Abstract
Increased risk of herpes zoster (HZ) has been observed in patients with immune-mediated diseases, including rheumatoid arthritis (RA), psoriasis (PsO), and inflammatory bowel disease; this risk can be further increased by the use of immunosuppressive therapy. One advancing modality of therapy for these diseases is Janus kinase (JAK) inhibition. Tofacitinib is an oral JAK inhibitor for the treatment of RA and psoriatic arthritis, which is currently under investigation for the treatment of ulcerative colitis (UC) and was previously investigated for psoriasis. JAK inhibitors have been associated with HZ events in patients across a number of indications. The pathogenesis underlying this risk of HZ is currently unknown. An increased risk of HZ has been noted in patients receiving immunosuppressive therapies for UC, including tofacitinib. In clinical trials, there was a dose-dependent risk of HZ (higher dose linked with increased risk). However, the majority of HZ cases are nonserious and noncomplicated, mild to moderate in severity, and manageable without permanent discontinuation of treatment. This review will discuss HZ risk in patients receiving JAK inhibitors, focusing on tofacitinib with respect to the potential mechanisms and epidemiology of HZ. Current guidelines for the prevention of HZ will be highlighted, and proposed management reviewed.
Keywords: herpes zoster, JAK, shingles, ulcerative colitis, tofacitinib
INTRODUCTION
Herpes zoster (HZ), also known as shingles, occurs due to reactivation of the varicella zoster virus (VZV), which establishes latency in the dorsal root ganglia or cranial nerve after primary infection.1 Classic presentation of HZ (noncomplicated) involves a rash, usually confined to 1 or 2 unilateral dermatomes, commonly on the chest.2 Complications of HZ include events with involvement of more than 2 dermatomes (multidermatomal), postherpetic neuralgia (PHN), disseminated skin disease, neurologic complications, ophthalmologic complications, verrucous skin lesions, and development of acyclovir-resistant VZV.1 PHN is the most common complication of HZ, with reported risk ranging from 2.6% to 52.0% depending upon study design and population.3
In the general population, the most common risk factor for HZ is increasing age.4 Several other potential factors have also been identified, including female sex, race, and immunosuppression.4 Patients with autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD) including ulcerative colitis (UC), and psoriasis (PsO), have an increased risk of HZ compared with the general population; this risk is further increased by the use of immunosuppressive therapy. The risk of HZ may be reduced by vaccination before immunosuppression therapy. However, real-world data show that vaccination rates for HZ are currently low, with only 30.6% of adults ≥60 years of age in the United States being vaccinated against HZ in 2014,5 and even lower rates observed for patients with IBD (5.3% of 300 patients in Canada).6
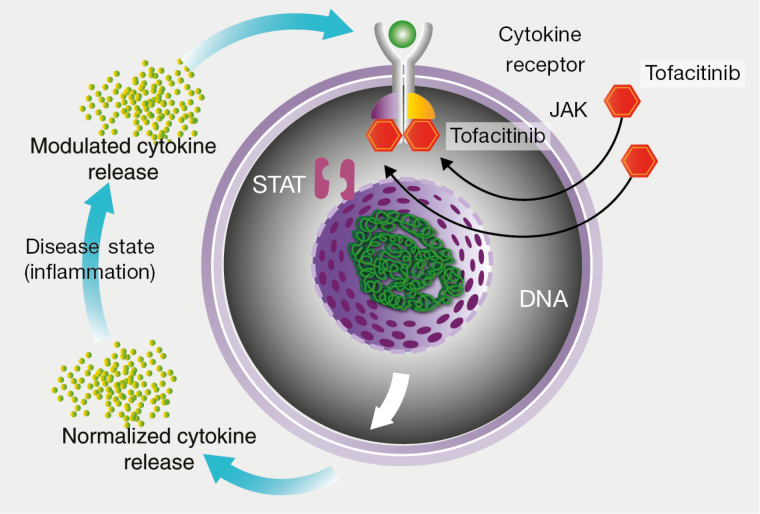
There are limitations with the current UC treatment options in regards to efficacy, resulting in a high unmet need.7–9 As there are currently only 2 classes of approved therapies for patients with moderately to severely active UC, agents with a novel mechanism of action could provide a much-needed mechanistic diversity for a multifaceted disease. This review will focus on patients receiving Janus kinase (JAK) inhibitors, small molecules that target intracellular cytokine signaling (Fig. 1) and seem to accentuate HZ risk more than other immunosuppressive therapies. There are a number of JAK inhibitors currently being investigated for use in IBD, some of which are also approved or under investigation in other indications. Tofacitinib is an oral JAK inhibitor for the treatment of RA and psoriatic arthritis, which is currently under investigation for the treatment of UC and was previously investigated for PsO. The objective of this review is to discuss HZ risk in patients with UC receiving JAK inhibitors (focusing on tofacitinib), with respect to the potential mechanisms, epidemiology, prevention, and management. Data will also be put into perspective with the experience gained from the use of these therapies in other indications.
FIGURE 1.
Mechanistic target of tofacitinib.
MECHANISM OF ACTION
JAK Signaling in IBD
The immunopathogenesis of IBD has been extensively reviewed elsewhere.10–13 The significant role that JAK-signal transducer and activator of transcription (STAT) pathways and associated cytokines have in the regulation of immunity and inflammation14 enables JAK inhibitors to be a promising therapeutic approach for the treatment of the IBD. A number of JAK inhibitors are currently approved or under investigation for immune-mediated diseases (Table 1).
