Abstract
Objective
Previous studies have suggested that cytokines and growth factors may predict ventricular recovery following aortic valve replacement (AVR). The primary objective of this study was to identify cytokines that predict ventricular recovery following transcatheter AVR (TAVR).
Methods
We prospectively enrolled 121 consecutive patients who underwent TAVR. Standard echocardiographic assessment at baseline, 1-month and 1-year after TAVR included left ventricular (LV) mass index (LVMI) and global longitudinal strain (GLS). Blood samples were obtained at the time of the procedure to measure cytokines using a 63-plex Luminex platform. Partial least squares-discriminant analysis was performed to identify cytokines associated with ventricular remodeling and function at baseline as well as 1 year after TAVR.
Results
The mean age was 84±9 years, with a majority of male subjects (59%), a mean LVMI of 120.4±45.1 g/m2 and LVGLS of −13.0±3.2%. On average, LV mass decreased by 8.1% and GLS improved by 20.3% at 1 year following TAVR. Among cytokines assayed, elevated hepatocyte growth factor (HGF) emerged as a common factor significantly associated with worse baseline LVMI and GLS as well as reduced ventricular recovery (p<0.005). Other factors associated with ventricular recovery included a select group of vascular growth factors, inflammatory mediators and tumor necrosis factors, including VEGF-D, ICAM-1, TNFβ, and IL1β.
Conclusion
We identified a network of cytokines, including HGF, that are significantly correlated with baseline LVMI and GLS, and ventricular recovery following TAVR.
Keywords: transcatheter aortic valve replacement, ventricular adaptation, cytokines
INTRODUCTION
With the aging population, degenerative calcific aortic stenosis (AS) has become more prevalent.1 AS is a progressive disease associated with inflammation and calcium deposition on the valve leaflets.2 In recent years, transcatheter aortic valve replacement (TAVR) has emerged as a safe and effective treatment option for patients with severe aortic stenosis (AS) who are at intermediate or high risk for surgery. Ventricular recovery following TAVR is, however, variable with some patients demonstrating greater improvement than others.
While several studies have reported that cytokines and growth factors are involved in myocardial hypertrophy, myocardial fibrosis, and myocardial dysfunction,3–5 their role in ventricular recovery following TAVR has not been extensively studied. Several circulating factors have been associated with adverse ventricular remodeling in pressure overload states including inflammasome associated cytokines (interleukin-18 and interleukin-1β), hepatic growth factor (HGF), and interferon-gamma pathway cytokines, while others have been associated with better adaptation such as vascular growth factors or tumor necrosis factors.6–8 Based on these findings, we hypothesize that these factors could also be associated with adverse ventricular remodeling and less ventricular recovery after TAVR. Therefore, in this prospective cohort study, we sought to determine the circulating cytokines and growth factors associated with ventricular function in patients with severe AS, as well as structural and functional ventricular recovery after TAVR.
METHODS
Study Population
We prospectively recruited consecutive patients with symptomatic, severe AS who agreed to participate and were deemed to be at high surgical risk and therefore underwent TAVR between October 2013 and April 2015 at Stanford University Medical Center as part of an ongoing registry. Operative risk was determined by our Heart Valve Evaluation Team. Patients were deemed high-risk or inoperable if the Society of Thoracic Surgeons (STS) risk score was >8% or the Heart Team considered the patient to be high-risk or inoperable due to other factors not accounted for by the STS risk calculator. Patients with recent myocardial infarction, active cancer, and advanced liver disease were not considered for TAVR. Patients were excluded if they were currently on immunomodulatory therapy such as prednisone or other immunosuppressive therapy or on dialysis.
Study protocol
Echocardiography was performed at baseline before TAVR and repeated at 1-month and at 1-year following TAVR per usual protocol and reanalyzed by the Stanford Cardiovascular Institute Biomarker and Phenotypic Core Laboratory. Blood samples were obtained at the time of the procedure prior to deployment of the valve. Serum and plasma were stored at −80°C until assayed. The protocol was approved by the Stanford Institutional Review Board, and written informed consent was obtained from each participant.
Echocardiographic Assessment
Echocardiography was performed using commercially available echocardiographic systems (Sonos 7500, iE33, and EPIQ 7C; Philips Medical Imaging, Eindhoven, the Netherlands), according to the American Society Echocardiography guideline recommendations.9 Aortic valve area was calculated using the continuity equation. Peak and mean systolic transaortic pressure gradients were calculated using the simplified Bernoulli equation from the same angle, either apical 5- or 3-chamber view.10 Severe aortic stenosis was defined as an aortic valve area (AVA) ≤ 1.0 cm2 or indexed AVA (AVAI) ≤ 0.6 cm2/m2 and/or mean systolic aortic gradient > 40 mmHg or peak velocity across the aortic valve > 4 m/sec.11 In the setting of LV systolic dysfunction and low-flow, low-gradient AS, the severity of AS was confirmed by low-dose dobutamine stress echocardiography.
