Abstract
Objective
To evaluate the use of interictal high-frequency oscillations (HFOs) in epilepsy surgery for prediction of postsurgical seizure outcome in a prospective multicenter trial.
Methods
We hypothesized that a seizure-free outcome could be expected in patients in whom the surgical planning included the majority of HFO-generating brain tissue while a poor seizure outcome could be expected in patients in whom only a few such areas were planned to be resected. Fifty-two patients were included from 3 tertiary epilepsy centers during a 1-year period. Ripples (80–250 Hz) and fast ripples (250–500 Hz) were automatically detected during slow-wave sleep with chronic intracranial EEG in 2 centers and acute intraoperative electrocorticography in 1 patient.
Results
There was a correlation between the removal of HFO-generating regions and seizure-free outcome at the group level for all patients. No correlation was found, however, for the center-specific analysis, and an individual prognostication of seizure outcome was true in only 36 patients (67%). Moreover, some patients became seizure-free without removal of the majority of HFO-generating tissue. The investigation of influencing factors, including comparisons of visual and automatic analysis, using a threshold analysis for areas with high HFO activity, and excluding contacts bordering the resection, did not result in improved prognostication.
Conclusions
On an individual patient level, a prediction of outcome was not possible in all patients. This may be due to the analysis techniques used. Alternatively, HFOs may be less specific for epileptic tissue than earlier studies have indicated.
For patients with drug-resistant focal epilepsy, epilepsy surgery is the most efficient treatment to attain seizure freedom.1 Noninvasive diagnostics such as MRI, neuropsychology, and EEG do not always lead to a definite localization of the seizure focus.2 In a subset of these cases, intracranial video-EEG monitoring is performed for several days with EEG electrodes placed subdurally as a grid or stereotactically with intracerebral probes.3 Alternatively, acute intraoperative subdural EEG recordings (electrocorticography [ECoG]) are obtained during the surgery.
High-frequency oscillations (HFOs) can be recorded with these different intracranial EEG (iEEG) methods4–7 and were proposed as new biological markers of epileptic tissue.7 HFOs commonly occur in brain areas showing the first ictal EEG activity, the seizure onset zone (SOZ),4,8 and the removal of HFO-generating tissue is correlated with a seizure-free postsurgical outcome.5,9–12 This evidence derives from several epilepsy centers and includes data from different patients, adults, and children, distinct types of epilepsy, mesiotemporal and neocortical, and different analysis techniques.13 These studies were retrospective and included small patient samples.10 The correlation between removal of HFO-generating tissue and seizure outcome was usually found on a group level, but studies also reported single patients in whom no correlation could be seen.9,14 In a group using ECoG, it was shown that the extent of presurgically identified HFOs is less predictive of the postsurgical seizure outcome than the residual HFOs found after the resection.10 On a single patient level, HFOs failed to identify the SOZ more precisely than spikes in another study.15
Even if interictal HFOs are thought to be better biomarkers for epileptic tissue than epileptic spikes, they have not been used in routine presurgical diagnostic workup for 2 main reasons: analysis methods are complicated, and the clinical evidence for using HFOs to tailor epilepsy surgery is too weak.16 The present study is a prospective, international, multicenter trial combining data from 3 experienced epilepsy centers, each using different iEEG methods. The data were acquired prospectively: interictal HFOs were marked and scored after the recording and before the seizure outcome was known. For ethics reasons, resections were not tailored to the HFO results. We hypothesized that the proportion of resected HFO-generating brain tissue correlates positively with postsurgical seizure freedom independently of the type of recording technique and epilepsy center.
Methods
Patient recruitment
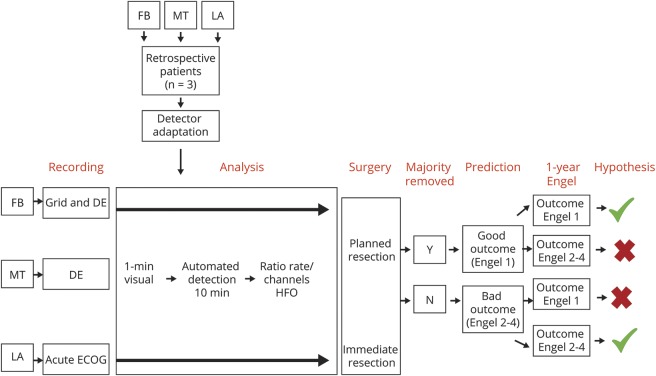
Between March 2011 and June 2012, the Montreal Neurologic Institute (Quebec, Canada), the Epilepsy Center Freiburg (Germany), and the Pediatric Epilepsy Program of the University of California Los Angeles (UCLA) each included up to 20 consecutive patients who underwent intraoperative or chronic iEEG and then surgery. All centers received ethics approval for HFO data analysis and anonymized data transfer between institutions. An overview of the methods can be found in figure 1.
Figure 1. Methodologic approach used in the present study.
The 3 retrospective patients have been used only for detector adaptation and validation and were not part of the statistical analysis. Patients who underwent acute electrocorticography (ECoG) underwent immediate resection after the ECoG, while patients with depth electrode had surgery some weeks after the intracranial EEG recording. DE = depth electrode; FB = Freiburg; HFO = high-frequency oscillation; LA = Los Angeles; MT = Montreal.
Inclusion criteria were surgical resection after the intracranial investigation and a good-quality recording of at least 10 minutes with a 2,000-Hz sampling rate. No restrictions were applied with respect to epilepsy type, extent of the epileptic activity, or age of the patients.
Clinical information
At the time of study inclusion, data on the patients' noninvasive presurgical workup were collected from medical charts. At the end of the clinical iEEG recording, the SOZ and the area of resection were defined as part of the clinical investigation independently of this study. The neurophysiologists marking the HFOs were blinded to these results. After a minimum of 12 months, postsurgical outcome was classified according to Engel by a physician unaware of the results of HFO analysis. All patients underwent a postsurgical MRI within a year of surgery to confirm the extent of the resection.
