Abstract
Introduction:
Older adults demonstrate poorer prospective memory (PM) performance as compared to younger adults, particularly for time-based cues and other strategically demanding PM tasks. Intraindividual variability (IIV) in neurocognitive test performance is an index of cognitive control that may be related to the execution of strategically demanding PM tasks.
Method:
Participants included 194 older Australian adults (age 50 to 88) who completed the Memory for Intentions Screening Test (MIST), the Prospective and Retrospective Memory Questionnaire (PRMQ), and clinical measures of executive functions. A measure of naturalistic time-based PM was also administered, in which participants were asked to call the examiner 24 hours after their appointment to report how many hours they slept. IIV was calculated as the mean-adjusted coefficient of variation (CoV) across subtests of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
Results:
IIV was significantly associated with time-based PM in the laboratory, independent of demographics. Additionally, IIV significantly predicted performance on a naturalistic time-based PM trial, independent of demographics and chronic medical conditions. IIV was not related to event-based laboratory PM or self-reported PM symptoms in daily life. Clinical measures of executive functions were similarly associated with time-based PM and the naturalistic PM task, but not with event-based PM or subjective PM symptoms.
Conclusions:
These results indicate that cognitive control, as indexed by IIV in neurocognitive performance, may play a role in naturalistic PM, as well as in highly strategic, but not automatic, laboratory-based PM among older adults.
Keywords: aging, memory for intentions, dispersion, coefficient of variation, executive function
Prospective memory (PM), or “remembering to remember,” is a complex cognitive function that involves executing a deferred intended action (McDaniel & Einstein, 2000). Older adults reliably demonstrate lower performance on PM tasks in the laboratory compared to younger individuals (e.g., Henry, MacLeod, Phillips, & Crawford, 2004). Of practical relevance, PM plays an important role in the day-to-day functioning of older adults, as the age-associated PM decrements measured in the laboratory are independently associated with poorer PM in daily life (Kamat et al., 2014), increased dependence in instrumental activities of daily living (e.g., Woods, Weinborn, Velnoweth, Rooney, & Bucks, 2012), poorer medication management (e.g., Woods et al., 2014), and lower quality of life (Woods et al., 2015). As such, it is important to gain a better understanding of the cognitive mechanisms that might underlie age-related declines in PM in nonclinical adults.
According to McDaniel and Einstein’s (2000) multiprocess view, PM can occur across a continuum, relying on: 1) strategic processes, in which individuals must exert considerable resources to monitor for the PM cue, and/or; 2) automatic processes, in which individuals spontaneously remember the deferred intended action upon processing the PM cue. Traditionally, PM cues are classified broadly as either time-based (e.g., take medication at 6:00 p.m.) or event-based (e.g., buy milk on the way home from work), with the former generally relying more heavily on strategic processes than the latter. However, event-based PM cues can vary in their strategic demands; for example, event-based cues may be more demanding of strategic monitoring when they are nonfocal to the ongoing task (Kliegel, Jäger, & Phillips, 2008). Relative to their younger counterparts, older adults tend to experience more difficulty with strategically demanding laboratory PM tasks, including time-based and nonfocal event-based PM tasks (e.g., Henry et al., 2004).
These differential effects of aging on laboratory PM may be attributable to the different neural and cognitive mechanisms underlying performance on strategic versus automatic PM tasks. Strategic PM has been shown to rely on prefrontal neural systems and executive functions, in contrast to more automatic PM tasks, which depend more heavily on medial temporal systems and retrospective memory retrieval (e.g., Burgess, Scott, & Frith, 2003; Gordon, Shelton, Bugg, McDaniel, & Head, 2011). In line with these findings, laboratory PM studies with older adults show that scores on the time-based PM tasks of the Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010) correlate with measures of executive functions, while scores on the more automatic event-based PM tasks are associated with performance on retrospective memory tasks (Kamat et al., 2014). Executive functions are also associated with performance on strategic PM tasks in clinical populations, including HIV infection (Woods, Dawson, Weber, Grant, & The HIV Neurobehavioral Research Center Group, 2010), Parkinson’s disease (Raskin et al., 2011), and schizophrenia (Ungvari, Xiang, Tang, & Shum, 2008).
Recent studies have raised the possibility that intraindividual variability (IIV), an aspect of executive functions that involves cognitive control, may also be associated with PM. IIV measures a person’s fluctuations in cognitive performance over time. While some variability in performance over time is quite normal, higher levels of variability are thought to reflect dysregulation of cognitive control. IIV is most commonly measured using trials of a single neurocognitive task (e.g., response time [RT]; Jensen, 1992); however, it can also be measured across a battery of neurocognitive tasks (i.e., dispersion; Lindenberger & Baltes, 1997) or on the same neurocognitive task administered at different time points (e.g., Hertzog, Dixon, & Hultsch, 1992). IIV in RTs has been associated with activation in prefrontal areas (e.g., Bellgrove, Hester, & Garavan, 2004), correlates with measures of executive dysfunction (e.g., Bellgrove et al., 2004), and is elevated among populations with frontal systems dysregulation, including persons with discrete frontal lobe lesions (e.g., Stuss, Murphy, Binns, & Alexander, 2003). As such, it is not surprising that older adults demonstrate higher RT IIV when compared to their younger counterparts (e.g., Bielak, Cherbuin, Bunce, & Anstey, 2014). The clinical relevance of such IIV elevations in older adults is illustrated by their association with everyday problem solving (Burton, Strauss, Hultsch, & Hunter, 2009) and cognitive decline (Bielak, Hultsch, Strauss, MacDonald, & Hunter, 2010).