Standard echocardiographic views were obtained in M-mode, two-dimensional (2D) and color tissue Doppler modes. LV end-systolic and end-diastolic volumes and ejection fraction (LVEF) were calculated using biplane Simpson’s method. LV internal diameter and interventricular septal and posterior wall thicknesses were obtained at end-diastole from the 2D image. LV mass was obtained by area-length method and LV mass index was calculated as LV mass normalized by body surface area. LV global longitudinal strain (GLS) was measured using Lagrangian strain by the average values of longitudinal strain obtained from the apical 4-, 3-, and 2-chamber views.12 We measured the myocardial length in end-diastole (L0) and in end-systole (L1) and calculated strain values as 100 × (L1−−L0)/ L0.13 The coefficient of variation was 2.2 for LS for intra-observer variability and 7.6 for LS for inter-observer variability in our Stanford Biomarker and Phenotypic Core Laboratory.12 In this study, ventricular remodeling (or cardiac remodeling) refers to changes in the size, shape, structure, and function of the heart. Ventricular size in our study was defined by using the diastolic left ventricular internal dimension scaled to height or BSA, geometrical remodeling of the heart was mainly assessed using relative wall thickness; and ventricular function was assessed with LV longitudinal strain. Furthermore, significant ventricular recovery was defined as improved LV mass index (relative change ≥ 20%), or increased GLS (relative change ≥ 15%).
Blood sample preparation and cytokine analysis
Blood sampling was performed after anesthesia had been administered but before the aortic valve was treated. We used a 63-plex Luminex bead kit (Affymetrix, Santa Clara, CA) customized at Stanford University Human Immune Monitoring Core facility. Each sample was measured in duplicates. Plates were read using a Luminex LabMap200 instrument.14 The Luminex LabMap200 outputs the fluorescence intensity of each bead measured for a given cytokine in a sample. For each well, we considered the median fluorescence intensity (MFI) of all beads measured for a given cytokine and averaged the MFI of the two replicates. By design, samples of patients with AS were matched on each plate to minimize inter-plate variability. Five plates were used for the assays and the coefficient of variation between assays for all biomarkers was < 15% for all cytokines. The complete list of cytokines assayed is shown in Supplementary Table 1.
Statistical methods
Categorical variables were compared using Pearson’s chi square test or Fisher’s exact test, as appropriate. Normality of the continuous variables was confirmed with the Shapiro-Wilk test. Comparisons of continuous variables between baseline and follow-up were performed using either the paired t-test or the Wilcoxon signed rank sum test, as appropriate. Repeated ANOVA was used to compare echocardiography data from the three time points (baseline, 1-month and 1-year). Univariate analysis was performed to determine the clinical variables associated with LV function parameters such as LV mass index and GLS. Then, parameters with p value < 0.15 were entered to multivariate analysis.
For the cytokine analysis, Partial Least Squares (PLS) regression analysis was used to identify groups of cytokines associated with baseline and ventricular remodeling and function at 1 year after TAVR, accounting for age, sex, aortic valve stenosis severity and history of ischemic heart disease as these parameters contribute to LV function. PLS creates several linear combinations (latent factors) and then uses the composites as principal components in discrimination. The importance of each cytokine in the construction of the latent factors is assessed from the variable’s importance in projection (VIP) scores of Wold. Cytokines with VIP > 1.5 were considered influential. A p value <0.05 was defined as statistically significant. SAS software, version 9.3 and JMP Genomics (SAS Institute, Cary, NC), SPSS version 21 (SPSS Inc, Chicago, Illinois), and MedCalc version 15.8 (MedCalc Software, Belgium) were used for the analysis. Correlation matrix plot was created using Hmisc, and ggcorrplot packages in R (version 3.3.2). Partial correlation analysis was performed using MedCalc version 15.8.
RESULTS
One hundred and twenty one consecutive patients were enrolled in this study. The mean age was 84±9 years and 56% were men (Table 1). Table 1 and 2 summarize the clinical and echocardiographic characteristics of enrolled patients. Transfemoral, transaortic, and transapical approaches were used in 101 (83%), 15 (12%), and 5 (4%) patients, respectively. Baseline echocardiographic examination was performed in all patients at Stanford University Medical Center and repeated in 83 patients at 1-year after TAVR. Nineteen patients (16%) died at 1-year and 19 patients were followed by their local cardiologist, as follow-up echocardiogram at 1 year at Stanford University Medical Center was recommended but not required per protocol. All serum samples were successfully analyzed with the multiplex Luminex panel and passed all quality control criteria.