Recording techniques
Details on the recording techniques are given in the supplementary materials (data available from Dryad, methods, doi.org/10.5061/dryad.hp591kt). In summary, all centers recorded with their local equipment. In Freiburg and Montreal, chronic stereotactic-depth electrodes were used; subdural grids also were used in Freiburg. At UCLA, acute intraoperative ECoG data were acquired. All centers used Stellate Harmonie for the analysis of HFOs and EEG data.
Visual HFO and baseline identification
HFO annotation was performed in the same way in all 3 centers. The period for annotation in the chronic iEEG (2 centers) was taken from slow-wave sleep. Sleep scoring was performed with the help of additional electrooculography and EMG electrodes. A period from the second night of recording at least 2 hours after and before ictal activity was chosen. In the intraoperative ECoG (1 center), the period with the least artifacts and interference by anesthesia was selected. The EEGs at all 3 centers were visually identified for a 1-minute epoch per channel. The methods for visual identification of HFOs have been published previously.6 Events were identified using an extended time scale of 0.8 second per page and high-pass finite impulse response filters (order 63). A ripple was marked if clearly visible with an 80-Hz high-pass filter and did not occur when filtering with 250 Hz. An event was regarded as a fast ripple (FR) if it was visible with a 250-Hz high-pass filter. Events had to have a minimum length of 4 oscillations, and 2 events had to be separated by at least 2 nonoscillatory baseline crossings, similar to what was done in several studies16
Automatic HFO detection
For the automatic detection of interictal HFOs, we used the detector developed by Zelmann et al.17 This requires visual markings of HFOs and true baseline periods during a short period; it can then be trained for optimal performance. We aimed to have the best detection conditions for the highly varying recording methods involved in the study. In each center, 3 patients recorded before the recruitment period were used for detector training, and then optimal detector settings were adapted with those results. Results and rates in these retrospective patients were not used for the statistical analysis in the study.
During 1 minute of slow-wave sleep in chronic recordings and in a period of good EEG quality in intraoperative ECoG, HFOs and baseline segments were marked by 2 independent reviewers from separate centers. After that, optimized detector settings derived from the retrospective patients were applied to the prospectively recruited patients on 10 minutes of EEG. Visual HFO and baseline markings of the first minute were used to evaluate detector performance. κ Coefficients were used to evaluate the agreement between visual and automatic markings.18
Statistical analysis
Automatically marked detections were used to calculate rates (events per minute) for ripples and FRs for each channel. For chronic recordings, an overlapping image was aligned between the MRI/CT with electrodes and the postsurgical MRI to define which contacts were in the tissue subsequently removed during surgery. Contacts were grouped as removed, not removed, and unclear. The last group refers to brain areas that were functionally isolated after surgery or at the border of the resection (hence, it was unclear if these areas contributed to seizure generation). For intraoperative ECoG, the electrode contacts over the resection were defined directly during surgery.
Statistical analyses were performed for the whole group of patients and for each center separately. To calculate whether the majority of events were removed during surgery, we used previously described methods9 (formula data available from Dryad, methods, doi.org/10.5061/dryad.hp591kt). We quantified first the HFO rates in removed and nonremoved contacts and then the number of removed contacts with HFOs regardless of HFO rates (i.e., the extent of tissue showing HFOs). A ratio was calculated between rates of HFOs in removed contacts and rates in nonremoved contacts, as well as the ratio between the number of removed and nonremoved contacts showing HFOs. With the use of these ratios, a value close to +1 identifies patients in whom the majority of HFOs/HFO channels were removed, whereas a value close to −1 indicates that the majority HFOs/HFO channels were untouched. For the prognostication of outcomes, patients with values between 0.1 and 1 were considered as majority removed, while patients with −0.1 to −1 were considered as majority untouched. Ratios were compared by the Spearman correlation. Statistical analysis compared patients with seizure-free outcome (Engel 1) and patients with seizures (Engel 2–4). A Spearman rank correlation analysis was used to assess correlations between the extent of removal and outcome for the 4 outcome groups (Engel 1–4).
Several subtests of the Spearman correlation were performed to evaluate the effect of potentially influencing factors on the results: (1) using ratios of the visually marked data separately from the automatic rate results, (2) considering “borderline” channels as either removed or not removed, and (3) with application of a threshold for HFO rates and their ratios considering only high-rate channels. The threshold was established with the upper fence (UF) method.19 The UF is calculated by using the following formula: UF = Q3 + (1.5 × IQR), in which Q3 is the third quartile and IQR is the interquartile range, i.e., the difference between the first and third quartiles. In the present study, this measure was used to identify outliers, i.e., channels with very high HFO rates for each individual patient. The significance level of all analysis was set at p < 0.05.
Standard protocol approvals, registrations, and patient consents
All patients or legal caregivers signed a written informed consent and Health Insurance Portability and Accountability Act authorizations before participation. This study has been approved and registered with the Freiburg Ethics Board (No. 131/08).
Data availability
Data not included in the article will be made available to qualified researchers on request subject to ethics approval.
