Abstract
Purpose
The incidence of colorectal cancer (CRC) in Korea has increased remarkably during the past few decades. The present study investigated the characteristics and survival of patients with CRC in Korea as a function of time, tumor distribution, stage, sex, and age.
Methods
We retrieved clinical data on 326,712 CRC patients diagnosed between 1996 and 2015 from the Korea Central Cancer Registry. The incidence and the 5-year relative survival rates were compared across time period, tumor distribution, stage, sex, and age group.
Results
The percentage of patients with colon cancer increased from 49.5% in 1996–2000 to 66.4% in 2011–2015 while the percentage of patients with rectal cancer decreased from 50.5% to 33.6%. The 5-year relative survival rates for all CRCs improved from 58.7% in 1996–2000 to 75.0% in 2011–2015. For 1996–2000, survival rates were highest for patients with left-sided colon cancers, followed by those with right-sided, transverse, rectal, rectosigmoid cancers. For 2011–2015, the survival rates for patients with left-sided cancers were highest, followed by those with rectosigmoid, rectal, transverse, and right-sided colon cancers. Patients with local and regional, but not distant, SEER (Surveillance, Epidemiology, and End Results) stage tumors experienced significantly increased survival rates for 2006–2010 and 2011–2015. The proportion of CRC patients by age decreased in the order ≥70, 60–69, 50–59, 40–49, ≤39 years whereas survival rates decreased in the order 50–59, 60–69, 40–49, ≤39, ≥70 years.
Conclusion
Korean CRC has some distinct characteristics and survival patterns in terms of tumor distribution, stage, sex, and age. With time, survival outcomes have improved for both local and regional, but not distant, stage tumors.
Keywords: Colorectal neoplasms, Korea, Character, Survival rate
INTRODUCTION
According to 2015 Korean cancer statistics [1], colorectal cancer (CRC) is the 2nd most common type of cancer, followed by stomach cancer, with 26,790 cases nationwide in 2015. The annual standardized incidence rate of CRC increased by 6.0% annually between 1999 and 2010 and then decreased from 2010 to 2015 by 6.1%. The age-standardized incidence rate in 2015 was 30.4/
100,000, making this the third most common cancer during this period. The age-standardized mortality rate for CRCs increased until 2004 by 5.5% annually and then began to decrease between 2004 and 2015 by 1.3% annually. The age-standardized rate of death from CRC per 100,000 in 2016 was 16.5, with CRC serving as the 3rd leading cause of death across all cancers in that year. Not only have the incidence and the mortality rates associated with these cancers changed over time, but so have the diagnosis and the treatment strategies for patients with CRCs, leading to significant improvements in the oncologic outcomes in Korea [2-4].
While others (e.g., Park et al. [5]) have previously reported the characteristics and the survival rates for patients with CRCs from 1993 to 2010, current rates should also be assessed as they have changed [1]. In addition, previous work did not investigate changes in frequency or survival rates per SEER (Surveillance, Epidemiology, and End Results) summary stage as the Korea Central Cancer Registry (KCCR) only began to report SEER summary stage data in 2006.
Since 1999, nationwide cancer incidence data have been recorded in the KCCR, which serves to collect information on cancer patients from registered hospitals. Additionally, the KCCR and regional cancer registries have directly surveyed information on previously unreported cancer patients by using a national insurance database. The KCCR is thought to include data from all cancer patients in Korea, making it an ideal tool for the analyses conducted here [1]. The purpose of this study was to investigate the characteristics of Korean patients with CRC, focusing on survival outcomes across tumor distribution, stage, and patient sex and age by using data from a nationwide cancer registry. Changes in survival rates were also assessed across discrete time periods or epochs.
METHODS
We retrieved clinical data on 326,712 patients with CRC diagnosed between 1996 and 2015. Pathologically-identified adenocarcinomas of the colon and rectum were included in the present study. Patient age, sex, and clinical data (such as tumor distribution and stage) were retrieved from the Korea National Cancer Incidence Database and used for analyses. This study was exempted from approval and informed consent by the Institutional Review Board of Severance Hospital Yonsei University Health System.
Relative survival rates (RSRs) were calculated across predefined 5-year time periods: 1996–2000, 2001–2005, 2006–2010, 2006–2010, and 2011–2015. Trends in relative survival from CRC by diagnostic year were illustrated using the Kaplan-Meier method. Tumor location was classified according to the following categories: the right side of the colon (from the cecum, including the appendix, to the colon’s hepatic flexure), transverse colon, left side of the colon (from the splenic flexure to the sigmoid colon), rectosigmoid, and the rectum. SEER stages (local, regional, and distant) were available for patients identified from 2006 to 2015. In brief, SEER local stage data included tumors confined to their origin organ (i.e., colon or rectum) without lymph-node metastasis. Regional disease included tumors with adjacent organ invasion or regional lymph-node metastasis. Distant disease included those with distant site metastasis. A comparison of SEER stages across TNM stages is shown in supplementary Table 1. The 5-year RSR was adjusted for the mortality expected among others of the same age and sex. The RSRs were calculated using the Ederer II method [6]. Log-rank tests were used to test for differences in survival rates.
Table 1.