TABLE 1:
JAK Inhibitors Currently Approved or Under Investigation
| Compound | In Vitro JAK Target | Indication and Trial Phase | Compound | In Vitro JAK Target | Indication and Trial Phase |
|---|---|---|---|---|---|
| Upadacitinib (ABT-494) | JAK1 | Atopic dermatitis (phase II) Crohn’s disease (phase II) PsA (phase III) RA (phase III) UC (phase III) |
Tofacitinibc | Preferential JAK1/JAK3 | JIA (phase III) PsA (phase III) PsO (phase III; development completed) RA (approved) UC (phase III) |
| Baricitiniba | JAK1/JAK2 | Atopic dermatitis (phase II/III) Diabetic kidney disease (phase II) PsO (phase II) RA (phase III) SLE (phase I) |
|||
| Decernotinib (VX-509) | JAK3 | RA (phase II/III) | Momelotinib (CYT387) | JAK1/JAK2 | Myelofibrosis (phase II/III) |
| Filgotinib | JAK1 | Crohn’s disease (phase III) RA (phase III) UC (phase III) |
Peficitinib | JAK1/JAK3 | PsO (phase II) RA (phase III) UC (phase II) |
| Pacritinib | JAK2 | Myelofibrosis (phase III) | Ruxolitinibb (INCB018424) | JAK1/JAK2 | PsO (phase II) Leukemia (phase I/II) Myelofibrosis (phase III) Polycythemia vera (phase III) |
ClinicalTrials.gov search carried out in November 2017 based upon compounds previously published in Winthrop et al. (2017)22; list is not exhaustive.
Abbreviations: JIA, juvenile idiopathic arthritis; SLE, systemic lupus erythematosus.
aUnder additional monitoring by the European Medicines Agency (EMA), after initial approval was received on February 13, 2017.
bApproved by the US FDA to treat myelofibrosis (November 16, 2011) and polycythemia vera (December 4, 2014); approved by the EMA for myeloproliferative disorders (August 23, 2012).
cApproved by the US FDA (November 6, 2012) and the EMA (March 22, 2017).
Four different JAKs form complexes to mediate specific signaling pathways: JAK1, JAK2, JAK3, and nonreceptor tyrosine-protein kinase (TYK2).15 All JAKs play an important role in immune response and development, with JAK1 and JAK2 also involved in hematopoiesis.15 JAK1 is required for both type I (α and β) and type II (γ) interferon (IFN) signaling pathways, whereas JAK3 is essential for lymphocyte development and function.15, 16
Molecular Mechanisms of HZ Infection
Cell-mediated immunity has been reported to play a bigger role than humoral response in the prevention of VZV infection and reactivation.17 This has been supported by the findings that patients with multiple myeloma (generally associated with humoral immunity defects; patients do not have an increased risk of HZ) present with increased HZ incidence after treatment with bortezomib, which interferes with cell-mediated immunity.18 Increased incidence of HZ has also been associated with a sphingosine 1-phosphate (S1P) receptor modulator in the treatment of multiple sclerosis, potentially due to the important role that S1P plays in lymphocyte trafficking, including egression from lymph nodes.19 In addition, T-cell-mediated immunity declines as individuals age, and the proportion of VZV-specific early effector and cytokine-producing CD4+ and CD8+ T cells is lower in older individuals, increasing the risk of reactivation and HZ occurrence.20, 21
Early host response through innate immunity, including type I and type II IFNs, is important in limiting VZV spread,23 and downregulation of IFNα has been shown to permit focal replication during VZV skin infection.24 The increased incidence of HZ associated with JAK inhibitors and other therapies that target innate immunity through these signaling pathways—for example, anti-IFN antibodies used for treating systemic lupus erythematous, including anifrolumab,25 and sifalimumab26—further supports this mechanistic link.
Molecular Mechanisms of HZ in Immune-Mediated Diseases
The precise mechanisms by which VZV reactivation occurs in those with immune-mediated diseases are currently unknown. Similar changes in VZV-specific early effector and cytokine-producing CD4+ and CD8+ T cells, as observed in aging individuals, are also associated with immunosuppression.27 In the immune response to HZ, signaling occurs via the JAK-STAT pathway, including type I (regulated by JAK1-TYK2 complexes) and type II IFNs (mediated via JAK1-JAK2 complexes).15 Therapies targeting these JAK complexes may therefore disrupt normal VZV response and may potentially increase the risk of VZV reactivation. The impact of different JAK inhibitors on the immunogenic network seem to overlap, even for those compounds that target individual JAKs with high specificity.28 This suggests that increased HZ risk will be observed for a number of JAK inhibitors and that it is not specific to those treatments targeting a particular JAK and/or JAK complexes. It is worth noting that this increased risk has not been observed for other viruses. A study in patients with PsO did not identify a relationship between cytomegalovirus (CMV) or Epstein-Barr virus (EBV) viral load and tofacitinib treatment,29 although sporadic cases of CMV infection had been observed in patients with RA receiving tofacitinib.30 These findings suggest that there are features that are unique to VZV reactivation, vs CMV and EBV, that are not yet fully understood.
Throughout the tofacitinib UC clinical development program (comprising 1157 patients, 1613 patient-years [PY], and 4.4 years of follow-up, as of December 2016), serious infections and opportunistic infections other than HZ were infrequent (W. J. Sandborn, et al., unpublished data, 2018). Serious infections were defined as any infection event that required hospitalization, parenteral antibiotics, or met any other serious adverse event criteria; patients with serious infections were required to discontinue from the UC clinical development program. Potential opportunistic infections in the UC clinical development program were reviewed by an independent adjudication committee. HZ cases were confirmed as opportunistic infections if they were determined to be multidermatomal (2 nonadjacent or 3–6 adjacent dermatomes) or disseminated (any of the following: diffuse rash >6 dermatomes, encephalitis, pneumonia, other nonskin organ involvement).
Serious infections occurred in ≤1.0% of patients in any treatment group in the Induction Cohort, with similar incidence rates (IRs; patients with events per 100 PY) for the Overall Cohort and between treatment groups in the Maintenance Cohort. There were a total of 22 opportunistic infections, and the majority (18/22) were due to HZ. Non-HZ opportunistic infections were infrequent. They included CMV colitis, pulmonary cryptococcosis, histoplasmosis, and CMV hepatitis (W. J. Sandborn, et al., unpublished data, 2018).