Results
Patient inclusion
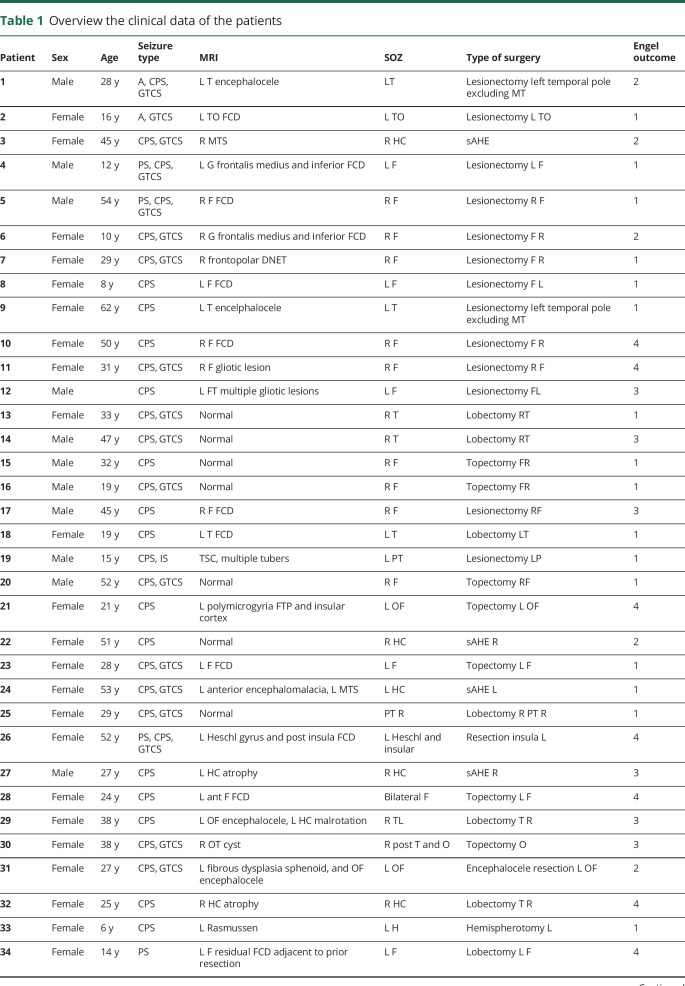
Fifty-two patients were recruited: 20 from Freiburg and Los Angeles each and 12 from Montreal (clinical details can be found in table 1).
Table 1.
Overview the clinical data of the patients
For Freiburg, patients were on average 31 years old. Four had nonlesional and 16 had lesional epilepsy. Eight patients had subdural grids, and 12 had stereotactically implanted electrodes. EEG was recorded from an average of 93.3 electrode contacts. Two patients underwent a selective amygdalohippocampectomy; 5 had a lobectomy; and 13 had a lesionectomy. According to the resection size, on average, 42.7% of the contacts were in removed tissue. Outcome was Engel 1 in 12 (60%) patients, Engel 2 in 3 patients, Engel 3 in 3 patients, and Engel 4 in 2 patients.
For Montreal, patients were on average 36 years old, and all were lesional. All patients were recorded with intracerebral electrodes, from an average of 47.2 contacts. Four patients underwent a selective amygdalohippocampectomy; 4 had a lobectomy; and 6 had a lesionectomy. According to the resection size, on average, 23.1% of the contacts were in removed tissue. Outcome was Engel 1 in 3 (25%) patients, Engel 2 in 2 patients, Engel 3 in 3 patients, and Engel 4 in 4 patients.
In Los Angeles, the average age at the time of surgery was 8.6 years (range 6 months–17 years 11 months), and all were lesional. All patients underwent intraoperative subdural ECoG recordings from an average of 23.7 contacts. Twelve patients underwent a hemispherectomy; 3 patients had a lobectomy; 4 had a lesionectomy; and 1 patient had an amygdalohippocampectomy. According to the resection size, on average, 84.9% of the recording contacts were over removed tissue. Outcome was Engel 1 in 15 (75%) patients, Engel 2 in 1 patient, Engel 3 in 2 patients, and Engel 4 in 2 patients.
HFOs rates and surgical outcome
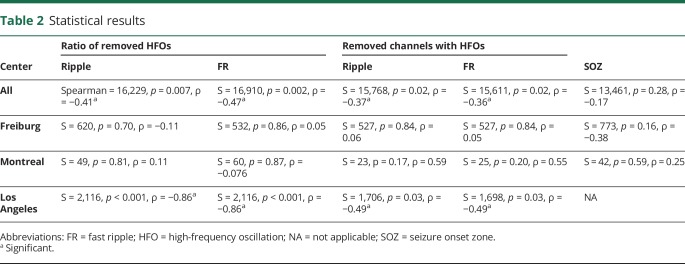
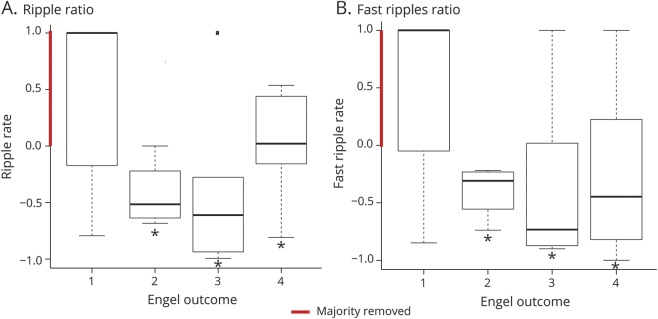
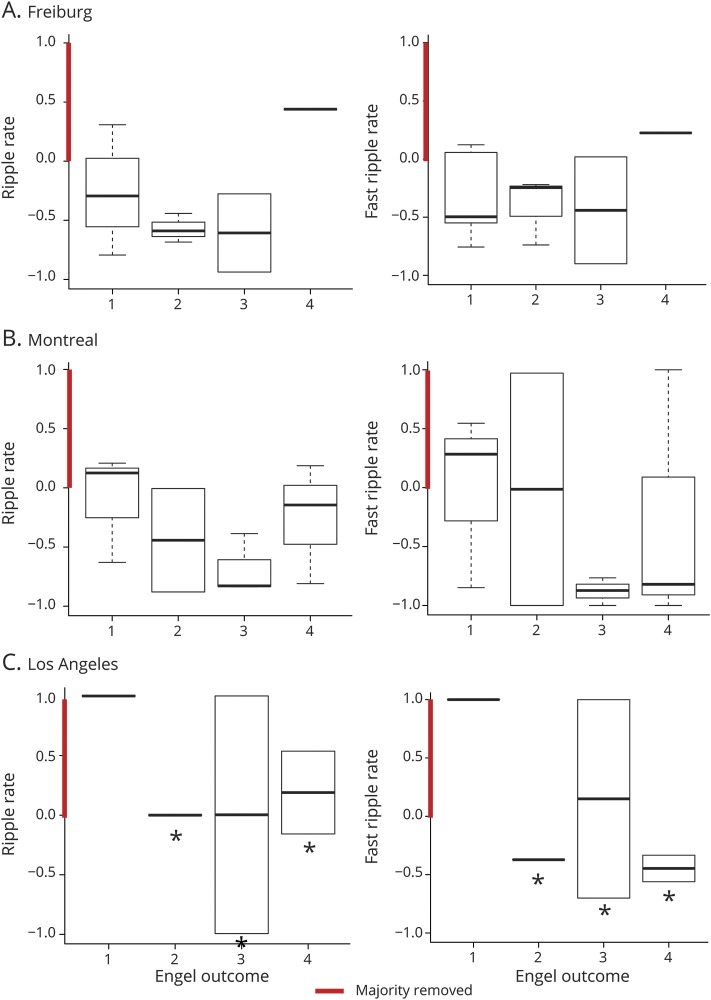
Statistical results for entire cohort and each center are shown in table 2. Significant results are indicated. Results were in the direction of the hypothesis: removal of HFOs or channels with HFOs was higher in patients with Engel 1 outcome than in those with Engel 2 to 4 outcome for the overall group (figure 2) and for UCLA but not for Freiburg and Montreal as single centers (figure 3).
