Abstract
Introduction:
The objectives of this study were to determine how HCV infection affects placental drug transporters, and to determine the role of drug transporters on the cellular accumulation of direct-acting antiviral drugs in human trophoblasts.
Methods:
Eighty-four ABC and SLC transporter genes were first screened in normal and HCV infected pregnant women using PCR profiler array. The changes in expression were confirmed by qPCR and Western blot. The impact of selected drug transporters on the cellular accumulation of radiolabeled antiviral drugs sofosbuvir, entecavir, and tenofovir was measured in primary human trophoblasts (PHT) and BeWo b30 cells in the presence or absence of transporter-specific inhibitors. PHT were then treated with CL097, ssRNA40, and imquimod to determine the impact of Toll-like receptor (TLR) 7/8 activation on drug transporter expression.
Results:
The expression of the ABC efflux transporters ABCB1/P-gp and ABCG2/BCRP was increased in placenta of women with HCV, while the nucleoside transporters SLC29A1/ENT1 and SLC29A2/ENT2 remained unchanged. The accumulation of sofosbuvir and tenofovir was unaffected by inhibition of these transporters in trophoblast cells. Entecavir accumulation was decreased by the inhibition of ENT2. P-gp and BCRP inhibition enhanced entecavir accumulation in BeWo b30, but not PHT. Overall, there was little effect of TLR7/8 activation on these drug transporters, and the accumulation of entecavir in PHT.
Discussion:
The data suggest that expression of placental drug transporters and selection of antiviral drug may impact fetal drug exposure in pregnancies complicated by HCV infections.
Keywords: antiviral drugs, HCV, placental drug transporters, toll-like receptors
INTRODUCTION
Chronic hepatitis C virus (HCV) affects between 1 and 8% of pregnant women, 3 to 10% of whom will transmit the disease to their baby [1]. The odds of transmission are 90% higher in the presence of HIV co-infection [2]. Antiviral drugs have been used to prevent mother-to-child transmission of human immunodeficiency virus (HIV) and hepatitis B virus (HBV), but has not been possible for HCV because of safety concerns with the medications currently used for treatment.
Direct-acting antiviral agents (DAAs) against HCV proteins have dramatically improved clinical outcomes. However, there is limited published experience with DAAs during pregnancy, and it is unclear how and to what extent they cross the human placenta. In order to facilitate safe and effective antiviral therapy or prophylaxis for pregnant women with chronic viral infections, it is important to understand the transfer mechanisms of these drugs to the fetus.
The ATP-binding cassette (ABC) and solute carrier (SLC) drug transporters play an important role in determining the plasma membrane permeability of antiviral drugs [3]. It is known that changes in the membrane expression of these transporters impact a drug’s disposition and pharmacokinetics. Drug transporter proteins are regulated by many factors including infection and inflammation [4–6], but there is little information regarding drug transporter regulation in placentas from HCV-infected mothers.
Inflammatory responses to infection are primarily mediated by Toll-like receptor (TLR)-signaling [7]. Various human and rodent studies identified regulation of drug transporters during activation of TLRs by bacterial or viral pathogens [4, 5, 8–10]. Single stranded RNA (ssRNA) viruses like HCV and HIV are sensed by TLR7 and TLR8 [11], but it is unknown whether receptor activation affects drug transporter expression.
The objectives of this study were to identify placental drug transporter regulation by HCV infection, to understand how drug transporters affect the accumulation of DAA drugs in placental cells, and to determine the role of TLR7/8 activation in the regulation of transporter expression.
MATERIALS AND METHODS
Chemicals and Reagents
Elacridar, Ko143, dipyridamole, and 6-S-[(4-Nitrophenyl)methyl]-6-thioinosine (NBMPR) were purchased from Tocris (Minneapolis, MN). Verapamil was purchased from Sigma-Aldrich (St. Louis, MO). All compounds were dissolved in DMSO and stored at −20°C until use. CL097, ssRNA40/LyoVec and ssRNA41/LyoVec, and imiquimod were purchased from InvivoGen (San Diego, CA). The anti-MDR1/ABCB1 monoclonal antibody (D3H1Q) was obtained from Cell Signaling Technology (Danvers, MA). The anti-BCRP antibody (clone BXP-21) was obtained from Millipore Sigma (Burlington, MA). ENT1 (clone F-12) and ENT2 (clone A-8) monoclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). [3H]-Entecavir (6.4 Ci/mmol) and [adenine-2,8-3H]-Tenofovir (17.1 Ci/mmol) were acquired from Moravek Biochemicals, Inc. (Brea, CA). [N-methyl-3H]-Sofosbuvir (80 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Corning Gentest ABC transporter membranes were purchased from Corning (Corning, Woburn, MA).
Human placenta and isolation of cytotrophoblasts
Human placentas were obtained with informed consent under a protocol approved by the Institutional Review Board at the University of Kansas Medical Center (Study #1530). Placental tissue samples were collected from women with chronic HCV infection (n=7; mean gestation 38.1 ± 2.2 weeks; mean plasma HCV RNA prior to delivery 4272319 ± 3515361 IU/mL; range 63920–7853243). Two HCV-positive patients had a history of tetrahydrocannabinol and amphetamine use. Additionally, one patient had a history of IV drug abuse and three patients were prescribed methadone maintenance therapy. Control samples were devoid of any significant underlying pathology. Two of the control patients endorsed tobacco use during pregnancy. Clinical characteristics of patient samples are listed in Supplementary Tables 1–2 [11].
Cytotrophoblasts were isolated from healthy placenta delivered at term by cesarean section and immunopurified as previously described [12, 13]. Cell purity was determined by immunofluorescence staining using anti-cytokeratin 7 and anti-vimentin antibodies. A proportion of 90% cytokeratin 7-positive cells (cytotrophoblasts) was of sufficient purity.
