in male meiosis. J. Cell Biol. 205, 663-675. 10.1083/jcb.201311050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering T. J., Wang Y.-L., Pandey R. N., Hegde R. S., Wang S.-C. and Namekawa S. H. (2015). BAZ1B is dispensable for H2AX phosphorylation on Tyrosine 142 during spermatogenesis. Biol Open 4, 873-884. 10.1242/bio.011734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F. W., Kirsh A. L., Sharma M., McLean D. J., Morris J. L., Griswold M. D., de Rooij D. G. and Braun R. E. (2004). Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 36, 647-652. 10.1038/ng1366 [DOI] [PubMed] [Google Scholar]
- Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S. and Zhang Y. (2002). Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039-1043. 10.1126/science.1076997 [DOI] [PubMed] [Google Scholar]
- Carone B. R., Hung J.-H., Hainer S. J., Chou M.-T., Carone D. M., Weng Z., Fazzio T. G. and Rando O. J. (2014). High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm. Dev. Cell 30, 11-22. 10.1016/j.devcel.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Dienstbier M., Hassan R., Schermelleh L., Sharif J., Blackledge N. P., De Marco V., Elderkin S., Koseki H., Klose R. et al. (2014). Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Rep. 7, 1456-1470. 10.1016/j.celrep.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya J. A., Hobbs R. M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K. E., Wolgemuth D. J. and Pandolfi P. P. (2004). Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653-659. 10.1038/ng1367 [DOI] [PubMed] [Google Scholar]
- Dechat T., Pfleghaar K., Sengupta K., Shimi T., Shumaker D. K., Solimando L. and Goldman R. D. (2008). Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 22, 832-853. 10.1101/gad.1652708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson M. J., Pearlman R. E., Karaiskakis A., Spyropoulos B. and Moens P. B. (1994). Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J. Cell Sci. 107, 2749-2760. [DOI] [PubMed] [Google Scholar]
- Geisler S. J. and Paro R. (2015). Trithorax and Polycomb group-dependent regulation: a tale of opposing activities. Development 142, 2876-2887. 10.1242/dev.120030 [DOI] [PubMed] [Google Scholar]
- Govin J., Escoffier E., Rousseaux S., Kuhn L., Ferro M., Thevenon J., Catena R., Davidson I., Garin J., Khochbin S. et al. (2007). Pericentric heterochromatin reprogramming by new histone variants during mouse spermiogenesis. J. Cell Biol. 176, 283-294. 10.1083/jcb.200604141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaf T. and Ward D. C. (1995). Higher order nuclear structure in mammalian sperm revealed by in situ hybridization and extended chromatin fibers. Exp. Cell Res. 219, 604-611. 10.1006/excr.1995.1270 [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Sin H.-S., Maezawa S., Broering T. J., Kartashov A. V., Alavattam K. G., Ichijima Y., Zhang F., Bacon W. C., Greis K. D. et al. (2015). SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev. Cell 32, 574-588. 10.1016/j.devcel.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijima Y., Sin H.-S. and Namekawa S. H. (2012). Sex chromosome inactivation in germ cells: emerging roles of DNA damage response pathways. Cell. Mol. Life Sci. 69, 2559-2572. 10.1007/s00018-012-0941-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselman A., Eaker S. and Handel M. A. (2003). Temporal expression of cell cycle-related proteins during spermatogenesis: establishing a timeline for onset of the meiotic divisions. Cytogenet Genome Res. 103, 277-284. 10.1159/000076813 [DOI] [PubMed] [Google Scholar]
- Kahn T. G., Dorafshan E., Schultheis D., Zare A., Stenberg P., Reim I., Pirrotta V. and Schwartz Y. B. (2016). Interdependence of PRC1 and PRC2 for recruitment to Polycomb Response Elements. Nucleic Acids Res. 44, 10132-10149. 10.1093/nar/gkw701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartashov A. V. and Barski A. (2015). BioWardrobe: an integrated platform for analysis of epigenomics and transcriptomics data. Genome Biol. 16, 158 10.1186/s13059-015-0720-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmins S. and Sassone-Corsi P. (2005). Chromatin remodelling and epigenetic features of germ cells. Nature 434, 583-589. 10.1038/nature03368 [DOI] [PubMed] [Google Scholar]
