Abstract
Current vitamin E requirements are uniformly applied across the population for those >14 y of age. However, aging is associated with alterations in cellular and physiologic functions, which are affected by vitamin E. Therefore, it is questionable whether vitamin E requirements can be uniformly applied to all adult age categories. With aging, there is dysregulation of the immune system in which there are decreased cell-mediated and pathogen defense responses coupled with an overactive, prolonged inflammatory state. Both animal and human studies in the aged suggest that intake above currently recommended levels of vitamin E may improve immune and inflammatory responses and be associated with a reduced risk of infectious disease. We review the evidence that was considered in establishing the current requirements for vitamin E and highlight data that should be considered in determining the vitamin E requirements in older adults, particularly focusing on the evidence suggesting a benefit of increased vitamin E intake on immune function and inflammatory processes and resistance to infection. The main objective of this Perspective is to initiate the discussion of whether the current Dietary Reference Intake for vitamin E should be increased for the older population. We make this suggestion on the basis of mechanistic studies showing biological plausibility, correction of a major cellular dysfunction in older adults, and strong evidence from several animal and a few human studies indicating a reduction in risk and morbidity from infections.
Keywords: vitamin E requirements, older adults, immune system, inflammation, infection
Introduction
Vitamin E functions as a lipid-soluble antioxidant capable of scavenging oxygen species to reduce oxidative stress and protect cell membranes from oxidative damage. Vitamin E is a generic term that describes all naturally occurring, structurally related tocopherols and tocotrienols, each with 4 homologs designated as α, β, γ, and δ. Among them, α-tocopherol is the most abundant form of vitamin E in the diet and plasma, and it has also been shown to be the most biologically active form (1, 2). For these reasons, α-tocopherol is the form of vitamin E for which the DRIs are established (3). Vitamin E supplements are made exclusively from α-tocopherol, primarily as the synthetic form dl-α-tocopherol, often esterified as dl-α-tocopheryl acetate. To determine the number of milligrams in supplements and comparisons between studies that use natural or synthetic forms of vitamin E, International Units are used. One IU of vitamin E is equivalent to 0.67 mg d-α-tocopherol (“natural” vitamin E) and 1 mg dl-α-tocopheryl acetate (“synthetic” vitamin E). The referral of vitamin E for Estimated Average Requirements (EARs), RDAs, and Adequate Intakes applies only to intake of the 2R-stereoisomeric forms of α-tocopherol from food, fortified food, and multivitamins, whereas the Tolerable Upper Intake Level applies to any forms of supplemental α-tocopherol. The current DRIs for vitamin E are 12 mg/d (EAR) and 15 mg/d (RDA) for all individuals ages ≥14 y, presented by age and sex categories (3). However, it is questionable whether vitamin E requirements can be uniformly applied to all adult age categories because its bioavailability and utilization is influenced by a variety of factors that are influenced by age, including dietary intake, absorption, transport, and metabolism [reviewed in (4, 5)].
Although overt vitamin E deficiency, characterized by sensory neuropathy and increased erythrocyte fragility, is rare, it has been reported that >60% of adults in the United States have vitamin E intakes below the EAR (<12 mg/d) (6). The 2015 Dietary Guidelines Advisory Committee characterized vitamin E as a “shortfall” nutrient, as determined by the EAR. However, because vitamin E underconsumption was not linked to biomarkers or health outcomes, it was not considered a nutrient of public health concern. Arguably, rather than focusing on vitamin E dietary insufficiency, perhaps the benefits of increasing consumption and improved health outcomes should be the focus, particularly in older adults. The Institute of Medicine (IOM) recommendations make no distinction between adult age categories or between sexes, which is based on the conclusion that there is no evidence that the aging process impairs vitamin E absorption or utilization or scientific evidence that vitamin E requirements vary in men and women (3). However, it is well documented that older adults (>65 y of age) have compromised immune and inflammatory responses, as well as enzymatic antioxidant defensive mechanisms, which increase their risk of both infectious and noninfectious chronic diseases (7–10) compared with younger adults. Furthermore, studies have provided evidence that older adults who consume vitamin E above the current DRI may increase their susceptibility to age-associated biological changes, particularly those related to immune function, inflammation, and resistance to infection.
We review the evidence that was considered in establishing the current DRI for vitamin E and highlight data that should be considered in determining the vitamin E requirements in the older adults, particularly focusing on the evidence that suggests a benefit of increased vitamin E intake on immune function and inflammatory processes and resistance to infection. We discuss variations in outcomes from studies examining vitamin E and immune function and address some of the controversy related to high vitamin E intake in the adult population. The main objective of this Perspective article is to initiate the discussion on whether the current DRI for vitamin E should be increased for the older population.
Current Status of Knowledge
Evidence that was considered in establishing the current requirements for vitamin E
Because overt human vitamin E deficiency is rare, there are limited clinical data available to establish daily requirements. Therefore, the IOM derived requirements for vitamin E based almost exclusively on experimental data from the Elgin project conducted, which took place between 1953 and 1967 and has been described in detail in the IOM DRI 2000 (3). Briefly, healthy men (age not specified) were depleted of vitamin E for >6 y, then repleted, and blood was drawn to test in vitro hydrogen peroxide–induced hemolysis in addition to plasma concentrations of vitamin E (α-tocopherol) (11–13). With vitamin E depletion, erythrocyte fragility, as assessed by in vitro hydrogen peroxide–induced hemolysis, was observed, and was subsequently reversed upon vitamin E repletion. Cutoffs were set on the basis of the plasma α-tocopherol concentration that could limit hydrogen peroxide–induced hemolysis to ≤12%. A plasma concentration of 12 µmol/L was determined to be the lowest concentration that could prevent hydrogen peroxide–induced hemolysis. Vitamin E adequacy was then determined on the basis of intake sufficient to increase plasma α-tocopherol concentrations to 12 µmol/L. When vitamin E–depleted subjects were repleted, plasma α-tocopherol concentrations were in linear range with α-tocopherol intake. On the basis of the criteria of a minimum of 12 µmol α-tocopherol/L in plasma, the EAR for vitamin E requirement was set as 12 mg α-tocopherol/d. The RDA was then established by assuming a CV of 10%, calculated as the EAR plus 2 CVs to meet requirements of 97–98% of individuals. Therefore, the current RDA for individuals ages ≥14 y was set at 15 mg/d.
Issues with criteria used for determining vitamin E requirements
As mentioned above, the current requirements for vitamin E for individuals aged ≥14 y were established on the basis of the in vitro assay of hydrogen peroxide–induced RBC hemolysis. It is questionable whether this assay represents the best choice to assess redox status in both technical and conceptual contexts and if it should be the main criterion to assess vitamin E requirement. In addition to the choice of biomarker, there are several issues that are important in discussing the appropriateness of the current vitamin E requirements for older populations (see below).