Cell culture
The choriocarcinoma cell line BeWo (clone b30) was obtained from Dr. Erik Rytting (University of Texas Medical Branch, Galveston, Texas) with permission from Dr. Alan Schwartz (Washington University, St Louis, MO). Cells were cultured at 37°C with 5% CO2 in a humidified atmosphere in DMEM supplemented with 2 mM glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin, and 10% FBS.
Primary human trophoblasts (PHT) were cultured in M199 media, supplemented with 10% FCS, epidermal growth factor (10 ng/ml), insulin (5 ng/ml), transferrin (10 ng/ml), sodium selenite (0.2 nM), penicillin/streptomycin (100 U/ml), and nystatin in a 95% air/5% CO2 humidified atmosphere at 37 °C.
RNA isolation and purification
Total cellular RNA was isolated from placental tissue using the TRIzol® reagent (Invitrogen) per manufacturer’s instructions. Total RNA concentration was determined by spectrophotometry (NanoDrop; Thermo Scientific) at 260nm and RNA integrity and purity confirmed by 260/280 ratio (>1.8). RNA integrity and purity was confirmed on the Agilent® Bioanalyzer using an RNA 6000 Nano LabChip®. All samples had RIN values > 7. Total RNA was isolated from PHT using RNeasy mini kit (Qiagen; Hilden, Germany) according to the manufacturer’s protocol.
qPCR profiler array
The mRNA of each placental tissue sample was converted into cDNA using the RT2 First Strand Kit (SABiosciences, Frederick, USA). The cDNA was then added to the RT2SYBR Green qPCR Master Mix (SABiosciences). Each sample was then added in duplicate on Human Drug Transporters RT2 Profiler™ PCR Array (384-well; SABiosciences). All steps were done according to the manufacturer’s protocol for the ABI Vii7 Detection System (Applied Biosystems, Foster City, CA).
The RT2 Profiler PCR Array data analysis software (http://www.sabiosciences.com/pcrarraydataanalysis.php) was used to analyze the PCR array data. Each array contained 5 separate housekeeping genes (RPLP1, HPRT, RPL13A, LDHA, and ACTB). The software determined the best reference gene(s) for normalization based on recommendations by Vandesompele et al. [14]. The reference genes were HPRT1 and ACTB for comparison of HCV-positive (n=4) and negative (n=4) samples. Any Cq value >35 was considered a negative call. The software calculates the fold change based on the widely used ΔΔ Cq method [15]. The p-value was calculated using a Student’s t-test (two tail distribution and equal variances between the two sample groups).
Real Time Quantitative PCR
Quantitative real-time PCR (qPCR) was performed as previously described [12]. Primers were selected based on previously published sequences to span at least one intron boundary to avoid false positive signals from residual genomic DNA. All primer sets were tested to ensure efficiency of amplification over a wide range of template concentrations. SYBR Green (Bio-Rad Laboratories, Hercules, CA) was used for amplicon detection. A melt curve was performed after amplification to ensure that all samples exhibited a single amplicon. PCR grade water served as a negative control for genomic contamination and displayed no amplification. Gene stability analysis of eight commonly used housekeeping genes in placental research (18s, CYC1, TOP1, UBC1, B2M, PKG1, YWHAZ, GAPDH) was compared across experimental and control groups using NormFinder [16]. The gene pair of 18s and PKG1 was used as the endogenous reference gene(s). Each sample (n=7/group) was assayed in duplicate. Relative changes in mRNA expression of the target genes were analyzed using the ΔΔCq method (2-ΔΔCq) [15].
Western blot assay
Total membrane protein from human placenta (n=7/group) was isolated using ProteoExract Native Membrane Protein Extraction kit (Millipore Sigma). Equal amounts of protein (30 ug) were loaded onto 7.5% polyacrylamide gels and separated by electrophoresis. Proteins were transferred to PVDF membranes, blocked for 1h, and probed overnight at 4 °C with primary antibodies. The membranes were probed with the respective secondary antibody. Protein bands were detected by ECL Western Blotting Detection Reagents (GE Healthcare Life Sciences; Pittsburgh, PA) and quantified using Image Lab software version 4.0 (Biorad). β-actin served as a loading control.
ATPase assay
Drug-stimulated transporter activity was estimated for DAAs by measuring inorganic phosphate released from ATP as previously described [17]. DAAs were tested at 20 and 100 μM concentration. Only those concentrations of compounds that demonstrate 2-fold stimulation were evaluated for being significantly different from the no compound control [18].
Accumulation of direct-acting antiviral drugs in trophoblasts
The influence of drug transporters P-gp, BCRP, ENT1, and ENT2 on the intracellular accumulation of DAAs was determined in the presence or absence of specific inhibitors. BeWo b30 cells were seeded on 24-well tissue culture dishes at a density of 2.5 × 105 cells/well in complete media and PHT were plated at a density of 5 × 105 cells/well. PHT were cultured for 3 to 4 days in growth medium. Syncytialization was confirmed by measuring β-hCG via ELISA as previously described [12]. β-hCG secretion increased significantly by 72 hours (data not shown).
Cellular accumulation studies were performed at 37 °C. Cells were pre-incubated at 37 °C for 30 minutes in buffer (Hanks’ balanced salt solution containing 25 mM glucose and 10 mM HEPES, adjusted to pH 7.4) containing Verapamil [100 μM], Ko143 (1 μM), Dypridamole (0.2 μM, 100 μM), or NBMPR (0.1 μM, 100 μM). Radiolabeled drugs were then added at concentrations of 40 nM for 3H-Sofosbuvir, 31.25 nM for 3H-Entecavir, 23.4 nM for 3H-Tenofovir. These concentrations were selected because they fall within the therapeutic range of antiviral activity. According to the time-dependent uptake of sofosbuvir, entecavir, and tenofovir in these cells, 5 minutes was selected as the linear uptake time (data not shown). The cells were washed three times with cold PBS, and lysed in 0.2N NaOH-0.5% Triton X-100 (pH 7.4). The amount of radioactivity in cell lysates was quantitated using a Beckman liquid scintillation counter (model LS 6000IC; Beckman Coulter, Brea, CA). Cellular protein content was determined by the bicinchoninic acid method. Inhibitors were prepared in DMSO at final concentrations <0.1%. An identical amount of DMSO was incorporated in the bathing media of controls.