Table 2.
Statistical results
Figure 2. Boxplots for the overall results of all centers.
There is a correlation between the removal of high-frequency oscillations (HFOs) and postsurgical outcome for (A) ripples and (B) fast ripples. Results are shown for the ratio of removed HFOs to nonremoved HFOs.
Figure 3. Boxplots showing results of single-center comparison between HFO removal and postsurgical seizure outcome.
No correlation/differences were found for patients in (A) Freiburg and (B) Montreal. There were differences for ripples and fast ripples in patients from the University of California at Los Angeles. Results are shown for the ratio of removed high-frequency oscillations (HFOs).
Analysis of potentially influencing factors on results
Visually vs automatically marked data
The average κ value between visual and automatic markings for all channels and patients was 0.62 ± 0.12 and considered sufficient. Nevertheless, all data were reanalyzed using the rates from the first EEG minute, which was visually marked, to exclude the influence of detector performance on the overall results of the study. The results were similar to those of the automatic detection, with a correlation for the data from UCLA and no correlation for the data from Freiburg and Montreal.
Borderline channels
Data were reanalyzed including channels at the border of a resection or considered functionally disconnected. Results in all centers remained unaltered in regard to correlations.
HFO rate thresholds
The UF was calculated using a threshold for each center and applying it to single patients. The analysis including the data from all centers regarding the removal of areas with the highest ripple counts stayed unchanged (S = 15,162.73, p = 0.04, ρ = −0.32), but there was no correlation between the removal of areas expressing the highest FR counts and surgical outcome. In addition, single-center analysis showed no correlation for the removal of HFOs and surgical outcome for any center. Therefore, even for UCLA data, applying the UF reduced the ability of HFOs to predict surgical outcome.
The UF was used in calculating a threshold for each patient and applying it to this same patient. The analysis including the data from all centers showed a correlation between the removal of areas expressing the highest ripple (S = 15,113.02, p = 0.044, ρ = −0.32) and FR counts (S = 15,419.5, p = 0.03, ρ = −0.34) and surgical outcome. However, single-center analyses for Freiburg and Montreal showed no correlation. For UCLA, there were higher ratios for ripples in removed regions in patients with seizure-free outcome (S = 1,103.43, p = 0.01, ρ = −0.62) but not for FR.
Prognostication of seizure outcome in individual patients
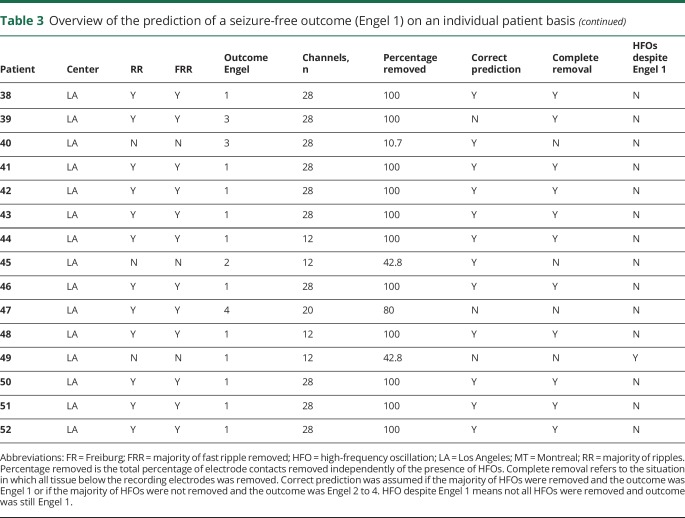
Table 3 provides an overview of the individual patients and the ability to provide an outcome prognostication with the use of ripples and FR. This table takes into account areas with the highest rates of HFOs as defined in the UF measurement: these areas were completely removed and predicted a good outcome, or the majority of HFOs remained, which predicted a poor outcome. The table also points out limitations of our study such as large resections in which the resection included many areas not expressing HFOs, as well as patients in whom many HFOs remained despite a good outcome.
Table 3.
Overview of the prediction of a seizure-free outcome (Engel 1) on an individual patient basis
Our hypothesis that HFOs predicts either good or poor surgical outcome was correct in 36 of 52 patients (69.2%, marked with Y in correct prediction in table 3). In all but 1 patient (patient 8), areas of ripples and FR were overlapping to an extent that removal of the majority of ripples also meant removal of the majority of FR and vice versa. Prognostication was correct in 10 of 20 (50%) patients from Freiburg, 9 of 12 (75%) from Montreal, and 17 of 20 (85%) from Los Angeles.