Hydrogen peroxide–induced RBC hemolysis assay is outdated
From the technical perspective, there are various limitations of the hydrogen peroxide–induced RBC hemolysis assay that has been recognized by the IOM. In the DRI document, it is stated that “hydrogen peroxide–induced RBC hemolysis is the best marker at [the] present time,” acknowledging that “it should be emphasized that research is urgently needed to explore the use of other biomarkers to assess vitamin E requirements” (3). The use of this assay as the best method to assess redox status has been widely debated. Horwitt (14) and other investigators (15) recognized the great variability of this procedure, stating that many variables, including the concentration of hydrogen peroxide and the rate at which it is added to the cell suspension, the incubation time, and temperature, can all affect the hemolysis rate. Although this classic hemolysis assay might have been the best method available at the time, these older technologies for redox status detection have been replaced by newer, more advanced, and comprehensive methods to allow for further verification and update of the methodology for assessing redox status [reviewed by Dikalov and Harrison (16)] and potentially vitamin E requirements. This includes new immunospin and mitochondrial-specific fluorescent probes and immunoassays that are more sensitive than previous methods (16).
The relevance of RBC hemolysis assay to the function of other cells is not clear
Assuming that the RBC, or erythrocyte, hemolysis assay accurately assesses the vitamin E requirement to prevent oxidative stress–induced hemolysis in RBCs, the relevance of this test, in an atypical cell, to the function of other cells is not clear. The vitamin E membrane content required to maintain normal cellular functions varies greatly depending on cell type. Within the blood component, the vitamin E content of erythrocytes is ∽0.2 µg/109 cells (17). However, immune cells are particularly enriched in vitamin E, likely because these cells contain high concentrations of PUFAs and are highly metabolically active, making them prone to oxidative damage (18, 19). Monocytes contain ∽125-fold more vitamin E (25 µg/109 cells) compared with erythrocytes (17), and neutrophils, lymphocytes, and platelets contain 23-fold, 20-fold, and 3-fold more vitamin E compared with erythrocytes, respectively (17). This broadly varied vitamin E content between cell types may well reflect the higher amount of vitamin E needed to maintain optimal immune function, as discussed in a study by Bendich et al. (20). Arguably, if an individual cell is to be used to determine requirement, then the cell with the highest requirement should be considered.
Nonantioxidant functions of vitamin E were not considered
As stated in the DRI report, the requirements for vitamin E were based on the biomarker of plasma α-tocopherol concentrations and corresponding hydrogen peroxide–induced erythrocyte lysis. Although this test reflects the antioxidant property of vitamin E, it fails to consider important nonantioxidant functions of vitamin E. These unique nonantioxidant functions of vitamin E were thoroughly reviewed by Azzi (21) and Zingg and Azzi (22). Relevant to this perspective is the immunomodulatory actions of vitamin E, which extend beyond its antioxidant role [e.g., in lymphocyte proliferation, prostaglandin E2 (PGE2) production, gene transcription, translation, cell membrane, and signal transduction] (23, 24). Results from these studies showed that the efficacy of different forms of tocopherols in affecting immune cell function is not predicted by their antioxidant capacity.
No physiologic or clinical significance was considered
The DRI report mentions that hydrogen peroxide–induced RBC hemolysis is one of a limited number of tests in which the marker, erythrocyte lysis, has been correlated with an actual health deficit, decreased erythrocyte survival. This assay focuses primarily on deficiency rather on than improving cellular function, prevention of disease, or health promotion, which are the criteria more recently used by the DRI committee to establish nutrient requirements (3). Furthermore, the relevance of erythrocyte hemolysis to age-associated cellular dysfunction and diseases is not clear. Thus, more age-specific, carefully selected biomarkers and outcomes such as the immune response should be used to define the optimal intake of vitamin E for older adults (see below). These variables should represent those that have physiologic significance and clinical relevance linking to the health status and risk of developing age-associated diseases.
Age was not considered when determining vitamin E requirements
The current vitamin E requirements are uniformly applied across the population, with the exception of those individuals <14 y of age. The requirements for certain dietary components for the elderly may need to be increased to reflect their special needs because of the age-associated molecular and cellular changes resulting in less than optimal function and increased risk of diseases. For example, the need for antioxidant nutrients may increase with age to counteract the age-associated reduced enzymatic antioxidant defense leading to an increase in oxidative stress (25). It is questionable whether the vitamin E requirements determined from a study in men can be widely applied to adults of all ages. As previously mentioned, the IOM recommendations make no distinction between adult age categories on the basis of the conclusion that there is no evidence that the aging process impairs vitamin E absorption or utilization (3). Indeed, although tissue concentrations of vitamin E in older individuals are comparable to those in younger individuals (19, 26) older adults have increased oxidative stress and resulting cell damage (27–29), which is believed to be one of the mechanisms underlying the age-related dysregulation of the immune and inflammatory responses (30), resulting in an increase in the incidence, morbidity, and mortality from infection and noninfectious chronic diseases (31, 32). Some of these age-associated changes have been shown to be improved by vitamin E supplementation, which should be an important consideration in determining if the current DRI is appropriate for the elderly.
Evidence That Should Be Considered in Revising the Current Dietary Requirements for Vitamin E in Older Adults
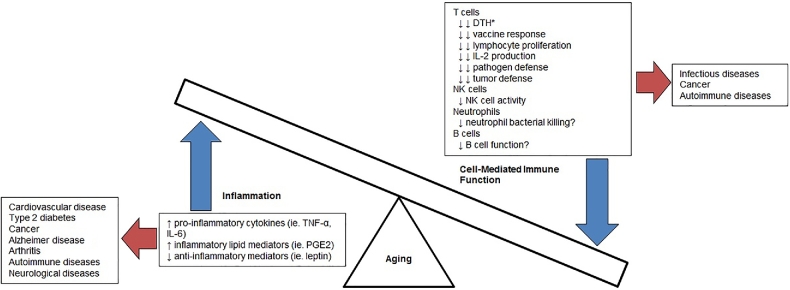
Since 2000, when vitamin E requirements were last evaluated, there has been growing evidence to suggest that older adults may benefit from a higher-than-recommended level of vitamin E. In aging, there is a dysregulation of the immune function, a phenomenon often termed “immunosenescence” (33–36). Dysregulation of the immune system that occurs with age is marked by hyporeactive cell-mediated and pathogen defense–related immune responses, particularly of T cell–mediated function, coupled with an overactive, prolonged inflammatory state referred to as “inflammaging” (Figure 1). The decline in T cell–mediated immunity is a key contributor to the increased susceptibility of older adults to infection and cancer, whereas the age-associated chronic inflammation is thought to contribute to increased risk of and susceptibility to chronic diseases such as cardiovascular disease, cancer, diabetes, and Alzheimer disease [reviewed by Michaud et al. (37)]. Alterations in inherent functions of the immune system are confounded by systemic factors including metabolic and hormonal changes that also affect the aging immune system. The general functions and age-associated changes in the major cell populations in the innate and adaptive immune systems have been reviewed thoroughly by Wu and Meydani (38), Shaw et al. (39), Panda et al. (40), and Nikolich-Zugich (41).
FIGURE 1.
Dysregulation of inflammation and cell-mediated immune function that occurs with aging and the relation to the pathogenesis of various age-associated diseases. Number of arrows indicate the strength of evidence, with double arrows indicating strong evidence and single arrow indicating moderate evidence. *Indicates skin response that is an in vivo measure of cell-mediated immune function and assessment of cell-mediated immune function. DTH, delayed-type hypersensitivity; PGE2, prostaglandin E2.