TLR7/8 Activation in primary human trophoblasts
TLR7 and TLR8 were activated using CL097 (TLR7/8 ligand), viral ssRNA (ssRNA40, TLR8 ligand), or imiquimod (TLR7 ligand). PHT were seeded in 35 mm tissue culture dishes at a density of 2–3 × 106 cells/well in complete medium. Cells were then treated with CL097 (25 μg/mL), ssRNA40 (5 μg/ml), ssRNA41 (5 μg/ml), imiquimod (5 μg/ml), or vehicle control for 12 h. The doses were selected based on previously published data in human trophoblasts [19–21]. Analysis of downstream target genes including IL8, IL6, and TNFα were used to evaluate TLR pathway activation.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism version 6.0 (Graphpad Software, Inc., San Diego, CA, USA). Statistical differences between groups were determined by unpaired f-test or one-way ANOVA followed by Dunnett’s post-test. For studies in isolated PHT, statistical analyses were carried out by paired t-tests or repeated measures one-way ANOVA. Differences were considered statistically significant when P ≤ 0.05.
RESULTS
Changes in expression of drug transporter proteins in placenta of HCV-infected women
We first screened a panel of drug transporter genes in a subset of women (n=4) with chronic HCV infection at term delivery using a pcr profiler array. We identified increased mRNA expression (⩾1.5-fold; P-value ⩽ 0.05) in 8 of the 84 transporter genes in placenta of women with HCV (Table 1). Seven of these genes encoded members of the SLC family of transporters including folate (SLC19A1, 1.8-fold) and thiamine (SLC19A2, 2.0-fold) transporters, amino acid transporters (SLC7A11, 1.5-fold; SLC7A6, 1.6-fold), nucleoside transporters (SLC29A1, 1.8-fold; SLC29A2, 2.0-fold), and the mitochondrial carrier protein citrin (SLC25A13, 1.5-fold). The gene encoding the ABC efflux transporter P-gp (ABCB1) was increased 3-fold. The complete list of genes is shown in Supplementary Table 3. We selected SLC29A1, SLC29A2, and ABCB1 for further analysis because of their role in the transport of antiviral drugs. BCRP (ABCG2) is another quintessential placental ABC transporter involved in antiviral drug transport. ABCG2 increased 1.8-fold, but did not achieve statistical significance (P = 0.11).
Table 1.
Significant changes in drug transporter genes between normal (n=4) and HCV-infected (n=4) human placenta samples.
| Average raw CT | ||||
|---|---|---|---|---|
| Gene | Fold Change | p-value | HCV | Control |
| ABCB1 | 2.92 | 0.05 | 23.02 | 24.29 |
| SLC19A1 | 1.75 | 0.05 | 26.27 | 26.80 |
| SLC19A2 | 2.00 | 0.05 | 22.04 | 22.76 |
| SLC29A1 | 1.80 | 0.00 | 23.37 | 23.94 |
| SLC29A2 | 2.03 | 0.03 | 26.50 | 27.23 |
| SLC7A11 | 1.52 | 0.02 | 29.11 | 29.44 |
| SLC7A6 | 1.58 | 0.01 | 24.13 | 24.51 |
| SLC25A13 | 1.50 | 0.03 | 24.83 | 25.11 |
We expanded on these findings to confirm changes in SLC29A1, SLC29A2, ABCB1, and ABCG2 mRNA and protein (n=7). Although more variable, ABCB1 (2.5-fold, P < 0.05) and P-gp (3.1 ± 0.85, P < 0.05) expression was higher in placenta of women with HCV compared to the control group. BCRP expression was also greater in placenta of HCV positive patients (2.3 ± 0.37, P < 0.05), even though the mean values of ABCG2 did not reach statistical significance. Neither SLC29A1/ENT1 nor SLC29A2/ENT2 expression changes could be confirmed in placenta from HCV positive women (Figure 1).
Figure 1:
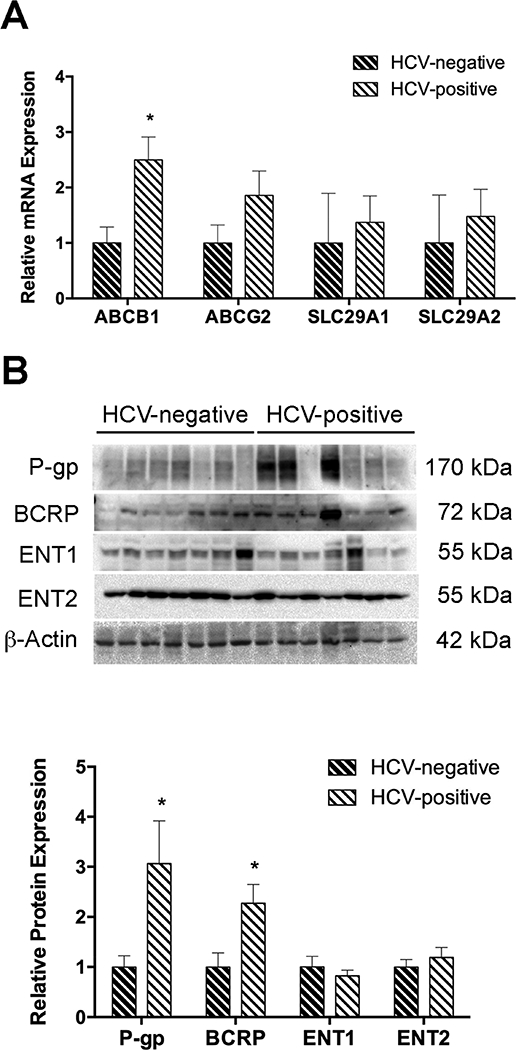
Expression levels of ABC and SLC drug transporters in placenta of HCV infected women. [A] ABCB1, ABCG2, SLC29A1, and SLC29A2 levels were determined by qPCR. The geometric mean of 18s and PKG1 was used in normalization. [B] P-gp, BCRP, ENT1 and ENT2 protein levels were evaluated by Western blot. β-actin served as the loading control. Data were represented as mean ± S.E.M from seven independent samples. Statistical differences were determined by an unpaired Student’s t-test, *P ⩽ 0.05.