Table 1.
Clinical characteristics and echocardiographic parameters
| Patients (n=121) |
Patients with follow-up echocardiogram (n=83) |
|
|---|---|---|
| Age (years) | 83.7±9.0 | 82.7±9.1 |
| Male sex, n (%) | 71 (59) | 48 (58) |
| BSA (m2) | 1.86±0.24 | 1.88±0.26 |
| BMI (kg/m2) | 27.1± 6.1 | 27.5± 6.6 |
| STS-score | 8.0±4.1 | 7.4±3.4 |
| Frailty ≥ II, n (%) | 74 (61) | 63 (76) |
| NYHA functional class ≥ III, n (%) | 64 (53) | 42 (51) |
| Comorbidity | ||
| Hypertension, n (%) | 101 (84) | 67 (81) |
| Dyslipidemia, n (%) | 86 (71) | 59 (71) |
| Diabetes mellitus, n (%) | 38 (31) | 25 (30) |
| History of coronary artery disease*, n (%) | 70 (58) | 48 (58) |
| CKD grade ≥ III, n (%) | 94 (78) | 63 (76) |
| 1-year mortality, n (%) | 19 (16) | NA |
| Echocardiographic parameters at baseline and 1-year follow-up | ||||
|---|---|---|---|---|
|
| ||||
| Baseline (n=121) |
Baseline (n=83) |
1-year (n=83) |
P value** | |
| Heart rate (bpm) | 73±13 | 73±14 | 73±13 | 0.93 |
| Systolic blood pressure (mmHg) | 125±17 | 124±16 | 135±16 | <0.001 |
| Diastolic blood pressure (mmHg) | 69±12 | 70±11 | 68±11 | 0.20 |
| Left ventricular internal diameter (cm) | 4.7±0.9 | 4.7±0.9 | 4.8±0.8 | 0.24 |
| Interventricular septum (cm) | 1.2±0.2 | 1.2±0.2 | 1.1±0.2 | 0.001 |
| Posterior wall (cm) | 1.2±0.2 | 1.2±0.2 | 1.1±0.2 | <0.001 |
| LV mass index (g/m2) | 120.4±45.1 | 116.0±42.8 | 103.4±34.6 | <0.001 |
| LVEF (%) | 54.1±12.7 | 54.2±13.0 | 59.6±9.1 | <0.001 |
| GLS (%) | −13.0±3.2 | −12.9±3.3 | −14.9±2.7 | <0.001 |
| e’ (cm/s) | 5.2±1.4 | 5.3±1.6 | 5.8±1.8 | 0.03 |
| a’ (cm/s) | 6.2±2.6 | 6.5±2.8 | 6.6±2.4 | 0.88 |
| E/e’ | 24.9±10.9 | 24.5±11.5 | 20.4±9.9 | 0.001 |
| LAVI (ml/m2) | 47.8±14.8 | 48.1±15.9 | 47.0±17.8 | 0.36 |
| AS severity | ||||
| Aortic valve area (cm2) | 0.62±0.18 | 0.62±0.16 | 1.38±0.41 | <0.001 |
| Aortic valve area index (cm2/m2) | 0.34±0.09 | 0.33±0.08 | 0.74±0.23 | <0.001 |
| Mean trans-aortic pressure gradient (mmHg) | 50.2±15.5 | 51.1±14.2 | 10.5±5.3 | <0.001 |
includes history of myocardial infarction in 28 patients (23%), bypass surgery in 38 patients (31%), and percutaneous coronary intervention in 36 patients (30%).
p values are between echocardiograms at Baseline (n=83) and 1-year (n=83).
BMI, body mass index; BSA, body surface area; CKD, chronic kidney disease; LA, left atrial; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; STS-score, Society of Thoracic Surgeons risk score; GLS, global longitudinal strain; LAVI, left atrial volume index; LV, left ventricular.
Table 2.