Trends in 5-year relative survival by tumor distribution according to time period
| Sex and tumor sites | 1996-2000 | 2001-2005 | 2006-2010 | 2011-2015 | Absolute change (%)a |
|---|---|---|---|---|---|
| Both sexes | |||||
| Colon & rectum (C18-C20) | 38,739 (58.7) | 66,935 (66.2) | 101,579 (72.6) | 119,459 (75.0) | 16.3 |
| Right side colon (C18.0-18.3) | 5,577 (63.1) | 10,519 (65.6) | 17,323 (71.0) | 22,664 (73.0) | 9.9 |
| Transverse colon (C18.4) | 1,430 (62.1) | 2,518 (65.4) | 4,261 (71.4) | 5,505 (74.6) | 12.5 |
| Left side colon (C18.5-18.7) | 8,013 (64.0) | 16,099 (70.0) | 29,487 (75.9) | 36,155 (78.3) | 14.3 |
| Rectosigmoid (C19.9) | 2,318 (56.9) | 4,067 (67.1) | 7,142 (74.4) | 10,906 (75.1) | 18.2 |
| Rectum (C20.9) | 17,653 (57.7) | 28,290 (66.7) | 36,748 (72.3) | 38,030 (74.6) | 16.9 |
| Men | |||||
| Colon & rectum (C18-C20) | 21,269 (59.7) | 38,266 (68.0) | 60,504 (74.5) | 71,522 (76.8) | 17.1 |
| Right side colon (C18.0-18.3) | 2,797 (66.0) | 5,229 (69.2) | 8,838 (74.9) | 11,079 (76.2) | 10.2 |
| Transverse colon (C18.4) | 758 (66.1) | 1,381 (68.4) | 2,444 (74.7) | 3,141 (78.8) | 12.7 |
| Left side colon (C18.5-18.7) | 4,566 (66.4) | 9,567 (72.1) | 18,151 (77.7) | 22,550 (79.4) | 13.0 |
| Rectosigmoid (C19.9) | 1,322 (57.2) | 2,416 (68.5) | 4,404 (75.3) | 6,825 (75.8) | 18.6 |
| Rectum (C20.9) | 9,878 (56.9) | 16,628 (66.7) | 22,649 (72.7) | 24,158 (75.4) | 18.5 |
| Women | |||||
| Colon & rectum (C18-C20) | 17,470 (57.6) | 28,669 (63.8) | 41,075 (69.8) | 47,937 (72.3) | 14.7 |
| Right side colon (C18.0-18.3) | 2,780 (60.1) | 5,290 (62.0) | 8,485 (67.0) | 11,585 (69.9) | 9.8 |
| Transverse colon (C18.4) | 672 (57.7) | 1,137 (61.8) | 1,817 (67.0) | 2,364 (68.9) | 11.2 |
| Left side colon (C18.5-18.7) | 3,447 (60.9) | 6,532 (66.9) | 11,336 (73.2) | 13,605 (76.5) | 15.6 |
| Rectosigmoid (C19.9) | 996 (56.4) | 1,651 (65.2) | 2,738 (73.0) | 4,081 (73.9) | 17.5 |
| Rectum (C20.9) | 7,775 (58.6) | 11,662 (66.7) | 14,099 (71.5) | 13,872 (73.1) | 14.5 |
Values are presented number (%).
Percentage change in 5-year relative survival from 1996 to 2000 and 2011 to 2015.
Estimated survival rates were compared across tumor distribution, stage, patient’s sex (male and female), and patient’s age at diagnosis (≤39, 40–49, 50–59, 60–69, and ≥70 years). All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA), and statistically significance was considered to be indicated by a P-value of less than 0.05.
RESULTS
Distribution of CRC according to tumor site, stage, and patient’s sex and age
Korean CRC tumor distribution statistics have changed annually. The rate of colon cancer increased from 49.5% in 1996–2000 to 66.4% in 2011–2015. Right-sided colon cancers increased from 15.9% to 20.0%, transverse colon cancers from 4.1% to 4.9%, left-sided colon cancers from 22.9% to 31.9%, and rectosigmoid junction cancers from 6.6% to 9.6%. However, the total rate of rectal cancer decreased from 50.5% to 33.6% during the same period. This change was observed in both males and females. The proportion of right-sided colon cancers in females increased steeply and was higher than that among males (25.4% vs. 16.4%) between 2011 and 2015. The proportion of left-sided colon cancers in females had a similar trend from 1996 to 2015. The proportion of left-sided colon and rectal cancers in males was higher than in females between 2001 and 2015 (Fig. 1).
Fig. 1.
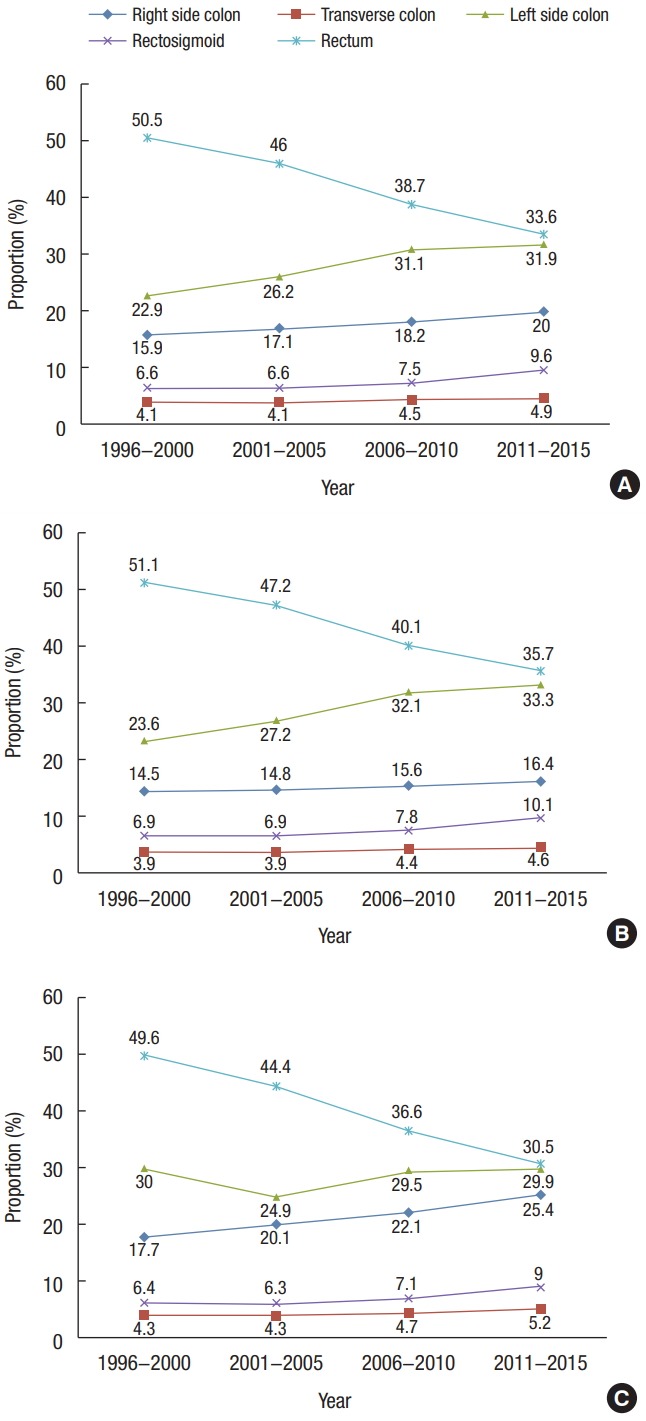
Trends in the tumor distribution according to time period from 1996 to 2015 in Korea. (A) Both sexes, (B) male, and (C) female.