Although HZ has been observed in those receiving JAK inhibitors, other immunosuppression therapies also increase the risk of HZ across a number of indications. In a recent systematic review and meta-analysis of HZ risk, an overall increased risk for biologics was observed compared with controls (Table 2).31 Higher risk of HZ has been attributed to tofacitinib, nontumor necrosis factor (TNF) biologics, and biologic and thiopurine combination therapy.
TABLE 2:
Treatment Type and HZ Risk from Meta-Analysis and Nested Case-Control Studies
| Treatment Type | Indication(s) | HZ Risk, OR (95% CI) | Reference |
|---|---|---|---|
| TNFi biologicsa | IBD (Crohn’s disease and UC)/PsO/RA | 1.28 (0.69–2.40)b | Marra et al. (2016)31 |
| IBD (Crohn’s disease and UC) | 1.81 (1.48–2.21)e | Long et al. (2013)35 | |
| Non-TNF biologicsa,b | Crohn’s disease/PsO/RA/SLE | 2.19 (1.20–4.02) | Marra et al. (2016)31 |
| All biologicsa,c (TNFi and non-TNF) | IBD (Crohn’s disease and UC)/PsO/RA/SLE | 1.71 (1.11–2.64)b | Marra et al. (2016)31 |
| IBD/PsO/RA | 1.58 (1.39–1.81)f | ||
| All nonbiologic DMARDsd | PsO/RA/SLE | 1.61 (0.84–3.10)b | Marra et al. (2016)31 |
| IBD/PsA/PsO/RA/AS | 1.21 (1.15–1.28)f | ||
| Tofacitinibb (5 and 10 mg BID) | Pso/RA | 2.16 (0.84–5.58) | Marra et al. (2016)31 |
| 5 mg BID | PsO/RA | 2.10 (0.83–5.34) | |
| 10 mg BID | PsO/RA | 3.01 (1.15–7.87) | |
| MTXb | RA | 0.89 (0.24–3.29) | Marra et al. (2016)31 |
| Thiopurines | SLE | 1.35 (0.33–5.61)b | Marra et al. (2016)31 |
| IBD (Crohn’s disease and UC) | 1.85 (1.61–2.13)e | Long et al. (2013)35 | |
| IBD (Crohn’s disease and UC) | 3.1 (1.7–5.6)e | Gupta et al. (2006)33 | |
| Corticosteroidse | IBD (Crohn’s disease and UC) | 1.5 (1.1–2.2) | Gupta et al. (2006)33 |
| IBD (Crohn’s disease and UC) | 1.73 (1.51–1.99) | Long et al. (2013)35 | |
| Biologic and thiopurine combination therapye | IBD (Crohn’s disease and UC) | 3.29 (2.33–4.65) | Long et al. (2013)35 |
Abbreviations: AS, ankylosing spondylitis; BID, twice daily; DMARD, disease-modifying antirheumatic drug; SLE, systemic lupus erythematous.
aIncludes monotherapy and combination therapy with MTX.
bRandomized controlled trials.
cCombined biologics included TNFi biologics (etanercept, adalimumab, Anbainuo, infliximab, certolizumab pegol, and golimumab) and non-TNF biologics (abetimus, interleukin-1 receptor antagonist, abatacept, tocilizumab, ustekinumab, sifalimumab, and natalizumab).
dCombined nonbiologics included tofacitinib, MTX, and azathioprine/cyclophosphamide.
eNested case-control studies.
fObservational studies.
For patients with IBD, risk factors of HZ have recently been reviewed,2 and HZ events have been observed in patients with UC receiving a range of treatment types, including tofacitinib and biologics. The varying mechanisms of action for these therapies could explain the differences in risk observed. For agents that target and disrupt cell-mediated responses (ie, TNF-inhibitor [TNFi] biologics and JAK inhibitors), higher risks of HZ have been observed compared with those compounds that demonstrate other mechanistic impacts, such as purine metabolism (methotrexate [MTX]).31
RISK AND EPIDEMIOLOGY OF HZ IN IMMUNE-MEDIATED DISEASES
In the general US population, the estimated lifetime risk of HZ is ~30%, which sharply increases with age, particularly for those ≥50 years of age.32 IRs of HZ range from 0.3 to 0.5 per 100 person-years across North America, Europe, and Asia-Pacific.3 Patients with IBD have an increased risk of HZ compared with age-matched individuals in the general UK population,33 regardless of disease duration.34 HZ IRs for patients with IBD (Crohn’s disease and UC) range from 0.66 to 1.29 per 100 person-years in those aged >45 years.33, 35, 36 HZ IRs in patients with IBD are comparable to (or lower than) other autoimmune diseases such as RA, multiple sclerosis, and PsO.37
As previously highlighted, increased risk of HZ has been observed for a number of immunosuppression therapies across indications.31 In stratified analyses of randomized controlled trial data, a greater risk of HZ was noted for non-TNFi agents overall (abetimus, interleukin-1 receptor antagonist, abatacept, tocilizumab, ustekinumab, sifalimumab, and natalizumab data combined) compared with TNFi agents (etanercept, adalimumab, Anbainuo, infliximab, certolizumab pegol, and golimumab data combined) (Table 2). Similar risks of HZ have been noted for patients with Crohn’s disease and UC recei ving thiopurine therapy; however, after biologic (infliximab, adalimumab, or certolizumab pegol) or combination (both thiopurine and biologic) therapy, slightly increased risks were observed for patients with UC compared with Crohn’s disease.35 These differential risks for HZ may be due to the mechanism of action for treatment types, as previously discussed.
