Abstract
Background
In thyroid nodules with indeterminate cytology, further clinical assessment aimed at ruling out malignancy is often mandatory. Ancillary imaging techniques and genetic mutation analysis can improve the risk stratification of such lesions, thereby facilitating the clinician’s decision to undertaken surgery or simple follow-up. The aim of this study was to evaluate the diagnostic performance of shear-wave elastography (SW), strain elastography (ELX 2/1), conventional ultrasound (US), contrast-enhanced ultrasound (CEUS), and BRAF V600E mutation analysis in the aforementioned lesions.
Material/Methods
We enrolled 81 patients, each with 1 indeterminate-cytology thyroid nodule. Thyroid function, thyroperoxidase antibodies and calcitonin were known in each case. SW, ELX 2/1, US, CEUS, and BRAF mutation analysis were subsequently performed, followed by a second FNAB. If the lesion was not downgraded to benign, surgery was recommended and histological reports collected.
Results
There were 28 nodules (34%) that proved benign on the second FNAB; 38 nodules (47%) underwent surgery (17 benign, 21 malignant), and 15 nodules (19%) refused surgery. The only techniques related to histological outcome were US (AUC=0,766), ELX 2/1 (AUC=0.701), and BRAF analysis (AUC=0.762). ELX 2/1 and SW reports were not correlated with each other (P=0.45). A scoring system taking into account all the variables considered performed better than the single variables alone (AUC=0.831).
Conclusions
In indeterminate-cytology thyroid lesions, repeating FNAB can avoid unnecessary surgery. ELX 2/1 seems to perform better than SW in distinguishing malignancy; these techniques could, however, be complementary in describing such lesions. A multiparametric approach appears the most accurate in predicting nodule histology.
MeSH Keywords: Elasticity Imaging Techniques, Thyroid Nodule, Ultrasonography
Background
The clinical management of thyroid nodules with indeterminate cytology is still controversial [1,2]. Despite continuous updating of cytological classifications in order to improve the risk stratification of these lesions, a significant number of nodules still do not reach a cytological definition [3–8]. In such cases, physicians have to adopt a clinical approach that can balance the risk of malignancy and the need to avoid useless surgery. Several ancillary techniques are now available to support this choice; molecular tests for genetic mutations have shown considerable specificity and positive predictive value in detecting malignancies [3,9–11], and the use of combined tests has been seen to enhance their negative predictive value [11]. However, the routine use of complete genetic profiling may not be feasible [9].
Although many imaging techniques can be used to assess thyroid nodules, none is yet able to distinguish malignant lesions from benign lesions in the event of indeterminate cytology. Moreover, the use of nuclear medicine techniques, such as 18FDG-PET, adds no further information in such cases [12]. Conventional ultrasound (US) has proved helpful in the management of these lesions, and several scoring systems with satisfactory accuracy and predictive value are now available [3,6,13–18]. While few data are available on the role of contrast-enhanced ultrasound (CEUS) in indeterminate-cytology nodules [19], its utility in distinguishing benign from malignant thyroid lesions and in the evaluation of cervical lymph nodes has already been proved [20–22]. Elastosonography is an emerging tool in the field of neck imaging and is particularly useful for the evaluation of thyroid nodules, especially if combined with conventional ultrasound [19,23–25]. Different elastosonographic techniques can be used to estimate nodule stiffness [26]; the shear-wave (SW) technique allows quantitative assessment but has not proved superior to the semi-quantitative techniques (strain index, ELX 2/1) in identifying malignancies [27]. The aim of the present study was to determine the clinical utility and diagnostic performance of BRAF gene mutation analysis, conventional US, CEUS, SW, and ELX 2/1 in thyroid nodules classified as indeterminate on previous fine-needle aspiration biopsy (FNAB).
Material and Methods
Patients
All consecutive patients with at least one thyroid nodule with indeterminate cytology (Thy 3a, Thy3f, or Thy4 according to the BTA classification) on FNAB performed between March 2014 and May 2017 in our Institute were enrolled in the study. Thyroid function tests, thyroid antibodies and calcitonin were recorded in all patients.