Cytokines related to baseline and change in LVMI and GLS as Identified by PLS Analysis
| Characteristics | LV mass index | Absolute GLS | Change in LV mass index (g/m2) |
Change in absolute GLS (%) |
|---|---|---|---|---|
| % variation explained by latent factors | ||||
| For predictor variables (cytokines) | 14.8 | 45.3 | 36.4 | 48.7 |
| For outcome variables (LV indexes) | 12.2 | 16.2 | 9.42 | 26.0 |
| N of used latent factors | 1 | 2 | 1 | 2 |
| P value | 0.0009 | 0.0012 | 0.0037 | 0.0002 |
| Factor | VIP | +/− | Factor | VIP | +/− | Factor | VIP | +/− | Factor | VIP | +/− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Top Cytokines responsible for outcome | HGF | 2.91 | + | VEGF-D | 2.11 | − | HGF | 2.36 | + | IL21 | 1.73 | − |
| ICAM-1 | 1.88 | + | IL15 | 1.54 | − | TNFβ | 2.66 | − | TRAIL | 1.72 | − | |
| TNFβ | 1.79 | + | MCP3 | 1.42 | − | IL9 | 2.16 | − | MIP1A | 1.48 | − | |
| Eotaxin | 2.54 | − | HGF | 1.37 | − | IFNα | 1.90 | − | HGF | 1.47 | − | |
| CD40L | 1.80 | − | ENA78 | 2.17 | + | FGF | 1.85 | − | IL1A | 1.47 | − | |
| IL1B | 1.64 | − | EGF | 1.75 | − | |||||||
| ENA78 | 1.55 | − | ||||||||||
LV mass index and GLS were adjusted for age and sex. Changes in LV mass index and GLS were adjusted for age and sex and baseline variable (LV mass index or GLS). Baseline and changes in GLS was additionally adjusted for BMI. P-values are for the associations between outcome and latent factors. VIP, variance importance in projection; GLS, global longitudinal strain. +/− = positive/negative association.
refers to cytokines, chemokines, growth factors, colony-stimulating factors and interferons (list of abbreviation is provided in Supplementary Table 1)
LV remodeling and function at baseline and its association with cytokines
The mean AVA, AVAI, peak transaortic pressure gradient, and mean transaortic pressure gradient of the population confirmed severe AS (Table 1). As shown in Supplementary Figure 2, echocardiographic parameters were distributed widely. In contrast to LVEF, GLS was abnormal in the majority of patients (95%) (Supplementary Figure 2-A and B). As expected, we found positive correlations between baseline LV function parameters, including GLS, LVMI, LAVI and E/e’ (Supplementary Figure 3). Furthermore, we found male sex to be correlated to LV mass (r=0.27, p=0.003), and that male sex (beta=−0.32, p<0.001) and AVAI (beta=0.20, p=0.02) independently correlated with absolute value of GLS in multivariate analysis (R2=0.18).
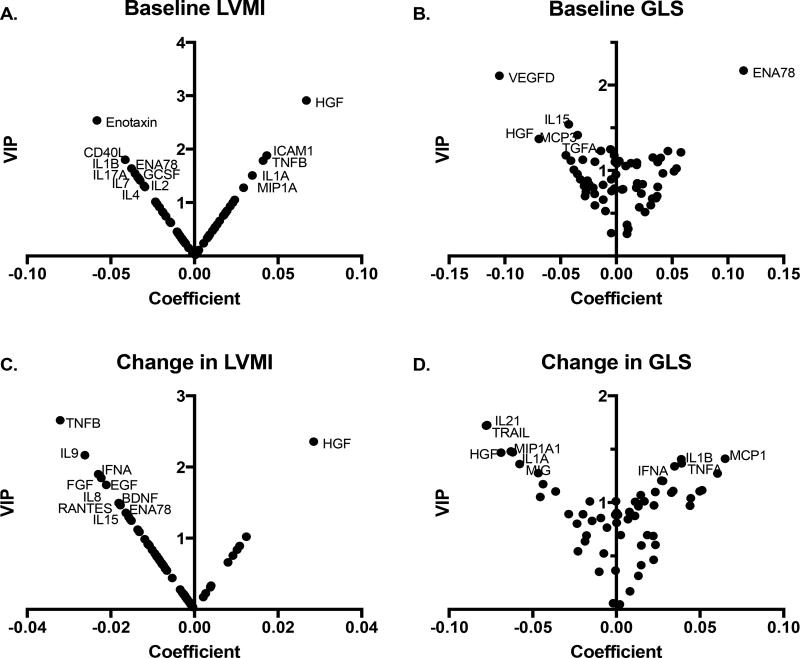
Cytokine and growth factor network explained part of the variance in LV mass index (12.2% of variance) and GLS (16.2% of variance) as summarized in Table 2. Higher hepatocyte growth factor (HGF) was related to higher LV mass index (variance importance in projection, VIP=2.91) and lower GLS (VIP=1.37) (Figure 1A–B). Higher intercellular adhesion molecule (ICAM) 1, and tumor necrosis factor (TNF) β were related to higher LV mass index, while lower interleukin (IL) 1β, Eotaxin, epithelial neutrophil (ENA) 78, and CD40 ligand (CD40L) were related to higher LV mass index. Higher IL-15, monocyte chemotactic protein (MCP)-3, vascular endothelial growth factor (VEGF)-D, and lower ENA78 levels were related to lower GLS.
Figure 1. V-plots for PLS-DA models generated with VIP and correlation coefficient values.