Detailed clinical presentation of HZ in patients receiving UC treatment is limited, with the majority of information available coming from individual case studies. In other indications, TNFi therapy, in particular, seems to be linked with more severe HZ events, with RA patients receiving infliximab or adalimumab (33% in combination with corticosteroids) having increased incidence of multidermatomal HZ compared with etanercept or control patients.38 Increased risk of hospitalization due to HZ has also been associated with TNFi treatment in patients with rheumatoid diseases (50% and 48% of patients also receiving MTX and corticosteroids, respectively) compared with the general population,39 and a high rate of severe HZ, including ophthalmic infections in RA patients and 1 occurrence of meningitis in a patient with ankylosing spondylitis.40 Two cases of VZV encephalitis have also been observed in patients with psoriatic arthritis (PsA) and RA receiving adalimumab (in combination with MTX for the patient with RA), and another case was noted for a patient with UC receiving infliximab.41–43
RISK AND EPIDEMIOLOGY OF HZ IN PATIENTS TREATED WITH JAK INHIBITORS
Aside from tofacitinib, published data for JAK inhibitors and HZ are somewhat lacking due to the current stage of development of many of these compounds (Table 1). The majority of available data describing HZ cases in patients on JAK inhibitors come from patients with RA, where HZ events have been reported for all JAK inhibitors under investigation: tofacitinib,44 baricitinib,45 decernotinib,46 filgotinib,47 peficitinib,48 upadacitinib,49 and tofacitinib combination therapy with MTX.50 IRs have only been reported for baricitinib (3.4 per 100 PY; 95% confidence interval [CI] not reported), peficitinib (6.3 per 100 PY in a Japanese population; 95% CI not reported),22 and tofacitinib (4.0 per 100 PY for patients with RA: 95% CI, 3.7–4.4; and 4.07 per 100 PY for patients with UC: 95% CI, 3.14–5.19).44,51 In the phase III study of baricitinib in patients with RA, HZ was observed in 7/648 patients, no cases of which were visceral or disseminated.45 For peficitinib, HZ occurred in 4/281 patients with RA in the phase II trial in Japan.48 There was a single HZ case (1/283) noted in the phase II study of filgotinib in patients with RA.47 Three occurrences (3/299) of HZ were observed in the phase II upadacitinib study of patients with RA, which were mild to moderate events involving a single dermatome,49 and 1 case (1/180) of HZ was reported in the CELEST trial.52 However, overall the publications relating to JAK inhibitors other than tofacitinib lack information regarding the severity, dermatome involvement, and associated risk factors for HZ events.
Peficitinib is another JAK inhibitor being investigated for the treatment of UC. However, the phase II trial (NCT01959282) results for peficitinib, including safety, are yet to be released.11 With respect to the treatment of Crohn’s disease, data have been published for filgotinib, where a single HZ event (1/174) was observed in a phase II trial of 20 weeks.53 Overall, the lack of information regarding clinical presentation of HZ for different JAK inhibitors means that the mechanism(s) of action is not yet fully understood.
RISK AND EPIDEMIOLOGY OF HZ IN TOFACITINIB-TREATED PATIENTS
Tofacitinib has been associated with increased HZ risk for all indications either approved or under investigation; there is evidence that this increased risk is dose-dependent, with numerically higher risk of HZ associated with tofacitinib 10 mg twice daily (BID) compared with 5 mg BID.31 Other risk factors for HZ in those patients receiving tofacitinib across indications include age and geographic location. IRs of HZ in Asia are more than double those observed in North America, Europe, and Latin American regions, driven by higher rates in the Japanese and Korean populations.54 HZ incidence observed in clinical trials of patients with UC treated with tofacitinib (4.07 per 100 PY)51 is similar to that previously observed in patients with RA who received tofacitinib (4.0 per 100 PY).44 As previously highlighted, there was a dose-dependent risk of HZ in patients with UC. IRs for tofacitinib 10 mg BID ranged from 4.25 per 100 PY for the phase II/III/open-label, long-term extension (OLE) cohort to 6.64 per 100 PY in the phase III Maintenance Cohort, although there was no treatment duration risk observed.51
In patients with RA, HZ incidence has recently been examined across 19 phase I, II, III, and OLE studies.44 The majority of these cases were nonserious (93%) and involved 1 dermatome (94%). PHN is a common HZ complication observed in immunocompromised patients2 and was noted in 47 (7.4%) patients with RA treated with tofacitinib.44 The IR of PHN within this tofacitinib cohort was at the lower end of the range reported for the general population (5%–15%).55
Independent risk factors for HZ within this cohort of patients with RA were increasing age, glucocorticoid use, patients enrolled in Asia, smoking status (non- or ex-smoker), and increasing tofacitinib dose (10 mg BID compared with 5 mg BID).44 Although concomitant conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy seemed to be associated with higher rates of HZ, it was not statistically identified as a risk factor. HZ events were lowest in those patients with RA receiving tofacitinib 5 mg BID without glucocorticoid and csDMARD use (0.56 per 100 PY) and highest for tofacitinib 10 mg BID with csDMARD and glucocorticoid use (5.44 per 100 PY).44
For patients with PsO, a recent analysis of 7 studies, including phase II, III, and an OLE study, evaluated 3623 patients treated with tofacitinib, with 130 reporting HZ.56 The majority of events involved <2 dermatomes, with 8 patients demonstrating multidermatomal HZ (6.0%). There was 1 case each of skin dissemination and eye involvement. Similar rates of PHN were observed in patients treated with PsO (7.7%), compared with RA (7.4%).44,56 PHN rates increased with age, with the lowest rates observed for those <50 years of age, increasing up to 50% for HZ patients ≥70 years of age.57 In patients with PsO receiving tofacitinib, a low rate of PHN was observed in those ≥65 years of age (16.7% of patients).56 Heightened HZ risk factors in these patients with PsO were similar to those demonstrated in RA, including Asian race, increasing age, increasing tofacitinib dose, and prior biologic exposure.56