At the time of the first FNAB, a US score based on 5 items (solidity, echogenicity, presence of irregular margins, internal vascularization and microcalcifications) was attributed to each nodule. All FNAB were performed under sonographic guidance. BRAF V600E codon mutation was also investigated. After the first FNAB, all patients underwent both strain and shear-wave elastosonography and contrast-enhanced ultrasound examination (CEUS). A second FNAB was performed 1 to 5 months after the previous one. Patients whose nodules proved cytologically benign started a follow-up program and dropped out from the present study; if the second FNAB was indicative of malignancy or yielded another indeterminate result, surgery was performed. Histology reports were collected and used as outcome for statistical purposes. Written informed consent to participate in the in the study was obtained from all patients.
Blood tests
Blood samples were analyzed by means of ultra-sensitive chemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany) for the evaluation of TSH and fT4 (normal ranges 0.3–4.2 mU/L for TSH, 15.4–28.3 pmol/L for fT4). The Dia Sorin assay (Saluggia, Italy) was used for thyroperoxidase antibodies (TPOAb), and concentrations <100 U/mL were considered negative. For serum calcitonin, a chemiluminescence immunoassay (Dia Sorin) was used, with 10 ng/L being taken as the upper limit of the normal range.
Ultrasound assessment and FNAB
All FNAB were performed at the Endocrine Unit by an endocrinologist with over 20 years of experience in thyroid nodule diagnosis and management, who used a 22-gauge needle. The same physician validated all the conventional US examinations, which were carried out by means of a conventional high-resolution device with a color Doppler module equipped with a ML 4–13 linear probe working at 7.5 MHz (MyLab Five, Esaote Biomedica, Genoa, Italy).
Elastosonographic and CEUS assessment
Elastosonographic and CEUS examinations were performed as previously reported [3] at the Interventional Radiology Unit and validated by the same radiologist with more than 5 years of experience in elastosonography. Conventional US and USE were performed by means of a hand-held GE Logiq E9 US scanner (GE Medical, Milwaukee, IL, USA) equipped with ML 6–15 linear probe working in the range of 6–15 MHz. In all elastosonography procedures, the USE features of the tissues were quantified by means of software pre-installed on the US machine. The same radiologist performed both strain and shear-wave USE in the same session. The strain USE was assessed by means of a semi-quantitative method, in which the elasticity score was calculated as the ratio between the elasticity of the nodule and that of a region of interest (ROI) located in the normal thyroid parenchyma at the same depth as the nodule [15].
Shear-wave USE yields a quantitative assessment of the elasticity of a nodule by evaluating the speed of propagation of the shear-wave in the tissues, and provides a numerical value expressed in kilopascals (KPa) or meters per second (m/s). A single value was recorded after setting a ROI in the center of the nodule and excluding potential confounders, such as cystic or calcific elements.
CEUS images were collected by a MyLab 70 US scanner after injection of a bolus of 4.8 mL SonoVue (Bracco, Milan, Italy). Time-to-peak (TTP) and Peak-index (PI) were calculated from the ratio between the nodule and the selected ROI enhancement by means of the software installed on the US scanner.
Pathology and molecular biology analyses
All cytological and histological evaluations were carried out by the same pathologist, who had more than 10 years of experience in analyzing thyroid cancer. Results were reported according to the 2014 BTA classification. Once the adequacy of the sample had been checked (>50% of neoplastic cells) and DNA extracted, BRAF V600E mutation was investigated by means of the direct Sanger sequencing method, in accordance with the recommendations of the Italian Association of Medical Oncology (AIOM) and the Italian Society of Pathology and Cytology (SIAPEC), or real-time polymerase chain reaction (PCR) with commercial kits approved for clinical use. The laboratory was accredited by Bureau Veritas International Organization for Standardization (ISO) 9001: 2008 and the external quality control for the determination of BRAF and RAS mutations was approved by the AIOM in 2012.
Surgery
Surgery was carried out by a team of endocrine surgeons at San Martino Hospital, each of whom had more than 10 years of experience in thyroid cancer management and performed at least 100 thyroidectomies annually for thyroid malignancies.
Statistical analysis
MedCalc Portable Launcher software version 2.2.0.0 was used for statistical analyses: correlation between ELX 2/1 and SW was evaluated through linear regression analysis, the Mann-Whitney test was used to compare groups of variables with non-parametric distribution, and ROC curves were used to establish cut-off values, sensitivity and specificity for each diagnostic test.