Variable’s importance in projection (VIP) scores and correlation coefficients are plotted as v-plots for PLS-regression analysis performed against (A) baseline LVMI, (B) baseline GLS, (C) absolute change in LVMI, and (D) absolute change in GLS at 1 year. The cytokines with VIP >1.3 are labeled, which represent the cytokines most responsible for construction of the latent factor in PLS analysis.
Dynamic change of cardiac function after TAVR
Echocardiographic parameters at 1-year after TAVR are shown in Table 1. After TAVR, mean and peak transaortic gradient decreased, and AVAI increased. Forty-three patients (52%) had no or trivial perivalvular aortic regurgitation, 31 (37%) had mild, and 9 (11%) had mild to moderate. 13 patients received a Corevalve, 5 patients received Portico valves, and the remaining patients received Sapien valves (XT and S3). LV function parameters such as LV mass index, GLS, and E/e’ ratio improved at 1-year, while LA volume index did not change significantly. Supplementary Figure 2-A and 2-B show the change at 1-month and 1-year in LV mass index and GLS, respectively, after TAVR in 83 patients with echocardiograms available at all 3 time points. Among patients who completed 1-year follow-up echocardiography after TAVR, LV mass index and GLS changed significantly (116±42 vs. 103±35 g/m2, p<0.001 for LV mass index and −12.9±3.3 vs. −14.9±2.7%, p<0.001 for GLS). As shown in Supplementary Figure 2-C, in 32% of patients LV mass index improved (relative change ≥ 20%) and in 66% of patients it remained stable (−20% < relative change < 20%) at 1-year, while in 47% GLS increased (relative change ≥ 15%) and in 52% of patients it remained stable (−15% < relative change < 15%). The cut off value; relative 15% change, was defined according to the intravariability in this study. An exploratory analysis of clinical outcomes among patients with more or less ventricular recovery at 1 month following TAVR showed that GLS improvement at 1 month correlates with improved mortality (median follow up 12.5 months, Cox regression p=0.008; Supplementary Table 2).
Association between baseline cytokine and structural and functional recovery post TAVR
Table 2 summarizes the cytokines related to changes in LV mass index and GLS. The values were adjusted for age, sex, and baseline values of LV mass index or GLS respectively. Change in GLS was additionally adjusted for body mass index as it emerged as one of its correlates. Higher HGF was associated with less improvement in LV mass index (VIP=2.36) and less improvement in GLS (VIP=1.47) (Figure 1). Higher levels of growth factors such as fibroblast growth factor (FGF) and epidermal growth factor (EGF) were related to a greater reduction in LV mass index (VIP=1.85 and 1.75, respectively). Other factors included TNF-β, IL-9, and IFN-α for change in LV mass, and IL-21, IL-1A, TRAIL, and macrophage inflammatory protein-1A (MIP-1 α) for change in GLS.
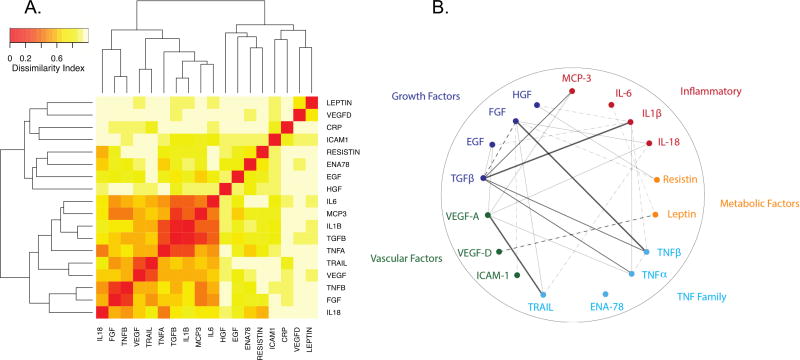
The identified cytokines showed a highly correlated pattern of expression, consistent with multiple cytokines in the assay belonging to common pathways (Figure 2A). A partial correlation diagram was created focused on the different pathways that emerged in the analysis including selected growth factors, inflammatory factors, factors associated with tumor necrosis factor pathway; we also included leptin and Resistin as BMI was associated with GLS changes (Figure 2B). Growth factors mainly had positive correlations with TNF family or inflammatory cytokines while they had negative correlations with vascular factors.
Figure 2. Correlation matrix plot and Partial correlation diagram between cytokines.