At first, these results appear satisfactory. However, in Freiburg and Montreal, only 3 of 19 predicted outcomes were correct prognostications of a seizure-free condition. Moreover, 14 patients had a seizure-free outcome despite the fact that the majority of HFOs were not removed (figure 4, patients marked with Y in the HFOs despite Engel 1 column in table 3). In these patients, removal of HFO-generating areas would have led to an extension of the surgery without any benefit for the patient.
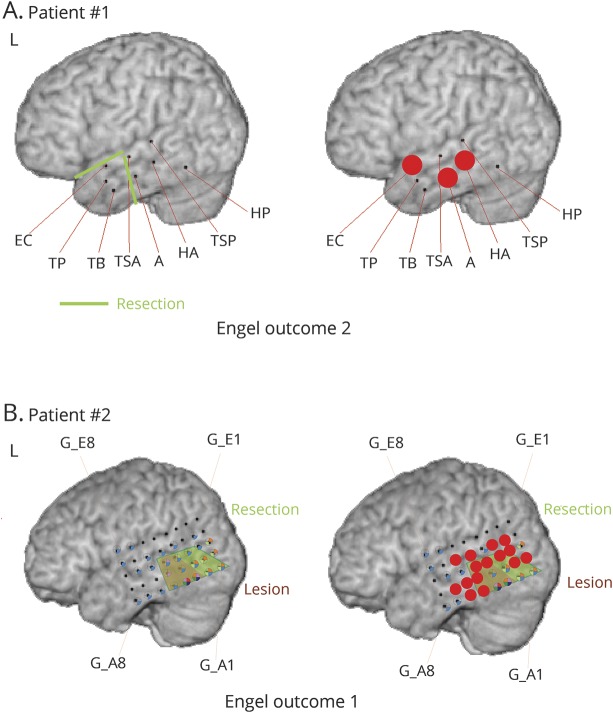
Figure 4. Two patient examples from the Freiburg cohort.
In both patients, not all high-frequency oscillations (HFOs) were removed. (A) In patient 1, mesial temporal contacts in the hippocampus (HA) and amygdala (A) had HFOs in the deepest contacts and were not removed because the patient had a pole resection, including an encephalocele, in this area and the mesial temporal structures were spared. The patient had Engel 2 outcome. Therefore, the prognostication was judged to be correct. (B) The second patient had a lesionectomy (brown) as shown. Red dots indicate contacts with highest fast ripple rates. Not all of these contacts were removed, as shown in the resection line (green). The patient was seizure-free after surgery; therefore, HFOs failed to predict outcome. EC = encephalocele; G = gyrus; HP = parahippocampal gyrus; TB = temporobasal; TP = temporopolar; TSA = temporalis superior anterior; TSP = temporalis superior posterior.
All other prognostications were for poor outcome. The prognostication of poor outcome could be by chance because all patients in Freiburg had remaining HFOs (a poor outcome was expected according to our hypothesis) and resections were in the majority smaller than the area generating HFOs.
In the patients from UCLA, the table demonstrates that removals were larger. In 15 patients (85%, including all with hemispherotomy), all areas underlying electrodes were removed, and in 14 patients, this resulted in a successful prognostication of postsurgical seizure freedom. Of the 5 remaining patients who underwent restricted surgery, 2 (patients 39 and 47) continued to have seizures despite resection of all HFOs, and in another (patient 49), most HFOs remained, but the patient became seizure-free.
Discussion
We prospectively evaluated the use of HFOs as a biomarker for epileptic tissue. An association between the removal of HFO-generating tissue and surgical seizure outcome has been suggested by retrospective13,20 and prospective single-center studies.21 Evidence has suggested that HFO measures are independent of recording technique and patient population. For this reason, the current study aimed to include a variety of patients from 3 centers with different traditions of acquiring iEEGs. The group analysis combining results of the 3 centers and the overall statistics (very similar to those used in previously published studies from these centers) seemed at first to confirm our hypothesis. However, a deeper analysis of the predictive value in each center and at the individual level revealed that HFOs did not reliably predict postsurgical outcome, with the exception of the Los Angeles site. The discussion focuses on the possible confounders that might explain the failure of the study to confirm our hypothesis.
The method chosen to identify HFOs is a first factor. Visual analysis has been used in some studies22,23 with the advantage of avoiding false detections, as often occurs with automatic detection. Moreover, it provides a better understanding of unknown data. Drawbacks of visual identification are potential reviewer bias and the time needed for identification.18 Automatic detection is much faster and, if validated, can be used by epileptologists inexperienced in identifying HFOs.18,24 For this reason, we adopted a hybrid approach in which visual markings were used for detector training and validation. The improved detector was then applied. To ensure that poor detector performance did not influence our results, we reanalyzed all data with the visual marking of the first minute and found no differences for visual and automatically evaluated HFO counts. We assumed that the 1-minute visually marked segment is representative of the HFO count in a given region.18 There are no guidelines for how long iEEGs should be visually analyzed for HFOs. It is conceivable that our epoch for visual analysis was insufficient,5 especially for such a multicenter study using different patient populations, ages, and recording techniques.