Given these intriguing findings, two recent studies have posited that IIV may be associated with PM. Specifically, it has been proposed that fluctuations in cognitive control may lead to both decreased strategic PM performance and increased IIV in neurocognitive tasks. In strategic PM, lapses in cognitive control may interfere with the individual’s ability to monitor the environment for the PM cue. In contrast, automatic PM tasks, which require minimal cue monitoring, may not be affected by such fluctuations in cognitive control. Lapses in cognitive control may also lead to greater inconsistency in neurocognitive task performance, as measured by IIV (e.g., Bunce, MacDonald, & Hultsch, 2004). Thus, it is hypothesized that IIV and strategic PM errors may reflect similar types of executive dysfunction. One recent study of 336 older adults (Haynes, Kliegel, Zimprich, & Bunce, 2018) found that IIV in RTs was associated with failures on focal and nonfocal event-based laboratory PM tasks (mean r = .12), independent of age. However, this study did not investigate time-based PM, and arguably even the focal event-based tasks used by Haynes and colleagues (2018) required some cue monitoring. Another recent study examined the relationship between IIV in RTs and performance on the non-PM ongoing task trials of an event-based PM experiment in 150 older adults (Ihle, Ghisletta, & Kliegel, 2017). This study observed that higher costs to ongoing task IIV, as a function of PM demands, were associated with failures in strategically demanding nonfocal (r = .23), but not focal (r = .04), PM. Taken together, these studies provide support for the notion that cognitive control, as indexed by IIV, may play a role in strategic PM.
The present study was designed to address four unanswered questions regarding the possible association between IIV and PM in older adults. First, we sought to test the hypothesis that IIV is more strongly associated with time-based compared to event-based PM, given the elevated strategic demands of the former. To our knowledge, the relationship between IIV and time-based PM has not previously been examined. Time-based PM is disproportionately affected relative to event-based PM in older adults and shows greater reliance on the prefrontal executive systems responsible for cognitive control, which underlie IIV. Therefore, if IIV is found to associate more strongly with time-based compared to event-based PM, it would provide greater support for the notion that there are distinct mechanisms behind strategic and automatic PM processes. Second, we expand the existing literature by including a much wider range of PM assessments covering laboratory, naturalistic, and symptom-based measures, which allow us to determine whether the association between IIV and PM generalizes to PM tasks in everyday life. Indeed, despite their poorer PM performance in the laboratory, older adults typically demonstrate comparable (or sometimes even better) performance on naturalistic PM tasks as compared to their younger counterparts. This phenomenon, known as the “age−PM paradox” (e.g., Rendell & Thomson, 1999), may be due to a variety of factors, such as increased motivation or use of compensatory strategies in older adults. Moreover, self-reported PM symptoms are not reliably associated with performance-based ability (e.g., Rönnlund, Vestergren, Mäntylä, & Nilsson, 2011), but are still independently predictive of functional outcomes in older adults (e.g., Woods et al., 2012). Therefore, a multifaceted evaluation of PM is important for understanding the underlying mechanisms of PM and their relevance to everyday life. Third, we explicitly examine the value of considering IIV alongside traditional clinical measures of executive functions as a cognitive contributor to PM. This approach provides valuable information about the convergent and concurrent validity of IIV as a contributing factor to PM. Fourth, we extend prior studies by utilizing an arguably more naturalistic assessment of IIV that measured performance variability across a battery of well-validated neurocognitive tasks (i.e., dispersion) rather than IIV in RTs. Dispersion correlates reliably with RT IIV (Hilborn, Strauss, Hultsch, & Hunter, 2009; Hultsch, MacDonald, & Dixon, 2002), is sensitive to aging (e.g., Christensen et al., 1999), and is associated with prefrontal gray matter atrophy (Hines et al., 2016). Dispersion is also consistently associated with aspects of daily functioning, including activities of daily living (Christensen et al., 1999), functional disability (Rapp, Schnaider-Beeri, Sano, Silverman, & Haroutunian, 2005), and naturalistic multitasking (Fellows & Schmitter-Edgecombe, 2015). Furthermore, dispersion is associated with important neurocognitive health outcomes, including cognitive decline (Hilborn et al., 2009), incident dementia (Holtzer, Verghese, Wang, Hall, & Lipton, 2008), and mortality (Thaler, Hill, Duff, Mold, & Scott, 2015). In daily life, individuals who experience declines in cognitive control may encounter everyday problems due to inconsistent performance across different daily tasks, rather than variability in speeded responses alone. Therefore, dispersion across tasks may be a more naturalistic, ecologically relevant measure of IIV. Although many prior studies have measured IIV across RTs, dispersion across tasks is also a well-validated approach to measuring this construct and may provide further information about the relationship between cognitive control and various measures of PM.
Method
Participants
Community dwelling adults aged 50 years or older were recruited via flyers and word of mouth and enrolled in the Healthy Ageing Research Program (HARP) at the University of Western Australia. Participants were excluded if they scored less than 24 on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) or reported a history of significant neurological (e.g. traumatic brain injury, stroke, seizure disorder, dementia, mild cognitive impairment) or psychiatric (e.g., schizophrenia, bipolar disorder) conditions. The final sample included 194 individuals who were 50 to 88 years of age. Basic descriptive data regarding the demographic and neurocognitive characteristics of these participants are provided in Table 1. This study was approved by the University of Western Australia human research ethics office, and all participants provided written, informed consent.
Table 1.