Selected cytokines based on the associations emerged from the PLS analyses and literature were used to create a (A) correlation matrix plot and a (B) partial correlation diagram. (A) The matrix plot was created using a dissimilarity matrix of 1-abs(correlation) and heatmap.2 function in R with hierarchical clustering with r values for individual correlations plotted in color grade. Highly co-regulated pattern of expression is found among the cytokines. (B) Multiple pathways appear to be involved in ventricular remodeling, and these pathways correlated with each other. All lines represent statistically significant partial correlations (p<0.05). The full lines represent direct correlations and dashed lines show inverse correlations. Thicker lines show stronger relationship (r>0.18: very pale line, r>0.30: thin, r>0.50, normal caliber line, r > 0.70: very thick thin).
DISCUSSION
In this study, we measured in detail the ventricular structure and function before and after TAVR, and identified a group of circulating cytokines that correlate with reverse remodeling and functional recovery following TAVR in patients with severe AS. Several different classes of cytokines were involved, including growth factors, vascular factors and inflammatory cytokines.
As previous studies have shown, we found patients undergoing TAVR to have a variable degree of ventricular hypertrophy and dysfunction at baseline. It is unclear what factors lead to the variability in LV remodeling. Severity of the aortic stenosis is reported to have little effect on the variability.15 Consistent with other studies16, 17, we also found that sex accounts for part of the difference in ventricular adaptation following TAVR.
In order to assess for ventricular recovery, a sensitive method of ventricular function measurement is required. Global longitudinal strain is emerging as a useful metric of ventricular function in AS. As highlighted by our study, ventricular strain was more sensitive in identifying ventricular dysfunction than bi-plane measurement of LVEF. In fact, most patients who are undergoing TAVR due to symptomatic AS appear to have LV dysfunction based on GLS. Mechanistically, ventricular strain can be altered because of the increased ventricular afterload, the presence of myocardial ischemia or interstitial fibrosis.18 Fabiani et al. also demonstrated that myocardial fibrosis was associated with alteration of regional and global longitudinal strain.19
Although the process of ventricular recovery following TAVR is likely multifactorial and complex, it appears to be prognostic for improved outcome following TAVR.20 Weidemann et al. demonstrated that fibrosis was associated with adverse ventricular remodeling, incomplete LV functional recovery, and worse cardiovascular outcomes after AVR.21 The fibrotic process can be dependent on multiple signaling processes, however, inflammation is known to be a critical driver of the process.22, 23 Despite the importance of inflammation in progression of cardiomyopathy, there are limited data on the role of cytokines in the progression of subclinical LV remodeling in AS. Our study is the first to explore the cytokine and growth factor networks associated with ventricular adaptation in patients with AS and its recovery following TAVR.
Among cytokines that emerged from this study, HGF was most significantly associated with both baseline LV mass index and GLS as well as their dynamic changes (Table 2). HGF is a growth factor excreted by tissue of mesenchymal origin, and is specifically expressed in smooth muscle cells and cardiac fibroblasts in the cardiovascular system. Previous studies found HGF levels to correlate with adverse outcomes in patients with heart failure.7, 24, 25 Experimental studies have shown that HGF and its receptor c-Met exert beneficial effects in the setting of cardiovascular injuries by counteracting apoptosis, excessive autophagy and oxidative stress through pro-survival and antioxidant activities, and forming new vessels from the pre-existing vascular bed and increasing blood flow.26, 27 Furthermore, HGF have been shown to reduce cardiac fibrosis in mice by inhibiting endothelial-mesenchymal transition and conversion of fibroblasts to myofibroblasts.28 Given the likely beneficial effects of HGF in response to cardiac injury, the correlation found in this study is likely a reflection of the degree of cardiac injury and fibrosis in AS, and the subsequent restorative response.
An association between HGF and ventricular remodeling has also been reported in patients with other disease processes. Kuznetsova et al. recently investigated ventricular adaptation among patients with systemic hypertension and demonstrated that the level of HGF was increased in patients with hypertension who had evidence of adverse LV remodeling or diastolic dysfunction.29 Lamblin et al. also showed that elevated level of HGF was associated with LV remodeling after myocardial infarction.25 The identification of HGF across these different disease states suggest that aortic stenosis, hypertension and infarction-induced LV remodeling may in part occur through a common pathway. Identification of such pathways may advance our understanding of LV remodeling and dysfunction and lead to novel therapeutics. In fact, treatment with HGF proteins and overexpression plasmids in animal models have shown improvement in LV function exposed to pressure overloaded states.28
Our exploratory analysis identified several vascular growth factors, inflammatory mediators and tumor necrosis factor pathways that could contribute to a ventricular recovery network and will require further validation. These identified cytokines form a principle component, based on our method of PLS which cluster cytokines depending on the measurement patterns to identify a latent factor, and hence are likely functionally connected. Based on search of publicly available data, we found evidence that HGF containing module was distinct to the inflammasome-related cytokines and was connected to the other modules of inflammatory cytokines identified from our analysis at the gene expression and protein levels (Supplementary Figures 5 and 6). In contrast, C-reactive protein was the least associated with other identified cytokines consistent with its lack of association with baseline ventricular remodeling or functional recovery in our study. Further study is needed to test if these factors associated with improved LV function following TAVR can meaningfully improve prediction of patients who will benefit from TAVR. HGF and other cytokines have been shown to improve reclassification of risk for adverse outcomes in heart failure,30 but our selected panel of cytokines may be able to improve the risk classification further specific to the TAVR candidates.