Data collection and subsequent analysis were performed in compliance with the Helsinki Declaration and approved by the Ethics Committee of the Interdisciplinary Group for Thyroid Pathology of Policlinico San Martino.
Results
We enrolled 81 patients (24 males, 57 females) with a median age of 59.4±15.3 years at the time of the first FNAB; each patient had one nodule.
Subclinical hyperthyroidism (TSH <0.5 mU/L) was diagnosed in 9 patients. TPOAb exceeded 100 U/mL in 8 patients: out of them 2 presented subclinical hypothyroidism (TSH >5.0 mU/L). Calcitonin was not elevated (>5 pg/mL) in any patient.
Of these 81 nodules, 28 proved benign (Thy2) on the second FNAB. In the remaining 53 cases, cytology was again indeterminate in 46 and was non-diagnostic in 7, and they were advised to surgery; out of these patients, 14 (8 indeterminate, 6 non-diagnostic) chose to join a follow-up program instead of undergoing surgery and 1 patient (Thy 3a at the second FNAB) was lost to follow-up. Thus, 38 patients (47%) underwent surgery. Of these, 17 had a benign histological report (nodular hyperplasia or follicular adenoma) while 21 had a diagnosis of malignancy (16 papillary thyroid cancers, 2 medullary thyroid cancers, 1 follicular variant of papillary thyroid cancer, 1 follicular thyroid cancer, 1 metastasis from lung carcinoma).
Of the 46 lesions resulted as Thy 3a on the first FNAB, 13 underwent surgery; 7 of these proved malignant (54%). Surgery was performed in 11 of the 18 Thy 3f nodules, only one of which proved malignant (9%); 17 lesions were classified as Thy 4 on the first FNAB, and all but 3 underwent surgery. Of these 14 lesions, 13 proved malignant and the remaining 1 was follicular hyperplasia (malignancy 92.8%).
In 7 patients, ELX 2/1 was not feasible owing to the lack of normal parenchyma in which to set a ROI, the prevalence of the liquid component or the position of the nodule; SW was always possible. No significant correlation was found between ELX 2/1 and SW values in the 70 cases in which they were both available (P=0.45) (Figure 1). A significant difference was seen between the ELX 2/1 values recorded in benign and malignant nodules (P=0.05; Figure 2), but not between the SW values (P=0.20; Figure 3). The difference in US scores between benign and malignant nodules was much more significant (P=0.005; Figure 4).
Figure 1.
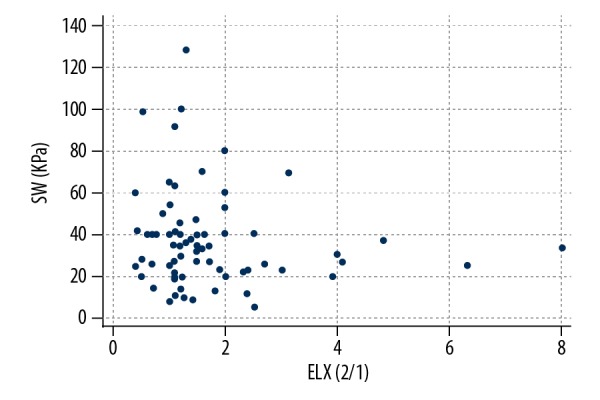
Linear regression graph showing ELX 2/1 and SW values. No significant correlation was found between the 2 variables (n=81, P=0.45).
Figure 2.

Comparison between ELX 2/1 values in benign and malignant nodules (Mann-Whitney test). The former proved to be significantly softer than the latter (P=0.05).
Figure 3.

Comparison between SW values in benign and malignant nodules (Mann-Whitney test) expressed in kilopascals (KPa). No significant difference was noted between the 2 groups (P=0.20).
Figure 4.

Comparison between US scores in benign and malignant nodules (Mann-Whitney test). Malignant lesions had significantly higher values than benign lesions (P=0.005).
ROC curve analysis of the specificity and sensitivity of each tool in distinguishing benign from malignant nodules yielded a cut-off of 2 for the US score (sensitivity 73.68%, specificity 75%, P=0.002) and of 1.73 for ELX 2/1 (sensitivity 50%, specificity 86.7%, P=0.02), while SW showed no statistical significance (P=0.09, cut-off 20 KPa). With regard to CEUS, TTP and PI did not prove useful in distinguishing benign from malignant lesions (P=0.97 and P=0.72, respectively).