The extent of the areas generating HFOs and the types of HFOs also represent confounding factors. In the Freiburg data, the extent of areas generating HFOs was larger than expected and exceeded the resection margin. One reason might be that we included physiologic and epileptic HFOs. Recent studies suggest that physiologic HFOs are not limited to mesial temporal structures, as initially thought, but also occur over occipital and central regions.25,26 These studies demonstrated that physiologic and epileptic HFOs have a large frequency overlap and cannot be separated by frequency alone.27,28 In the Freiburg data, HFOs showed the largest extent in the subdural grid recordings and at contacts covering regions known to generate physiologic HFOs, findings that might have interfered with our analysis. Moreover, HFOs were more spatially limited in the data from Los Angeles, data acquired under general anesthesia, a condition probably resulting in fewer physiologic HFOs.29 It is important to note that resection margins were determined differently between the centers; while SOZ definitions were available for Montreal and Freiburg, Los Angeles relied on interictal EEG data and performed larger resections if interictal activity was visible at the borders of the ECoG recordings. Thus, resections were more extensive even if HFO extent was more restricted in these recordings.
Spontaneous short physiologic HFOs cannot be excluded with the current methods and might explain our nonspecific results for epileptic areas. Recent studies aimed to distinguish physiologic HFOs by using amplitude and frequency values.28,30 In contrast to physiologic HFO, epileptic events might be more repetitive and monomorphic and can be identified by searching for events that do not change their morphology.31 Another method is analyzing the co-occurrence of HFOs with physiologic phenomena such as sleep slow waves or sleep spindles to identify physiologic and co-occurrence with epileptic spikes to identify epileptic HFOs. Applying one of these methods to our data in the future might improve prognostication.8,32,33
To prospectively predict surgical outcome, it is important to know which proportion of HFOs have to be removed to gain seizure freedom. In our study, we decided to use the measurement of majority of HFOs removed. This fits the concept that more HFO-generating areas have to be removed than remain for a patient to be seizure-free.9,14 There is, however, no clear evidence that this assumption is right, and it may be necessary to remove all areas generating epileptic HFOs.
In a prospective study, it is often difficult to judge which HFO-generating tissue is relevant. A solution to this problem is to use, as in retrospective studies, individual thresholds to determine the area of highest HFO activity within an individual patient.11,19 For instance, there is controversial evidence for the influence of electrode types and size of contacts on the measurable rates of HFOs.34,35 HFO counts in our study were more similar within the same center than between centers. For this reason, we used a threshold called UF, which identifies extreme outliers derived from the average HFO count of each center and established for each individual patient. None of these analyses improved the predictive value of HFO removal, possibly because the actual count of HFOs is not the most important factor or because areas with medium activity are important in the prognostication.
In the current study, some patients became seizure-free even when most of the HFO-generating tissue remained untouched. This is strong evidence that HFOs are not reliable markers of epileptic activity. However, studies on intraoperative ECoG introduced the analysis of postsurgically remaining FR as a measure to predict surgical outcome.10 After surgery, residual HFOs could not be predicted by the presurgical HFO distribution, and some areas stopped showing HFOs postoperatively even if they were not resected or disconnected. Prognostication of outcome with FR was accurate in patients showing presurgical HFO that vanished the postsurgical ECoG.36,37 The combination of presurgical and postsurgical ECoG analysis may be better because it gives an idea about the remaining networks still able to generate FRs. For this reason, information on postsurgically remaining FRs might represent a more direct measure of epileptogenicity than presurgically recorded FRs. This measure is limited to intraoperative ECoG and is not usable in chronic recordings. An explanation for our patients who became seizure-free despite residual HFO areas is that the network necessary to generate HFOs was successfully interrupted.
The results of this study are unexpected. We are especially perplexed by the fact that the majority of HFO-generating tissue remained untouched in several patients who became seizure-free after surgery. Prior data from 2 of our centers have shown very different results.9,21,38 Two meta-analyses of the existing studies, however, have already suggested that the evidence of HFOs as a predictor of surgical outcome is rather weak.13,39 A retrospective study from Montreal showed that small changes in the patient sample might alter the statistics in a way that HFO removal and surgical outcome were correlated only with temporal but not extratemporal epilepsies.14 In the present study, we could not perform this comparison without risking that a bias between recording methods would influence the results because patients undergoing ECoG were all neocortical while those with depth contacts were often mesiotemporal.
In another recent study,36 the removal of regions generating FRs was not predictive of surgical outcome. In a previous dataset from Freiburg, pattern analysis was necessary to obtain significant differences in HFO removal between patients with good and those with poor outcome.40 The varying results and rather weak evidence in the meta-analysis suggest that patient inclusion had influenced the results of these studies. It would also be important to evaluate which frequency band from gamma to FRs is most promising for predicting surgical outcome.36,41 Finally, some pediatric studies included patients with large resections such as hemispherotomies and multilobar removals5,21,42 and faced the same challenge seen in our Los Angeles data. It might therefore be that results from the group data of our study are more an effect of resection size than removal of HFO-generating tissue. Overall, we believe that although our findings do not exclude the possibility that HFOs indicate the epileptogenic zone, they suggest that more knowledge about individual differences in HFO generation and recording techniques is needed before this measure is used as a valid clinical tool.
The difference in the results between the Los Angeles and the Freiburg/Montreal subgroups could be explained by several factors mentioned above. In addition, the intraoperative ECoG approach under anesthesia in Los Angeles differs significantly from the chronic recording in the epilepsy units. The entirely neocortical epilepsy cohort in Los Angeles is another major difference from the mixed epilepsy groups in Freiburg and Montreal. Lastly, resections of the SOZ after chronic iEEG are vastly different from resections guided by the irritative zone during ECoG.