General Descriptive Data for the Study Sample (N = 194)
| Variable | M (SD) | Range |
|---|---|---|
| Demographic and medical characteristics | ||
| Age (years) | 70.2 (7.6) | 50−88 |
| Education (years) | 14.1 (3.3) | 5−27 |
| Sex (% women) | 65.0 | |
| Elevated depression (%) | 13.4 | |
| Elevated anxiety (%) | 8.8 | |
| Number of chronic medical conditions | 1.1 (1.1) | 0−5 |
| RBANS | ||
| Coefficient of variation (CoV) | 0.2 (0.1) | 0.1−0.4 |
| Total Score | 102.2 (12.9) | 70−148 |
| List Learning | 27.8 (4.7) | 11−40 |
| Story Memory | 16.7 (3.6) | 6−24 |
| Figure Copy | 16.0 (2.7) | 8−20 |
| Line Orientation | 17.8 (2.3) | 10−22 |
| Picture Naming | 9.4 (0.7) | 7−10 |
| Semantic Fluency | 23.0 (5.5) | 6−40 |
| Digit Span | 11.2 (2.6) | 6−22 |
| Coding | 45.8 (9.2) | 24−68 |
| List Recall | 6.3 (2.2) | 0−10 |
| Story Recall | 8.7 (2.3) | 2−12 |
| Figure Recall | 11.6 (3.8) | 0−20 |
| Executive functions measures | ||
| Executive functions T-score | 50.0 (6.6) | 32.5−69.1 |
| Trailmaking Test B (s) | 81.2 (37.2) | 33−300 |
| CLOX executive index | 1.7 (2.4) | –2−14 |
| Letter C fluency | 17.1 (5.0) | 5−33 |
| Action (verb) fluency | 18.5 (5.3) | 3−34 |
Note. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; CLOX = executive clock-drawing task.
Intraindividual Variability (i.e., Dispersion) Measure
Participants completed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, 1998), which is a brief, well-validated measure of cognitive functioning. The RBANS consists of five domain subscales (Immediate Memory, Visuospatial/Constructional, Language, Attention, and Delayed Memory) derived from 12 subtests. We are aware of one prior study using IIV on the RBANS, which found that higher dispersion, calculated using the coefficient of variation (CoV) across the RBANS, was associated with self-reported memory problems and greater risk of mortality in older adults (Thaler et al., 2015). This study set the precedent for the construct validity of RBANS CoV as a means by which to measure dispersion.
In the present study, raw scores on the primary 11 RBANS subtests (List Learning, Story Memory, Figure Copy, Line Orientation, Picture Naming, Semantic Fluency, Digit Span, Coding, List Recall, Story Recall, and Figure Recall) were used to generate a measure of IIV. Specifically, the CoV was calculated as the dispersion of an individual’s scores across the 11 RBANS subtests in a single testing session, adjusting for overall RBANS performance. Raw scores on the 11 subtests were converted to sample-based T-scores to provide a common metric from which to derive IIV. Next, the intraindividual mean (IM) and intraindividual standard deviation (ISD) of the 11 subtest T-scores were calculated for each participant. There was a moderate negative correlation between RBANS subtest IM and ISD (ρ = –.28, p < .001). The CoV ratio score was calculated as ISD ⁄ IM for each individual. Therefore, a higher CoV indicated greater within-person variability across the RBANS subtests.
Prospective Memory Measures
Participants completed the research version (Woods et al., 2008) of the MIST (Raskin et al., 2010), which is a standardized, laboratory-based measure of PM. Participants were instructed that the test would measure their ability to remember tasks and carry them out. During the MIST, participants completed an ongoing word search distractor task and were asked to stop the word search when it was time to perform an assigned PM task. Although participants were told that they could use a digital clock behind them to keep track of time, they were not explicitly encouraged to do so. Throughout the test, participants were asked to complete four time-based (e.g., “In 15 minutes, tell me that it is time to take a break.”) and four event-based (e.g., “When I show you a postcard, self-address it.”) PM tasks. Each PM task either had a 2-minute or 15-minute delay between the task instruction and execution. For each task, participants earned 1 point for responding at the correct time (for time-based trials) or to the appropriate cue (for event-based trials) and 1 point for providing the correct response. In this study, we focused on the time-based and event-based scales, which were calculated as the number of points earned (out of 8) for each cue type. In general, participants performed better on the event-based MIST PM scale than the time-based MIST PM scale (Wilcoxon signed-rank S = 8005.5, p < .001, d = 1.19) which is consistent with the greater strategic demands of the time-based PM tasks.
Additional analyses were conducted using error scores on the time-based and event-based PM scales of the MIST. Errors in which participants failed to respond to the PM cue (i.e., received 0 of 2 points for that task) were coded as omission errors. For participants who recalled some portion of the PM task but executed the task incorrectly (i.e., received 1 of 2 points for that task), errors were coded as task substitution errors (i.e., responding with the incorrect task), loss of content errors (i.e., recognizing the PM cue but forgetting the task), loss of time errors (i.e., responding with the correct intention at the incorrect time), place losing omission errors (i.e., performing only part of the task), modality interference errors (i.e., responding with an action instead of a verbal response, or vice versa) or random errors (i.e., providing a random response at a random time).
Additionally, a 24-hr naturalistic task was administered following the laboratory trials of the MIST. This is a time-based PM task in which participants were instructed to leave a telephone message for the examiner the following day, specifying how many hours they slept the previous night. Participants were permitted, but not explicitly encouraged, to use mnemonic strategies (e.g., adding an event to their calendar) for this task. In order to capture the PM-specific components of this naturalistic task, participants were separated into three groups based on whether they correctly completed the task (n = 43), called the examiner but made an error (e.g., called at the incorrect time or provided the wrong information; n = 51), or failed to call (n = 98). Note that, naturalistic task data were not available for two participants.
Participants also completed the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith, Della Sala, Logie, & Maylor, 2000). The PRMQ is a self-report questionnaire with 16 questions about everyday prospective and retrospective memory symptoms rated from 1 (Never) to 5 (Very Often). A sum of responses for the eight items assessing frequency of PM lapses (range = 8−40) was used to measure PM complaints (Cronbach’s α = .83). Self-reported PM symptoms were missing for seven participants. Participant data on the laboratory, naturalistic, and self-reported PM measures are provided in Table 2.
Table 2.