Given the well-established dysregulated immune and inflammatory responses with age across all species, the key role these immune and inflammatory responses play in the risk of and morbidity and mortality from diseases associated with aging, the immune system presents itself as a meaningful and clinically relevant biological system to determine requirements for a nutrient such as vitamin E in older adults. In establishing the requirement for vitamin E, the criteria were set primarily on the basis of the prevention of cellular dysfunction leading to disease (3). Yet, data related to improvements in immune dysfunction, such as that of the immune response with age, were not considered.
Mechanisms for Vitamin E's Effects on Immune Function
The mechanisms underlying the immunomodulatory actions of vitamin E have been studied in cell culture and animal models, which were reviewed extensively by Wu and Meydani (42) and Meydani et al. (43). Briefly, vitamin E can affect T cell function directly by influencing membrane integrity and signal transduction, or indirectly by reducing the production of suppressive and inflammatory factors such as PGE2. In addition, because vitamin E is a potent antioxidant, localized in lipid compartments, it may play a critical role in correcting the age-associated dysregulation of redox status and altered cellular functions. This is particularly important for immune cells because of their high membrane content of PUFAs, and because their activity and function is highly dependent on cell membrane composition and structure. By preventing lipid peroxidation and the associated damage to cell membranes, vitamin E may help maintain the integrity and function of immune cell membranes. In addition, vitamin E may directly modulate certain properties of cell membranes, including lipid raft mobility, which, in turn, may influence movement and activation of surface signaling molecules [reviewed by Wang and Quinn (44)]. Thus, it is proposed that vitamin E may counteract the age-associated impairement in the immune function through its impact on immune cell membrane function and structure.
A number of animal and human studies have indicated that vitamin E deficiency impairs both humoral and cell-mediated immune functions [reviewed by Han and Meydani (45)]. Although overt vitamin E deficiency is rare, the 2015 Dietary Guidelines Advisory Committee recognized vitamin E as an underconsumed nutrient, determined by the DRI (46). This dietary insufficiency is of greater concern for older adults, who are already at higher risk of nutritional deficiencies and inadequate intake (47, 48) due to a variety of factors, including alterations in biological and physiologic functions, illness, hospitalization, and polypharmacy as well as socioeconomic determinants [reviewed in Brownie (49)]. Thus, providing vitamin E beyond what is currently recommended may be beneficial in improving vitamin E intake as well the immune function in older adults.
Vitamin E improves dysregulated immune and inflammatory responses in the aged
There is evidence from both animal and human studies that higher than currently recommended levels of vitamin E are necessary to balance the immune and inflammatory responses in the aged. The impact of vitamin E supplementation on age-associated changes in immune and inflammatory responses and risk of infection has been thoroughly reviewed by Wu and Meydani (42) and Pae and Wu (50).
Studies in animal models have shown a strong beneficial effect of vitamin E on age-associated deterioration in immune and inflammatory responses (summarized in Table 1). Meydani et al. (51) showed that old mice (24 mo) had significantly lower T cell proliferation, IL-2 production, and delayed-type hypersensitivity (DTH) response compared with young mice (3 mo). Old mice fed 500 parts per million (ppm) vitamin E (dl-α-tocopheryl acetate) had significantly higher T cell–mediated responses, including lymphocyte proliferation, IL-2 production, and DTH response, compared with mice fed a diet containing adequate amounts of vitamin E (30 ppm). Of note, the impaired T cell–mediated responses observed in old mice improved with vitamin E supplementation to levels comparable to those seen in young mice (51). Vitamin E supplementation also reversed the age-associated increase in the production of the inflammatory eicosanoid PGE2, as well as lipid peroxidation (51, 67). In subsequent experiments, Beharka et al. (68), Hayek et al. (60), and Wu et al. (67) showed that vitamin E reduced PGE2 production by inhibiting the activity of enzyme cyclooxygenase 2. Furthermore, these studies showed that vitamin E enhanced T cell–mediated function in the older animals through 2 different mechanisms: 1) indirectly by inhibiting PGE2 production (a T cell–suppressor factor) from macrophages and 2) directly enhancing the function of naïve CD4+ T cells, the subset of T cells that exhibit the most age-related defects (59, 68). Isolated naïve T cells from old mice had a significantly lower ability to divide and to produce IL-2 compared with those from young mice. Supplementation of naïve cells from old mice with vitamin E, at levels comparable to plasma concentrations of vitamin E in humans taking 200 IU d-α-tocopherol/d, significantly increased the ability of old naïve cells to divide and produce IL-2, reaching concentrations similar to those in T cells from young animals (59). Vitamin E had no significant effect on PGE2 production or T cell function of young mice, indicating that the effect of vitamin E was specific to old mice with compromised immune and inflammatory responses. Further molecular studies showed that vitamin E's enhancement of naïve T cell function was due to the correction of membrane-associated key signaling molecules, including tyrosine-protein kinase ZAP70, linker for activation of T cells family member 1 (LAT), phospholipase-Cγ, and Vav proteins (93). In addition to reducing the production of inflammatory PGE2, vitamin E has been shown to reduce the production of other inflammatory markers such TNF-α and IL-6 in the aged, particularly in response to pathogens (61, 67, 83).
TABLE 1.
Effect of age and vitamin E supplementation on immune variables associated with cell-mediated immune functions in animals and humans1
| Immune variable | Effect of age | References | Effect of vitamin E supplementation | References |
|---|---|---|---|---|
| Lymphocyte proliferation | ||||
| Animals | ↓ After stimulation | (51, 52) | ↑ After stimulation | (51–53) |
| Humans | ↓ After stimulation | (54–56) | ↑ After stimulation | (57, 58) |
| IL-2 | ||||
| Animals | ↓ Production | (51–53, 59–62) | ↑ Production | ( 51–53, 59–61) |
| Humans | ↓ Production | (54, 63, 64) | ↔ Production | (65) |
| ↑ Production | (58, 66) | |||
| PGE2 | ||||
| Animals | ↑ Production | (51, 52, 67, 68) | ↓ Production | (51, 52, 67, 68) |
| Humans | ↑ Production | (66, 69–71) | ↓ Production | (66) |
| DTH | ||||
| Animals | ↓ Response | (51, 62) | ↑ Response | (51) |
| Humans | ↓ Response | (72–74) | ↑ Response | (66, 75) |
| ↔ Response | (65) | |||
| Vaccine response | ||||
| Animals | ↓ Response to tetanus vaccine | (76, 77) | ||
| Humans | ↓ Response to tetanus vaccine | (78) | ↑ Response to hepatitis B vaccine | (75) |
| ↑ Response to tetanus vaccine | ||||
| ↔ Response to diphtheria vaccine | ||||
| ↔ Response to pneumococcal vaccine | (79) | |||
| ↓ Response to influenza vaccine (meta-analysis of 3 antigens) | (80) | |||
| NK cells | ||||
| Animals | ↓ NK cell activity | (81) | ↔ NK cell activity in unchallenged mice | (81) |
| ↑ NK cell activity after challenge (SRBC injection) | ||||
| Humans | ↓ NK cell activity | (63, 82) | ↑ NK cell activity | (58) |
| Respiratory infections | ||||
| Animals | ↑ Susceptibility to Streptococcus pneumonia infection | (83) | ↓ Susceptibility to S. pneumonia infection | (83) |
| ↑ Susceptibility to influenza infection | (84, 85) | ↓ Susceptibility to influenza infection | (84, 85) | |
| Humans | ↑ Risk of pneumonia, tuberculosis, and influenza | (10) | ↔ Incidence and severity of acute RTI | (86) |
| ↑ Risk of mortality from lower RI | (8) | ↓ Number of subjects with all or upper RI | (87) | |
| ↓ Incidence of common cold | ||||
| ↔ Incidence and number of days with infection for all or upper or lower RI | ||||
| ↔ Antibiotic use | ||||
| ↑ Recovery period, higher morbidity and mortality from RI | (88) | ↓ Pneumonia risk for male smokers over the age of 60 | (89) | |
| ↑ Risk of hospitalization from RI | (90) | ↓ Risk of cold for male smokers over the age of 65 | (89, 91) | |
| ↓ Risk of rehospitalization within 90 d after pneumonia | (92) | |||
DTH, delayed-type hypersensitivity; PGE2, prostaglandin E2; RI, respiratory infection; SRBC, sheep RBC; ↓, decrease; ↑, increase; ↔ , no change.