In both time periods (2006–2010 and 2011–2015), a regional tumor stage designation was more common than a local stage designation. From 2011 to 2015, the proportion of all tumors with a regional stage designation across both sexes was 43.4%; 35.3% were local stage tumors, and 15.9% were distant stage tumors. This order was evident among both male and female patients when analyzed independently (Fig. 2).
Fig. 2.
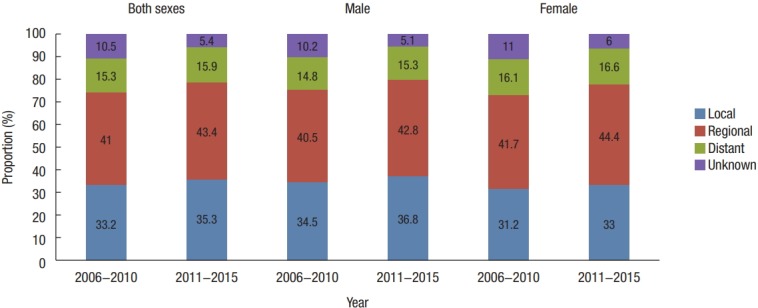
Trends in the proportion of SEER (Surveillance, Epidemiology, and End Results) stage according to time period from 2006 to 2015 in Korea.
The incidence of CRC by age in both time periods decreased in the following order: ≥70, 60–69, 50–59, 40–49, and ≤39 years (Fig. 3). In patients aged 50–59 and 60–69, the proportion of local stage tumors was higher than in the other age groups. Those aged 40–49 and ≥70 years had similar proportions of tumors distributed across SEER stages. Those aged less than 39 had the highest proportion of advanced stage tumors (regional, 47.1% and distant, 20.8%) (Fig. 4). Those over 70 years of age had the highest proportion of right-sided colon cancers of all age groups (24.2% vs. 18.9% in those 60–69, 16.1% in those 50–59, 15.5% in those 40–49, and 18.6% in those ≤39 years), though the proportion of rectal cancers was lower in these patients than in those of a younger age (31.6% vs. 32.6% in those 60–69, 36.2% in those 50–59, 38.4% in those 40–49, and 34.9% in those ≤39 years) (Fig. 5).
Fig. 3.
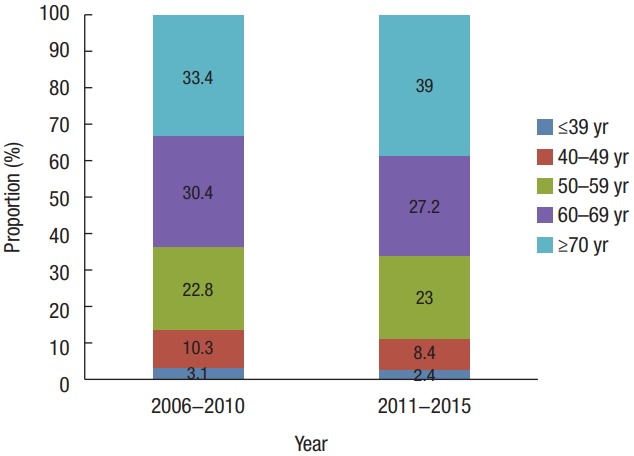
Trends in the proportion of age according to time period from 2006 to 2015 in Korea.
Fig. 4.
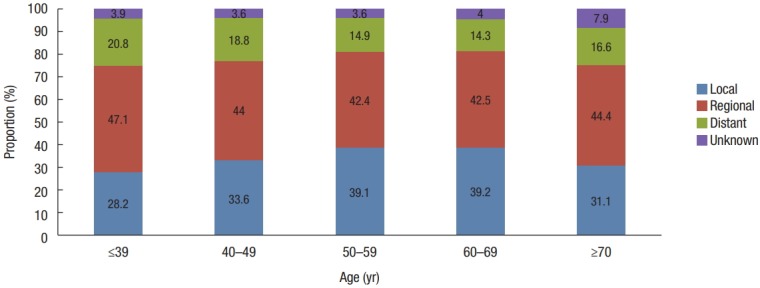
Trends in the proportion of SEER (Surveillance, Epidemiology, and End Results) stage according to age from 2011 to 2015 in Korea.
Fig. 5.
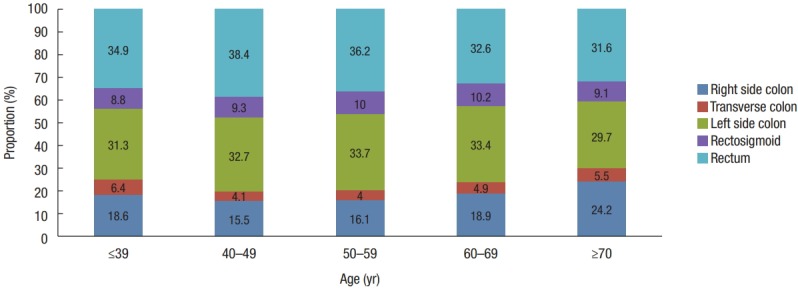
Trends in the tumor distribution according to age from 2011 to 2015 in Korea.