To the best of our knowledge, none of the published studies aimed to obtain a prognostication in outcomes on an individual level. One study compared the identification of SOZ areas with spikes and HFOs on an individual patient level. HFOs were not better in identifying seizure onset compared to spikes in individual patients, contrary to what had been found in group-level studies.15 The current results may therefore be limited to our specific patient groups. Nevertheless, the results question the value of HFOs as biomarkers of epileptic tissue, at least when combined from different centers, different age groups, and different techniques. As discussed, several variables in our study design might have contributed to the lack of significant results. The body of evidence that supports a connection between epilepsy and HFO occurrence is, however, large. Studies in rodents with mesial temporal and posttraumatic epilepsy have shown that HFOs are closely linked to epileptogenicity.43,44 HFOs occurred only in animals that developed chronic epilepsy in contrast to those that remained healthy after initial injury. HFOs were found to be closely linked to the SOZ in a large variety of epilepsies associated with developmental malformations, hippocampal sclerosis, and genetic disorders.16,45 HFOs not only are recorded interictally but strongly increase directly before or during seizures.46,47 A reduction of antiepileptic medication results in an increase of HFOs, and successful treatment can be measured as a reduction of HFOs.22,48 Many studies suggest a strong link between HFOs and epilepsy. It is possible that current techniques to identify HFOs and separate different types of HFOs and the inability to distinguish which types of HFOs truly matter in regard to seizure outcome may have influenced our results. Further studies including larger patient samples and better measures to distinguish physiologic and epileptic HFOs are needed. Current techniques for HFO identification and separation are still time-consuming, but recent publications suggest that real-time analysis may become available soon.49 It is still possible, however, that HFOs are not as specific a biomarker of the epileptogenic zone as early studies have led us to believe.
Glossary
- ECoG
electrocorticography
- FR
fast ripple
- HFO
high-frequency oscillation
- iEEG
intracranial EEG
- SOZ
seizure onset zone
- UCLA
University of California Los Angeles
- UF
upper fence
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Dr. Jacobs designed the study, visually analyzed EEGs from the other centers, and wrote the manuscript. Dr. Wu recorded patients at UCLA, visually analyzed EEG from the other centers, and edited the manuscript. Dr. Perucca selected the patients at the Montreal Neurologic Institute, visually analyzed EEGs from the other centers, and edited the manuscript. Dr. Zelmann performed the automatic detection of all patients' EEGs and programmed the necessary adaptations of the detectors for each center. Ms. Mader performed the statistics on this project. Dr. Dubeau advised on the analysis of the iEEG, clinically defined the respective SOZs, and edited the manuscript. Dr. Mathern analyzed the extent of the surgical resections at his side, supervised the project at UCLA, and edited the manuscript. Dr. Schulze-Bonhage advised on the analysis of the iEEG, clinically defined the respective SOZs, and edited the manuscript. Dr. Gotman supervised the technical analysis of the project, supported the development of the study design, and edited the manuscript.
Study funding
J.J. was supported by the German Research Foundation (DFG grant JA1725/2-1). J.Y.W. was supported by the Tuberous Sclerosis Alliance, Novartis Pharmaceuticals Inc, Today's and Tomorrow's Children Fund, Department of Defense/Congressionally Directed Medical Research Program, and the NIH (P20-NS080199, U01-NS082320, R01-NS082649, U54-NS092090, and U01-NS092595). The Montreal Neurologic Institute group was supported by grant FDN 143208 of the Canadian Institutes of Health Research.
Disclosure
J. Jacobs reports no disclosures relevant to the manuscript. J. Wu has received research funding from Novartis and GW Pharmaceutical, is an editorial board member of the Journal Pediatric Investigation, and has been supported by the National Institute of Neurological Disorders and Stroke/NIH (R01 NS 082649) and the Today's and Tomorrow's Children Fund from UCLA Mattel Children's Hospital at the University of California Los Angeles. P. Perucca, R. Zelmann, M. Mader, F. Dubeau, and G. Mathern report no disclosures relevant to the manuscript. A. Schulze-Bonhage has received honoraria for lectures or advice from Bial, EISAI, PRECISIS, and UCB and has received research support from Deutsche Forschungsgemeinschaft, BMBF, European Union, NIH, and Bundesministerium für Bildung und Forschung. J. Gotman reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain 2001;124:1683–1700. [DOI] [PubMed] [Google Scholar]
- 2.Boling W, Aghakhani Y, Andermann F, Sziklas V, Olivier A. Surgical treatment of independent bitemporal lobe epilepsy defined by invasive recordings. J Neurol Neurosurg Psychiatry 2009;80:533–538. [DOI] [PubMed] [Google Scholar]
- 3.Crépon B, Navarro V, Hasboun D, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain 2010;133:33–45. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs J, LeVan P, Châtillon CÉ, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 2009;132:1022–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology 2010;75:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 2008;49:1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragin A, Engel J, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol 2010;23:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Wang IZ, Bulacio JC, et al. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia 2013;54:370–376. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol 2010;67:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Klink NEC, van't Klooster MA, Zelmann R, et al. High frequency oscillations in intra-operative electrocorticography before and after epilepsy surgery. Clin Neurophysiol 2014;125:2212–2219. [DOI] [PubMed] [Google Scholar]
- 11.Okanishi T, Akiyama T, Tanaka SI, et al. Interictal high frequency oscillations correlating with seizure outcome in patients with widespread epileptic networks in tuberous sclerosis complex. Epilepsia 2014;55:1602–1610. [DOI] [PubMed] [Google Scholar]
- 12.Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia 2011;52:1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höller Y, Kutil R, Klaffenböck L, et al. High-frequency oscillations in epilepsy and surgical outcome: a meta-analysis. Front Hum Neurosci 2015;9:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haegelen C, Perucca P, Châtillon C-E, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia 2013;54:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roehri N, Pizzo F, Lagarde S, et al. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann Neurol 2018;83:84–97. [DOI] [PubMed] [Google Scholar]