Effect of drug transporter inhibition on the accumulation of antiviral drugs in human trophoblasts
The next objective was to determine how drug transporters alter the disposition of direct-acting antiviral drugs sofosbuvir, entecavir, and tenofovir in placental cells. P-gp and BCRP are abundantly expressed in the apical membrane of the syncytiotrophoblasts. Together, these transporters function to efflux drugs out of cells and protect the developing fetus from potentially harmful substances. An ATPase assay was performed to determine if sofosbuvir, entecavir, and tenofovir interact with P-gp and/or BCRP. The positive control compound in each transporter assay significantly stimulated inorganic phosphate release with a fold stimulation more than two. Treatment with sofosbuvir, but not entecavir and tenofovir, produced at least a 2-fold stimulation of human P-gp and BCRP (Figure 2).
Figure 2:
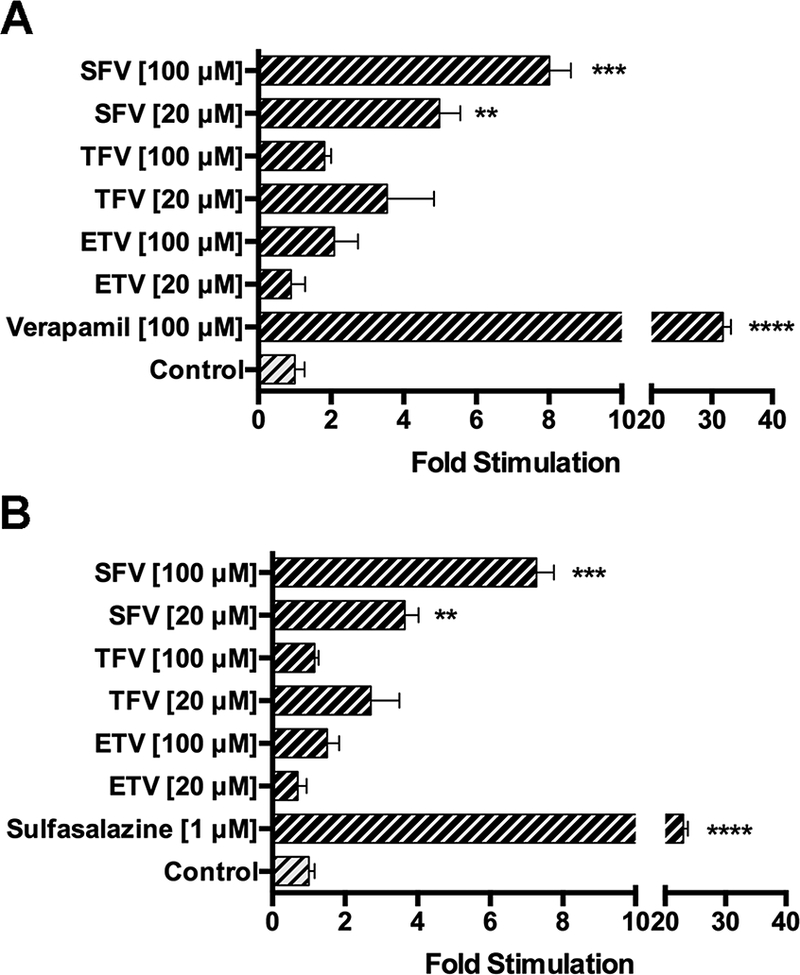
Stimulation of human P-gp and human BCRP ATPase activity by direct-acting antiviral drugs. Stimulation of ATPase activity was estimated by measuring inorganic phosphate released from ATP in the presence or absence of their respective controls for sofosbuvir, entecavir, and tenofovir (20 and 100 μM). The drug-stimulated ATPase activity was reported as fold-stimulation relative to the basal ATPase activity in the absence of drug (DMSO control). Data are represented as mean fold stimulation of the average control from triplicate reactions. *P ⩽ 0.05.
A series of accumulation assays were then performed to determine how P-gp, BCRP, ENT1, and ENT2 regulate the transfer of sofosbuvir, entecavir, and tenofovir into placental cells. The inhibition of P-gp, BCRP, ENT1, and ENT2 had no effect on sofosbuvir accumulation in either BeWo b30 cells (Figure 3A) or PHT (Figure 3B).
Figure 3:
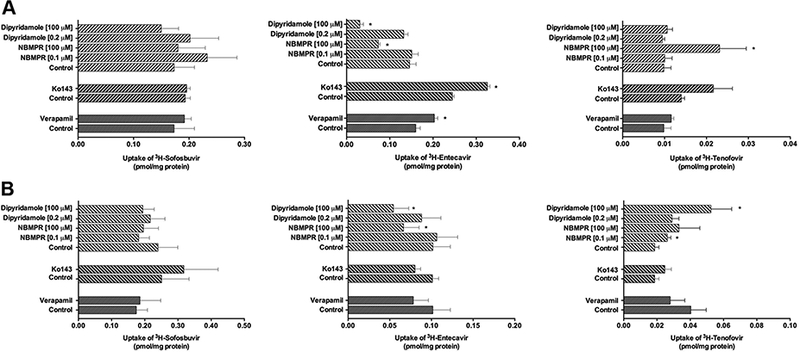
Transport of direct acting antiviral drugs by ABC and SLC transporters in placental cells. The cellular accumulation of radiolabeled sofosbuvir, entecavir, and tenofovir was evaluated in [A] BeWo b30 cells (n=3) and [B] PHT (n=4–5) in the presence of chemical inhibitors of P-gp, BCRP, and ENT1/2. Samples were run in triplicate and data represented as mean ± S.E.M. Statistical differences were determine by either unpaired or paired t-test, *P ⩽ 0.05.