RSRs for CRCs by tumor site, stage, and patient’s sex and age
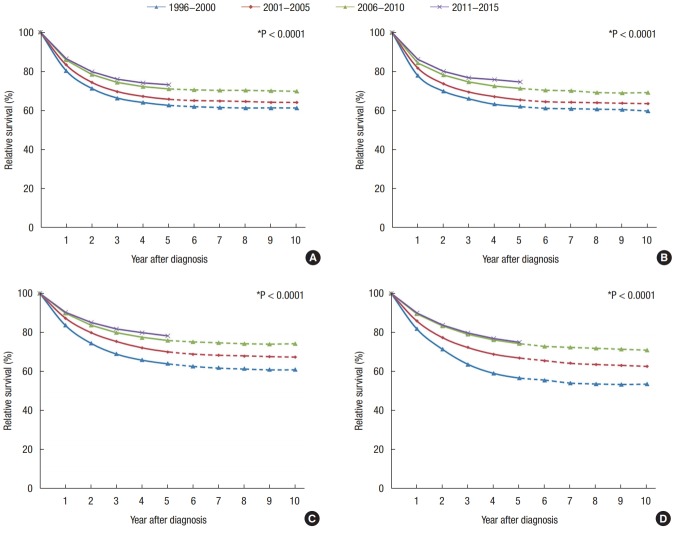
The 5-year RSRs for patients with all CRCs and for those with right-sided colon, transverse colon, left-sided colon, rectosigmoid, and rectum cancers by time period are summarized in Table 1. Survival improved significantly across all tumor sites, with left-sided colon cancers having the most favorable outcomes across all time periods (Fig. 6). In both the total cohort and among female patients, right-sided and transverse colon cancers had more favorable outcomes than did rectosigmoid and rectal cancers from 1996 to 2000. In contrast, from 2001 to 2015, rectosigmoid and rectal cancers had more favorable survival outcomes than did right-sided or transverse colon cancers. In male patients, survival rates for right-sided and transverse colon cancers were higher than they were for rectosigmoid or rectal cancers across all time periods. Females had worse survival rates across the entire assessed time period (Table 1).
Fig. 6.
Trends in relative survival of patients with colorectal cancer according to tumor distribution by year after diagnosis from 1996 to 2015 in Korea. (A) Right side colon (C18.0–18.3), (B) transverse colon (C18.4), (C) left side colon (C18.5–18.7), (D) rectosigmoid (C19.9), and (E) rectum (C20.9).
Survival rates according to SEER stage were compared between the 2006–2010 and the 2011–2015 time periods. Survival with local or regional SEER stage tumors significantly increased from 2006–2010 to 2011–2015 (92.8% vs. 94.7%, P < 0.0001 for local SEER stage; 78.8% vs. 81.6%, P < 0.0001 for regional SEER stage) and did not differ for distant stage tumors (19.7% vs. 19.6%, P = 0.9859) (Table 2). Survival rates for patients with local stage tumors increased between the 2006–2010 and the 2011–2015 time periods by more than 90% while survival rates for those with regional stage CRC tumors increased annually, with survival rates for patients with regional stage colon cancer tumors increasing by more than 80% from 2011 to 2015. However, survival rates for patients with rectal cancers were 76.1% for the 2006–2010 time period and 78.9% for the 2011–2015 period. Survival rates for patients with distant stage colon cancer tumors on the left side of the colon increased from 21.8% in 2006–2010 to 23.3% in 2011–2015. Right-sided colon, transverse colon, rectosigmoid, and rectal cancer tumors with a distant stage classification were associated with decreased survival rates from 2006–2010 to 2011–2015. This was especially true for right-sided and transverse colon cancers of a distant stage, which had lower survival than left-sided colon, rectosigmoid, and rectal cancers (Table 3).
Table 2.
Five-year relative survival by SEER stage between 2006–2010 and 2011–2015
| SEER stage | Both sexes |
Changea | Male |
Changea | Female |
Changea | |||
|---|---|---|---|---|---|---|---|---|---|
| 2006-2010 | 2011-2015 | 2006-2010 | 2011-2015 | 2006-2010 | 2011-2015 | ||||
| Overall | 101,579 (72.6) | 119,459 (75.0) | 2.4 | 60,504 (74.5) | 71,522 (76.8) | 2.3 | 41,075 (69.8) | 47,937 (72.3) | 2.5 |
| Local | 33,734 (92.8) | 42,142 (94.7) | 1.9 | 20,904 (94.1) | 26,319 (95.9) | 1.8 | 12,830 (90.6) | 15,823 (92.7) | 2.1 |
| Regional | 41,618 (78.8) | 51,901 (81.6) | 2.8 | 24,479 (80.0) | 30,602 (82.4) | 2.4 | 17,139 (77.2) | 21,299 (80.5) | 3.3 |
| Distant | 15,557 (19.7) | 18,943 (19.6) | -0.1 | 8,954 (20.0) | 10,976 (20.5) | 0.5 | 6,603 (19.3) | 7,967 (18.3) | -1 |
| Unknown | 10,670 (61.3) | 6,473 (54.0) | -7.3 | 6,167 (65.0) | 3,625 (58.8) | -6.2 | 4,503 (56.3) | 2,848 (47.9) | -8.4 |
Values are presented number (%).
SEER, Surveillance, Epidemiology, and End Results.
Percentage change in 5-year relative survival from 1996 to 2000 and 2011 to 2015.
Table 3.