Circulating levels of ICAM1 has also been shown to correlate with cardiac dysfunction and HF.31, 32 Experimental evidence suggests that ICAM1 becomes up-regulated, mediating T-cell infiltration in the LV in response to pressure overloaded states to regulated cardiac remodeling. Further, ICAM1-deficient mice models were protected from adverse cardiac remodeling following transverse aortic constriction (TAC) through mechanism that include reduced fibrosis and monocyte and T-cell mediated inflammation.33 VEGF-D is a member of the vascular endothelial growth factor family, that is known to promote lymphangiogenesis and angiogenesis, and was also found to be significantly up-regulated in mouse models of pressure overload HF and ischemic cardiomyopathy in response to injury.34–36
Several limitations in our study should be taken into account. First, although supported by previous studies and mechanistic plausibility, this study is underpowered to analyze the association between cytokine network and overall mortality and thus is intended to be exploratory and warrants validation in large independent cohorts. The study is also underpowered for any subgroup analyses due to the small cohort. Further studies will be important to identify whether these circulating biomarker profiles will be able to improve risk stratification and selection of patients who will benefit most from TAVR. Second, only the baseline cytokines profile was included in this study, not allowing for serial assessment. Finally, we only analyzed resting ventricular recovery parameters, which fail to capture the extent of functional recovery that not only depends on ventricular response to exercise but also peripheral muscle physiology.
In conclusion, we found that sex and baseline AVAI only explain a small part of the variability in LV function in patients with AS. Among circulating cytokine and growth factors, HGF emerges prominently as a factor associated with both baseline ventricular remodeling and function as well as ventricular structural and functional recovery following TAVR. Future studies are needed to validate these findings and to identify the mechanism of ventricular adaptation associated with TAVR.
Supplementary Material
Highlights.
Ventricular recovery following TAVR can be assessed by echocardiogram.
Ventricular recovery following TAVR correlates with improved survival.
Cytokines and growth factors correlate with ventricular recovery following TAVR.
Acknowledgments
Thu Vu, RN for help with coordinating sample collections and processing.
Funding
We thank funding support from the Stanford Cardiovascular Institute, Stanford Department of Medicine, NIH T32 EB009035 (JCW), NIH R01 HL132875 (JCW), Translational Research and Applied Medicine (JBK, FH, WFF), Women’s Sex-Difference in Medicine Grant (JBK, YK, ROM, FH, WFF), and Pai Chan Lee Research Fund (FH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- 1.Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: Preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290. [DOI] [PubMed] [Google Scholar]
- 2.Gavina C, Falcao-Pires I, Rocha-Goncalves F, Leite-Moreira A. Left ventricular hypertrophy in isolated aortic stenosis: Primetime for the ventricle. Curr Pharm Biotechnol. 2012;13:2503–2514. [PubMed] [Google Scholar]
- 3.Woldbaek PR, Sande JB, Stromme TA, et al. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol. 2005;289:H708–714. doi: 10.1152/ajpheart.01179.2004. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide- dependent kinase-1-akt-gata4 signaling pathway in cardiomyocytes. J Biol Chem. 2005;280:4553–4567. doi: 10.1074/jbc.M411787200. [DOI] [PubMed] [Google Scholar]
- 5.Platis A, Yu Q, Moore D, Khojeini E, Tsau P, Larson D. The effect of daily administration of il-18 on cardiac structure and function. Perfusion. 2008;23:237–242. doi: 10.1177/0267659108101511. [DOI] [PubMed] [Google Scholar]
- 6.Shimokawahara H, Jougasaki M, Setoguchi M, et al. Relationship between vascular endothelial growth factor and left ventricular dimension in patients with acute myocardial infarction. J Cardiol. 2014;64:360–365. doi: 10.1016/j.jjcc.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Lamblin N, Susen S, Dagorn J, et al. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. Am Heart J. 2005;150:137–143. doi: 10.1016/j.ahj.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Butts B, Gary RA, Dunbar SB, Butler J. The importance of nlrp3 inflammasome in heart failure. J Card Fail. 2015;21:586–593. doi: 10.1016/j.cardfail.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: Eae/ase recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 11.American College of Cardiology/American Heart Association Task Force on Practice G, Society of Cardiovascular A, Society for Cardiovascular A et al. Acc/aha 2006 guidelines for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): Developed in collaboration with the society of cardiovascular anesthesiologists: Endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Ariyama M, Kobayashi Y, et al. Comparison of left ventricular manual versus automated derived longitudinal strain: Implications for clinical practice and research. Int J Cardiovasc Imaging. 2016;32:429–437. doi: 10.1007/s10554-015-0804-x. [DOI] [PubMed] [Google Scholar]