The inhibition of P-gp with Verapamil [100 μM] and BCRP with Ko143 [1 μM] increased the cellular accumulation of entecavir in BeWo b30 cells (Figure 3A) but not PHT (Figure 3B). Similar results were obtained when cells were pretreated with the dual P-gp and BCRP inhibitor Elacridar [5 μΜ] (Supplementary Figure 1). The inhibition of ENT1 and ENT2 transporters often results in decreased cellular accumulation of their substrates. NBMPR specifically inhibits ENT1 at low concentrations (⩽ 0.1 μM), but also inhibits ENT2 at higher concentrations (⩾ 100 μM). The inhibition by NBMPR at high concentration (100 μM) diminished the in vitro uptake of ETV in BeWo b30 (Figure 3A) and PHT (Figure 3B). Similar results were found when cells were treated with another known ENT inhibitor, dipyridamole.
TFV accumulation in the BeWo b30 and PHT was 5 to 10 times lower than ETV despite dosing with relatively similar drug concentrations. TFV was not transported by P-gp or BCRP. Surprisingly, the inhibition with NBMPR at 100 μM enhanced the accumulation of TFV in BeWo b30 cells (Figure 3A), while NBMPR and dipyridamole at 0.1 μM and 100 μM, respectively, increased accumulation in PHT compared to the control (Figure 3B).
Effect of Toll-Like Receptors 7 and 8 Activation on Drug Transporters in Primary Human Trophoblasts
To better understand the potential pathways of drug transporter regulation in HCV infections, we assessed whether the activation of TLR7 and/or TLR8 alters drug transporter expression. Stimulation with the TLR7 and/or TLR8 ligands increased mRNA levels of the downstream target genes IL8, IL6, and/or TNFα, supporting activation of the TLR signaling pathways (Figure 4A). There was no difference in ABCB1 (Figure 4B), ABCG2 (Figure 4C), and SLC29A1 (Figure 4D) in PHT exposed to the TLR7/8 agonists. SLC29A2 levels were increased in cells treated with CLO97 (TLR7/8) and imiquimod (TLR7) (Figure 4D). Although ENT2 protein levels were on average higher in cells stimulated with imiquimod, the difference was not statistically significant (Figure 4F).
Figure 4:
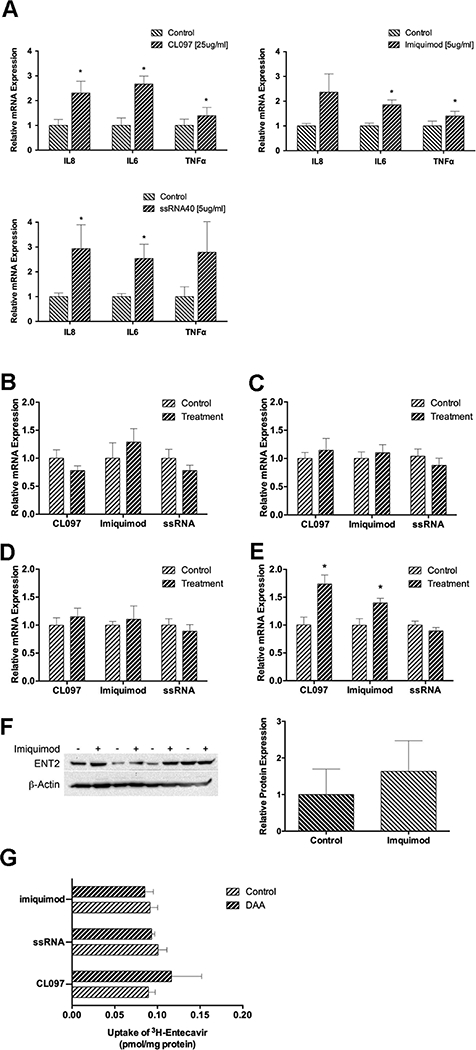
The effect of TLR7/8 agonists on drug transporter expression in PHT. Trophoblasts from 4–5 independent placentas were cultured for 3 days and treated with CL097 [25 μg/mL], Imiquimod [5 μg/mL], or ssRNA40 [5 μg/mL] and ssRNA41 (negative control) for 12 h. [A] The activation of TLR signaling pathways was evaluated by measuring the expression cytokine target genes. The relative mRNA expression of [B] ABCB1, [C] ABCG2, [D] SLC29A1, and [E] SLC29A2 was determined by qPCR. [F] The effect of TLR7 activation by imiquimod on ENT2 protein expression was determined by Western blot followed by densitometry. β-Actin was used as a loading control. [G] The effect of TLR7/8 activation on entecavir accumulation in PHT was assessed by measuring the intracellular levels of radiolabeled entecavir after a 48 hr treatement with CL097 [25 μg/mL], Imiquimod [5 μg/mL], or ssRNA40 [5 μg/mL]. Statistical differences were determined by paired t-test. Data are presented as mean ± S.E.M, *P ⩽ 0.05 relative to the control.
Since entecavir transport was affected by drug transporter inhibition, we performed accumulation studies to determine whether the activation of TLR7/8 signaling affects the disposition of entecavir through modulation of drug transporters in PHT. There was no difference in entecavir accumulation when cells were pretreated for 48h with CL097, imiquimod, or ssRNA40 compared to control (Figure 4G).