Five-year relative survival of all patients by tumor distribution and SEER stage between 2006–2010 and 2011–2015
| SEER stage | Right side colon (C18.0–C18.3) |
Transverse colon (C18.4) |
Left side colon (C18.5–18.7) |
Rectosigmoid (C19.9) |
Rectum (C20.9) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2006-2010 | 2011-2015 | 2006-2010 | 2011-2015 | 2006-2010 | 2011-2015 | 2006-2010 | 2011-2015 | 2006-2010 | 2011-2015 | |
| Overall | 17,323 (71.0) | 22,664 (730) | 4,261 (71.4) | 5,505 (74.6) | 29,487 (75.9) | 36,155 (78.3) | 7,142 (74.4) | 10,906 (75.1) | 36,748 (72.3) | 38,030 (74.6) |
| Local | 4,975 (93.8) | 7,027 (95.0) | 1,308 (93.8) | 1,854 (95.4) | 10,025 (95.0) | 13,255 (96.5) | 2,074 (93.5) | 3,169 (96.0) | 12,882 (91.0) | 13,962 (93.4) |
| Regional | 8,036 (77.8) | 10,837 (80.5) | 1,914 (78.0) | 2,604 (80.8) | 12,141 (83.5) | 15,427 (85.7) | 3,323 (81.6) | 5,523 (83.5) | 14,963 (76.1) | 16,381 (78.9) |
| Distant | 2,924 (18.0) | 3,951 (17.8) | 675 (16.7) | 819 (15.1) | 4,831 (21.8) | 5,942 (23.3) | 1,073 (21.3) | 1,835 (19.5) | 4,907 (20.6) | 5,356 (19.6) |
| Unknown | 1,388 (62.0) | 849 (49.7) | 364 (57.2) | 228 (49.5) | 2,490 (67.5) | 1,531 (58.6) | 672 (64.2) | 379 (45.3) | 3,996 (60.4) | 2,331 (55.6) |
Values are presented number (%).
SEER, Surveillance, Epidemiology, and End Results.
More favorable overall survival rates were found in certain age groups, with the survival rate decreasing in the following order in both the 2006–2010 and the 2011–2015 time periods: 50–59, 60–69, 40–49, ≤39, and ≥70 years. This order was the same for those with local stage tumors. Those aged 50–59 and 60–69 had the most favorable overall survival outcomes, with rates over 80% in the 2011–2015 time period (82.1% and 81.2%, respectively). Those above 70 years of age had the worst overall survival rate at 64.8% in 2011–2015. For patients with regional stage tumors, those aged 40–49 had more favorable survival outcomes than those aged 60–69. For patients with distant stage tumors, the most favorable survival outcome was found among those aged 40–49 (Table 4).
Table 4.
Five-year relative survival of all patients by age group and SEER stage between 2006–2010 and 2011–2015
| SEER stage | Year | ≤39 yr |
40-49 yr |
50-59 yr |
60-69 yr |
≥70 yr |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | ||
| Overall | 2006-2010 | 3,161 | 73.6 (72.1-75.2) | 10,471 | 77.4 (76.6-78.2) | 23,113 | 79.9 (79.4-80.5) | 30,885 | 77.6 (77.0-78.1) | 33,949 | 60.8 (60.1-61.4) |
| 2011-2015 | 2,848 | 75.6 (73.5-77.5) | 10,037 | 79.4 (78.3-80.3) | 27,474 | 82.1 (81.5-82.7) | 32,533 | 81.2 (80.6-81.8) | 46,567 | 64.8 (64.1-65.5) | |
| Local | 2006-2010 | 877 | 95. 9(94.3-97.1) | 3,349 | 97.4 (96.7-98.0) | 8,334 | 97.4 (96.9-97.9) | 10,957 | 96.3 (95.6-96.9) | 10,217 | 82.7 (81.6-83.9) |
| 2011-2015 | 804 | 96.8 (94.6-98.2) | 3,370 | 97.1 (96.2-97.9) | 10,729 | 97.9 (97.4-98.4) | 12,767 | 97.6 (96.9-98.2) | 14,472 | 88.6 (87.4-89.8) | |
| Regional | 2006-2010 | 1,384 | 80.4 (78.2-82.4) | 4,446 | 84.2 (83.0-85.2) | 9,510 | 84.3 (83.5-85.1) | 12,730 | 81.7 (80.9-82.5) | 13,548 | 69.8 (68.7-70.8) |
| 2011-2015 | 1,341 | 85.6 (82.8-88.0) | 4,416 | 86.8 (85.3-88.1) | 11,639 | 87.2 (86.3-88.1) | 13,838 | 85.9 (84.9-86.7) | 20,667 | 73.8 (72.6-74.9) | |
| Distant | 2006-1010 | 626 | 24.2 (20.9-27.7) | 1,801 | 24.5 (22.5-26.6) | 3,356 | 24.7 (23.2-26.2) | 4,362 | 21.9 (20.6-23.2) | 5,412 | 12.5 (11.5-13.5) |
| 2011-2015 | 592 | 21.1 (16.7-25.9) | 1,884 | 28.0 (25.2-30.8) | 4,102 | 25.8 (23.9-27.7) | 4,633 | 23.0 (21.3-24.6) | 7,732 | 11.8 (10.7-12.9) | |
| Unknown | 2006-2010 | 274 | 81.1 (75.9-85.3) | 875 | 75.6 (72.5-78.4) | 1,913 | 78.9 (76.9-80.8) | 2,836 | 72.4 (70.5-74.2) | 4,772 | 42.4 (40.7-44.1) |
| 2011-2015 | 111 | 79.1 (68.3-86.6) | 367 | 82.1 (76.8-86.4) | 1,004 | 79.2 (75.9-82.1) | 1,295 | 75.8 (72.7-78.7) | 3,696 | 33.6 (31.3-35.9) | |
SEER, Surveillance, Epidemiology, and End Results; CI, confidence interval.
In patients with transverse colon, left-sided colon, rectosigmoid, and rectal cancers, those aged 50–59 had more favorable survival rates, with the order of survival by age group proceeding in the same order as it did for the overall cohort (listed above). Those younger than 39 had the best survival rates for right-sided colon cancers while those older than 40 had the highest survival rates for left-sided colon cancers (Table 5).
Table 5.