Prospective Memory (PM) Measures for the Study Sample (N = 194)
| PM Measure | M (SD) | Range |
|---|---|---|
| MIST time-based PM | 5.3 (1.4) | 1−8 |
| Omission errors | 0.6 (0.7) | 0−3 |
| Task substitution errors | 0.3 (0.6) | 0−3 |
| Loss of content errors | 1.0 (0.9) | 0−4 |
| Loss of time errors | 0.2 (0.4) | 0−2 |
| Place losing omission errors | 0.0 (0.1) | 0−1 |
| Modality interference errors | 0.0 (0.1) | 0−1 |
| Random errors | 0.0 (0.2) | 0−3 |
| MIST event-based PM | 6.8 (1.2) | 2−8 |
| Omission errors | 0.2 (0.5) | 0−4 |
| Task substitution errors | 0.5 (0.7) | 0−3 |
| Loss of content errors | 0.3 (0.6) | 0−3 |
| Loss of time errors | 0.0 (0.2) | 0−2 |
| Place losing omission errors | 0.0 (0.1) | 0−1 |
| Modality interference errors | 0.0 (0.1) | 0−1 |
| Random errors | 0.0 (0.1) | 0−1 |
| MIST ongoing word search task (# words) | 13.2 (5.1) | 2−36 |
| MIST 24-hr naturalistic task (n = 192) | ||
| Called with correct message (%) | 22.4 | |
| Call error (%) | 26.6 | |
| Failed to call (%) | 51.0 | |
| PRMQ PM scale (n = 187) | 18.7 (4.4) | 9−36 |
Note. MIST = Memory for Intentions Screening Test; PRMQ = Prospective and Retrospective Memory Questionnaire.
Executive Functions Measures
Participants were administered several clinical measures of executive functions, including the Trailmaking Test (TMT) parts A and B (Reitan & Wolfson, 1985), the executive clock-drawing task (CLOX; Royall, Cordes, & Polk, 1998), letter “C” fluency (Benton, Hamsher, & Sivan, 1994), and action (verb) fluency (Woods et al., 2005). Raw total scores on TMT B, CLOX executive index (calculated as CLOX part 2 − CLOX part 1), letter fluency, and action fluency were converted to sample-based T-scores and then averaged to create an executive functions composite score. Higher mean T-scores reflected better executive functions.
Mood and Demographic Measures
To assess depression, participants completed either the Patient Health Questionnaire-9 (n = 124; PHQ-9; Kroenke, Spitzer, & Williams, 2001) or the Geriatric Depression Scale 15-item Short Version (n = 70; GDS-15; Sheikh & Yesavage, 1986), as a function of some changes in the parent protocol. These measures were dichotomized and collapsed into a single variable for the purpose of the current study. Specifically, elevated depression (yes/no) was defined as a score of 5 or greater on either the PHQ-9 or the GDS-15. To assess anxiety, participants completed either the Generalized Anxiety Disorder 7-item scale (n = 124; GAD-7; Spitzer, Kroenke, Williams, & Löwe, 2006) or the 20-item Geriatric Anxiety Inventory (n = 70; GAI; Pachana et al., 2007). Elevated anxiety (yes/no) was defined as a score of 5 or greater on the GAD-7 or a score of 9 or greater on the GAI. Participants also completed a demographic and medical history questionnaire.
Results
Higher CoV was associated with poorer time-based MIST PM performance (ρ = –.16, p = .023) but not event-based MIST PM (ρ = –.05, p = .519; Figure 1). Error analyses for the time-based MIST PM tasks demonstrated that higher CoV was significantly correlated with more omission errors (ρ = .19, p = .007), but CoV was not related to task substitution (ρ = .04, p = .535), loss of content (ρ = –.02, p = .752), loss of time (ρ = .02, p = .776), place losing omission (ρ = –.07, p = .319), modality interference (ρ = –.09, p = .209), or random (ρ = –.04, p = .574) errors. As might be expected, CoV was correlated with older age (ρ = .21, p = .003) but was only marginally associated with fewer years of education (ρ = –.14, p = .056) and did not differ by sex (p = .510, d = .05). Importantly, the association between higher CoV and poorer time-based MIST PM performance remained significant when demographics (i.e., age and education) were considered. Specifically, in a multiple regression with CoV, age, and education, the overall model was significant, R2 = .11, F(3, 190) = 7.92, p < .001, and demonstrated that both CoV (β = –0.15, p = .042) and age (β = –0.23, p = .002), but not education (β = 0.10, p = .157), significantly predicted time-based MIST PM.
Figure 1.
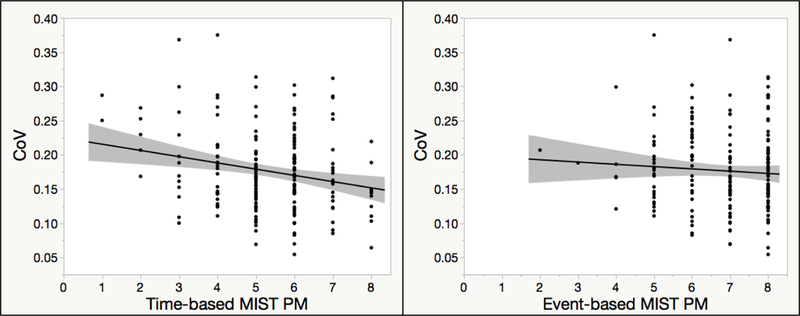
Correlations of the coefficient of variation (CoV) with performance on the time-based and event-based prospective memory (PM) scales of the Memory for Intentions Screening Test (MIST).
Executive functions demonstrated a similar pattern of associations with PM as did CoV: Specifically, better executive functions were correlated with higher time-based MIST PM (ρ = .35, p < .001) but not event-based MIST PM (ρ = .06, p = .370). Likewise, better executive functions were significantly correlated with fewer time-based PM omission errors (ρ = –.28, p < .001), but were not associated with any other types of errors on the time-based MIST PM tasks (ps > .10). Executive functions were also associated with CoV (ρ = –.32, p < .001; Table 3). In a multiple regression with CoV and executive functions predicting time-based MIST PM, the overall model was significant, R2 = .13, F(2, 191) = 13.92, p < .001, and executive functions significantly predicted time-based MIST PM (β = 0.30, p < .001), while CoV did not (β = –0.12, p = .106).