DISCUSSION
The drug transporters that regulate the passage of DAAs in the placenta have not been systematically investigated, nor has the impact of viremia on placental drug transport. In the present study, we evaluated the regulation of ABCB1/P-gp, ABCG2/BCRP, SLC29A1/ENT1, and SLC29A2/ENT2 in placenta of women with chronic HCV and focused on their role in the cellular disposition of DAAs.
Multiple studies have investigated the effect of HCV and HCV-induced inflammation on hepatic transporter expression. Liver biopsies from patients with HCV exhibited increased P-gp expression compared to uninfected controls [22]. Other reports indicated that the expression and function of BCRP increased in hepatocyte derived cellular carcinoma cell lines Huh7 expressing the HCV non-structural 5A (NS5A) protein and Huh7.5 containing either full-length- or subgenomic-HCV replicon systems [23]. In the present study, we provide new evidence to support increased expression of P-gp and BCRP in the placenta of women with HCV. It should be noted that several of the HCV patients reported a history of using drugs of abuse or were prescribed methadone maintenance therapy. A prior report indicated that methadone upregulates BCRP expression in human trophoblasts [24]. However, the HCV samples with the greatest expression levels of BCRP and P-gp were not associated with those patients on methadone maintenance therapy. Nonetheless, we cannot rule out the possibility that these drugs may contribute, in part, to the observed changes in drug transporters.
The placental transcription and immunoreactivity of P-gp was also found to be increased in patients with HIV [6]. The risk of vertical transmission of HCV is enhanced with high viral load and/or co-infection with HIV. Due to the higher expression of P-gp and/or BCRP in women affected by HCV and HIV, direct-acting antiviral drugs that are substrates of these transporters would prevent perinatal HCV transmission during pregnancy while limiting the risk of fetal drug exposure.
The equilibrative nucleoside transporters (ENT1–4) facilitate vectorial transport of nucleosides and nucleoside analogs which are commonly used as anti-cancer drugs, immunosuppressants, and antiviral agents. Human ENT1 and ENT2 are the best-characterized of the ENT isoforms, and are expressed at the placental barrier. In syncytiotrophoblasts, ENT1 was localized on the apical membrane whereas ENT2 was expressed on apical and basal membrane [25–27]. Although SLC29A1/ENT1 and SLC29A2/ENT2 expression remained unchanged in the placenta of HCV-infected women, their interaction with nucleoside analogs could have critical implications on the safety and efficacy of direct-acting antiviral drugs during pregnancy.
For years, ribavirin has been used in combination therapy for the treatment of HCV infection. However, due its teratogenic effects in animals, ribavirin is contraindicated during pregnancy (FDA pregnancy category X) [28]. Currently, there is no recommended medication for treatment of HCV in pregnant women. The improved efficacy and tolerability of new drugs like sofosbuvir opens possibilities for treatment of HCV in pregnancy [29]. Sofosbuvir is classified as Pregnancy Category B but there is little evidence regarding its safety in pregnant women. It is unknown whether sofosbuvir crosses the human placenta and affects the fetus. GS-461 203, an active metabolite, and GS-331 007, its predominant metabolite which represents >90% of the exposure [30], cross the placenta of rats and rabbits [31]. Preclinical studies report that sofosbuvir, but not GS-331 007, is a substrate of intestinal P-gp and BCRP [31]. Indeed, sofosbuvir interacts with these transporters as determined by ATPase assays (Figure 2). However, inhibiting P-gp and BCRP in placental cells had no effect on the uptake of sofosbuvir (Figure 3A,B). This suggests that sofosbuvir interacts with P-gp and BCRP but is not effluxed by these transporters in the placenta. It is also possible that other nucleoside transporters are involved in sofosbuvir transport thereby overwhelming the impact of P-gp and BCRP on sofosbuvir efflux. Our data indicates that sofosbuvir is not a substrate for ENT1 and ENT2. Additional studies are required to understand the accumulation mechanisms or transporters that participate in the transfer of sofosbuvir into placenta cells.
P-gp, BCRP, and ENT1/2 are among several transporters involved in the transport of entecavir in placenta [32]. Entecavir (pregnancy risk factor C) is an effective HBV drug but should only be used during pregnancy if the potential benefits outweigh the possible risks. Our findings confirmed that entecavir is transported by ENT2 and agreed with a prior report that showed inhibiting P-gp and BCRP enhanced entecavir uptake into BeWo b30, but not PHT. Ma et al [32] speculated that this may be due to the high-affinity influx transporters in PHT eclipsing the role of these efflux transporters.
Tenofovir is a nucleoside analog with a pregnancy category B designation that is currently approved for the treatment of HBV and HIV. Our data agrees with initial results indicating that tenofovir is not transported by placental P-gp or BCRP [33]. We demonstrated that it is also not a substrate for ENT1 or ENT2. In some instances, cells treated with either dipyridamole or NBMPR exhibited increased tenofovir accumulation. Tenofovir is known to be transported by OAT1, OAT3, and MRP4. OAT1 and OAT3 are not present in placenta [34]. However, MRP4 is expressed in human placenta. One study localized MPR4 to the apical membrane of the syncytiotrophoblasts where it likely functions to limit the accumulation of its substrates in the placenta [35]. Both dipyridamole and NBMPR inhibit MRP4 [36]. Therefore, one explanation for increased accumulation of tenofovir observed in the placenta cells is the inhibition of MRP4 by dipyridamole and NBMPR.