Studies by others have also shown vitamin E to inhibit PGE2 production and to improve T cell–mediated immune response in older mice (52, 94). The age-specific nature of vitamin E's effect—improving specific age-associated cell and molecular defects—provides evidence for biological plausibility and correction of cellular dysfunction and strongly suggests that the vitamin E requirement for optimal immune and inflammatory response in the aged is higher than that in the young.
Similarly, in humans, there is strong evidence to suggest that vitamin E supplementation improves dysregulated immune functions in older adults (Table 1). Several double-blinded, placebo-controlled clinical trials have been conducted that showed the efficacy of vitamin E on improving T cell–mediated and innate immune functions in older adults. In a group of healthy older adults (≥60 y old), supplementation of 800 IU vitamin E/d (dl-α-tocopheryl acetate) for 1 mo improved in vivo and ex vivo indexes of T cell–mediated functions, which have been shown to decline with age, including lymphocyte proliferation, IL-2 production, and DTH response, while decreasing PGE2 production and plasma lipid peroxidation levels (66). In a subsequent double-blind randomized trial aimed to define the optimal level of vitamin E for immune enhancement, Meydani et al. (75) supplemented healthy older adults (>65 y of age) with placebo or 60, 200, and 800 IU vitamin E/d for 4.5 mo. They showed that daily intake of 200 IU vitamin E (dl-α-tocopherol) improved antibody titers to tetanus and hepatitis B vaccines as well as DTH response (Table 2). From this study, a dose of 200 IU vitamin E was shown to be most effective in improving T cell–mediated functions, compared with 60- or 800-IU/d doses (75) (Table 2). De la Fuente et al. (58) also showed that supplementation with 200 IU vitamin E (dl-α-tocopherol) for 3 mo enhanced T cell function in independently living, healthy elderly, including increased mitogen-stimulated lymphocyte proliferation and IL-2 production, compared with preintervention. The functions of neutrophils and NK cells in elderly subjects were also improved with vitamin E supplementation, comparable to that in adult controls (58). However, vitamin E supplementation in independently living elderly Dutch (65–80 y) at 100 IU/d, but not 50 IU/d, for 6 mo tended to increase the DTH response (P = 0.06) (65). This suggests that lower amounts of vitamin E may not be as effective in improving age-associated immune dysfunction, although the higher baseline plasma vitamin E concentrations in the elderly population in the Dutch study might have contributed to the nonsignificant effect of vitamin E at 100 IU/d.
TABLE 2.
Effect of vitamin E supplementation on antibody response to hepatitis B and DTH response in healthy elderly adults1
| Intervention group | Subjects, n | Change in antibody response to hepatitis B,2 U/mL | Change in DTH diameter of induration,3 % |
|---|---|---|---|
| Placebo | 14 | 3.3b | 18b |
| Vitamin E | |||
| 60 IU/d | 18 | 6.4b | 42b |
| 200 IU/d | 18 | 19.9a | 65a |
| 800 IU/d | 17 | 5.2a | 50b |
Data adapted from reference 75. Means within a column that do not share a common superscript letter differ, P < 0.05. DTH, delayed-type hypersensitivity.
A standard dose of hepatitis B vaccine (Recombivax HB, gift from Merck, Wesport, PA) was administered on day 156 of the study and 2 additional booster vaccinations were administered on days 186 and 216. Blood was collected before vaccination, 1 mo after vaccination, and 1 mo after each booster administration, and serum antibody concentrations were measured. Change in antibody response to hepatitis B vaccine represents the difference in antibody concentrations 1 mo after the third hepatitis B booster administration compared with before vaccination. Values represent a mean change from baseline.
DTH skin response was assessed with Multi-Test CMI (Merieux Institute Ince, Miami, FL) and compared a glycine control with 7 recall antigens: tetanus toxoid, diphtheria toxoid, Streptococcus (group C), Mycobacterium tuberculosis, Candida albicans, Trichophyton mentagrophytes, and Proteus mirabilis. The percentage change in DTH diameter of induration refers to the difference between baseline and 128 d postsupplementation, where the diameter of induration is representative of the current cell-mediated immunity. Values represent a mean change from baseline.
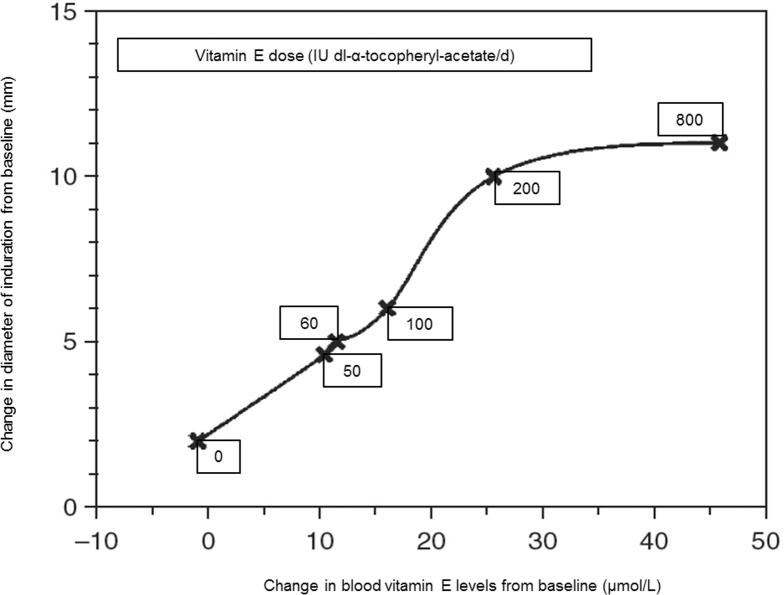
Of note, when results from these studies are considered together, a linear relation is observed between an increase in DTH response and elevation in plasma vitamin E concentrations up to 25 µmol/L (43, 65, 95) (Figure 2). Above this plasma concentration of vitamin E, no further increase in DTH was observed (43). Change in plasma vitamin E concentration of 25 µmol/L is achievable by consuming 200 IU vitamin E/d, whereas supplementation with doses of 50, 60, and 100 IU vitamin E/d resulted in a mean increase in plasma vitamin E concentrations by 10–15.8 µmol/L (43, 65). Thus, we suggest that sufficient change in plasma vitamin E concentrations for optimal immune response in older adults is achievable by consuming ∽200 IU vitamin E/d (43). This is supported by similar results observed in response to hepatitis B vaccine (75). The dose-response enhancement in DTH as well as improvement in vaccine response in older adults are of clinical significance because DTH has been shown to be a predictor of mortality in older adults (31, 96) and age-associated decreases in vaccine efficacy predisposes older adults to higher morbidity and mortality from infections.