In patients with UC treated with tofacitinib (phase II induction study, induction and maintenance phase III studies, and ongoing OLE studies), HZ incidence was 4.07 per 100 PY.51 The OCTAVE HZ publication reported 69 HZ events occurring in 65 patients, with the majority of events being nonserious (65, 94%) and involving ≤2 dermatomes (48, 74%).51 Cases were adjudicated and considered “multidermatomal” if they involved 2 nonadjacent or 3–6 adjacent dermatomes or “disseminated” if they involved ≥7 dermatomes or visceral disease. There were 18 events (in 17 patients) assessed by the adjudication committee to be multidermatomal (12 events in 11 patients) or disseminated (6 events in 6 patients). Of the disseminated cases, 3 were diffuse cutaneous rashes, 2 involved ocular and skin disease, and 1 was an invasive case of HZ meningitis. Three (4.6%) patients developed PHN. In 65 patients with HZ, 60 (92.3%) continued the study, 5 (7.7%) discontinued the study, and 16 (24.6%) had their tofacitinib dose either reduced or temporarily withheld. The majority of patients with HZ were treated with antiviral therapy (89.2%). Similar to patients with RA, there seemed to be a dose-dependent risk of HZ in UC patients treated with tofacitinib, but duration of treatment was not an influencing factor. Importantly, the majority of HZ events were noncomplicated and mild to moderate in severity. Risk factors for HZ in patients with UC included increasing age and prior TNFi failure, similar to those demonstrated in RA and PsO. Although not significant within the multivariate model, Asian patients also had a higher HZ risk.51
Being from Japan or Korea has been identified as an independent risk factor for HZ when receiving tofacitinib. This could be due to a genetic predisposition toward HZ, which is amplified under JAK inhibition in specific populations. A recent genetic analysis of 5246 patients treated with tofacitinib identified multiple loci associated with an increased risk of HZ, one of which was significantly more prevalent in Japanese populations.58 Irrespective of the mechanism behind these geographic differences, this underlying risk should be considered in all studies of JAK inhibitors, as HZ rates may be intrinsically higher than in trials of other treatment due to the different compositions of patient populations.
PREVENTION OF HZ
Guidelines for the Prevention of HZ
The live zoster vaccine (LZV) Zostavax (Merck Sharp & Dohme Limited; Hertfordshire, UK) was approved by the US Food and Drug Administration (FDA) in May 2006, and was initially recommended in individuals ≥60 years of age who were not immunosuppressed.59 As this is a live, attenuated vaccine, there is a risk of disseminated disease in those individuals who are immunocompromised or lack VZV-specific immunity.60 Current guidelines from the US Advisory Committee on Immunization Practices (ACIP) recommend that the LZV be offered to all immunocompetent adults ≥60 years of age.61 After the FDA approved the use of the LZV for adults ≥50 years of age in 2011, the ACIP reviewed their recommendations62 but did not lower the recommended age for vaccination in the general population. The LZV has been associated with a lower IR of HZ (6.7; 95% CI, 5.7–7.9) in patients with immune-mediated disorders (including RA, PsA, and IBD) than unvaccinated patients (11.6; 95% CI, 11.4–11.9) over a median follow-up of 2 years.63 HZ vaccination was associated with a hazard ratio of 0.61 (95% CI, 0.52–0.71) for HZ risk after 42 days.63
In patients with RA, vaccination for patients ≥50 years of age is recommended by the American College of Rheumatology (ACR) before treatment with biologic therapy or tofacitinib.64 Guidance for patients with IBD can vary according to source. Recent American College of Gastroenterology clinical guidelines for IBD recommend that patients ≥50 years of age, including those who are immunosuppressed, should consider vaccination against HZ.34 Individuals with UC not only have an increased risk of HZ that can be subsequently amplified by immunosuppressive therapy but also tend to be younger than patients with RA; the greatest hazard ratio for HZ is observed among patients 40–59 years of age with UC.65 Therefore, patients with UC may fall below the currently approved age of HZ vaccination; ACIP and ACR guidelines recommend vaccination in those aged ≥60 and ≥50 years, respectively. The European Crohn’s and Colitis Organisation has highlighted the need for more safety and efficacy data before making specific recommendations for IBD patients receiving immunosuppressive therapy.66 However, there is growing support for patients with IBD to be vaccinated against a range of illnesses (including HZ) regardless of age, especially those who are immunosuppressed.67 Although the vaccination rate for HZ among adults ≥60 years of age in the United States increased from 27.9% in 2014 to 30.6% in 2015,5 self-reported vaccination uptake rates for HZ in patients with IBD are extremely low (5.3% of 300 patients in Canada), potentially due to confusion over conflicting guidelines.6
The use of immunosuppresive therapy in patients with UC complicates the decision-making process of when to vaccinate patients. The Centers for Disease Control and Prevention (CDC) suggest that vaccination is safe for those patients receiving low-dose immunosuppression (<20 mg/d of prednisone or equivalent),61 although other studies have shown a dimimished antibody response to the LZV in patients on low-dose immunosuppressive therapy.68 HZ vaccination in patients with IBD taking anti-TNF medication has now been shown to be relatively safe.69 Due to the potential for prolonged viremia, a period of time between vaccination and immunosupression could theoretically decrease the risk of reactivation. Various recommendations for minimum time periods between vaccination and immunosuppression have been made, ranging from 3–4 weeks before commencement of immunosuppressive therapy66,70 to vaccination 1–6 months after discontinuation of immunosuppressive therapy.61 Such time periods are suggested to allow an adequate immune response after vaccination, before the individual is immunosuppressed. Current tofacitinib labeling states that live vaccines should not be taken while receiving tofacitinib71; the LZV vaccination should therefore be considered before treatment initiation, if feasible.