- 16.Frauscher B, Bartolomei F, Kobayashi K, et al. High-frequency oscillations: the state of clinical research. Epilepsia Epub 2017 Jun 30. [DOI] [PMC free article] [PubMed]
- 17.Zelmann R, Mari F, Jacobs J, Zijlmans M, Chander R, Gotman J. Automatic detector of high frequency oscillations for human recordings with macroelectrodes. Conf Proc IEEE Eng Med Biol Soc 2010;2010:2329–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelmann R, Zijlmans M, Jacobs J, Châtillon C-E, Gotman J. Improving the identification of high frequency oscillations. Clin Neurophysiol 2009;120:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama T, McCoy B, Go CY, et al. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia 2011;52:1802–1811. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs J, Staba R, Asano E, et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol 2012;98:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain SA, Mathern GW, Sankar R, Wu JY. Prospective and “live” fast ripple detection and localization in the operating room: impact on epilepsy surgery outcomes in children. Epilepsy Res 2016;127:344–351. [DOI] [PubMed] [Google Scholar]
- 22.Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology 2009;72:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerber K, LeVan P, Dümpelmann M, et al. High frequency oscillations mirror disease activity in patients with focal cortical dysplasia. Epilepsia 2013;54:1428–1436. [DOI] [PubMed] [Google Scholar]
- 24.Burnos S, Hilfiker P, Surucu O, et al. Human intracranial high frequency oscillations (HFOs) detected by automatic time-frequency analysis. PLoS One 2014;9:e94381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp 2012;33:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nonoda Y, Miyakoshi M, Ojeda A, et al. Interictal high-frequency oscillations generated by seizure onset and eloquent areas may be differentially coupled with different slow waves. Clin Neurophysiol 2016;127:2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia 2009;50:598–604. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto A, Brinkmann BH, Stead SM, et al. Pathological and physiological high-frequency oscillations in focal human epilepsy. J Neurophysiol 2013;110:1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zijlmans M, Huiskamp GM, Cremer OL, Ferrier CH, van Huffelen AC, Leijten FSS. Epileptic high-frequency oscillations in intraoperative electrocorticography: the effect of propofol. Epilepsia 2012;53:1799–1809. [DOI] [PubMed] [Google Scholar]
- 30.Amiri M, Frauscher B, Gotman J. Phase-amplitude coupling is elevated in deep sleep and in the onset zone of focal epileptic seizures. Front Hum Neurosci 2016;10:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Gurses C, Sha Z, et al. Stereotyped high-frequency oscillations discriminate seizure onset zones and critical functional cortex in focal epilepsy. Brain Epub 2018 Jan 30. [DOI] [PMC free article] [PubMed]
- 32.Weiss SA, Orosz I, Salamon N, et al. Ripples on spikes show increased phase-amplitude coupling in mesial temporal lobe epilepsy seizure-onset zones. Epilepsia 2016;57:1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piza DL, Bruder JC, Jacobs J, Schulze-Bonhage A, Stieglitz T, Dumpelmann M. Differentiation of spindle associated hippocampal HFOs based on a correlation analysis. Conf Proc IEEE Eng Med Biol Soc 2016;2016:5501–5504. [DOI] [PubMed] [Google Scholar]
- 34.Châtillon CE, Zelmann R, Hall JA, Olivier A, Dubeau F, Gotman J. Influence of contact size on the detection of HFOs in human intracerebral EEG recordings. Clin Neurophysiol 2013;124:1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain J Neurol 2008;131:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van't Klooster MA, van Klink NEC, Zweiphenning WJEM, et al. Tailoring epilepsy surgery with fast ripples in the intraoperative electrocorticogram. Ann Neurol 2017;81:664–676. [DOI] [PubMed] [Google Scholar]
- 37.van't Klooster MA, van Klink NEC, Leijten FSS, et al. Residual fast ripples in the intraoperative corticogram predict epilepsy surgery outcome. Neurology 2015;85:120–128. [DOI] [PubMed] [Google Scholar]
- 38.Hussain SA, Mathern GW, Hung P, Weng J, Sankar R, Wu JY. Intraoperative fast ripples independently predict postsurgical epilepsy outcome: comparison with other electrocorticographic phenomena. Epilepsy Res 2017;135:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gloss D, Nolan SJ, Staba R. The role of high-frequency oscillations in epilepsy surgery planning. Cochrane Database Syst Rev 2014:CD010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerber K, Dümpelmann M, Schelter B, et al. Differentiation of specific ripple patterns helps to identify epileptogenic areas for surgical procedures. Clin Neurophysiol 2014;125:1339–1345. [DOI] [PubMed] [Google Scholar]
- 41.Ren L, Kucewicz MT, Cimbalnik J, et al. Gamma oscillations precede interictal epileptiform spikes in the seizure onset zone. Neurology 2015;84:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiwara H, Greiner HM, Lee KH, et al. Resection of ictal high-frequency oscillations leads to favorable surgical outcome in pediatric epilepsy. Epilepsia 2012;53:1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bragin A, Wilson CL, Almajano J, Mody I, Engel J. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia 2004;45:1017–1023. [DOI] [PubMed] [Google Scholar]
- 44.Bragin A, Li L, Almajano J, et al. Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia 2016;57:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia 2011;52:1812–1819. [DOI] [PubMed] [Google Scholar]
- 46.Usui N, Terada K, Baba K, et al. Clinical significance of ictal high frequency oscillations in medial temporal lobe epilepsy. Clin Neurophysiol 2011;122:1693–1700. [DOI] [PubMed] [Google Scholar]
- 47.Sato Y, Doesburg SM, Wong SM, Ochi A, Otsubo H. Dynamic preictal relations in FCD type II: potential for early seizure detection in focal epilepsy. Epilepsy Res 2015;110:26–31. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi K, Akiyama T, Oka M, Endoh F, Yoshinaga H. A storm of fast (40–150 Hz) oscillations during hypsarrhythmia in West syndrome. Ann Neurol 2015;77:58–67. [DOI] [PubMed] [Google Scholar]
- 49.Fedele T, van't Klooster M, Burnos S, et al. Automatic detection of high frequency oscillations during epilepsy surgery predicts seizure outcome. Clin Neurophysiol 2016;127:3066–3074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not included in the article will be made available to qualified researchers on request subject to ethics approval.