FIGURE 2.
Relation between changes in delayed-type hypersensitivity skin response (diameter of induration) and changes in blood vitamin E concentrations from baseline after 30 d of supplementation with different doses of vitamin E in healthy older adults. Reproduced from reference 95 with permission.
Clinical benefits of vitamin E supplementation in the aged
The dysregulation of immune and inflammatory responses with age is believed to underlie the pathophysiology of many age-associated diseases and the morbidity and mortality associated with them, including infections, cardiovascular and neurologic diseases, diabetes, cancer, and arthritis (32, 97). Infectious diseases, including pneumonia, tuberculosis, Herpes zoster, and gastrointestinal and urinary tract infections, are among the leading causes of morbidity and mortality in elderly (7, 10), resulting in a large health care burden (98). Older adults also have a higher risk of certain autoimmune diseases and a higher incidence and risk of mortality from most cancers (31).
In addition to clinical benefits achieved through the enhancement of DTH and vaccine efficacy by vitamin E supplementation in the aged, evidence from animal models and human studies indicates that consuming higher-than-recommended levels of vitamin E may reduce the risk of respiratory infections in older adults. Respiratory infections, particularly influenza and streptococcus pneumonia, are among the leading causes of morbidity and mortality in older adults (7, 10). Older adults not only are more susceptible to respiratory infections but, once infected, they have a longer recovery period and exhibit higher morbidity, hospitalization, and mortality (10, 88). Therefore, respiratory infections significantly affect quality of life in older adults (90).
Several studies have shown that after influenza A/Port Chalmers/1/73 (H3N2) infection, old mice have impaired IL-2 and IFN-γ production, significantly higher lung viral titers, lung pathology, weight loss, and mortality compared with young mice (85, 99, 100). Hayek et al. (84) and Han et al. (85) showed that old mice infected with influenza virus and supplemented with 500 ppm vitamin E had significantly less lung viral titer, pathology, weight loss, and mortality compared with old mice supplemented with 30 ppm vitamin E for 30 d. These effects were associated with an improvement in IL-2 production, a key cytokine needed for T cell function and reduction in inflammatory cytokines (61, 84). Old mice are also more susceptible to Streptococcus pneumonia infection than are young mice. This is in part due to unregulated migration of neutrophils to lungs and their higher production of inflammatory cytokines (83). Vitamin E supplementation (500 ppm d-α-tocopheryl acetate) of old mice significantly reduced neutrophil migration and inflammatory cytokine production, leading to a significant reduction in mortality due to S. pneumonia infection (83). The protective effect of vitamin E was observed in old but not young mice, again suggesting age-specific requirements for vitamin E.
In humans, several studies have shown a clinical benefit of supplementation of vitamin E in the elderly (summarized in Table 1). Self-reported vitamin E supplementation was associated with a 63% lower rate of rehospitalization within 90 d in older adults who were previously hospitalized with pneumonia (92). In a double-blind, placebo-controlled trial, nursing home residents (≥65 y old) supplemented with an optimal level of vitamin E for immune response [i.e. 200 IU vitamin E/d (dl-α-tocopherol)] for 1 y had a significantly lower (22–30%) incidence of upper respiratory infection, particularly the common cold (87). A significant reduction in the duration of the common cold was also observed (87). A study conducted in Dutch elderly (≥60 y old) did not find a significant effect of vitamin E (200 IU/d) on respiratory infections (86). Differences in the study population, including genetic and nutritional status, may have contributed to differences in the results obtained between the 2 studies. Given that the DRIs are established for the North American population, results from studies conducted in the United States may be more applicable in determining recommendations for this population. A secondary analysis from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study examined the effect of 50 mg/d supplementation of vitamin E (dl-α-tocopherol) for 5–8 y in male Finnish smokers (50–69 y old). Results showed that vitamin E supplementation affected the risk of pneumonia but was dependent on the characteristics of the subgroup (e.g., age of initiation of smoking) and was largely influenced by age (89, 91). Hemila (89) concluded that vitamin E supplementation reduces pneumonia risk only when subjects were >60–65 y old, and nonsignificant for younger males. Similar to pneumonia risk, the effect of vitamin E supplementation on the risk of the common cold was largely influenced by the age of the subjects (after approximately age 65), as well as smoking status and whether they lived in urban or rural areas (91).
Although the effect of vitamin E on the risk of noncommunicable chronic diseases is controversial, it is interesting to note that in the Women's Health Study supplementation with 600 IU vitamin E (α-tocopherol) every other day for an average of 10.1 y significantly reduced the risk of major cardiovascular events only in those >65 y of age (26% risk reduction, P = 0.009) (101), suggesting different vitamin E requirements for disease risk reduction between young and older adults. Taken together, animal and human studies indicate that supplementation with higher than currently recommended levels of vitamin E is needed in order to correct dysregulation of immune and inflammatory responses, a major age-associated biological change that has been identified as a key contributor to infectious and chronic disease in older adults. These studies suggest that correcting these cell and molecular dysfunctions will result in a reduced risk of, and morbidity from, age-associated diseases, particularly infections.
Risks Associated with Higher-Than-Recommended Levels of Vitamin E Intake
It is important to recognize the potential risk of recommending increased vitamin E consumption, and to consider the safety margin when determining new dietary allowances for vitamin E. Concerns have been raised with regard to the safety of vitamin E supplementation because of the potential of vitamin E to affect platelet aggregation and blood clotting. However, various studies have confirmed that supplementation with vitamin E, within the Tolerable Upper Limit (i.e., 1000 mg/d of any form of supplementary α-tocopherol) posed no potential risk. Supplementation with vitamin E at doses between 60 and 800 IU/d (equivalent to 55–727 mg/d) for 30 d and 4 mo in healthy older adults had no adverse effects on health, including bleeding time, autoantibody production, and creatinine concentrations (102, 103). In addition, supplementation with 200 IU vitamin E/d for 1 y in nursing home residents with some pre-existing conditions did not cause any adverse effects, including mortality. In fact, those supplemented with 200 IU vitamin E/d had a 10% lower mortality than those who were treated with placebo for 1 y. Similarly, long-term prospective studies confirm that there is no increased risk in cardiovascular-related events with vitamin E supplementation (104). Furthermore, in a meta-analysis by Miller et al. (105), which included 19 clinical trials (mean age range: 47–84 y; mean vitamin E dose: 16.5–2000 IU/d), showed that vitamin E at doses ≤400 IU/d had no effect on all-cause mortality. In fact, this meta-analysis showed that, for dosages <150 IU/d, all-cause mortality slightly decreased (105). On the basis of evidence from clinical trials, Hathcock et al. (106) concluded that vitamin E supplements ≤1600 IU (1073 mg d-α-tocopherol/d) appear to be safe for most adults. Thus, the optimal level defined for improving immune response in older adults (i.e., 200 IU/d, which is 9-fold the currently recommended level) is both efficacious and safe, and well below the Tolerable Upper Level for α-tocopherol.