Five-year relative survival of all patients by age group and tumor distribution between 2006–2010 and 2011–2015
| Tumor distribution | Year | ≤39 yr |
40-49 yr |
50-59 yr |
60-69 yr |
≥70 yr |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | ||
| Right side colon (C18.0-18.3) | 2006-2010 | 626 | 78.5 (75.1-81.6) | 1,519 | 78.7 (76.5-80.7) | 3,363 | 76.9 (75.3-78.3) | 5,256 | 74.9 (73.6-76.2) | 6,559 | 62.0 (60.5-63.5) |
| 2011-2015 | 504 | 79.6 (74.8-83.5) | 1,493 | 78.8 (76.0-81.3) | 4,231 | 80.5 (79.0-82.0) | 5,864 | 79.3 (77.9-80.7) | 10,572 | 64.9 (63.4-66.4) | |
| Transverse colon (C18.4)) | 2006-2010 | 180 | 69.8 (62.5-76.0) | 436 | 76.3 (71.9-80.1) | 903 | 79.7 (76.8-82.4) | 1,297 | 78.2 (75.5-80.7) | 1,445 | 58.1 (54.8-61.3) |
| 2011-2015 | 174 | 78.5 (71.1-84.2) | 394 | 78.1 (72.7-82.7) | 1,038 | 83.3 (80.2-86.1) | 1,504 | 80.0 (77.1-82.6) | 2,395 | 66.1 (62.9-69.2) | |
| Left side colon (C18.5-18.7) | 2006-2010 | 940 | 70.1 (67.1-73.0) | 3,162 | 78.4 (76.9-79.9) | 7,066 | 81.8 (80.9-82.8) | 9,295 | 80.1 (79.2-81.1) | 9,024 | 66.3 (64.9-67.6) |
| 2011-2015 | 851 | 74.5 (70.6-77.9) | 3,137 | 81.0 (79.2-82.7) | 8,839 | 83.8 (82.8-84.8) | 10,345 | 83.9 (82.9-84.9) | 12,983 | 69.1 (67.7-70.5) | |
| Rectosigmoid (C19.9) | 2006-1010 | 175 | 72.4 (65.0-78.4) | 698 | 76.8 (73.4-79.9) | 1,683 | 80.1 (77.9-82.0) | 2,234 | 80.0 (78.0-81.9) | 2,352 | 63.9 (61.3-66.4) |
| 2011-2015 | 240 | 70.5 (62.0-77.4) | 895 | 77.7 (73.9-81.1) | 2,623 | 80.2 (78.0-82.2) | 3,155 | 79.0 (76.8-81.0) | 3,993 | 68.2 (65.7-70.7) | |
| Rectum (C20.9) | 2006-2010 | 1,068 | 74.2 (71.5-76.8) | 4,108 | 76.6 (75.2-77.9) | 8,796 | 79.7 (78.8-80.6) | 10,972 | 76.9 (76.0-77.8) | 11,804 | 59.8 (58.7-61.0) |
| 2011-2015 | 947 | 74.6 (70.7-78.1) | 3,684 | 78.4 (76.6-80.1) | 9,498 | 81.8 (80.7-82.8) | 10,092 | 80.6 (79.5-81.7) | 13,809 | 63.4 (62.0-64.7) | |
SEER, Surveillance, Epidemiology, and End Results; CI, confidence interval.
DISCUSSION
Due to advancements in the treatment of patients with CRC and the extraordinary efforts made by Korean clinicians, a steady improvement of CRC survival rates in Korea and globally, as evidenced in the present study and others, has occurred across the last several decades. A recent study of global cancer survival rates, including individual data from 25,676,887 patients across 279 population-based registries in 67 countries from 1995 to 2009, included data on colon cancer, specifically, for 3,613,067 patients [7]. For patients diagnosed with colon cancer between 2005 and 2009, the age-standardized 5-year net survival rates were 50%–59% in many countries. Specifically in Korea, the 5-year survival rate was 42.5% between 1995 and 1999, though this increased to 60.4% between 2000 and 2004 and further to 66.0% between 2005 and 2009. These survival outcomes were better than those in the other countries assessed, including a survival rate of 64.7% in the United States and 64.4% in Japan. Rectal cancer data from this large study are also available for 1,413,861 patients. For patients diagnosed with rectal cancer between 2005 and 2009, the age-standardized 5-year net survival rates were 50%–59% in many countries. Survival rates were especially high in Korea, at 51.6% between 1995 and 1999, 60.8% between 2000 and 2004, and 65.9% between 2005 and 2009. As with colon cancer, these rectal cancer survival rates are better than the other assessed rates, including the rate in the United States (64.0%) or Japan (60.3%).
An anatomical rightward-shift in CRC incidence rates has also been reported in recent studies [8-12]. For example, the American Cancer Society reported a higher proportion of right-sided colon cancers (41%) than left-sided ones (22%) in the United States from 2009 to 2013. However, a striking variation in this distribution is seen by patient’s sex and age at diagnosis. In the United States, the likelihood of a proximal tumor location is greater in women than in men and increases with age. For example, 57% of CRCs in women aged 80 years and older occur in the proximal colon versus just 26% in men younger than 50 years of age. Among both men and women younger than 50, of all the regions assessed, CRC tumors are most commonly diagnosed in the rectum (41% and 36%, respectively) [12]. In the present study, females and those older than 70 years of age had a higher proportion of right-sided colon cancers than did males and those in younger age groups. Otherwise, men and those aged below 60 had the highest proportion of rectal cancers.
Data revealed in the present study compare well to those reported previously. A steep increase in right-sided colon cancers in females was observed in this study. Similarly, in Europe, the proportion of CRCs presenting in the proximal colon has increased significantly from 27% to 32% in men and from 35% to 41% in women between the period from 1994 to 1997 and that from 2010 to 2012 [13]. The increase in distal colon cancers, followed by proximal colon cancers, reported by previous studies [9-11] was also observed in the present study. Additionally, a steady decrease in the incidence of rectal cancers reported in the present study has also been reported by others. Overall, the Japanese and the Israeli populations have been found to have the highest incidence rates for cancers in the proximal and distal colon while those in Israel and China have the lowest incidence rates of rectal cancers [3, 14].