- 13.Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024–1034. doi: 10.1161/01.cir.59.5.1024. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg-Hasson Y, Hansmann L, Liedtke M, Herschmann I, Maecker HT. Effects of serum and plasma matrices on multiplex immunoassays. Immunol Res. 2014;58:224–233. doi: 10.1007/s12026-014-8491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dweck MR, Joshi S, Murigu T, et al. Left ventricular remodeling and hypertrophy in patients with aortic stenosis: Insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:50. doi: 10.1186/1532-429X-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll JD, Carroll EP, Feldman T, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 17.Williams M, Kodali SK, Hahn RT, et al. Sex-related differences in outcomes after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis: Insights from the partner trial (placement of aortic transcatheter valve) J Am Coll Cardiol. 2014;63:1522–1528. doi: 10.1016/j.jacc.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Heymans S, Schroen B, Vermeersch P, et al. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 19.Fabiani I, Scatena C, Mazzanti CM, et al. Micro-rna-21 (biomarker) and global longitudinal strain (functional marker) in detection of myocardial fibrotic burden in severe aortic valve stenosis: A pilot study. J Transl Med. 2016;14:248. doi: 10.1186/s12967-016-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauerman HL, Reardon MJ, Popma JJ, et al. Early recovery of left ventricular systolic function after corevalve transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003425. [DOI] [PubMed] [Google Scholar]
- 21.Weidemann F, Herrmann S, Stork S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 22.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: Interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41:532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 23.Hsue PY, Tawakol A. Inflammation and fibrosis in hiv: Getting to the heart of the matter. Circ Cardiovasc Imaging. 2016;9:e004427. doi: 10.1161/CIRCIMAGING.116.004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rychli K, Richter B, Hohensinner PJ, et al. Hepatocyte growth factor is a strong predictor of mortality in patients with advanced heart failure. Heart. 2011;97:1158–1163. doi: 10.1136/hrt.2010.220228. [DOI] [PubMed] [Google Scholar]
- 25.Lamblin N, Bauters A, Fertin M, de Groote P, Pinet F, Bauters C. Circulating levels of hepatocyte growth factor and left ventricular remodelling after acute myocardial infarction (from the reve-2 study) Eur J Heart Fail. 2011;13:1314–1322. doi: 10.1093/eurjhf/hfr137. [DOI] [PubMed] [Google Scholar]
- 26.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant DS, Kleinman HK, Goldberg ID, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okayama K, Azuma J, Dosaka N, et al. Hepatocyte growth factor reduces cardiac fibrosis by inhibiting endothelial-mesenchymal transition. Hypertension. 2012;59:958–965. doi: 10.1161/HYPERTENSIONAHA.111.183905. [DOI] [PubMed] [Google Scholar]
- 29.Kuznetsova T, Haddad F, Knez J, et al. Cytokines profile in hypertensive patients with left ventricular remodeling and dysfunction. J Am Soc Hypertens. 2015;9:975–984. e973. doi: 10.1016/j.jash.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Richter B, Koller L, Hohensinner PJ, et al. A multi-biomarker risk score improves prediction of long-term mortality in patients with advanced heart failure. Int J Cardiol. 2013;168:1251–1257. doi: 10.1016/j.ijcard.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 31.Yin WH, Chen JW, Jen HL, et al. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail. 2003;5:507–516. doi: 10.1016/s1388-9842(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 32.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: The health abc (health, aging, and body composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvador AM, Nevers T, Velazquez F, et al. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J Am Heart Assoc. 2016;5:e003126. doi: 10.1161/JAHA.115.003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rissanen TT, Markkanen JE, Gruchala M, et al. Vegf-d is the strongest angiogenic and lymphangiogenic effector among vegfs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 35.Huusko J, Lottonen L, Merentie M, et al. Aav9-mediated vegf-b gene transfer improves systolic function in progressive left ventricular hypertrophy. Mol Ther. 2012;20:2212–2221. doi: 10.1038/mt.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JH, Yoon JY, Ko SM, et al. Endothelial progenitor cell transplantation decreases lymphangiogenesis and adverse myocardial remodeling in a mouse model of acute myocardial infarction. Exp Mol Med. 2011;43:479–485. doi: 10.3858/emm.2011.43.8.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.