Table 3.
Intercorrelations Between Neurocognitive and Demographic Variables (N = 194)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. CoV | − | |||||
| 2. ISD | .94*** | − | ||||
| 3. IM | –.56*** | –.28*** | − | |||
| 4. Executive functions | –.32*** | –.19** | .49*** | − | ||
| 5. Age | .21** | .12 | –.31*** | –.13* | − | |
| 6. Education | –.14 | –.06 | .25*** | .22** | –.11 | − |
Note. Boldface correlation represents Pearson’s r. All other correlations are Spearman’s ρ. CoV = coefficient of variation; ISD = intraindividual SD of Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) subtest T-scores; IM = intraindividual M of RBANS subtest T-scores.
p < .05
p < .01
p < .001.
A multinomial logistic regression showed that CoV predicted performance on the 24-hr task above relevant covariates. Of the potential demographic and medical covariates listed in Table 1, only age and number of medical conditions were associated with the naturalistic PM task at p < .10 and were therefore included in this model. The overall logistic regression model with CoV, age, and number of chronic medical conditions predicting 24-hr PM task group was significant, χ2(6) = 23.46, p < .001, and showed that both CoV, χ2(2) = 6.52, p = .038, and age, χ2(2) = 11.56, p = .003, were significant predictors of naturalistic PM task group. Number of chronic medical conditions was not a significant predictor in this model, χ2(2) = 5.55, p = .062. Figure 2 shows descriptively that participants who failed to complete the 24-hr call had the highest CoV (M = 0.19, SD = 0.06), followed by those who correctly completed the task (M = 0.17, SD = 0.06, Hedges’ g = .29 vs. call fail group) and those who called but made an error (M = 0.16, SD = 0.05, Hedges’ g = .42 vs. call fail group). Note that, executive functions were related to the 24-hr task at the level of a trend, χ2(2) = 5.01, p = .082. Nonetheless, the effect size for executive functions predicting failure on the naturalistic PM task (average Hedges’ g = .27) was similar to that for CoV (average Hedges’ g = .35), further supporting the concurrent validity of CoV. In a logistic regression with CoV and executive functions predicting 24-hr PM task, the overall model was significant, χ2(4) = 10.38, p = .035, but neither CoV, χ2(2) = 5.38, p = .068, nor executive functions, χ2(2) = 3.66, p = .160, significantly predicted naturalistic PM task group.
Figure 2.
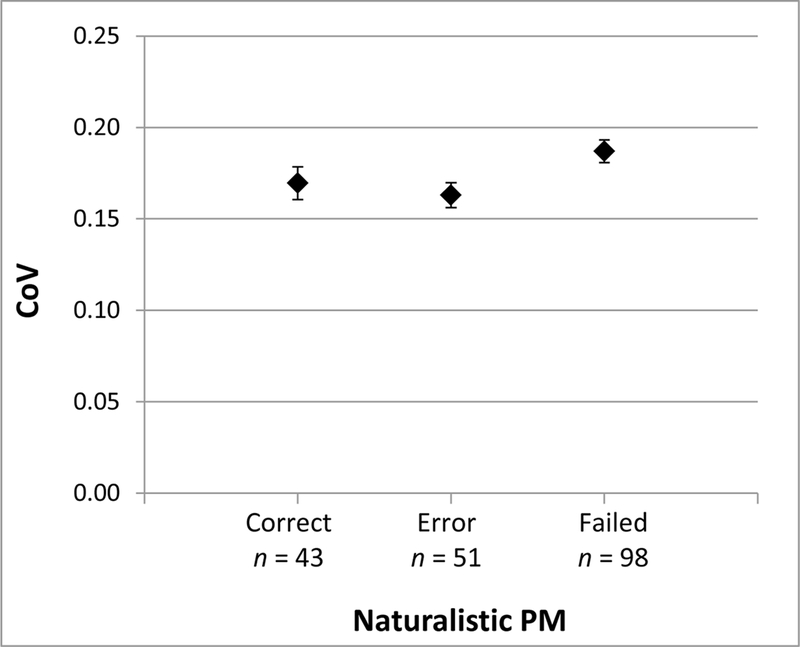
Mean coefficient of variation (CoV) by performance on the 24-hr naturalistic prospective memory (PM) task. Error bars represent standard error. Correct = called at the correct time with the correct message; Error = called but made an error; Failed = did not call
Finally, PM complaints on the PRMQ were not significantly related to either CoV (ρ = –.00, p = .973) or executive functions (ρ = .07, p = .336).
Discussion
PM declines with age and can be associated with adverse health outcomes among older adults (e.g., Woods et al., 2015). This study extends our understanding of the cognitive architecture of PM by elucidating its relationship to IIV, a measure of cognitive control. Specifically, the current study investigated this relationship using a variability index from a battery of neurocognitive tasks (i.e., dispersion) and several measures of PM, including time-based and event-based laboratory PM, naturalistic PM, and self-reported PM symptoms, in a large sample of community-dwelling older adults. Results demonstrated that CoV was significantly related to time-based, but not event-based, PM, as measured in the laboratory. While the correlation between CoV and time-based MIST PM was of a small magnitude (ρ = –.16), it was nevertheless independent of demographic variables. By virtue of being a clinical measure, the time-based MIST PM scale includes both PM and retrospective memory components. Error analyses confirmed that CoV was specifically related to omission errors in the time-based tasks (i.e., failure to respond to a PM cue) rather than retrospective memory errors (e.g., task substitutions or loss of content errors). CoV also uniquely predicted performance on the naturalistic time-based PM task, over and above demographic variables and chronic medical conditions. However, CoV was not related to self-reported PM symptoms in daily life.