Results from influenza and pneumococcal vaccine studies in patients with RA provide evidence that tofacitinib treatment (short- or long-term) has no major effect on B-cell and T-cell function, which is required for humoral immune responses to vaccination.72,73 Recent data have been published from the RA LZV study, in which the objective was to assess immunologic response to the LZV in an RA population receiving tofacitinib. In total, 112 patients started tofacitinib 5 mg BID treatment 2–3 weeks after the LZV.72,73 Six weeks postvaccination, the geometric fold rise (GMFR) from baseline in VZV-specific immunoglobulin G levels was 2.11 (80% CI, 1.87–2.37) and 1.74 (80% CI, 1.55–1.95) for patients receiving tofacitinib and placebo, respectively. VZV-specific T-cell GMFR increased for tofacitinib (1.50; 80% CI, 1.31–1.70) similarly to placebo-treated patients (1.29; 80% CI, 1.14–1.46).74 This suggests that adequate immune response to LZV is observed for patients subsequently receiving tofacitinib. The vaccination seemed safe in all but 1 patient, who lacked preexisting VZV immunity. This patient developed cutaneous vaccine dissemination, which resolved upon discontinuation of tofacitinib and commencing antiviral treatment. Other studies of VZV in healthy individuals75,76 have reported comparable immune responses to those patients with RA receiving tofacitinib 5 mg BID or placebo.74 However, these studies examined a different patient population (those with RA instead of UC) and evaluated a lower tofacitinib dose (5 mg BID vs 10 mg BID).
A nonlive vaccine for HZ, Shingrix (herpes zoster subunit vaccine), has recently been approved in the United States and Canada,77,78 after promising phase III trial results from ~39,000 people across 18 countries.79,80 Efficacy against HZ was >90% for the general population, sustained over the duration of the trial (4 years), and seemed to be independent of age (97.2%80 and 91.3%79 efficacy observed for patients ≥50 and ≥70 years of age, respectively). Phase III trials in other patient populations, including those with renal transplant (NCT02058589) and solid tumors (NCT01798056), have been recently completed; results are yet to be published. Use of a nonlive vaccine would enable a more accessible vaccination against HZ, as this could be administered to a range of patient populations receiving different treatment types and does not pose the risk of contracting HZ from the vaccine. Results from a revaccination study of patients ≥50 years of age have recently been presented at the US CDC Advisory ACIP meeting.81 Patients who received LZV at least 5 years before the HZ subunit vaccine had similar responses to those patients without prior LZV exposure. These data demonstrated that the incidence of HZ was reduced after vaccination with the HZ subunit vaccine, as was the overall incidence of PHN. A potential limitation of this newly developed vaccine is that safety in patients with autoimmune diseases is currently unknown, including the risk of HZ reactivation. Moving forward, these data suggest that patients of any age with IBD could be safely vaccinated against HZ; further research is required to confirm this.
Management of HZ in Patients With UC
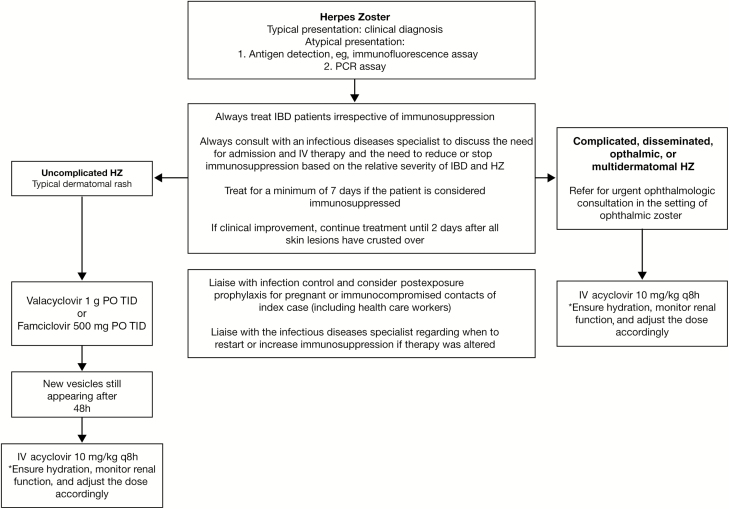
Treatment options for HZ events in patients with IBD vary depending upon clinical presentation (Fig. 2). The recommended treatment for noncomplicated HZ is valacyclovir (1 g orally) or famciclovir (500 mg orally), 3 times daily for 7–14 days.2 Treatment for complicated HZ is intravenous (IV) acyclovir (10 mg/kg adjusted for renal function, every 8 hours for 7–14 days) or foscarnet (40 mg/kg IV, every 8 hours until lesions have healed) for acyclovir-resistant HZ.2 IV acyclovir is the preferred treatment in IBD patients with disseminated disease or visceral involvement.2 Treatment for HZ should be prescribed within 72 hours of rash onset and should continue for a minimum of 7–10 days.1
FIGURE 2.
Management of herpes zoster in patients with IBD receiving immunosuppressant treatment. Abbreviations: PCR, polymerase chain reaction; PO, orally; q8h, every 8 hours; TID, 3 times a day.
Management of HZ in Patients Receiving Tofacitinib
HZ events observed after treatment with tofacitinib across all indications tend to be noncomplicated and can typically be managed without permanent discontinuation of therapy. Recommended management for HZ events in patients with tofacitinib aligns with current guidelines for patients with UC (Fig. 2). During clinical trials of tofacitinib in patients with RA, antiviral therapy was provided to 89.6% of patients during exposure to tofacitinib.44 In this same cohort, 43.1% of patients with an event of HZ continued tofacitinib treatment, 42.0% stopped tofacitinib temporarily, 0.8% reduced their tofacitinib dose, 8.0% permanently discontinued tofacitinib, and the remaining 6.1% did not report treatment changes.44 Similar trends were noted for those patients with PsO receiving tofacitinib. Antiviral treatment was given to 80.0% of patients, and the majority (93.0%) continued or resumed tofacitinib use after HZ resolved.56
As reported in parallel with this review,51 HZ events in the OCTAVE clinical program in patients with UC were generally nonserious, and the majority of cases did not result in permanent discontinuation of the study. There were 65 patients with an event of HZ, most of whom were able to either continue tofacitinib treatment (n = 44) or temporarily stop/reduce their tofacitinib dose (n = 16). Only 5 patients discontinued the study.51 Most importantly, across all indications, HZ events were generally noncomplicated and manageable with standard antiviral treatment, and the majority of patients were able to continue or resume tofacitinib treatment.