Recommendations for Revising Vitamin E Recommendations for Older Adults and Suggestions for Future Research
Considering the revision of the current recommendations for vitamin E, it is useful to take into account the criteria that were used to determine the vitamin E requirement and compare these with the criteria the 2000 DRI committee put forward to re-evaluate nutrient recommendations. In establishing the requirement, the criteria were set on the basis of prevention of cellular dysfunction leading to disease (3). However, the evaluation mainly focused on chronic diseases and data related to improvement of cellular dysfunction, such as that of the immune response or infection, were not considered. Furthermore, the criteria used to establish vitamin E requirement (prevention of RBC hemolysis) are not appropriate to determine vitamin E requirements to prevent relevant cellular dysfunction leading to the prevention of disease in older adults. In an editorial, Traber (107) commented that establishing the function of vitamin E is key to establishing requirements. Since the 2000 DRIs, there have been many studies that have aimed to establish the functions of vitamin E. On the basis of the studies reviewed and the discussion put forward above, one of the major functions of vitamin E is correction of the dysregulated immune cell and inflammatory functions in older adults, which should be considered in determining recommendations for vitamin E in this population. In particular, the dose-response studies conducted in humans defining the optimal level of vitamin E in older adults are worthy of consideration. Cell and molecular studies also provide strong support for biological plausibility with regard to specific vitamin E requirements for older adults. Furthermore, the totality of evidence from animal and human studies suggests a reduction in risk of infectious disease, a major cause of morbidity and mortality, with higher than currently recommended levels of vitamin E in older adults.
Thus, we propose that vitamin E recommendations for older adults need to be re-evaluated. In the past 18 y since the DRIs were evaluated, there has been growing evidence to suggest that, for older adults, there is a benefit to increased vitamin E intakes. This Perspective focuses on the benefits to immune and inflammatory variables. In order to consider revision of recommendations of vitamin E, a panel of vitamin E experts should come together and consider the totality of evidence, including other health outcomes (cardiovascular, metabolic) and functions of vitamin E. The panel of experts can determine if the existing information is adequate to revise recommendations or identify gaps that need to be addressed before such change can be made. Among questions to consider are the efficacy of other forms of tocopherol and the role of other forms in consideration in revising recommendations. Along these lines, there is emerging evidence that other homologs of vitamin E are unique and may potentially have higher efficacy (21). Furthermore, the utilization of synthetic (dl-α-tocopherol) compared with natural vitamin E (d-α-tocopherol), which could differentially affect T lymphocyte gene transcription (24), should be considered.
Conclusions
The appropriateness of the criteria on which vitamin E recommendations were based is outdated and questionable when applied broadly to all individuals >14 y of age. Evidence, based on biological plausibility as well as data from animal studies and clinical trials that show the efficacy of vitamin E in improving immune response and reducing the risk of infection in the aged, strongly suggests that higher than currently recommended levels of vitamin E might be needed for the elderly. The current literature suggests that 200 IU/d is most effective for improving immune function in the elderly. Low dietary consumption of vitamin E (6), coupled with compelling evidence that increased intake of vitamin E above current recommendations for the general population may benefit older individuals, presents a strong rationale for suggesting a revision of the dietary recommendations for vitamin E.
Acknowledgments
All authors read and approved the final manuscript.
Notes
Perspectives articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or points of view. Opinions expressed in Perspectives articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Supported by the United States Department of Agriculture (USDA), Agriculture Research Service (SNM and DW), the National Institute on Aging (SNM and DW) and the Canadian Institutes of Health Research (EDL).
Author disclosures: SNM, EDL, and DW, no conflicts of interest. An earlier study conducted by Meydani et al. (1990) received partial support from Hoffman-LaRoche, Inc., a vitamin supplier. Supplements were provided by Hoffman-LaRoche, Inc., for previous studies conducted by Meydani et al. (1997; 2004; 2005).
Abbreviations used:
- DTH
delayed-type hypersensitivity
- EAR
Estimated Average Requirement
- IOM
Institute of Medicine
- PGE2
prostaglandin E2
- ppm
parts per million
References
- 1. Burton GW, Traber MG. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr 1990;10:357–82. [DOI] [PubMed] [Google Scholar]
- 2. Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm 2007;76:1–21. [DOI] [PubMed] [Google Scholar]
- 3. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids Institute of Medicine National Academies Press, DC, USA: 2000. [PubMed] [Google Scholar]
- 4. Schmolz L, Birringer M, Lorkowski S, Wallert M. Complexity of vitamin E metabolism. World J Biol Chem 2016;7(1):14–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med 2007;28(5-6):423–36. [DOI] [PubMed] [Google Scholar]
- 6. Fulgoni VL III, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J Nutr 2011;141(10):1847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crossley KB, Peterson PK. Infections in the elderly. Clin Infect Dis 1996;22(2):209–15. [DOI] [PubMed] [Google Scholar]
- 8. Mehr DR, Foxman B, Colombo P. Risk factors for mortality from lower respiratory infections in nursing home patients. J Fam Pract 1992;34(5):585–91. [PubMed] [Google Scholar]
- 9. Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL. Aging and CD8+ T cell immunity to respiratory virus infections. Exp Gerontol 2007;42(5):427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis 2002;2(11):659–66. [DOI] [PubMed] [Google Scholar]
- 11. Horwitt MK. Vitamin E and lipid metabolism in man. Am J Clin Nutr 1960;8:451–61. [DOI] [PubMed] [Google Scholar]
- 12. Horwitt MK, Liebert E, Kreisler O, Wittman P. Studies of vitamin deficiency. Science 1946;104(2705):407. [DOI] [PubMed] [Google Scholar]
- 13. Horwitt MK. Vitamin E in human nutrition—an interpretative review. Bordens Rev Nutr Res 1961;22:1–17. [PubMed] [Google Scholar]
- 14. Horwitt MK. Therapeutic uses of vitamin E in medicine. Nutr Rev 1980;38(3):105–13. [DOI] [PubMed] [Google Scholar]
- 15. Farrell P. Human health and disease. In: Machlin LJ, editor. Vitamin E, a comprehensive treatise. New York: Marcel Dekker;1980. p. 520–620. [Google Scholar]
- 16. Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal 2014;20(2):372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bendich A, editor Antioxidant vitamins and their functions in immune responses. New York: Plenum Press; 1990. [DOI] [PubMed] [Google Scholar]