The differential relationship between CoV and the time- and event-based laboratory PM tasks suggests that cognitive control plays a role in strategic, but not automatic, PM processes in older adults. In other words, greater variability in neurocognitive performance across a battery of clinical tasks was associated with poorer ability to execute future intentions that required time monitoring. In particular, greater variability was associated with omission errors, i.e., the frequency that participants failed to prospectively respond to time-based cues. CoV was not related to other types of errors, such as performing the PM task at the incorrect time or substituting a different PM task. Indeed, the time-based MIST PM scale has been shown to correlate moderately with time monitoring (e.g., clock checks; Doyle et al., 2013). Therefore, fluctuations in cognitive control seem to be particularly associated with a disruption to the strategic PM process of monitoring for the target cue.
The specificity of these findings was supported by the observation that fluctuations in cognitive control as measured by IIV did not interfere with the execution of future intentions when the PM tasks were event-based and thus more automatic and requiring less cue monitoring. This finding is consistent with prior research regarding the cognitive mechanisms underlying IIV and PM. IIV represents a type of cognitive control, which is thought to be necessary for strategic monitoring in PM tasks. More automatic PM tasks, such as the event-based trials of the MIST, rely more on automatic retrieval and retrospective memory processes (e.g., Kamat et al., 2014) and thus may not suffer as much in relation to lapses of cognitive control. Indeed, our findings provide neuropsychological support for the hypothesis that IIV and strategic PM conditions rely on cognitive processes that arise from similar (i.e., prefrontal) brain regions, while more automatic PM tasks do not. While these findings are consistent with the experimental literature, they contrast with prior studies that have found associations between IIV and event-based PM tasks (Haynes et al., 2018; Ihle et al., 2017). In prior studies, IIV was measured using RTs, which may be more sensitive to brief lapses in cognitive control than our measure of IIV that was calculated using overall neurocognitive task performance. Experimental measures of PM may show even stronger associations with IIV measures derived from RTs than those that were observed herein. Additionally, prior studies used different event-based tasks that may have required more cue monitoring than the event-based PM trials of the MIST. Therefore, while IIV may be related to performance on some event-based PM tasks, our results suggest that IIV is not strongly related to automatic PM tasks that require minimal cue monitoring.
Concurrent evidence for this hypothesis was provided by analyses showing that the executive functions composite was similarly related to time-based, but not event-based, laboratory PM tasks. Specifically, executive functions (e.g., cognitive flexibility and generativity) were associated with PM omission errors, but not retrospective memory errors, on the time-based laboratory PM tasks. In other words, while decreased performance on tasks of executive functions was associated with decreased accuracy on PM tasks that required time monitoring, it was not associated with PM task performance when a more salient, event-based cue was present. These results, along with the moderate correlation between CoV and executive functions, support the concurrent validity of IIV as a measure of cognitive control that is associated with time-based PM performance.
A broadly similar pattern of results was found using a naturalistic, performance-based PM task. Specifically, CoV was associated with performance on the 24-hr naturalistic task, independent of demographics and chronic medical conditions. Thus, fluctuations in cognitive control may underlie PM difficulties not only in the laboratory, but also in some aspects of daily life. As everyday life is accompanied by many distractors that could interfere with time monitoring, high neurocognitive variability may be particularly detrimental to strategic monitoring outside of the laboratory. In fact, the range odds ratio for RBANS CoV in predicting failure to call on the naturalistic PM task was 7.94, 95% confidence interval [1.52–41.57]. Contrary to expectations, participants who correctly completed the 24-hr task demonstrated higher CoV than those who called but made an error. This between-group difference in CoV was not significant, but was still associated with a small-to-medium effect size. In contrast, clinical measures of executive functions were only related to performance on the naturalistic task at the trend level. However, the effect size for executive functions as a predictor of failure on the 24-hr task was similar to that for CoV. It is also important to note that our executive functions composite variable is limited in its measurement of the construct. Our composite measures cognitive flexibility, generativity, and visuoconstructional planning and organization; however, other aspects of executive functions, such as inhibition, abstraction, and novel problem solving, may also play a role in PM.
Neither CoV nor the executive functions composite was related to self-reported PM symptoms. These findings are consistent with prior research suggesting that there is not a reliable association between objective and self-reported PM (Uttl & Kibreab, 2011; Woods et al., 2007). While PM symptoms were associated with decreased event-based PM performance (ρ = –.21, p = .004), they were not related to laboratory (ρ = –.05, p = .461) or naturalistic (p = .536) time-based PM performance. Despite this lack of association, self-reported PM is an independent predictor of functional outcomes in older adults (e.g., Woods et al., 2012; Woods et al., 2014; Woods et al., 2015). There may be many reasons for this discrepancy between subjective and objective PM; for example, one study found that subjective PM was only related to objective PM in older adults who had few depressive symptoms and few memory concerns (Zeintl, Kliegel, Rast, & Zimprich, 2006). Self-reported PM in our sample may have been similarly influenced by psychiatric or neurocognitive factors, which may moderate the association between cognitive control, as measured by IIV, and subjective PM symptoms. Indeed, post hoc analyses revealed that PM symptoms were associated with elevated anxiety (p = .024, d = 0.71) and marginally associated with elevated depression (p = .079, d = 0.54). Older adults may also use compensatory strategies, such as alarms or other reminders, which buffer the effects of neurocognitive variability on PM tasks in everyday life (e.g., Uttl & Kibreab, 2011). Another possibility is that older adults may lack metacognitive awareness of their PM abilities (e.g., Meeks, Hicks, & Marsh, 2007). Subjective PM measures may also pick up on more subtle PM problems, which older adults are able to compensate for in the structured laboratory environment, but which nonetheless interfere with PM in everyday life.