A mutation of the BRAF V600E exon was found in 11 nodules (13.5%). As expected, this test displayed perfect specificity but low sensitivity in detecting malignancies (sensitivity 40%, specificity 100%, P=0.0004). On the other hand, calcitonin was very specific in detecting medullary thyroid carcinomas, with values above 45 ng/L in the only 2 cases found.
In order to assess the relative weight of each tool, odds ratios were calculated: SW and BRAF had the highest OR (30.7, P=0.020 and 38.33, P=0.015, respectively), the US score and ELX 2/1 showed similar values (8.12, P=0.005 and 7.72, P=0.018), while CEUS had the lowest ones (1.62, P=0.050 and 1.9, P=0.329 for TTP and PI, respectively). In order to improve the accuracy of the examination, we created a scoring system that assigned one point to each of the following items: ELX 2/1 >1.73, US score>2, BRAF V600E mutated. This score displayed 100% specificity and 68.4% sensitivity for values >1 (P<0.0001, Figure 5). Lastly, a scoring system that included all the variables we considered (US score >2, ELX 2/1 >1.73, SW >20 KPa, TTP ≥1.10 s, PI ≤0.97 s and BRAF V 600E mutation) was applied to the 35 cases in which all these variables were available. This score showed good sensitivity in detecting malignant lesions (criterion >2, sensitivity 89.5%, specificity 75%, P<0.0001; Figure 5).
Figure 5.
ROC curves showing sensitivity and specificity of combined scores. A three-item score (A) (ELX 2/1 >1.73, US score>2, BRAF V600E mutated) had 100% specificity and 68.4% sensitivity for values >1 (P<0.001). The score which took into account all the available items (B) (US score >2, ELX 2/1 >1.73, SW >20 KPa, TTP ≥1.10 s, PI ≤0.97 s and BRAF V 600E mutation) showed 89.5% sensitivity and 75% specificity for values >2 (P<0.0001).
Discussion
FNAB is the gold standard examination for characterizing thyroid nodules after a primary ultrasonographic assessment [28]. In the event of an indeterminate cytology result, the repetition of FNAB can rule out malignancy in 30–60% of cases [28,29] and could therefore reduce unnecessary thyroidectomies [31]. One study reported an increased prevalence of malignancy when FNAB was repeated [30]; however, this finding may have been due to the fact that, in that study, the second FNAB was performed in those nodules in which clinical and ultrasonographic risk was higher. Indeed, similar findings did not emerge from previous studies [28–30]. If the second FNAB yields an indeterminate or non-diagnostic result, a follow-up program should always be considered; this will also depend on the age and clinical conditions of the single patient [29]. In patients with no absolute contraindication to surgery, however, further assessment is mandatory.
We particularly focused on the use of elastography, as several studies have found that low elasticity of the lesion is related with a higher incidence of malignancy [32–34], though it is also related with other histological features, such as the presence of fibrosis and the expression of galectin-3 and fibronectin-1 [33]. Although elastography can be performed in various ways and by means of various techniques [25], there is no consensus as to which approach is most able to rule out malignancy. In many cases, indeed, the semi-quantitative and operator-dependent real-time elastosonography (ELX 2/1) technique has shown a better performance than the more recent shear-wave technique [24, 26, 35]. Our data confirm this notion, since the ELX 2/1 score was the only parameter that correlated significantly with histological outcomes. We also noted that the results of ELX 2/1 and SW were not related to each other. This may be due to the fact they are based on 2 completely different physical parameters – i.e. the dimensional change in diameters under external pressure in the case of ELX 2/1, versus the characteristics of shear-wave propagation in the case of SW. On the other hand, ELX 2/1 is not always feasible, owing to the lack of normal thyroid tissue with which to compare the nodule, or in those situations in which physical compression cannot be applied.
In some cases, an approach that combines different ancillary techniques has proved to offer greater diagnostic accuracy than a single technique alone [18,36–39]. As reported in previous studies [15–17,40], our data confirmed the role of conventional ultrasound as the basis for the assessment, owing to its advantages in terms of cost and time. However, the need for a scoring system that performs better than the separate evaluation of single items is increasingly evident. Many different systems have been created in order to meet this need [16,17]; hypo-echogenicity, irregular margins, and the presence of microcalcifications are acknowledged to be risk factors for malignancy, while the role of intra-nodular vascularization is still debated [3,22,27,41].