- 18. Coquette A, Vray B, Vanderpas J. Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Arch Int Physiol Biochim 1986;94(5):S29–34. [PubMed] [Google Scholar]
- 19. Hatam LJ, Kayden HJ. A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res 1979;20(5):639–45. [PubMed] [Google Scholar]
- 20. Bendich A, Gabriel E, Machlin LJ. Dietary vitamin E requirement for optimum immune responses in the rat. J Nutr 1986;116(4):675–81. [DOI] [PubMed] [Google Scholar]
- 21. Azzi A. Many tocopherols, one vitamin E. Mol Aspects Med 2017;61:92–103. [DOI] [PubMed] [Google Scholar]
- 22. Zingg JM, Azzi A. Non-antioxidant activities of vitamin E. Curr Med Chem 2004;11(9):1113–33. [DOI] [PubMed] [Google Scholar]
- 23. Wu D, Meydani M, Beharka AA, Serafini M, Martin KR, Meydani SN. In vitro supplementation with different tocopherol homologues can affect the function of immune cells in old mice. Free Radic Biol Med 2000;28(4):643–51. [DOI] [PubMed] [Google Scholar]
- 24. Han SN, Pang E, Zingg JM, Meydani SN, Meydani M, Azzi A. Differential effects of natural and synthetic vitamin E on gene transcription in murine T lymphocytes. Arch Biochem Biophys 2010;495(1):49–55. [DOI] [PubMed] [Google Scholar]
- 25. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408(6809):239–47. [DOI] [PubMed] [Google Scholar]
- 26. Hayek MG, Meydani SN, Meydani M, Blumberg JB. Age differences in eicosanoid production of mouse splenocytes: effects on mitogen-induced T-cell proliferation. J Gerontol 1994;49(5):B197–207. [DOI] [PubMed] [Google Scholar]
- 27. Hernanz A, Fernandez-Vivancos E, Montiel C, Vazquez JJ, Arnalich F. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci 2000;67(11):1317–24. [DOI] [PubMed] [Google Scholar]
- 28. Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA 2004;101(10):3381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toroser D, Sohal RS. Age-associated perturbations in glutathione synthesis in mouse liver. Biochem J 2007;405(Part 3):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fulop T, Witkowski JM, Pawelec G, Alan C, Larbi A. On the immunological theory of aging. Interdiscip Top Gerontol 2014;39:163–76. [DOI] [PubMed] [Google Scholar]
- 31. Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol 1990;45(2):M45–8. [DOI] [PubMed] [Google Scholar]
- 32. Fulop T, Dupuis G, Witkowski JM, Larbi A. The role of immunosenescence in the development of age-related diseases. Rev Invest Clin 2016;68(2):84–91. [PubMed] [Google Scholar]
- 33. Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today 2000;21(10):515–21. [DOI] [PubMed] [Google Scholar]
- 34. Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol 2007;211(2):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004;5(2):133–9. [DOI] [PubMed] [Google Scholar]
- 36. Miller RA. Aging and immune function. Int Rev Cytol 1991;124:187–215. [DOI] [PubMed] [Google Scholar]
- 37. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 2013;14(12):877–82. [DOI] [PubMed] [Google Scholar]
- 38. Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets 2014;14(4):283–9. [DOI] [PubMed] [Google Scholar]
- 39. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013;13(12):875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol 2009;30(7):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nikolich-Zugich J. The aging immune system: challenges for the 21st century. Semin Immunol 2012;24(5):301–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu D, Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol 2008;84(4):900–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev 2005;205:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res 1999;38(4):309–36. [DOI] [PubMed] [Google Scholar]
- 45. Han SN, Meydani SN. Impact of vitamin E on immune function and its clinical implications. Expert Rev Clin Immunol 2006;2(4):561–7. [DOI] [PubMed] [Google Scholar]
- 46. Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: development and major conclusions. Adv Nutr 2016;7(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ryan AS, Craig LD, Finn SC. Nutrient intakes and dietary patterns of older Americans: a national study. J Gerontol 1992;47(5):M145–50. [DOI] [PubMed] [Google Scholar]
- 48. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol 1992;47(3):B98–104. [DOI] [PubMed] [Google Scholar]
- 49. Brownie S. Why are elderly individuals at risk of nutritional deficiency? Int J Nurs Pract 2006;12(2):110–8. [DOI] [PubMed] [Google Scholar]
- 50. Pae M, Wu D. Nutritional modulation of age-related changes in the immune system and risk of infection. Nutr Res 2017;41:14–35. [DOI] [PubMed] [Google Scholar]
- 51. Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E1(2) synthesis and enhances the immune response of aged mice. Mech Ageing Dev 1986;34(2):191–201. [DOI] [PubMed] [Google Scholar]
- 52. Moriguchi S, Hamada M, Yamauchi K, Sakai K, Yamamoto S. The role of vitamin E in T-cell differentiation and the decrease of cellular immunity with aging. J Med Invest 1998;45(1-4):1–8. [PubMed] [Google Scholar]
- 53. Ren Z, Pae M, Dao MC, Smith D, Meydani SN, Wu D. Dietary supplementation with tocotrienols enhances immune function in C57BL/6 mice. J Nutr 2010;140(7):1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ, Adler WH. Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest 1988;81(4):1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Douziech N, Seres I, Larbi A, Szikszay E, Roy PM, Arcand M, Dupuis G, Fulop T. Modulation of human lymphocyte proliferative response with aging. Exp Gerontol 2002;37(2-3):369–87. [DOI] [PubMed] [Google Scholar]
- 56. Gillis S, Kozak R, Durante M, Weksler ME. Immunological studies of aging: decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest 1981;67(4):937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meydani SN, Lipman R, Blumberg JB, Taylor A. Dietary energy restriction decreases ex vivo spleen prostaglandin E2 synthesis in Emory mice. J Nutr 1990;120(1):112–5. [DOI] [PubMed] [Google Scholar]
- 58. De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res 2008;42(3):272–80. [DOI] [PubMed] [Google Scholar]
- 59. Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol 2001;167(7):3809–17. [DOI] [PubMed] [Google Scholar]
- 60. Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol 1997;159(5):2445–51. [PubMed] [Google Scholar]
- 61. Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BS, Meydani SN. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology 2000;100(4):487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vissinga CS, Dirven CJ, Steinmeyer FA, Benner R, Boersma WJ. Deterioration of cellular immunity during aging. The relationship between age-dependent impairment of delayed-type hypersensitivity reactivity, interleukin-2 production capacity, and frequency of Thy-1+,Lyt-2- cells in C57BL/Ka and CBA/Rij mice. Cell Immunol 1987;108(2):323–34. [DOI] [PubMed] [Google Scholar]
- 63. Rabinowich H, Goses Y, Reshef T, Klajman A. Interleukin-2 production and activity in aged humans. Mech Ageing Dev 1985;32(2-3):213–26. [DOI] [PubMed] [Google Scholar]
- 64. Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol 1996;169(2):185–95. [DOI] [PubMed] [Google Scholar]
- 65. Pallast EG, Schouten EG, de Waart FG, Fonk HC, Doekes G, von Blomberg BM, Kok FJ. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr 1999;69(6):1273–81. [DOI] [PubMed] [Google Scholar]