This study adds to our understanding of the relationship between IIV and strategic PM, but it is not without limitations. Time-based PM is multifactorial, and IIV explains only a very small amount of variance in this complex cognitive process. Conceptual models of PM (e.g., McDaniel & Einstein, 2000) and prior studies (e.g., Jäger & Kliegel, 2008) indicate that time-based PM is influenced by an array of cognitive factors such as time perception, inhibition, cognitive flexibility, planning, and processing speed. Thus, our data show that IIV is one small, but significant piece of the time-based PM puzzle. Although we hypothesize that the relationship of CoV with time-based, but not event-based, PM tasks is a function of greater strategic monitoring demands in the time-based tasks, we did not explicitly measure monitoring. Future studies that include measures of time monitoring (e.g., clock checks) could further clarify the relationship between IIV and different types of PM tasks. Furthermore, the event-based scale of the MIST is sometimes criticized for ceiling effects in healthy samples (e.g., Raskin et al., 2011); however, it is unlikely that ceiling effects restricted our ability to observe significant correlations between the event-based PM scale and IIV in this sample. Although the distribution of the event-based scale of the MIST was negatively skewed, it nevertheless had sufficient variability to demonstrate correlation with the RBANS Total Score (ρ = .16, p = .024). Our understanding of the relationship between IIV and PM in naturalistic settings is also limited, since our measure of naturalistic PM included only one trial (see Uttl, White, Gonzalez, McDouall, & Leonard, 2013). Future studies that include a more comprehensive assessment of naturalistic PM may be helpful in providing additional information about how neurocognitive variability may impact the completion of PM tasks in everyday life. It is also important to note that our sample included a small number of participants (n = 6, 3.1%) who endorsed moderate-to-severe depressive symptoms, as indicated by a score of 10 or greater on the PHQ-9 or 9 or greater on the GDS-15. These six participants with elevated symptoms of depression did not differ significantly from the rest of the cohort on CoV, time-based MIST PM, the 24-hr PM task, or executive functions (ps > .10). Moreover, depression measured as a continuous variable was not correlated with RBANS CoV, time-based MIST PM, the 24-hr trial, or executive functions (ps > .10). Therefore, it is unlikely that moderate-to-severe depression influenced the study results. Lastly, while CoV is widely used in studies of IIV, not all authors agree that it is the best method of calculating variability (e.g., Lindenberger & Baltes, 1997). RBANS CoV was chosen for this study because it adjusts for the influence of mean performance and is associated with longitudinal outcomes (Thaler et al., 2015). In our study as well, RBANS CoV shows evidence of construct validity by way of its associations in the expected directions with older age and executive dysfunction. Notably, a conservative post hoc model which included both CoV and RBANS Total Score as predictors of time-based MIST PM remained significant, R2 = 0.07, F(2, 191) = 6.89, p = .001, and CoV was a significant predictor of time-based PM (β = –0.17, p = .024), while RBANS Total Score (β = 0.14, p = .059) demonstrated marginal significance. Thus, the association between CoV and time-based MIST PM is unlikely to be an artifact of overall neurocognitive performance. However, RBANS CoV is only one measure of IIV. Other convergent approaches, such as measuring IIV in RTs or over a test-retest period, are needed to further explore the hypotheses tested in this study. Despite these limitations, this study provides important insights into the cognitive architecture of PM among older adults.
Acknowledgements
Steven Paul Woods is partially supported by the National Institute of Mental Health Grant R01-MH073419.
Footnotes
The authors report no conflicts of interest.
References
- Bellgrove MA, Hester R, & Garavan H (2004). The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia, 42(14), 1910–1916. [DOI] [PubMed] [Google Scholar]
- Benton LA, Hamsher KD, & Sivan AB (1994). Controlled Oral Word Association Test Multilingual Aphasia Examination. Iowa City, IA: AJA. [Google Scholar]
- Bielak AA, Cherbuin N, Bunce D, & Anstey KJ (2014). Intraindividual variability is a fundamental phenomenon of aging: Evidence from an 8-year longitudinal study across young, middle, and older adulthood. Developmental Psychology, 50(1), 143–151. [DOI] [PubMed] [Google Scholar]
- Bielak AA, Hultsch DF, Strauss E, MacDonald SW, & Hunter MA (2010). Intraindividual variability in reaction time predicts cognitive outcomes 5 years later. Neuropsychology, 24(6), 731–741. [DOI] [PubMed] [Google Scholar]
- Bunce D, MacDonald SW, & Hultsch DF (2004). Inconsistency in serial choice decision and motor reaction times dissociate in younger and older adults. Brain and Cognition, 56(3), 320–327. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, & Frith CD (2003). The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia, 41(8), 906–918. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, & Hunter MA (2009). The relationship between everyday problem solving and inconsistency in reaction time in older adults. Aging, Neuropsychology, and Cognition, 16(5), 607–632. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, & Jacomb P (1999). Dispersion in cognitive ability as a function of age: A longitudinal study of an elderly community sample. Aging, Neuropsychology, and Cognition, 6(3), 214–228. [Google Scholar]
- Doyle KL, Loft S, Morgan EE, Weber E, Cushman C, Johnston E, ... & HIV Neurobehavioral Research Program (HNRP) Group. (2013). Prospective memory in HIV-associated neurocognitive disorders (HAND): The neuropsychological dynamics of time monitoring. Journal of Clinical and Experimental Neuropsychology, 35(4), 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RP, & Schmitter-Edgecombe M (2015). Between-domain cognitive dispersion and functional abilities in older adults. Journal of Clinical and Experimental Neuropsychology, 37(10), 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Shelton JT, Bugg JM, McDaniel MA, & Head D (2011). Structural correlates of prospective memory. Neuropsychologia, 49(14), 3795–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BI, Kliegel M, Zimprich D, & Bunce D (2018). Intraindividual reaction time variability predicts prospective memory failures in older adults. Aging, Neuropsychology, and Cognition, 25(1), 132–145. [DOI] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, & Crawford JR (2004). A meta-analytic review of prospective memory and aging. Psychology and Aging, 19(1), 27–39. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, & Hultsch DF (1992). Intraindividual change in text recall of the elderly. Brain and Language, 42(3), 248–269. [DOI] [PubMed] [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, & Hunter MA (2009). Intraindividual variability across cognitive domains: Investigation of dispersion levels and performance profiles in older adults. Journal of Clinical and Experimental Neuropsychology, 31(4), 412–424. [DOI] [PubMed] [Google Scholar]