In this regard, we found that, when the only tools we found to be significantly related to malignancy (US score, ELX 2/1 and BRAF mutation) were considered together, specificity was improved, but sensitivity was lower than that of the US score alone. However, on combining all the data we had, a high level of sensitivity was reached, while specificity remained acceptable.
The present study was certainly limited by the relatively small number of patients involved and, in particular, by the lack of histological diagnoses in many cases; this was due to the small number of FNAB that proved indeterminate. Nodules classified as benign on the second biopsy, however, were assigned to a follow-up program, in order to verify the absence of evolution of the lesion over time. Cut-off values obtained through ROC curve analysis could be influenced by the sample size and, sometimes, by the operator who performs the examination. For this reason, it is important for every center which carries out this kind of diagnostic investigation to have its own reference values, given the absence of universally standardized ones.
At last, it is important to specify that since a great inter-observer variability has widely been described both for US [42] and for cytological [43] evaluation of thyroid nodules, the presence of a single operator who analyzed all the cases in our study could be a limitation for the application of such data to the routine clinical practice.
Conclusions
The management of thyroid nodules with indeterminate cytology remains an area of diagnostic uncertainty. In about half of these cases, a second FNAB can rule out surgery. Many ancillary tools for the further assessment of these patients are available; each technique has its own peculiarity and can provide the clinician with particular information. A good knowledge of each technique allows physicians to maximize diagnostic performance and to combine the various techniques in order to obtain better sensitivity or specificity, according to their needs. ELX 2/1 seems to perform better than SW in distinguishing malignancy. Moreover, these 2 techniques do not appear to be related to each other nor interchangeable; they may instead be complementary in the context of a multiparametric assessment of lesions with indeterminate cytology.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Valderrabano P, McIver B. Evaluation and management of indeterminate thyroid nodules: The revolution of risk stratification beyond cytological diagnosis. Cancer Control. 2017;24(5):1073274817729231. doi: 10.1177/1073274817729231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract. 2016;22:622–39. doi: 10.4158/EP161208.GL. [DOI] [PubMed] [Google Scholar]
- 3.Giusti M, Massa B, Balestra M, et al. Retrospective cytological evaluation of indeterminate thyroid nodules according to the British Thyroid Association 2014 classification and comparison of clinical evaluation and outcomes. J Zhejiang Univ Sci B. 2017;18:555–66. doi: 10.1631/jzus.B1600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–65. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–46. doi: 10.1089/thy.2017.0500. [DOI] [PubMed] [Google Scholar]
- 6.Ulisse S, Bosco D, Nardi F, et al. Thyroid imaging reporting and data system score combined with the New Italian Classification for thyroid cytology improves the clinical management of indeterminate nodules. Int J Endocrinol. 2017;2017 doi: 10.1155/2017/9692304. 9692304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kocjan G, Chandra A, Cross P, et al. BSCC Code of Practice – fine needle aspiration cytology. Cytopathology. 2009;20:283–96. doi: 10.1111/j.1365-2303.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 8.Valderrabano P, Khazai L, Thompson ZJ, et al. Cancer risk associated with nuclear atypia in cytologically indeterminate thyroid nodules: A systematic review and meta-analysis. Thyroid. 2018;28(2):210–19. doi: 10.1089/thy.2017.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciacchitano S, Lavra L, Ulivieri A, et al. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget. 2017;25:49421–42. doi: 10.18632/oncotarget.17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko YS, Hwang TS, Kim JY, et al. Diagnostic limitation of fine-needle aspiration (FNA) on indeterminate thyroid nodules can be partially overcome by preoperative molecular analysis: Assessment of RET/PTC1 rearrangement in BRAF and RAS wild-type routine air-dried FNA specimens. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040806. pii: E806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaussin-Petrucci M, Descotes F, Depaepe L, et al. Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathology. 2017;28:482–87. doi: 10.1111/cyt.12493. [DOI] [PubMed] [Google Scholar]