CONCLUSION
Patients with UC have an increased risk of HZ compared with the general population, and this risk can be increased by the use of immunosuppressive therapy. JAK inhibitors, including tofacitinib, have been associated with HZ risk in patients across a number of indications. The role that JAK inhibitors have upon key inflammatory cytokines and the regulation of host defense and immune response could mechanistically increase the risk of HZ. There is also the potential for JAK inhibitors to have a differential role in HZ risk, depending upon the specific JAK complex targeted. Before comparisons can be made between JAK inhibitors, further information regarding HZ incidence and clinical presentation is required.
Although a dose-dependent risk for HZ has been observed for tofacitinib, the majority of HZ cases reported are noncomplicated, mild to moderate in severity, and manageable with standard antiviral therapy. Most importantly, tofacitinib may be safely continued after an event of HZ. The majority of patients in the tofacitinib clinical programs (including those with UC) were able to continue tofacitinib treatment during their HZ event, with a small number of patients temporarily stopping tofacitinib treatment and resuming after treatment for HZ.
To reduce the risk of HZ in patients receiving JAK inhibitors, vaccination before treatment initiation for all patients should be considered. The use of tofacitinib after LZV in the RA setting provided similar vaccine immune responses to those found in patients not receiving tofacitinib. Vaccination of patients with UC should be considered, regardless of age, to reduce the risk of HZ before immunosuppressive therapy, such as tofacitinib.
ACKNOWLEDGMENTS
The author would like to thank Amy Marren, Leonardo Salese, Chinyu Su, and Hernan Valdez of Pfizer Inc for their review of this manuscript. Medical writing support under the guidance of the author was provided by Kristina Harrison, PhD, at CMC Connect, a division of Complete Medical Communications Ltd, Macclesfield, UK and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Conflicts of interest: J.F.C. has been a consultant or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Pfizer, Protagonist, Second Genome, Seres, Shire, Takeda, Tigenix, and Theradiag; has been a speaker for AbbVie, Ferring, and Takeda; has been part of the speakers’ bureau for Amgen; has stock options with Intestinal Biotech Development, Genefit; and has received research grants from AbbVie, Takeda, and Janssen.
Supported by: Pfizer Inc.
REFERENCES
- 1. Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Côté-Daigneault J, Peerani F, MacMahon E, et al. Management and prevention of herpes zoster in the immunocompromised inflammatory bowel disease patient: a clinical quandary. Inflamm Bowel Dis. 2016;22:2538–2547. [DOI] [PubMed] [Google Scholar]
- 3. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster?Lancet Infect Dis. 2004;4:26–33. [DOI] [PubMed] [Google Scholar]
- 5. Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ. 2017;66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439–444. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;225:92–99. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.e1. [DOI] [PubMed] [Google Scholar]
- 10. Danese S, Grisham M, Hodge J, et al. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155–G162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Vries LCS, Wildenberg ME, De Jonge WJ, et al. The future of janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2017;11:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet. 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 13. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Shea JJ. Targeting the Jak/STAT pathway for immunosuppression. Ann Rheum Dis. 2004;63:ii67–ii71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharfe N, Dadi HK, O’Shea JJ, et al. Jak3 activation in human lymphocyte precursor cells. Clin Exp Immunol. 1997;108:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hambleton S, Gershon AA. Preventing varicella-zoster disease. Clin Microbiol Rev. 2005;18:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784–4790. [DOI] [PubMed] [Google Scholar]
- 19. Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNSS Drugs. 2015;29:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berger R, Florent G, Just M. Decrease of the lymphoproliferative response to varicella-zoster virus antigen in the aged. Infect Immun. 1981;32:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salam N, Rane S, Das R, et al. T cell ageing: effects of age on development, survival & function. Indian J Med Res. 2013;138:595–608. [PMC free article] [PubMed] [Google Scholar]
- 22. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234–243. [DOI] [PubMed] [Google Scholar]
- 23. Abendroth A, Arvin AM. Immune evasion as a pathogenic mechanism of varicella zoster virus. Semin Immunol. 2001;13:27–39. [DOI] [PubMed] [Google Scholar]
- 24. Ku CC, Zerboni L, Ito H, et al. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200:917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furie R, Khamashta M, Merrill JT, et al. ; CD1013 Study Investigators Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 2017;69:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khamashta M, Merrill JT, Werth VP, et al. ; CD1067 study investigators Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–357. [DOI] [PubMed] [Google Scholar]
- 28. Moodley D, Yoshida H, Mostafavi S, et al. Network pharmacology of JAK inhibitors. Proc Natl Acad Sci U S A. 2016;113:9852–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valenzuela F, Papp KA, Pariser D, et al. Effects of tofacitinib on lymphocyte sub-populations, CMV and EBV viral load in patients with plaque psoriasis. BMC Dermatol. 2015;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winthrop KL, Park SH, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marra F, Lo E, Kalashnikov V, et al. Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3:ofw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. [DOI] [PubMed] [Google Scholar]
- 33. Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–1490. [DOI] [PubMed] [Google Scholar]
- 34. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. [DOI] [PubMed] [Google Scholar]
- 35. Long MD, Martin C, Sandler RS, et al. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524–2533. [DOI] [PubMed] [Google Scholar]