HCV, like HIV, is a positive strand ssRNA virus sensed by TLR7/8 [37–40]. These receptors are present in human term placenta [41] but it is unknown whether these signaling pathways affect drug transport. We investigated whether TLR7/8 activation affects ABCB1, ABCG2, and SLC29A1/2 in placental cells. The expression SLC29A2 was moderately increased in response to TLR7 activation with CL097 and imiquimod (Figure 4E). Despite higher mean expression, imiquimod-induced changes in ENT2 were not significantly different from control at the protein level (Figure 4F). Furthermore, imiquimod treatment did not alter entecavir accumulation (Figure 4G) which we showed to be transported in part by SLC29A2. These results could be explained by the fact that TLR7 activation caused only a small increase in SLC29A2 expression, and that multiple transporters contribute to entecavir transport in placental cells. Taken together, it appears that the activation of TLR7/8 pathways in trophoblasts has little effect on the expression of P-gp, BCRP, and ENT transporters.
In summary, our findings suggest that expression of placental drug transporters and the selection of antiviral drug may impact fetal drug exposure in pregnancies complicated by HCV infections. For example, the increased expression of P-gp and BCRP in placenta from HCV-infected women would contribute to limit the maternal-fetal transport of antiviral drug substrates across the placental barrier. We acknowledge that a major limitation of this study is the relatively small placental sample size used in the analyses. Obviously, larger patient numbers could reveal significance in transporters with high inter-individual variability as well as those that showed a trend toward expression differences. Thus, future studies must expand on these findings. Furthermore, preclinical studies should be performed to test drug transporter interactions and the placental transfer of other novel direct-acting antiviral drugs currently used to treat HCV infections.
Supplementary Material
HIGHLIGHTS.
P-gp and BCRP mRNA and protein expression were increased in placenta of women with HCV.
Sofosbuvir accumulation in trophoblast cells was not affected by transporter inhibition.
ENT2 was involved in entecavir accumulation into trophoblast cells.
ENT1/2, P-gp, and BCRP were not involved in tenofovir accumulation in trophoblast cells.
Activation of TLR7 and TLR8 had little effect on transporter expression.
ACKNOWLEDGEMENTS
We would like to thank Dr. Erik Rytting for providing the BeWo b30 cells.
FUNDING
This work was supported in part by the National Institutes of Health [5R00HD068454–05], and an NIH Clinical and Translational Science Award grant (UL1 TR000001, formerly UL1RR033179) awarded to the University of Kansas Medical Center, and an internal Lied Basic Science Grant Program of the KUMC Research Institute.
Nonstandard Abbreviations:
- ABC
ATP-binding cassette
- BCRP
breast cancer resistance protein
- CNT
concentrative nucleoside transporters
- DAA
direct-acting antiviral agents
- ENT
equilibrative nucleoside transporter
- ETV
entecavir
- FBS
fetal bovine serum
- P-gp
p-glycoprotein
- PHT
primary human trophoblasts
- qPCR
quantitative real time polymerase chain reaction
- SFV
sofosbuvir
- SLC
solute carrier
- TFV
tenofovir
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY AND REFERENCES CITED
- [1].Le Campion A, Larouche A, Fauteux-Daniel S, Soudeyns H, Pathogenesis of hepatitis C during pregnancy and childhood, Viruses 4(12) (2012) 3531–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Polis CB, Shah SN, Johnson KE, Gupta A, Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis, Clin Infect Dis 44(8) (2007) 1123–31. [DOI] [PubMed] [Google Scholar]
- [3].Vahakangas K, Myllynen P, Drug transporters in the human blood-placental barrier, Br J Pharmacol 158(3) (2009) 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lye P, Bloise E, Javam M, Gibb W, Lye SJ, Matthews SG, Impact of bacterial and viral challenge on multidrug resistance in first-and third-trimester human placenta, Am J Pathol 185(6) (2015) 1666–75. [DOI] [PubMed] [Google Scholar]
- [5].Petrovic V, Piquette-Miller M, Impact of polyinosinic/polycytidylic acid on placental and hepatobiliary drug transporters in pregnant rats, Drug metabolism and disposition: the biological fate of chemicals 38(10) (2010) 1760–6. [DOI] [PubMed] [Google Scholar]
- [6].Camus M, Delomenie C, Didier N, Faye A, Gil S, Dauge MC, Mabondzo A, Farinotti R, Increased expression of MDR1 mRNAs and P-glycoprotein in placentas from HIV-1 infected women, Placenta 27(6–7) (2006) 699–706. [DOI] [PubMed] [Google Scholar]
- [7].Mogensen TH, Pathogen recognition and inflammatory signaling in innate immune defenses, Clin Microbiol Rev 22(2) (2009) 240–73, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shah P, Guo T, Moore DD, Ghose R, Role of constitutive androstane receptor in Toll-like receptor-mediated regulation of gene expression of hepatic drug-metabolizing enzymes and transporters, Drug metabolism and disposition: the biological fate of chemicals 42(1) (2014) 172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shah P, Omoluabi O, Moorthy B, Ghose R, Role of Adaptor Protein Toll-Like Interleukin Domain Containing Adaptor Inducing Interferon beta in Toll-Like Receptor 3- and 4-Mediated Regulation of Hepatic Drug Metabolizing Enzyme and Transporter Genes, Drug metabolism and disposition: the biological fate of chemicals 44(1) (2016) 61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Petrovic V, Wang JH, Piquette-Miller M, Effect of endotoxin on the expression of placental drug transporters and glyburide disposition in pregnant rats, Drug metabolism and disposition: the biological fate of chemicals 36(9) (2008) 1944–50. [DOI] [PubMed] [Google Scholar]
- [11].Nelson DM, Burton GJ, A technical note to improve the reporting of studies of the human placenta, Placenta 32(2) (2011) 195–6. [DOI] [PubMed] [Google Scholar]
- [12].Mason CW, Lee GT, Dong Y, Zhou H, He L, Weiner CP, Effect of prostaglandin E2 on multidrug resistance transporters in human placental cells, Drug metabolism and disposition: the biological fate of chemicals 42(12) (2014) 2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS, Isolation and culture of term human trophoblast cells, Methods Mol Med 121 (2006) 203–17. [DOI] [PubMed] [Google Scholar]