- 12.Valderrabano P, Montilla-Soler J, Mifsud M, et al. Hypermetabolism on (18)F-fluorodeoxyglucose positron emission tomography scan does not influence the interpretation of thyroid cytopathology, and nodules with a SUVmax <2.5 are not at increased risk for malignancy. Thyroid. 2016;26:1300–7. doi: 10.1089/thy.2015.0654. [DOI] [PubMed] [Google Scholar]
- 13.Trimboli P, Deandrea M, Mormile A, et al. American Thyroid Association ultrasound system for the initial assessment of thyroid nodules: Use in stratifying the risk of malignancy of indeterminate lesions. Head Neck. 2018;40(4):722–27. doi: 10.1002/hed.25038. [DOI] [PubMed] [Google Scholar]
- 14.He YP, Xu HX, Zhao CK, et al. Cytologically indeterminate thyroid nodules: Increased diagnostic performance with combination of US TI-RADS and a new scoring system. Sci Rep. 2017;7(1):6906. doi: 10.1038/s41598-017-07353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trimboli P, Fulciniti F, Zilioli V, et al. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn Cytopathol. 2017;45:113–17. doi: 10.1002/dc.23651. [DOI] [PubMed] [Google Scholar]
- 16.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JSA, Dmytriw AA, Yu E, et al. Investigation of thyroid nodules: A practical algorithm and review of guidelines. Head Neck. 2018 doi: 10.1002/hed.25160. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Durante C, Grani G, Lamartina L, et al. The diagnosis and management of thyroid nodules: A review. JAMA. 2018;319(9):914–24. doi: 10.1001/jama.2018.0898. [DOI] [PubMed] [Google Scholar]
- 19.Giusti M, Campomenosi C, Gay S, et al. The use of semi-quantitative ultrasound elastosonography in combination with conventional ultrasonography and contrast-enhanced ultrasonography in the assessment of malignancy risk of thyroid nodules with indeterminate cytology. Thyroid Res. 2014;7(1):9. doi: 10.1186/s13044-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Luo YK, Zhang MB, et al. Diagnostic accuracy of contrast-enhanced ultrasound enhancement patterns for thyroid nodules. Med Sci Monit. 2016;22:4755–64. doi: 10.12659/MSM.899834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Wang Y, Bai B, et al. Advantages of routine ultrasound combined with contrast-enhanced ultrasound in diagnosing papillary thyroid carcinoma. Ultrasound Q. 2017;33:213–18. doi: 10.1097/RUQ.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Zhou H, Yang P, et al. Contrast-enhanced ultrasonography features of papillary thyroid carcinoma for predicting cervical lymph node metastasis. Exp Ther Med. 2017;14:4321–27. doi: 10.3892/etm.2017.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang BR, Kim EK, Moon HJ, et al. Qualitative and semiquantitative elastography for the diagnosis of intermediate suspicious thyroid nodules based on the 2015 American Thyroid Association Guidelines. J Ultrasound Med. 2018;37(4):1007–14. doi: 10.1002/jum.14449. [DOI] [PubMed] [Google Scholar]
- 24.Wojtaszek-Nowicka M, Słowińska-Klencka D, Sporny S, et al. The efficiency of elastography in the diagnostics of follicular lesions and nodules with an unequivocal FNA result. Endokrynol Pol. 2017;68(6):610–22. doi: 10.5603/EP.a2017.0050. [DOI] [PubMed] [Google Scholar]
- 25.Bardet S, Ciappuccini R, Pellot-Barakat C, et al. Shear wave elastography in thyroid nodules with indeterminate cytology: Results of a prospective bicentric study. Thyroid. 2017;27:1441–49. doi: 10.1089/thy.2017.0293. [DOI] [PubMed] [Google Scholar]
- 26.Sigrist RMS, Liau J, Kaffas AE, et al. Ultrasound elastography: Review of techniques and clinical applications. Theranostics. 2017;7:1303–29. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Liu Y, Qian L. Diagnostic potential of real-time elastography (RTE) and shear wave elastography (SWE) to differentiate benign and malignant thyroid nodules: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(43):e8282. doi: 10.1097/MD.0000000000008282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baloch Z, LiVolsi VA, Jain P, et al. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol. 2003;29:203–6. doi: 10.1002/dc.10361. [DOI] [PubMed] [Google Scholar]