- 37. Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–744. [DOI] [PubMed] [Google Scholar]
- 39. García-Doval I, Pérez-Zafrilla B, Descalzo MA, et al. ; BIOBADASER 2.0 Study Group Incidence and risk of hospitalisation due to shingles and chickenpox in patients with rheumatic diseases treated with TNF antagonists. Ann Rheum Dis. 2010;69:1751–1755. [DOI] [PubMed] [Google Scholar]
- 40. Serac G, Tubach F, Mariette X, et al. Risk of herpes zoster in patients receiving anti-TNF-α in the prospective French RATIO registry. J Invest Dermatol. 2012;132:726–729. [DOI] [PubMed] [Google Scholar]
- 41. Buccoliero G, Lonero G, Romanelli C, et al. Varicella zoster virus encephalitis during treatment with anti-tumor necrosis factor-alpha agent in a psoriatic arthritis patient. New Microbiol. 2010;33:271–274. [PubMed] [Google Scholar]
- 42. Elwir SM, Shaffer CC, Arvan SW, et al. Disseminated varicella zoster virus infection with encephalitis in a UC patient receiving infliximab. Gastroenterol Hepatol (N Y). 2013;9:54–56. [PMC free article] [PubMed] [Google Scholar]
- 43. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. [DOI] [PubMed] [Google Scholar]
- 44. Winthrop KL, Curtis JR, Lindsey S, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 2017;69:1960–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Genovese MC, van Vollenhoven RF, Pacheco-Tena C, et al. VX-509 (decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 2016;68:46–55. [DOI] [PubMed] [Google Scholar]
- 47. Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis. 2017;76:1009–1019. [DOI] [PubMed] [Google Scholar]
- 48. Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68:2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fleischmann R, Mysler E, Hall S, et al. ; ORAL Strategy investigators Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–468. [DOI] [PubMed] [Google Scholar]
- 51. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sandborn WJ, Feagan BG, Panes J, et al. Safety and efficacy of ABT-494 (Upadacitinib), an oral Jak1 inhibitor, as induction therapy in patients with Crohn’s disease: results from Celest. Gastroenterol. 2017;152:1308. [Google Scholar]
- 53. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. [DOI] [PubMed] [Google Scholar]
- 54. Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Friesen KJ, Chateau D, Falk J, et al. Cost of shingles: population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis. 2017;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Winthrop KL, Lebwohl M, Cohen AD, et al. Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol. 2017;77:302–309. [DOI] [PubMed] [Google Scholar]
- 57. Javed S, Kamili QU, Mendoza N, et al. Possible association of lower rate of postherpetic neuralgia in patients on anti-tumor necrosis factor-α. J Med Virol. 2011;83:2051–2055. [DOI] [PubMed] [Google Scholar]
- 58. Bing N, Zhou H, Zhang B, et al. Genome-wide trans-ancestry meta-analysis of herpes zoster in RA and Pso patients treated with tofacitinib. Arthritis Rheumatol. 2015;67:Abstract566. [Google Scholar]
- 59. US Food and Drug Administration. Zostavax FDA approval. October 10, 2017. https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm136941.htm. Accessed May 02, 2018. [Google Scholar]
- 60. Electronic Medicines Compendium (eMC). Zostavax SmPC. August 18, 2017. https://www.medicines.org.uk/emc/product/6101. Accessed May 02, 2018. [Google Scholar]
- 61. Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57:1–30; quiz CE2. [PubMed] [Google Scholar]
- 62. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729–731. [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 65. Card T, Hubbard R, Logan RF. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 2003;125:1583–1590. [DOI] [PubMed] [Google Scholar]
- 66. Rahier JF, Magro F, Abreu C, et al. ; European Crohn’s and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. [DOI] [PubMed] [Google Scholar]
- 67. Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2016;12:540–546. [PMC free article] [PubMed] [Google Scholar]
- 68. Wasan SK, Zullow S, Berg A, et al. Herpes zoster vaccine response in inflammatory bowel disease patients on low-dose immunosuppression. Inflamm Bowel Dis. 2016;22:1391–1396. [DOI] [PubMed] [Google Scholar]
- 69. Khan N, Shah Y, Trivedi C, et al. Safety of herpes zoster vaccination among inflammatory bowel disease patients being treated with anti-TNF medications. Aliment Pharmacol Ther. 2017;46:668–672. [DOI] [PubMed] [Google Scholar]
- 70. Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–318. [DOI] [PubMed] [Google Scholar]
- 71. Pfizer Inc. XELJANZ summary of product characteristics. March 22, 2017. https://www.medicines.org.uk/emc/product/2500. Accessed May 02, 2018. [Google Scholar]
- 72. Winthrop KL, Neal J, Hrycaj P, et al. Evaluation of influenza and pneumococcal vaccine responses in rheumatoid arthritis patients using tofacitinib. Ann Rheum Dis. 2013;72:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol. 2017;69:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Levin MJ, Oxman MN, Zhang JH, et al. ; Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Levin MJ, Schmader KE, Gnann JW, et al. Varicella-zoster virus-specific antibody responses in 50-59-year-old recipients of zoster vaccine. J Infect Dis. 2013;208:1386–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. US Food and Drug Administration. SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) suspension for intramuscular injection, highlights of prescribing information. July 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdf. Accessed November 6, 2017. [Google Scholar]
- 78. Health Canada. SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) suspension for injection. October 2017. http://ca.gsk.com/media/1350785/patient- information-english.pdf. Accessed November 6, 2017. [Google Scholar]
- 79. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. [DOI] [PubMed] [Google Scholar]
- 80. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. [DOI] [PubMed] [Google Scholar]
- 81. GlaxoSmithKline. GSK presents positive results from phase III revaccination study of its candidate shingles vaccine Shingrix at CDC’s Advisory Meeting. June 21, 2017. http://www.gsk.com/en-gb/media/press-releases/gsk-presents-positive-results-from-phase-iii-revaccination-study-of-its-candidate-shingles-vaccine-shingrix-at-cdc-s-advisory-meeting/. Accessed October 17, 2017. [Google Scholar]