An increase in the survival rates across time in all tumor categories studied here was observed in the present study. Typically, left-sided colon cancers had more favorable associated outcomes than did right-sided colon cancers in all time periods assessed here. Notably, recent studies have also reported a worse prognosis associated with right-side colon cancers [8-11], though no clear explanation has been given for this trend. Other tumor differences may be less variable in terms of associated mortality. For instance, Weiss et al. [15] reported no difference between the 5-year mortality rates associated with proximal and distal colon cancer tumors. They found that while proximal colon tumors had a better prognosis in stage II, they had a worse prognosis in stage III. They suggested that this finding might be due to differences in tumor biology, including microsatellite instability (MSI). MSI-high cancers are more frequently found in stage II proximal colon cancers and are associated with a more favorable prognosis.
Primary tumors arising from the left and the right sides of the colon also differ in terms of their distinct chromosomal and molecular characteristics. High MSI, CIMP, and MLH1 methylation levels are more likely to occur in right-sided than left-sided colon cancers [16]. In contrast, higher chromosomal instability is observed in left-sided colon cancers [17]. Frequently, genetic mutations and distributions of CM5 subtypes differ between right- and left-sided CRCs (e.g., KRAS, BRAF, TGFbR2, and PI3KCA mutations are more common in right-sided CRCs while TP53, APC, and KRAS mutations are more common in left-sided CRCs) [18]. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor 1 expressions have also been reported to be significantly higher in left-sided than in right-sided CRCs [19].
A difference in molecular pathways may underlie differences in the presentation and the clinical outcomes associated with CRCs of differing locations, though the reasons for these differences remain mostly unknown. Differential distributions of genomic CRC subtypes and other biologic features between left- and right-sided CRCs may contribute to an inferior prognosis with advanced stage right-sided CRC and to an inferior prognosis with anti-EGFR therapy in right-sided CRC. In the present study, right-sided and transverse colon cancers were also associated with a poorer prognosis than were left-sided, rectosigmoid, and rectal cancers between 2001 and 2015. Tumors in the local and the regional stages were further associated with lower survival rates when they were right-sided in transverse and rectosigmoid colon cancers, but not in rectal cancer, than when they were left-sided. Right-sided and transverse colon cancers were associated with poorer survival rates than were left-sided, rectosigmoid, and rectal cancers of a distant stage.
Above all, the most promising outcome from the present study is the improvement of survival rates for patients with rectal cancers over time. From 1996–2000, rectal cancers were associated with worse survival rates than were right-sided colon cancers (56.9% vs. 63.1%, respectively). After 2001, rectal cancer was associated with better survival rates than were right-sided colon cancers (74.6% vs. 73.0%, respectively, from 2011–2015). This increased survival may be due to improvements in and standardizations of surgical techniques, including the total mesorectal excision and the combined radical resection, for the treatment of local, advanced rectal cancers [20, 21]. Furthermore, advances in pre- and postoperative adjuvant treatments, including chemotherapy and radiotherapy, have increased radical resection rates and decreased local recurrence rates in patients with CRC [22].
The SEER staging system used here differs slightly from the TNM staging system. Localized colon cancer corresponding to stage I or IIA requires no adjuvant chemotherapy. Regional CRCs corresponding to stages IIB or IIC and stage III require adjuvant chemotherapy for colon cancer and chemoradiotherapy for rectal cancer. Local stage cancers have a 5-year survival rate that is extremely high (94.7% between 2011 and 2015, increased from 92.8% between 2006 and 2010). Regional stage cancers have also experienced increased survival rates recently (from 78.8% between 2006 and 2010 to 81.6% between 2011 and 2015). Despite advances in chemotherapeutics over the past few decades, survival outcomes for distant stage CRCs have not improved between the 2006–2010 and the 2011–2015 epochs (2-year survival rate: 40.2% vs. 40.7%, 5-year survival rate: 19.7% vs. 19.6%, respectively). Improved surgical techniques and advanced instruments and systems may, however, further increase these survival rates and the qualities of life for patients with local and regional stage CRCs.
Chemotherapy plays an important role as an adjuvant treatment for patients with regional stage CRCs and is a first-line treatment for patients with distant stage CRCs. However, target agents, such as bevacizumab and cetuximab, have demonstrated no significant improvements in survival outcomes for those with distant stage CRCs. Greater efforts must be made and multidisciplinary approaches that combine surgery, chemotherapy, and radiotherapy must be taken if the survival outcomes for patients with distant stage CRCs are to be improved. Furthermore, early CRC screening and increased rates of examination should be encouraged in public health domains to improve patient prognoses.
Public health issues surrounding cancer screening and diagnoses often interact with the patient’s sex and other characteristics. For example, males may be exposed to more colon cancer risk factors, including cigarette smoke and alcohol [23, 24]. Meanwhile, women are more likely to develop MSI-high proximal colon cancers [25]. This may have led to improved prognoses in females, as has been reported previously [26]. However, the present study reports a contradictory result—worse survival rates in females. This difference was most prominent among those over 70, a finding which may be further attributed to poorer socioeconomic associations among elderly women [27]. For example, elderly women tend to pursue and receive less aggressive therapy for advanced diseases. Additionally, these patients may be presented with fewer opportunities for regular health checks and disease screening.
CRC prognoses among younger patients remain a controversial issue. While some studies have reported that younger patients, including those with later stage cancers and exhibiting more proximal, aggressive tumor histology [28], face a worse prognosis, others have reported no worse prognosis among the young [29]. In the present study, middle aged (40–69 years) patients had the most favorable survival outcomes, followed by the youngest (≤39 years) and the oldest (≥70 years) patients across all tumor stage categories. Those younger than 39 typically had the highest portion of advanced stage cancers among all age groups, an association that may have contributed to poorer survival outcomes in this group. The youngest patients had survival rates for right-sided and transverse colon cancers comparable to those for middle-aged patients (40–69 years), though these rates differed for left-sided, rectosigmoid, and rectal cancers, for which younger patients had poorer survival rates. Patients over 70 years of age had poorer survival rates than younger patients, regardless of SEER stage and tumor location.