- 29.Dincer N, Balci S, Yazgan A, et al. Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology. 2013;24:385–90. doi: 10.1111/cyt.12021. [DOI] [PubMed] [Google Scholar]
- 30.Kuru B, Atmaca A, Kefeli M. Malignancy rate associated with Bethesda category III (AUS/FLUS) with and without repeat fine needle aspiration biopsy. Diagn Cytopathol. 2016;44:394–98. doi: 10.1002/dc.23456. [DOI] [PubMed] [Google Scholar]
- 31.Chen JC, Pace SC, Chen BA, et al. Yield of repeat fine-needle aspiration biopsy and rate of malignancy in patients with atypia or follicular lesion of undetermined significance: The impact of the Bethesda System for Reporting Thyroid Cytopathology. Surgery. 2012;152:1037–44. doi: 10.1016/j.surg.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Pandey NN, Pradhan GS, Manchanda A, Garg A. Diagnostic value of acoustic radiation force impulse quantification in the differentiation of benign and malignant thyroid nodules. Ultrason Imaging. 2017;39:326–36. doi: 10.1177/0161734617706170. [DOI] [PubMed] [Google Scholar]
- 33.Rago T, Scutari M, Loiacono V, et al. Low elasticity of thyroid nodules on ultrasound elastography Is correlated with malignancy, degree of fibrosis, and high expression of galectin-3 and fibronectin-1. Thyroid. 2017;27:103–10. doi: 10.1089/thy.2016.0341. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Chang C, Gao Y, et al. Does shear wave elastography provide additional value in the evaluation of thyroid nodules that are suspicious for malignancy? J Ultrasound Med. 2016;35:2397–404. doi: 10.7863/ultra.15.09009. [DOI] [PubMed] [Google Scholar]
- 35.Tian W, Hao S, Gao B, et al. Comparison of diagnostic accuracy of real-time elastography and shear wave elastography in differentiation malignant from benign thyroid nodules. Medicine (Baltimore) 2015;94(52):e2312. doi: 10.1097/MD.0000000000002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccardo A, Puntoni M, Treglia G, et al. Thyroid nodules with indeterminate cytology: prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur J Endocrinol. 2016;174:693–703. doi: 10.1530/EJE-15-1199. [DOI] [PubMed] [Google Scholar]
- 37.Seshadri KG. A Pragmatic approach to the Indeterminate thyroid nodule. Indian J Endocrinol Metab. 2017;21:751–57. doi: 10.4103/ijem.IJEM_143_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn HS, Lee JB, Seo M, et al. Distinguishing benign from malignant thyroid nodules using thyroid ultrasonography: Utility of adding superb microvascular imaging and elastography. Radiol Med. 2018;123(4):260–70. doi: 10.1007/s11547-017-0839-2. [DOI] [PubMed] [Google Scholar]
- 39.Trimboli P, Guglielmi R, Monti S, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: A prospective multicenter study. J Clin Endocrinol Metab. 2012;97:4524–30. doi: 10.1210/jc.2012-2951. [DOI] [PubMed] [Google Scholar]
- 40.Deng XH, Tang LN, Liu SQ, et al. A proposal to stratify the Intermediate-risk thyroid nodules according to the AACE/ACE/AME guidelines with ultrasound features. Sci Rep. 2017;7(1):17901. doi: 10.1038/s41598-017-18207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakhshaee M, Davoudi Y, Mehrabi M, et al. Vascular pattern and spectral parameters of power Doppler ultrasound as predictors of malignancy risk in thyroid nodules. Laryngoscope. 2008;118:2182–86. doi: 10.1097/MLG.0b013e3181864ae7. [DOI] [PubMed] [Google Scholar]
- 42.Grani G, Lamartina L, Cantisani V, et al. Interobserver agreement of various thyroid imaging reporting and data systems. Endocr Connect. 2018;7(1):1–7. doi: 10.1530/EC-17-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padmanabhan V, Marshall CB, Akdas Barkan G, et al. Reproducibility of atypia of undetermined significance/follicular lesion of undetermined significance category using the bethesda system for reporting thyroid cytology when reviewing slides from different institutions: A study of interobserver variability among cytopathologists. Diagn Cytopatho. 2017;45(5):399–405. doi: 10.1002/dc.23681. [DOI] [PubMed] [Google Scholar]