- Hines LJ, Miller EN, Hinkin CH, Alger JR, Barker P, Goodkin K, ... & Sanders J (2016). Cortical brain atrophy and intra-individual variability in neuropsychological test performance in HIV disease. Brain Imaging and Behavior, 10(3), 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, & Lipton RB (2008). Within-person across-neuropsychological test variability and incident dementia. JAMA, 300(7), 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, & Dixon RA (2002). Variability in reaction time performance of younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(2), P101–P115. [DOI] [PubMed] [Google Scholar]
- Ihle A, Ghisletta P, & Kliegel M (2017). Prospective memory and intraindividual variability in ongoing task response times in an adult lifespan sample: the role of cue focality. Memory, 25(3), 370–376. [DOI] [PubMed] [Google Scholar]
- Jäger T, & Kliegel M (2008). Time-based and event-based prospective memory across adulthood: Underlying mechanisms and differential costs on the ongoing task. The Journal of General Psychology, 135(1), 4–22. [DOI] [PubMed] [Google Scholar]
- Jensen AR (1992). The importance of intraindividual variation in reaction time. Personality and Individual Differences, 13(8), 869–881. [Google Scholar]
- Kamat R, Weinborn M, Kellogg EJ, Bucks RS, Velnoweth A, & Woods SP (2014). Construct validity of the Memory for Intentions Screening Test (MIST) in healthy older adults. Assessment, 21(6), 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, & Phillips LH (2008). Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus nonfocal cues. Psychology and Aging, 23(1), 203–208. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ‐9. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, & Baltes PB (1997). Intellectual functioning in old and very old age: Cross-sectional results from the Berlin Aging Study. Psychology and Aging, 12(3), 410–432. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, & Einstein GO (2000). Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology, 14(7), S127–S144. [Google Scholar]
- Meeks JT, Hicks JL, & Marsh RL (2007). Metacognitive awareness of event-based prospective memory. Consciousness and Cognition, 16(4), 997–1004. [DOI] [PubMed] [Google Scholar]
- Pachana NA, Byrne GJ, Siddle H, Koloski N, Harley E, & Arnold E (2007). Development and validation of the Geriatric Anxiety Inventory. International Psychogeriatrics, 19(1), 103–114. [DOI] [PubMed] [Google Scholar]
- Randolph C (1998). Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Sano M, Silverman JM, & Haroutunian V (2005). Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: Relationship to functional status. Gerontology, 51(3), 206–212. [DOI] [PubMed] [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, & Tröster AI (2011). A differential deficit in time-versus event-based prospective memory in Parkinson’s disease. Neuropsychology, 25(2), 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin S, Buckheit C, & Sherrod C (2010). Memory for Intentions Test (MIsT). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Reitan RM, & Wolfson D (1985). The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation (Vol. 4). Reitan Neuropsychology. [Google Scholar]
- Rendell PG, & Thomson DM (1999). Aging and prospective memory: Differences between naturalistic and laboratory tasks. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 54(4), P256–P269. [DOI] [PubMed] [Google Scholar]
- Rönnlund M, Vestergren P, Mäntylä T, & Nilsson LG (2011). Predictors of self-reported prospective and retrospective memory in a population-based sample of older adults. The Journal of Genetic Psychology, 172(3), 266–284. [DOI] [PubMed] [Google Scholar]
- Royall DR, Cordes JA, & Polk M (1998). CLOX: an executive clock drawing task. Journal of Neurology, Neurosurgery & Psychiatry, 64(5), 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI & Yesavage JA, (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist, 5(1/2), 165–173. [Google Scholar]
- Smith G, Della Sala S, Logie RH, & Maylor EA (2000). Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory, 8(5), 311–321. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, & Alexander MP (2003). Staying on the job: The frontal lobes control individual performance variability. Brain, 126(11), 2363–2380. [DOI] [PubMed] [Google Scholar]
- Thaler NS, Hill BD, Duff K, Mold J, & Scott JG (2015). Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) intraindividual variability in older adults: associations with disease and mortality. Journal of Clinical and Experimental Neuropsychology, 37(6), 622–629. [DOI] [PubMed] [Google Scholar]
- Ungvari GS, Xiang YT, Tang WK, & Shum D (2008). Prospective memory and its correlates and predictors in schizophrenia: an extension of previous findings. Archives of Clinical Neuropsychology, 23(5), 613–622. [DOI] [PubMed] [Google Scholar]
- Uttl B, & Kibreab M (2011). Self-report measures of prospective memory are reliable but not valid. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 65(1), 57–68. [DOI] [PubMed] [Google Scholar]
- Uttl B, White CA, Gonzalez DW, McDouall J, & Leonard CA (2013). Prospective memory, personality, and individual differences. Frontiers in Psychology, 4:130, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group. (2007). Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology, 22(2), 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group. (2010). The semantic relatedness of cue–intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology, 32(4), 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group. (2008). Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist, 22(5), 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI, & HIV Neurobehavioral Research Center (HNRC) Group. (2005). Action (verb) fluency: Test-retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society, 11(4), 408–415. [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Li YR, Hodgson E, Ng AR, & Bucks RS (2015). Does prospective memory influence quality of life in community-dwelling older adults?. Aging, Neuropsychology, and Cognition, 22(6), 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Maxwell BR, Gummery A, Mo K, Ng AR, & Bucks RS (2014). Event-based prospective memory is independently associated with self-report of medication management in older adults. Aging & Mental Health, 18(6), 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Velnoweth A, Rooney A, & Bucks RS (2012). Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. Journal of the International Neuropsychological Society, 18, 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeintl M, Kliegel M, Rast P, & Zimprich D (2006). Prospective memory complaints can be predicted by prospective memory performance in older adults. Dementia and Geriatric Cognitive Disorders, 22(3), 209–215. [DOI] [PubMed] [Google Scholar]