- Lam A. J., St-Pierre F., Gong Y., Marshall J. D., Cranfill P. J., Baird M. A., McKeown M. R., Wiedenmann J., Davidson M. W., Schnitzer M. J. et al. (2012). Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005-1012. 10.1038/nmeth.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen A. and Helin K. (2014). Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 14, 735-751. 10.1016/j.stem.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Lesch B. J. and Page D. C. (2014). Poised chromatin in the mammalian germ line. Development 141, 3619-3626. 10.1242/dev.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch B. J., Dokshin G. A., Young R. A., McCarrey J. R. and Page D. C. (2013). A set of genes critical to development is epigenetically poised in mouse germ cells from fetal stages through completion of meiosis. Proc. Natl. Acad. Sci. USA 110, 16061-16066. 10.1073/pnas.1315204110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch B. J., Silber S. J., McCarrey J. R. and Page D. C. (2016). Parallel evolution of male germline epigenetic poising and somatic development in animals. Nat. Genet. 48, 888-894. 10.1038/ng.3591 [DOI] [PubMed] [Google Scholar]
- Luo M., Zhou J., Leu N. A., Abreu C. M., Wang J., Anguera M. C., de Rooij D. G., Jasin M. and Wang P. J. (2015). Polycomb protein SCML2 associates with USP7 and counteracts histone H2A ubiquitination in the XY chromatin during male meiosis. PLoS Genet. 11, e1004954 10.1371/journal.pgen.1004954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa S., Hasegawa K., Yukawa M., Sakashita A., Alavattam K. G., Andreassen P. R., Vidal M., Koseki H., Barski A. and Namekawa S. H. (2017). Polycomb directs timely activation of germline genes in spermatogenesis. Genes Dev. 31, 1693-1703. 10.1101/gad.302000.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa S., Hasegawa K., Yukawa M., Kubo N., Sakashita A., Alavattam K. G., Sin H.-S., Kartashov A. V., Sasaki H., Barski A. et al. (2018a). Polycomb protein SCML2 facilitates H3K27me3 to establish bivalent domains in the male germline. Proc. Natl. Acad. Sci. USA 115, 4957-4962. 10.1073/pnas.1804512115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa S., Yukawa M., Alavattam K. G., Barski A. and Namekawa S. H. (2018b). Dynamic reorganization of open chromatin underlies diverse transcriptomes during spermatogenesis. Nucleic Acids Res. 46, 593-608. 10.1093/nar/gkx1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J. and Stillman B. (2000). Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602-8612. 10.1128/MCB.20.22.8602-8612.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Ficca M., Muller-Navia J. and Scherthan H. (1998). Clustering of pericentromeres initiates in step 9 of spermiogenesis of the rat (Rattus norvegicus) and contributes to a well defined genome architecture in the sperm nucleus. J. Cell Sci. 111, 1363-1370. [DOI] [PubMed] [Google Scholar]
- Miyanari Y., Ziegler-Birling C. and Torres-Padilla M.-E. (2013). Live visualization of chromatin dynamics with fluorescent TALEs. Nat. Struct. Mol. Biol. 20, 1321-1324. 10.1038/nsmb.2680 [DOI] [PubMed] [Google Scholar]
- Mu W., Starmer J., Fedoriw A. M., Yee D. and Magnuson T. (2014). Repression of the soma-specific transcriptome by Polycomb-repressive complex 2 promotes male germ cell development. Genes Dev. 28, 2056-2069. 10.1101/gad.246124.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekawa S. H. (2014). Slide preparation method to preserve three-dimensional chromatin architecture of testicular germ cells. J. Vis. Exp. 83, e50819 10.3791/50819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekawa S. H. and Lee J. T. (2011). Detection of nascent RNA, single-copy DNA and protein localization by immunoFISH in mouse germ cells and preimplantation embryos. Nat. Protoc. 6, 270-284. 10.1038/nprot.2010.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekawa S. H., Park P. J., Zhang L.-F., Shima J. E., McCarrey J. R., Griswold M. D. and Lee J. T. (2006). Postmeiotic sex chromatin in the male germline of mice. Curr. Biol. 16, 660-667. 10.1016/j.cub.2006.01.066 [DOI] [PubMed] [Google Scholar]
- Namekawa S. H., VandeBerg J. L., McCarrey J. R. and Lee J. T. (2007). Sex chromosome silencing in the marsupial male germ line. Proc. Natl. Acad. Sci. USA 104, 9730-9735. 10.1073/pnas.0700323104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. H., O'Carroll D., Scherthan H., Mechtler K., Sauer S., Schöfer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A. et al. (2001). Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323-337. 10.1016/S0092-8674(01)00542-6 [DOI] [PubMed] [Google Scholar]
- Puschendorf M., Terranova R., Boutsma E., Mao X., Isono K., Brykczynska U., Kolb C., Otte A. P., Koseki H., Orkin S. H. et al. (2008). PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 40, 411-420. 10.1038/ng.99 [DOI] [PubMed] [Google Scholar]
- Raisner R. M., Hartley P. D., Meneghini M. D., Bao M. Z., Liu C. L., Schreiber S. L., Rando O. J. and Madhani H. D. (2005). Histone variant H2A.Z marks the 5’ ends of both active and inactive genes in euchromatin. Cell 123, 233-248. 10.1016/j.cell.2005.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux S., Reynoird N., Escoffier E., Thevenon J., Caron C. and Khochbin S. (2008). Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod. Biomed. Online 16, 492-503. 10.1016/S1472-6483(10)60456-7 [DOI] [PubMed] [Google Scholar]