- 66. Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr 1990;52(3):557–63. [DOI] [PubMed] [Google Scholar]
- 67. Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol 1998;275(3 Pt 1):C661–8. [DOI] [PubMed] [Google Scholar]
- 68. Beharka AA, Wu D, Han SN, Meydani SN. Macrophage PGE2 production contributes to the age-associated decrease in T cell function which is reversed by dietary antioxidants. Mech Ageing Dev 1997;93:59–77. [DOI] [PubMed] [Google Scholar]
- 69. Goodwin JS, Bankhurst AD, Messner RP. Suppression of human T-cell mitogenesis by prostaglandin: existence of a prostaglandin-producing suppressor cell. J Exp Med 1977;146(6):1719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goodwin JS. Modulation of conconavalin-A-induced suppressor cell activation by prostaglandin E2. Cell Immunol 1980;49(2):421–5. [DOI] [PubMed] [Google Scholar]
- 71. Bartocci A, Maggi FM, Welker RD, Veronese F. Age-related immunossuppresion: putative role of prostaglandins. In: Powles TJ, Backman RS, Honn KV, Ramwell P, Prostaglandins and cancer. New York: Alan R Liss; 1982. p. 725–30. [Google Scholar]
- 72. Waldorf DS, Willkens RF, Decker JL. Impaired delayed hypersensitivity in an aging population: association with antinuclear reactivity and rheumatoid factor. JAMA 1968;203(10):831–4. [PubMed] [Google Scholar]
- 73. Roberts-Thomson IC, Whittingham S, Youngchaiyud U, Mackay IR. Ageing, immune response, and mortality. Lancet 1974;2(7877):368–70. [DOI] [PubMed] [Google Scholar]
- 74. Rodysill KJ, Hansen L, O'Leary JJ. Cutaneous-delayed hypersensitivity in nursing home and geriatric clinic patients. Implications for the tuberculin test. J Am Geriatr Soc 1989;37(5):435–43. [DOI] [PubMed] [Google Scholar]
- 75. Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects: a randomized controlled trial. JAMA 1997;277(17):1380–6. [DOI] [PubMed] [Google Scholar]
- 76. Ershler WB, Hebert JC, Blow AJ, Granter SR, Lynch J. Effect of thymosin alpha one on specific antibody response and susceptibility to infection in young and aged mice. Int J Immunopharmacol 1985;7(4):465–71. [DOI] [PubMed] [Google Scholar]
- 77. Garg M, Luo W, Kaplan AM, Bondada S. Cellular basis of decreased immune responses to pneumococcal vaccines in aged mice. Infect Immun 1996;64(11):4456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kishimoto S, Tomino S, Mitsuya H, Fujiwara H, Tsuda H. Age-related decline in the in vitro and in vivo syntheses of anti-tetanus toxoid antibody in humans. J Immunol 1980;125(5):2347–52. [PubMed] [Google Scholar]
- 79. Ruben FL, Uhrin M. Specific immunoglobulin-class antibody responses in the elderly before and after 14-valent pneumococcal vaccine. J Infect Dis 1985;151(5):845–9. [DOI] [PubMed] [Google Scholar]
- 80. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24(8):1159–69. [DOI] [PubMed] [Google Scholar]
- 81. Meydani SN, Yogeeswaran G, Liu S, Baskar S, Meydani M. Fish oil and tocopherol-induced changes in natural killer cell-mediated cytotoxicity and PGE2 synthesis in young and old mice. J Nutr 1988;118(10):1245–52. [DOI] [PubMed] [Google Scholar]
- 82. Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol 1999;34(2):253–65. [DOI] [PubMed] [Google Scholar]
- 83. Bou Ghanem EN, Clark S, Du X, Wu D, Camilli A, Leong JM, Meydani SN. The alpha-tocopherol form of vitamin E reverses age-associated susceptibility to Streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment. J Immunol 2015;194(3):1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hayek MG, Taylor SF, Bender BS, Han SN, Meydani M, Smith DE, Eghtesada S, Meydani SN. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis 1997;176(1):273–6. [DOI] [PubMed] [Google Scholar]
- 85. Han SN, Meydani M, Wu D, Bender BS, Smith DE, Vina J, Cao G, Prior RL, Meydani SN. Effect of long-term dietary antioxidant supplementation on influenza virus infection. J Gerontol A Biol Sci Med Sci 2000;55(10):B496–503. [DOI] [PubMed] [Google Scholar]
- 86. Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA 2002;288(6):715–21. [DOI] [PubMed] [Google Scholar]
- 87. Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 2004;292(7):828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mouton CP, Bazaldua OV, Pierce B, Espino DV. Common infections in older adults. Am Fam Physician 2001;63(2):257–68. [PubMed] [Google Scholar]
- 89. Hemila H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging 2016;11:1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis 2009;49(7):1025–35. [DOI] [PubMed] [Google Scholar]
- 91. Hemila H, Virtamo J, Albanes D, Kaprio J. The effect of vitamin E on common cold incidence is modified by age, smoking and residential neighborhood. J Am Coll Nutr 2006;25(4):332–9. [DOI] [PubMed] [Google Scholar]
- 92. Neupane B, Walter SD, Krueger P, Marrie T, Loeb M. Predictors of inhospital mortality and re-hospitalization in older adults with community-acquired pneumonia: a prospective cohort study. BMC Geriatr 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J Immunol 2007;178(3):1443–9. [DOI] [PubMed] [Google Scholar]
- 94. Marko MG, Pang HJ, Ren Z, Azzi A, Huber BT, Bunnell SC, Meydani SN. Vitamin E reverses impaired linker for activation of T cells activation in T cells from aged C57BL/6 mice. J Nutr 2009;139(6):1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meydani SN, Han SN. Nutrient regulation of the immune response: the case of vitamin E. Present knowledge in nutrition. 8th ed Washington (DC): ILSI Press; 2001. [Google Scholar]
- 96. Christou NV, Tellado-Rodriguez J, Chartrand L, Giannas B, Kapadia B, Meakins J, Rode H, Gordon J. Estimating mortality risk in preoperative patients using immunologic, nutritional, and acute-phase response variables. Ann Surg 1989;210(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol 2014;29:23–8. [DOI] [PubMed] [Google Scholar]
- 98. Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010;28(31):4955–60. [DOI] [PubMed] [Google Scholar]
- 99. Bender BS, Johnson MP, Small PA. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology 1991;72(4):514–9. [PMC free article] [PubMed] [Google Scholar]
- 100. Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci 2005;60(6):688–94. [DOI] [PubMed] [Google Scholar]
- 101. Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294(1):56–65. [DOI] [PubMed] [Google Scholar]
- 102. Meydani SN, Meydani M, Rall LC, Morrow F, Blumberg JB. Assessment of the safety of high-dose, short-term supplementation with vitamin E in healthy older adults. Am J Clin Nutr 1994;60(5):704–9. [DOI] [PubMed] [Google Scholar]
- 103. Meydani SN, Meydani M, Blumberg JB, Leka LS, Pedrosa M, Diamond R, Schaefer EJ. Assessment of the safety of supplementation with different amounts of vitamin E in healthy older adults. Am J Clin Nutr 1998;68(2):311–8. [DOI] [PubMed] [Google Scholar]
- 104. Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 1993;328(20):1444–9. [DOI] [PubMed] [Google Scholar]
- 105. Miller ER III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142(1):37–46. [DOI] [PubMed] [Google Scholar]
- 106. Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Johnston CS, Kelly FJ, Kraemer K et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr 2005;81(4):736–45. [DOI] [PubMed] [Google Scholar]
- 107. Traber MG. Vitamin E: too much or not enough? Am J Clin Nutr 2001;73(6):997–8. [DOI] [PubMed] [Google Scholar]