The age-related associations revealed here may be attributed to several factors. In the present study, those older than 70 had the highest proportion of right-sided colon cancers and the lowest proportion of local stage tumors. Furthermore, elderly patients may receive less aggressive therapy, have a greater comorbidity burden, and have poorer performance scores. Treatment parity may underlie these differences. For instance, studies have demonstrated that, given the same level of treatment, elderly patients have survival rates comparable to those of the younger comparators [30]. Considering an ever-expanding elderly population, future studies should examine this phenomenon in more detail to identify its causes and, thus, improve CRC treatment outcomes.
In summary, the findings presented here reveal that survival rates among patients with CRCs, and especially those with rectal cancer, have improved dramatically in recent years. Survival rates among patients with a local or regional SEER CRC tumor stage have also improved. Our study findings may lead to further macroscopic insights into the nature of CRC and improved prognoses for patients with these cancers in South Korea.
Acknowledgments
The authors thank the members of the Korean Colorectal Cancer Study Group for conception and design, data analysis and interpretation, and final approval of the manuscript. This study was supported by a grant from the National Cancer Center (NCC-1610200).
Footnotes
The authors report no conflicts of interest related to this research.
REFERENCES
- 1.Jung KW, Won YJ, Kong HJ, Lee ES, Community of Population-Based Regional Cancer Registries Cancer Statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–16. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin A, Joo J, Bak J, Yang HR, Kim J, Park S, et al. Site-specific risk factors for colorectal cancer in a Korean population. PLoS One. 2011;6:e23196. doi: 10.1371/journal.pone.0023196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012;44:219–26. doi: 10.4143/crt.2012.44.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HC, Shin A, Kim BW, Jung KW, Won YJ, Oh JH, et al. Data on the characteristics and the survival of korean patients with colorectal cancer from the Korea central cancer registry. Ann Coloproctol. 2013;29:144–9. doi: 10.3393/ac.2013.29.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note No. 10. End results evaluation section. Bethesda (MD): National Cancer Institute; 1959. [Google Scholar]
- 7.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–40. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 9.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41:173–7. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 10.Singh H, Demers AA, Xue L, Turner D, Bernstein CN. Time trends in colon cancer incidence and distribution and lower gastrointestinal endoscopy utilization in Manitoba. Am J Gastroenterol. 2008;103:1249–56. doi: 10.1111/j.1572-0241.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen IK, Bray F. Trends in colorectal cancer incidence in Norway 1962-2006: an interpretation of the temporal patterns by anatomic subsite. Int J Cancer. 2010;126:721–32. doi: 10.1002/ijc.24839. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 13.McDevitt J, Comber H, Walsh PM. Colorectal cancer incidence and survival by sub-site and stage of diagnosis: a population-based study at the advent of national screening. Ir J Med Sci. 2017;186:113–21. doi: 10.1007/s11845-016-1513-8. [DOI] [PubMed] [Google Scholar]
- 14.Park HM, Woo H, Jung SJ, Jung KW, Shin HR, Shin A. Colorectal cancer incidence in 5 Asian countries by subsite: An analysis of Cancer Incidence in Five Continents (1998-2007) Cancer Epidemiol. 2016;45:65–70. doi: 10.1016/j.canep.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401–9. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MS, McGuffey EJ, Morris JS, Manyam G, Baladandayuthapani V, Wei W, et al. Association of CpG island methylator phenotype and EREG/AREG methylation and expression in colorectal cancer. Br J Cancer. 2016;114:1352–61. doi: 10.1038/bjc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen H, Yang J, Huang Q, Jiang MJ, Tan YN, Fu JF, et al. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21:6470–8. doi: 10.3748/wjg.v21.i21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan YT, Jen-Kou L, Lin CH, Yang SH, Lin CC, Wang HS, et al. Mutations in the RAS and PI3K pathways are associated with metastatic location in colorectal cancers. J Surg Oncol. 2015;111:905–10. doi: 10.1002/jso.23895. [DOI] [PubMed] [Google Scholar]
- 19.Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 20.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg. 1982;69:613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 21.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–32. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 22.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 23.Chung SJ, Kim YS, Yang SY, Song JH, Park MJ, Kim JS, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010;25:519–25. doi: 10.1111/j.1440-1746.2009.06147.x. [DOI] [PubMed] [Google Scholar]
- 24.Kontou N, Psaltopoulou T, Soupos N, Polychronopoulos E, Xinopoulos D, Linos A, et al. Alcohol consumption and colorectal cancer in a Mediterranean population: a case-control study. Dis Colon Rectum. 2012;55:703–10. doi: 10.1097/DCR.0b013e31824e612a. [DOI] [PubMed] [Google Scholar]
- 25.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–50. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 26.Paulson EC, Wirtalla C, Armstrong K, Mahmoud NN. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. 2009;52:1982–91. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 27.Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–7. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leff DR, Chen A, Roberts D, Grant K, Western C, Windsor AC, et al. Colorectal cancer in the young patient. Am Surg. 2007;73:42–7. [PubMed] [Google Scholar]
- 29.Schellerer VS, Merkel S, Schumann SC, Schlabrakowski A, Förtsch T, Schildberg C, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer : CRC in patients under 50 years of age. Int J Colorectal Dis. 2012;27:71–9. doi: 10.1007/s00384-011-1291-8. [DOI] [PubMed] [Google Scholar]
- 30.Khattak MA, Townsend AR, Beeke C, Karapetis CS, Luke C, Padbury R, et al. Impact of age on choice of chemotherapy and outcome in advanced colorectal cancer. Eur J Cancer. 2012;48:1293–8. doi: 10.1016/j.ejca.2011.09.029. [DOI] [PubMed] [Google Scholar]