- [14].Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes, Genome Biol 3(7) (2002) RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25(4) (2001) 402–8. [DOI] [PubMed] [Google Scholar]
- [16].Andersen CL, Jensen JL, Orntoft TF, Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets, Cancer Res 64(15) (2004) 5245–50. [DOI] [PubMed] [Google Scholar]
- [17].Mason CW, Hassan HE, Kim KP, Cao J, Eddington ND, Newman AH, Voulalas PJ, Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse, The Journal of pharmacology and experimental therapeutics 333(3) (2010) 854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS, Rational use of in vitro P-glycoprotein assays in drug discovery, The Journal of pharmacology and experimental therapeutics 299(2) (2001) 620–8. [PubMed] [Google Scholar]
- [19].Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, Narayanan AM, Young KJ, Jones KA, Kuehl TJ, Mitchell BM, Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice, PLoS One 7(7) (2012) e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Manlove-Simmons JM, Zaher FM, Tomai M, Gonik B, Svinarich DM, Effect of imiquimod on cytokine induction in first trimester trophoblasts, Infect Dis Obstet Gynecol 8(2) (2000) 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Potter JA, Garg M, Girard S, Abrahams VM, Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and - independent manner, Biol Reprod 92(1) (2015) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA, High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease, J Pathol 200(5) (2003) 553–60. [DOI] [PubMed] [Google Scholar]
- [23].C WR, Rosso N, Gentile E, Cuestas M, Tiribelli C, Oubina JR, Mathet VL, Dissimilar expression of multidrug resistance mdr1 and bcrp by the replication of hepatitis C virus: role of the nonstructural 5A protein, Journal of viral hepatitis 20(4) (2013) e127–30. [DOI] [PubMed] [Google Scholar]
- [24].Neradugomma NK, Liao MZ, Mao Q, Buprenorphine, Norbuprenorphine, R-Methadone, and S-Methadone Upregulate BCRP/ABCG2 Expression by Activating Aryl Hydrocarbon Receptor in Human Placental Trophoblasts, Molecular pharmacology 91(3) (2017) 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barros LF, Yudilevich DL, Jarvis SM, Beaumont N, Young JD, Baldwin SA, Immunolocalisation of nucleoside transporters in human placental trophoblast and endothelial cells: evidence for multiple transporter isoforms, Pflugers Arch 429(3) (1995) 394–9. [DOI] [PubMed] [Google Scholar]
- [26].Errasti-Murugarren E, Diaz P, Godoy V, Riquelme G, Pastor-Anglada M, Expression and distribution of nucleoside transporter proteins in the human syncytiotrophoblast, Molecular pharmacology 80(5) (2011) 809–17. [DOI] [PubMed] [Google Scholar]
- [27].Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, Tse CM, Hayashi J, Unadkat JD, In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta, American journal of physiology. Regulatory, integrative and comparative physiology 293(5) (2007) R1809–22. [DOI] [PubMed] [Google Scholar]
- [28].Roberts SS, Miller RK, Jones JK, Lindsay KL, Greene MF, Maddrey WC, Williams IT, Liu J, Spiegel RJ, The Ribavirin Pregnancy Registry: Findings after 5 years of enrollment, 2003–2009, Birth Defects Res A Clin Mol Teratol 88(7) (2010) 551–9. [DOI] [PubMed] [Google Scholar]
- [29].Spera AM, Eldin TK, Tosone G, Orlando R, Antiviral therapy for hepatitis C: Has anything changed for pregnant/lactating women?, World J Hepatol 8(12) (2016) 557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kirby BJ, Symonds WT, Kearney BP, Mathias AA, Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of the Hepatitis C Virus NS5B Polymerase Inhibitor Sofosbuvir, Clin Pharmacokinet 54(7) (2015) 677–90. [DOI] [PubMed] [Google Scholar]
- [31].Gilead, Sovaldi (sofosbuvir) Summary of Product Characteristics., in: Union E (Ed.) 2014. [Google Scholar]
- [32].Ma Z, Yang X, Jiang T, Bai M, Zheng C, Zeng S, Sun D, Jiang H, Multiple SLC and ABC transporters contribute to the placental transfer of entecavir, Drug metabolism and disposition: the biological fate of chemicals (2017). [DOI] [PubMed] [Google Scholar]
- [33].Neumanova Z, Cerveny L, Ceckova M, Staud F, Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta, Aids 28(1) (2014) 9–17. [DOI] [PubMed] [Google Scholar]
- [34].Roth M, Obaidat A, Hagenbuch B, OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies, Br J Pharmacol 165(5) (2012) 1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Azzaroli F, Mennone A, Feletti V, Simoni P, Baglivo E, Montagnani M, Rizzo N, Pelusi G, D DEA, Lodato F, Festi D, Colecchia A, Roda E, o JL, Mazzella G, Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid, Aliment Pharmacol Ther 26(8) (2007) 1139–46. [DOI] [PubMed] [Google Scholar]
- [36].Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P, Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5, Molecular pharmacology 63(5) (2003) 1094–103. [DOI] [PubMed] [Google Scholar]
- [37].Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M, Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells, J Immunol 178(12) (2007) 7658–66. [DOI] [PubMed] [Google Scholar]
- [38].Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N, Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions, The Journal of clinical investigation 115(11) (2005) 3265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee J, Tian Y, Chan ST, Kim JY, Cho C, Ou JH, TNF-alpha Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism, PLoS Pathog 11(5) (2015) e1004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee J, Wu CC, Lee KJ, Chuang TH, Katakura K, Liu YT, Chan M, Tawatao R, Chung M, Shen C, Cottam HB, Lai MM, Raz E, Carson DA, Activation of anti-hepatitis C virus responses via Toll-like receptor 7, Proc Natl Acad Sci U S A 103(6) (2006) 1828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA, Expression and activity of Toll-like receptors 1–9 in the human term placenta and changes associated with labor at term, Biol Reprod 80(2) (2009) 243–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