- Saksouk N., Barth T. K., Ziegler-Birling C., Olova N., Nowak A., Rey E., Mateos-Langerak J., Urbach S., Reik W., Torres-Padilla M. E. et al. (2014). Redundant mechanisms to form silent chromatin at pericentromeric regions rely on BEND3 and DNA methylation. Mol. Cell 56, 580-594. 10.1016/j.molcel.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Saksouk N., Simboeck E. and Dejardin J. (2015). Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin 8, 3 10.1186/1756-8935-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H., Schöfisch K., Dell T. and Illner D. (2014). Contrasting behavior of heterochromatic and euchromatic chromosome portions and pericentric genome separation in pre-bouquet spermatocytes of hybrid mice. Chromosoma 123, 609-624. 10.1007/s00412-014-0479-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz W., Alsheimer M., Ollinger R. and Benavente R. (2005). Nuclear envelope remodeling during mouse spermiogenesis: postmeiotic expression and redistribution of germline lamin B3. Exp. Cell Res. 307, 285-291. 10.1016/j.yexcr.2005.03.023 [DOI] [PubMed] [Google Scholar]
- Shirakawa T., Yaman-Deveci R., Tomizawa S., Kamizato Y., Nakajima K., Sone H., Sato Y., Sharif J., Yamashita A., Takada-Horisawa Y. et al. (2013). An epigenetic switch is crucial for spermatogonia to exit the undifferentiated state toward a Kit-positive identity. Development 140, 3565-3576. 10.1242/dev.094045 [DOI] [PubMed] [Google Scholar]
- Sin H.-S., Barski A., Zhang F., Kartashov A. V., Nussenzweig A., Chen J., Andreassen P. R. and Namekawa S. H. (2012). RNF8 regulates active epigenetic modifications and escape gene activation from inactive sex chromosomes in post-meiotic spermatids. Genes Dev. 26, 2737-2748. 10.1101/gad.202713.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin H.-S., Kartashov A. V., Hasegawa K., Barski A. and Namekawa S. H. (2015). Poised chromatin and bivalent domains facilitate the mitosis-to-meiosis transition in the male germline. BMC Biol. 13, 53 10.1186/s12915-015-0159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Nozaki M., Takeda N. and Shinkai Y. (2007). Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 26, 3346-3359. 10.1038/sj.emboj.7601767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadeu A. M. B., Ribeiro S., Johnston J., Goldberg I., Gerloff D. and Earnshaw W. C. (2008). CENP-V is required for centromere organization, chromosome alignment and cytokinesis. EMBO J. 27, 2510-2522. 10.1038/emboj.2008.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Naruse C., Costa Y., Shirakawa T., Tachibana M., Sharif J., Kezuka-Shiotani F., Kakiuchi D., Masumoto H., Shinkai Y. et al. (2011). HP1gamma links histone methylation marks to meiotic synapsis in mice. Development 138, 4207-4217. 10.1242/dev.064444 [DOI] [PubMed] [Google Scholar]
- Teneng I., Stribinskis V. and Ramos K. S. (2007). Context-specific regulation of LINE-1. Genes Cells 12, 1101-1110. 10.1111/j.1365-2443.2007.01117.x [DOI] [PubMed] [Google Scholar]
- Terasawa M., Shinohara A., Hotta Y., Ogawa H. and Ogawa T. (1995). Localization of RecA-like recombination proteins on chromosomes of the lily at various meiotic stages. Genes Dev. 9, 925-934. 10.1101/gad.9.8.925 [DOI] [PubMed] [Google Scholar]
- Trojer P. and Reinberg D. (2007). Facultative heterochromatin: is there a distinctive molecular signature? Mol. Cell 28, 1-13. 10.1016/j.molcel.2007.09.011 [DOI] [PubMed] [Google Scholar]
- Turner J. M. A. (2007). Meiotic sex chromosome inactivation. Development 134, 1823-1831. 10.1242/dev.000018 [DOI] [PubMed] [Google Scholar]
- Valerius M. T., Patterson L. T., Witte D. P. and Potter S. S. (2002). Microarray analysis of novel cell lines representing two stages of metanephric mesenchyme differentiation. Mech. Dev. 112, 219-232. 10.1016/S0925-4773(02)00008-4 [DOI] [PubMed] [Google Scholar]
- van Steensel B. and Belmont A. S. (2017). Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell 169, 780-791. 10.1016/j.cell.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver D. E., van Pelt A. M. M., Repping S. and Hamer G. (2013). Role for rodent Smc6 in pericentromeric heterochromatin domains during spermatogonial differentiation and meiosis. Cell Death Dis. 4, e749 10.1038/cddis.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zang C., Rosenfeld J. A., Schones D. E., Barski A., Cuddapah S., Cui K., Roh T.-Y., Peng W., Zhang M. Q. et al. (2008). Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40, 897-903. 10.1038/ng.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Hu Y.-C., Liu H. and Shi Y. (2009). Loss of YY1 impacts the heterochromatic state and meiotic double-strand breaks during mouse spermatogenesis. Mol. Cell. Biol. 29, 6245-6256. 10.1128/MCB.00679-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.